Abstract
Hospitalizations for acute decompensated heart failure are increasing in the United States. Moreover, the prevalence of heart failure is increasing consequent to an increased number of older individuals, as well as to improvement in therapies for coronary artery disease and sudden cardiac death that have enabled patients to live longer with cardiovascular disease. The main treatment goals in the hospitalized patient with heart failure are to restore euvolemia and to minimize adverse events. Common in-hospital treatments include intravenous diuretics, vasodilators, and inotropic agents. Novel pharmaceutical agents have shown promise in the treatment of acute decompensated heart failure and may simplify the treatment and reduce the morbidity associated with the disease. This review summarizes the contemporary management of patients with acute decompensated heart failure.
Key words: Acute disease; aged; cardiac output, low; disease progression; diuretics; furosemide; heart failure/classification/drug therapy/mortality; hospitalization; length of stay; milrinone; morbidity/trends; relaxin; tolvaptan; ultrafiltration; United States/epidemiology; vasodilator agents; ventricular dysfunction, left
Heart failure (HF) is a growing problem worldwide: more than 20 million people around the world are affected, and more than 5 million in the United States.1 The prevalence of HF follows an exponential pattern, and it rises with age. Heart failure affects 6% to 10% of people over the age of 65 years. Although the relative incidence is lower in women than in men, women constitute at least half of the cases of HF because of their longer life expectancy. In the U.S., the treatment of HF has a direct cost of over $34 billion per year, most of which results from hospitalization.1 There are over 1 million hospitalizations with a primary diagnosis of HF each year in the U.S., and HF is the most common diagnosis for hospital admissions in patients above 65 years of age.2 Furthermore, hospitalization for acute decompensated heart failure (ADHF) is a powerful predictor of readmission and post-discharge death in patients with chronic HF, with mortality rates as high as 20% after discharge.3,4 The incidence of HF hospitalizations has tripled over the last 3 decades, likely consequential to the aging population, improved survival after myocardial infarction, and prolonged survival of HF patients with current medical and device therapies.1 In this review, we focus on the contemporary management of the patient with ADHF and discuss future directions in the field.
Definition
Acute decompensated heart failure can be defined as the sudden or gradual onset of the signs or symptoms of heart failure requiring unplanned office visits, emergency room visits, or hospitalization. Regardless of the underlying precipitant of the exacerbation, pulmonary and systemic congestion due to increased left- and right-heart filling pressures is a nearly universal finding in ADHF.5
Characteristics of Patients
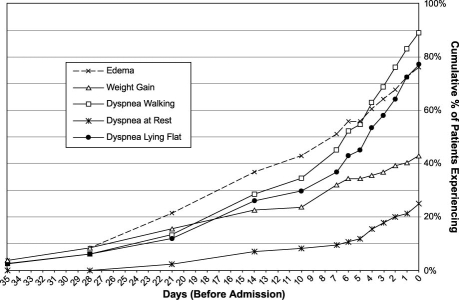
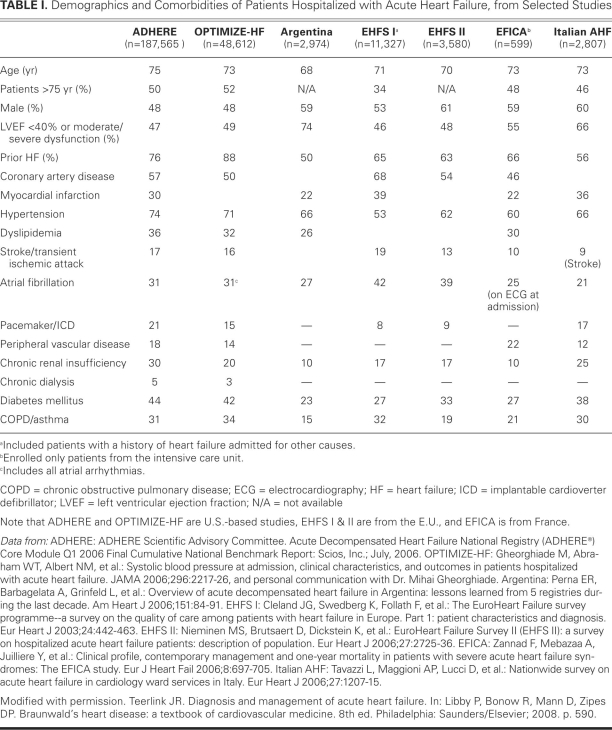
Most patients admitted to the hospital with ADHF have a worsening of chronic HF, although 15% to 20% of ADHF hospitalizations represent new diagnoses of HF. In the U.S., the age of hospitalized patients is generally 70 to 75 years old, with both sexes equally represented. Approximately half of hospitalized HF patients have moderately to severely reduced left ventricular (LV) systolic function, with an ejection fraction (LVEF) of <0.40. Patients with a new diagnosis of HF are much more likely to present with pulmonary edema or cardiogenic shock, while decompensation of chronic HF usually presents with other signs of congestion and fluid retention, such as weight gain, exertional dyspnea, or orthopnea. These symptoms can begin days or weeks before presentation (Fig. 1).6 Patients with ADHF have a remarkably high prevalence of atrial fibrillation or flutter (30%–46%), valvular disease (44%), and dilated cardiomyopathy (25%), consistent with the chronic nature of their underlying HF.7 A history of coronary artery disease (CAD) is present in 60% of patients, hypertension in 70%, diabetes in 40%, and renal impairment in 20% to 30%. At presentation, most HF patients are relatively normotensive. Approximately 25% of patients are hypertensive (systolic blood pressure, >160 mmHg), and fewer than 10% are hypotensive.8–10 Patients admitted with ADHF having a relatively preserved LVEF tend to be older, female, and more likely to present with severe hypertension.11 Table I summarizes the presenting comorbidities and other characteristics of patients hospitalized with acute HF.
Fig. 1. Number of days from onset of worsening of selected symptoms of heart failure to hospital admission in 83 patients admitted with heart failure. Most symptoms were present 1 week before admission, which suggests that earlier outpatient intervention might reduce hospitalizations.
Data from: Schiff GD, Fung S, Speroff T, McNutt RA. Decompensated heart failure: symptoms, patterns of onset, and contributing factors. Am J Med 2003;114(8):625–30. (Reproduced with permission.)
TABLE I. Demographics and Comorbidities of Patients Hospitalized with Acute Heart Failure, from Selected Studies
Precipitants for Heart-Failure Decompensation
Specific factors that precipitate HF hospitalization can often be identified, although studies suggest that up to 40% or 50% of ADHF episodes have no known cause.12 It is imperative that these precipitants, when identified, be defined and treated and that effective interventions be developed to prevent recurrence. The most common precipitants for HF hospitalization are noncompliance with medications or dietary restrictions, uncontrolled hypertension, ischemia, arrhythmias, and exacerbation of chronic obstructive pulmonary disease with or without pneumonia.13 Other contributors include noncardiac conditions such as renal dysfunction, diabetes mellitus, anemia, and the side effects of medications (nonsteroidal anti-inflammatory drugs, calcium-channel blockers, and thiazolidinediones).14
Clinical Classification
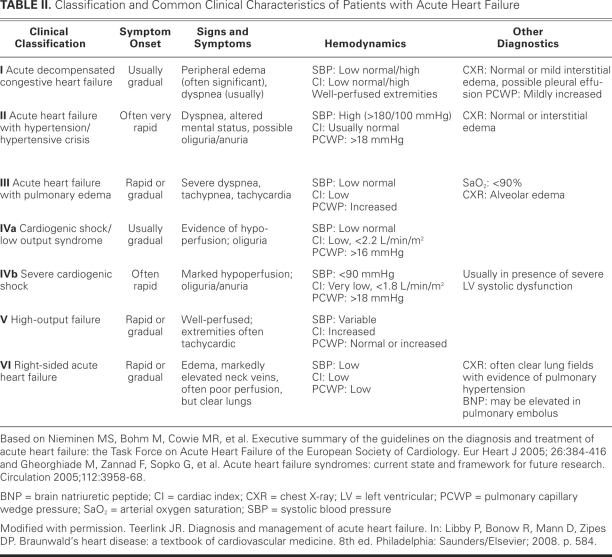
Several classification schemes have been developed for acute HF. Patients are generally divided into those who present with HF for the 1st time (de novo) and those whose chronic HF worsens. Of the approximately 80% of ADHF patients with worsening of chronic HF, less than 10% have advanced HF. The characteristics of advanced HF include low blood pressure, renal impairment, and signs or symptoms of HF that are refractory to standard therapy. The European Society of Cardiology guidelines for the diagnosis and treatment of acute HF classifies patients into 1 of 6 groups on the basis of typical clinical and hemodynamic characteristics15 (Table II), based on the work of Cotter,16 Gheorghiade, and colleagues. The first 3 categories of patients (those with ADHF, hypertensive acute heart failure [AHF], and AHF with pulmonary edema) comprise over 90% of AHF presentations. The patient with ADHF typically presents with mild-to-moderate signs and symptoms of congestion and does not meet the criteria for other categories. Hypertensive AHF patients are characterized by their relatively preserved LV systolic function (LVEF, >0.40), elevated blood pressure, and pulmonary edema. The 3rd group, patients who have AHF with pulmonary edema, has a clinical presentation that is dominated by severe respiratory distress, orthopnea, signs of pulmonary edema (verified by rales on physical examination and chest radiography), and hypoxemia (the oxygen saturation is usually <90% on room air). Patients with low-output syndrome have evidence of tissue hypoperfusion due to HF and display a continuum of severity ranging from a low-output state to cardiogenic shock. High-output failure remains an uncommon cause of AHF and generally presents with warm extremities, pulmonary congestion, and at times low blood pressure (that is, sepsis) with high cardiac output and usually an elevated heart rate. Underlying conditions associated with this type of ADHF include anemia, thyrotoxicosis, and Paget's disease. Right-sidedAHF occurs most commonly in patients with underlying lung disease, such as those who have chronic obstructive pulmonary disease and develop cor pulmonale, or those who have pulmonary hypertension for other reasons, including left-heart failure. Right-sided AHF patients generally present with increased jugular venous pressure, hepatomegaly, edema, low-output syndrome, and hypotension.
TABLE II. Classification and Common Clinical Characteristics of Patients with Acute Heart Failure
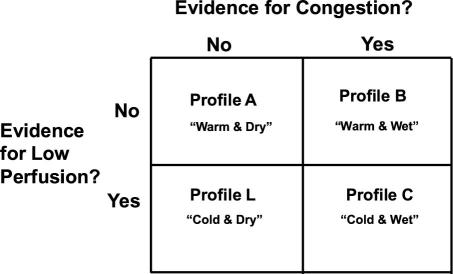
Another clinically relevant and widely used system for classifying ADHF was developed by Stevenson and colleagues.17 In contrast with the European Society of Cardiology system, this system focuses more on the severity of disease at presentation than on the cause of HF. It classifies patients on the basis of the clinical presence or absence of hypoperfusion (cold vs warm) and of congestion at rest (wet vs dry) (Fig. 2). Patients with clinical profile A (warm and dry) had a 6-month mortality rate of 11%, compared with 40% for profile C (cold and wet), which shows that these clinical profiles can have prognostic significance.
Fig. 2. Hemodynamic profiles of patients presenting with advanced heart failure, as described by a 2 × 2 table. Evaluation of clinical symptoms and signs enables the classification of a patient into a hemodynamic profile and may assist in selecting initial therapy and providing prognostic information. Although this classification scheme was developed for patients who have, predominantly, systolic dysfunction and advanced heart failure, it provides a useful construct for the evaluation of patients with ADHF as well.
Modified from: Nohria A, Mielniczuk LM, Stevenson LW. Evaluation and monitoring of patients with acute heart failure syndromes. Am J Cardiol 2005;96(6A):32G–40G.
Treatment
Hospitalization
Certain clinical presentations warrant hospitalization for patients with ADHF. Any patient with ADHF who has hypotension, worsening renal function, or altered mental status should be considered high risk and hospitalized. In addition, ADHF patients who present with dyspnea, tachypnea, or hypoxemia (again, oxygen saturation of <90%) at rest, or with any hemodynamically significant arrhythmia, including atrial fibrillation with rapid ventricular response, warrant hospital admission—as does any patient who presents with evidence of an acute coronary syndrome.18 Hospitalization should also be considered for HF patients with any of the following: severe weight gain, defined as >5 kg; signs and symptoms of pulmonary or systemic congestion; major electrolyte disturbances; repeated implantable cardioverter-defibrillator firings; or pneumonia. Furthermore, the clinician should be aware that a patient with ADHF has a poor systemic reserve for coping with other medical conditions. Disease processes that in the normal population could be treated adequately in the outpatient setting might require hospitalization in the ADHF patient.
Inpatient Monitoring
Hospitalized HF patients are at risk for hemodynamic instability and arrhythmias; therefore, close monitoring is necessary. Telemetry should be initiated and continued for at least 24 to 48 hours after admission. Vital signs should be monitored more than once daily, including measurement of orthostatic blood pressure and oxygen saturation. Patients should undergo at least daily monitoring of weight, fluid balance, electrolyte levels, serum creatinine levels, and signs and symptoms of congestion. Other serum tests, such as those for brain-type natriuretic peptide (BNP), liver function, D-dimer, international normalized ratio, and complete blood count, are recommended if clinically indicated.
Diuresis
Although acute pulmonary edema can develop without significant volume overload (for example, in cases of acute myocardial infarction, acute mitral or aortic insufficiency, or acute fulminant myocarditis), patients with ADHF usually display volume overload. In either situation, acute pulmonary edema is present and fluid removal is important to relieve the symptoms of HF and to improve oxygenation. In patients whose fluid overload is significant, diuretic therapy should be initiated without delay, because early intervention in this regard has been associated with better outcomes for patients hospitalized with ADHF.19,20 By reducing intravascular volume, diuresis lowers central venous and pulmonary capillary wedge pressures, which decreases pulmonary edema and often results in augmented forward-stroke volume and cardiac output. Other benefits include reductions in tricuspid and mitral regurgitation, consequent to decreases in filling volumes in the right and left ventricles.21 Fortunately, effective diuresis is straightforward in most patients, through the use of conventional diuretic agents.
In patients who are refractory to standard therapy, however, fluid management can be difficult and indicative of a more complicated course. Aggressive diuresis has diverse consequences, including electrolyte disturbances and consequent arrhythmias, intravascular depletion, hypotension, and renal dysfunction. Worsening renal function makes further diuresis more difficult and worsens the prognosis in HF patients.22 Diuretic therapy has also been shown to precipitate activation of neurohormones and the renin–angiotensin–aldosterone system, which is thought to be deleterious in cases of HF. Given the multiple side effects of diuretic therapy, it is not surprising that higher doses of diuretics have been associated with worse outcomes.23 However, patients who need higher doses of diuretics are generally the sickest, which makes a causal relationship difficult to establish. Although the safety and efficacy of diuretics have not been established in randomized, controlled trials, extensive observational experience has shown their efficacy in relieving congestive symptoms, which makes them a mainstay of therapy for the hospitalized patient with ADHF.
Loop Diuretic Agents. Loop diuretic agents are considered 1st-line diuretic therapy because they are effective and generally well tolerated. Even very high doses can be given safely without the induction of ototoxicity, which is a very rare complication of diuresis.24 In the patient who is hospitalized with ADHF, intravenous rather than oral administration is recommended, because of greater and more consistent bioavailability of the drug. Diuretic dosing should be individualized, although common initial doses of loop diuretic agents in patients with normal renal function include furosemide (40 mg, intravenously) bumetanide (1 mg, intravenously), or torsemide (10 to 20 mg, intravenously). Patients who chronically take diuretics usually need a higher dose in the acute setting. The initial intravenous dose should be at least the equivalent in milligrams to the maintenance oral dose. Peak diuresis generally occurs 30 to 60 minutes after administration, although intravenous loop diuretics also have an initial venodilating effect similar to that of morphine, which leads to decreased pulmonary congestion before the onset of diuresis.25 Furosemide is the most widely used diuretic agent in patients with HF. Interestingly, there are recent animal data to suggest that torsemide, but not furosemide, can block the aldosterone cascade—which leads to decreased fibrotic remodeling in the myocyte.26,27 There is also a suggestion that torsemide might have different sympathetic system activities than furosemide.28 In a nonrandomized clinical study, outcomes with torsemide were better than those with furosemide.29 The conclusions from these studies must be applied in clinical practice with some circumspection, however, because animal studies do not necessarily predict clinical diuretic effects; and the human study was only an observational analysis. Moreover, even if there are differences among agents when they are used in isolation, the concomitant use of angiotensin-converting enzyme (ACE) inhibitors and β-blockers might alter both the efficacy and toxicity of diuretics in individual patients. Until there is definitive proof of the superiority of an alternative, furosemide will clearly continue to be a necessary diuretic agent for patients with HF, due to its established use and relatively low price.
Other Diuretic Agents. Thiazide diuretic agents are less potent than are loop diuretics when used alone, and they cause more pronounced potassium losses for the same quantity of diuresis.30 Furthermore, they are ineffective in patients with advanced renal insufficiency (that is, in patients with a glomerular filtration rate below 30 mL/min). Spironolactone is a weak diuretic agent that is used in HF due to its inhibition of aldosterone, the beneficial neurohumoral effects of which have been shown to decrease myocardial remodeling and vascular fibrosis,31 thereby reducing the mortality rate among patients with severe HF and low LVEF.32
Inadequate Response to Diuretic Therapy. For patients who have more advanced HF or who respond inadequately to initial loop diuretic therapy, there are several considerations. First, it is important to eliminate the concurrent use of any drugs that adversely affect renal function, particularly nonsteroidal anti-inflammatory drugs. The potential adverse effects of these drugs are frequently underestimated by physicians who treat patients with HF. In patients with severe HF, a single dose of indomethacin has been shown to significantly lower the glomerular filtration rate.33 In general, clinicians should review all of the patient's maintenance medications and decide whether adjustments should be made as a result of the hospitalization. Although it has been shown that continuation of β-blockers for most patients is well tolerated and results in better outcomes,34 upward titration during an acute exacerbation of HF may reduce the efficacy of interventions that relieve congestion. Similarly, in patients admitted with azotemia or acute renal failure, temporary reduction or discontinuation of ACE inhibitors or angiotensin-receptor blockers may be necessary. The next consideration in the refractory patient is to use sufficient doses of diuretic agents, which often need to be aggressively augmented. The loop diuretic dose should be doubled until adequate diuresis occurs or until the maximum recommended dose is reached. The administration of loop diuretics as a continuous intravenous infusion, as opposed to intermittent bolus administration, may also be attempted in refractory patients. There are several possible advantages to this approach. Continuous delivery of the drug to the nephron avoids the compensatory renal sodium reabsorption that occurs when blood levels of the diuretic agent are low. Also, intermittent bolus delivery may lead to marked fluctuations in intravascular volume and to high peak serum levels of the diuretic agents, thereby increasing their toxicity. In a Cochrane review35 of 8 clinical trials involving 254 patients with ADHF randomized to continuous versus bolus loop-diuretic administration, those who received continuous-infusion diuretic administration had increased urine output, compared with patients who received equivalent doses through intermittent bolus administration. There was also less ototoxicity in the continuous-infusion group and no difference in electrolyte disturbance. Another therapeutic approach is the addition of a thiazide diuretic to a loop diuretic, which can potentiate the effects of the loop diuretic. In particular, the combination of metolazone with loop diuretic drugs has been shown to be very effective.36 Unfortunately, chlorothiazide is the only thiazide that can be administered intravenously. It should be noted that combining loop and thiazide diuretic agents has the potential to precipitate severe potassium-wasting, so this requires careful monitoring. Spironolactone can be added to combination diuretic therapy in order to prevent potassium-wasting, but, as indicated above, it is unlikely to enhance diuresis appreciably. Also, sodium and fluid restriction should be enforced, especially in hyponatremic patients. Finally, ultrafiltration for fluid removal may, in some cases, need to be considered (see below).
Vasodilation
After diuretics, intravenous vasodilators are probably the most useful medications for the management of ADHF. By stimulating guanylate cyclase within smooth-muscle cells, vasodilators such as nitroglycerin, nitroprusside, and nesiritide cause both arterial and venous dilation, which results in a lowering of LV filling pressure, improved stroke volume, and improved forward cardiac output, without increasing arrhythmias. When vasodilators are considered, early administration may be better than late. A recent analysis from the Acute Decompensated Heart Failure Registry (ADHERE) of over 35,000 hospitalized HF patients in need of vasoactive agents (vasodilators or inotropic agents) showed that patients who received vasoactive agents within 6 hours of hospital admission had a significantly lower in-hospital mortality rate and shorter lengths of stay than did those who received the drugs later.
Intravenous nitroglycerin is generally begun at 10 to 20 μg/min and is increased in 10-to 20-μg increments until the patient's symptoms are improved or pulmonary capillary wedge pressure is decreased to 16 mmHg without reducing systolic blood pressure below 80 mmHg. The most common side effect of intravenous or oral administration of nitrates is headache, which can be treated with analgesics and often resolves during continued therapy. Hemodynamic tolerance of nitroglycerin, or tachyphylaxis, occurs as early as 1 to 2 hours after initiation of the drug.
Nitroprusside is generally initiated at 10 μg/min and is increased by 10 to 20 μg every 10 to 20 minutes as tolerated, with the same hemodynamic goals as described above. The half-life is approximately 2 minutes, which facilitates early establishment, in the intensive care unit, of an individual patient's optimal level of vasodilation. The major limitation of nitroprusside is possible cyanide toxicity, which manifests itself predominantly in the form of gastrointestinal and central nervous system symptoms. Cyanide is most likely to accumulate in patients who have severely reduced hepatic perfusion and decreased hepatic function consequent to low cardiac output; and it is more likely to develop in patients who receive more than 250 μg/min for over 48 hours. Suspected cyanide toxicity is treated by decreasing or discontinuing the nitroprusside infusion. Like nitroglycerin, long-term (>48-hr) use of nitroprusside is also associated with hemodynamic tolerance.
Nesiritide is a recombinant form of BNP, which is an endogenous peptide secreted primarily from the LV in response to increased wall stress. Nesiritide is given as a loading dose of 2 μg/kg, followed by an infusion of 0.01 to 0.03 mg/kg/min. Nesiritide effectively lowers LV filling pressures and improves symptoms during the treatment of ADHF. Nesiritide is not indicated for diuresis, despite its classification as a natriuretic. However, it appears to potentiate the effect of concurrently administered diuretics in such a manner that the total required diuretic dose may be lower. Recently, there have been concerns about the potential adverse effects of nesiritide on renal function and about increased death among ADHF patients who receive nesiritide. There is an ongoing clinical trial in 7,000 patients (ASCEND-HF) that is evaluating the safety of this agent in patients with ADHF.
Hypotension is the most common side effect of all 3 vasodilating agents. All 3 drugs can cause pulmonary artery vasodilation, which can lead to worsening hypoxia or pulmonary edema in patients who have underlying ventilation–perfusion abnormalities.
Ultrafiltration
Ultrafiltration or other forms of dialysis may be useful for diuretic-refractory patients who have ADHF, and some investigators have advocated its early and more widespread use. Potential advantages of ultrafiltration include adjustable fluid-removal volume and rates, neutral effect on serum electrolytes, and decreased neurohormonal activation. In the RAPID-CHF trial,37 40 patients with ADHF and renal insufficiency (serum creatinine, ≥1.5 mg/dL) were randomly assigned to receive usual care with or without ultrafiltration. The ultrafiltration group had significantly greater fluid removal (4,650 vs 2,838 mL) and weight loss (2.5 vs 1.9 kg) after 24 hours. The largest randomized study to date that evaluated ultrafiltration as a treatment in this patient population is the UNLOAD trial, 38 in which 200 patients hospitalized for ADHF were randomized to ultrafiltration or standard care. The study showed that patients in the ultrafiltration group had both greater fluid loss at 48 hours and fewer HF rehospitalizations at 90 days. The rates of adverse events were similar in the 2 groups. Interestingly, however, there was a trend toward a higher incidence of worsening creatinine levels in patients who received ultrafiltration. There have also been other small trials39,40 that showed no benefit to ultrafiltration over furosemide alone. Therefore, at present, it is reasonable to use ultrafiltration in treating the diuretic-refractory patient, but widespread or pre-emptive use in the patient with ADHF will require more evidence of benefit and safety.
Inotropic Therapy
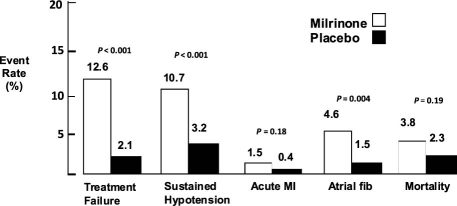
Inotropic agents are frequently used to alleviate the symptoms of severe HF—poor cardiac performance and systemic hypoperfusion. However, inotropic agents are known to have serious adverse effects, including increases in death rate. In the OPTIME-CHF trial,41 949 patients admitted to the hospital with ADHF were randomized to standard medical therapy with the addition of either milrinone or a placebo. Milrinone therapy was associated with a significantly higher incidence of hypotension and atrial arrhythmias and with nonsignificant increases in in-hospital death (Fig. 3). On the basis of these results, inotropic therapy should be reserved for patients who have advanced HF marked by severe LV systolic dysfunction with ventricular dilation and by manifest acute or chronic clinical symptoms due to low cardiac output—such as hypotension, diminished peripheral perfusion, and end-organ dysfunction—or by lack of response to vasodilator or diuretic therapy. The inotropic agents available for use in the United States include dobutamine (a β-adrenergic receptor agonist) and milrinone (a phosphodiesterase inhibitor). Intravenous administration of inotropic drugs is not recommended in HF patients who have preserved systolic function or preserved cardiac output, or in patients who have normal left-heart filling pressures.
Fig. 3. Adverse events in comparison of short-term milrinone infusion with standard medical therapy, from the OPTIME-CHF trial.41
fib = fibrillation; MI = myocardial infarction
Reproduced with permission from: Cuffe MS, Califf RM, Adams KF Jr, Benza R, Bourge R, Colucci WS, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA 2002;287(12):1541–7.41
Emerging Medical Therapies for ADHF
Vasopressin Receptor Antagonists
The importance of vasopressin as the cause of hyponatremia and fluid retention in patients with HF remains a topic of debate. Nevertheless, there are early indications that vasopressin antagonists can be effective aquaretic agents in the treatment of HF, without the neurohormonal activation caused by loop diuretic agents. Antidiuretic hormone antagonists have clearly been shown to reverse hyponatremia and to cause aquaresis acutely.42 Studies with longer follow-up periods, however, suggest that increased water intake and decreased responsiveness to the vasopressin antagonist might limit the chronic benefits of these drugs.
Tolvaptan, a vasopressin-2 antagonist, is the best-studied agent in the setting of HF with hyponatremia. When added to standard medical therapy in patients who have ADHF and reduced LVEF, it has been shown to modestly improve hemodynamics, dyspnea, body weight, and hyponatremia.43,44 Continuation of tolvaptan after hospital discharge, however, had no benefit on mortality or hospital readmission rates, compared with standard medical therapy. Tolvaptan is currently approved for the treatment of hyponatremia by the U.S. Food and Drug Administration (FDA), but not for the treatment of HF.
Conivaptan is a vasopressin-1 and −2 antagonist, also approved by the FDA for the treatment of hyponatremia. Although its hemodynamic profile is similar to that of tolvaptan, it has not been shown to improve signs and symptoms in patients with ADHF.45 On the basis of these results, the role of vasopressin antagonists in the management of ADHF remains to be determined.
Relaxin
Relaxin is a naturally occurring peptide hormone that plays a central role in mediating the hemodynamic and renovascular adaptive changes that occur during normal human pregnancy. The effects of relaxin include release of nitric oxide, inhibition of endothelin and angiotensin II, production of vascular endothelial growth factor, and production of matrix metalloproteinases.46 These effects lead to both systemic and renal vasodilation and increased arterial compliance, both of which are very desirable effects in the treatment of HF. An initial pilot study in human beings has shown favorable effects in patients with HF, including a reduction in ventricular filling pressures and increased cardiac output.47 A larger and more recent placebo-controlled, parallel-group, dose-ranging study of 234 patients with ADHF48 showed that relaxin was associated with relief of dyspnea and with a decrease in the combined endpoint of cardiovascular death or readmission at 60 days. In view of these and other promising initial results, relaxin continues to be investigated as a potential treatment of ADHF.
Adenosine Antagonists
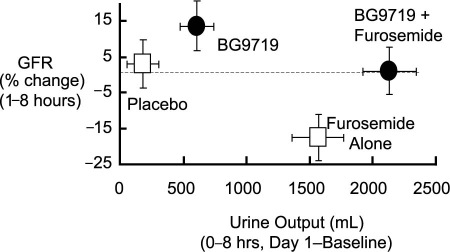
Plasma adenosine concentrations are elevated in chronic-HF patients. It is thought that adenosine contributes to decreased glomerular filtration, possibly as a result of dilation in efferent glomerular vessels49 or vasoconstriction in afferent vessels.50 Adenosine constricts the afferent arteriole via the A1 receptor, leading to decreased glomerular filtration. Adenosine antagonists induce diuresis both by inhibiting sodium absorption in the proximal tubule and by blocking tubuloglomerular feedback, thereby increasing the glomerular filtration rate in HF.51 In an early clinical study, the adenosine A1 antagonist BG9719 caused a dose-dependent increase in urine output. The addition of BG9719 to furosemide caused not only an increase in diuresis, but also prevented the decline in creatinine clearance associated with furosemide (Fig. 4).52 A pilot trial using the selective A1-receptor antagonist rolofylline suggested improvement in symptoms of HF, as well as post-discharge outcomes. However, in the pivotal PROTECT53 study, rolofylline did not improve symptoms of AHF when compared with placebo. Moreover, treatment with rolofylline 30 mg did not reduce the risk of death or cardiovascular or renal rehospitalization, which was the secondary endpoint of the trial. Efforts to develop this drug have been halted.
Fig. 4. Adenosine antagonists are being investigated as diuretics that improve renal function. In 1 study, the adenosine antagonist BG9719 in combination with furosemide increased urine output while preserving the glomerular filtration rate (GFR).52
Reproduced with permission from: Gottlieb SS, Brater DC, Thomas I, Havranek E, Bourge R, Goldman S, et al. BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy [published erratum appears in Circulation 2002;106(13):1743]. Circulation 2002;105(11):1348–53.52
Ularitide
Ularitide is a small natriuretic peptide composed of 32 amino-acid residues originally isolated from human urine. This peptide has been evaluated in early clinical trials.54 It improved hemodynamics as well as HF signs and symptoms, apparently with no worsening of renal function when compared with placebo.55 Important adverse effects included severe hypotension. Further studies are needed to determine the safety and efficacy of ularitide for treatment of ADHF.
Calcium Sensitizers
Calcium sensitizers are a new category of inotropic agents that enhance myocardial contractility by increasing the affinity of troponin C for calcium. Levosimendan is a member of this pharmacologic class that does not increase epinephrine or norepinephrine concentrations and therefore does not cause vasoconstriction, remodeling, or down-regulation of cardiac receptor sensitivity, in contrast with β-agonists such as dobutamine. In the LIDO study,56 levosimendan was superior to dobutamine in reducing pulmonary capillary wedge pressure and death at 6 months. However, in the SURVIVE trial,57 which randomized more than 1,300 patients with ADHF to intravenous dobutamine or levosimendan, there was no difference between the groups with regard to dyspnea or a global evaluation of symptoms. Furthermore, there was no difference between the groups in all-cause death, cardiovascular death, or days out of the hospital at 180 days. Concerns about the safety of levosimendan were raised in the REVIVE-2 trial (unpublished), where there was an increase in hypotension and atrial fibrillation in the treatment arm. Levosimendan is not approved in the United States. but is approved in Europe as a 2nd-line agent for severe low-output HF refractory to standard therapy, or as a 1st-line agent for cardiogenic shock.
Conclusion
The management of ADHF remains challenging, even for skilled clinicians. As noted, proper management generally requires a combination of diuretics, vasodilators, and occasionally inotropic support, to achieve the goal of a euvolemic and adequately perfused patient. Ultrafiltration is emerging as a promising therapy, especially for patients who become diuretic resistant. Proper management requires assiduous laboratory and physiologic monitoring to prevent renal failure, hypotension, and arrhythmias. Unfortunately, there is no regimen for ADHF that succeeds in all cases, but with knowledge of the available approaches and their shortcomings, the clinician is able to provide symptomatic relief to patients in the vast majority of cases. The increasing prevalence of HF in the U.S. population and the consequent higher economic and human burden posed by the disease require the development of new treatments. Current investigations of novel pharmaceutical agents provide promise for simplifying treatment and improving outcomes in patients with HF.
Footnotes
Address for reprints: Douglas L. Mann, MD, Division of Cardiology, Washington University School of Medicine, 660 S. Euclid Ave., Campus Box 8086, St. Louis, MO 63110.
E-mail: dmann@dom.wustl.edu
References
- 1.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol 2008;52(6):428–34. [DOI] [PubMed]
- 2.Nieminen MS, Harjola VP. Definition and epidemiology of acute heart failure syndromes. Am J Cardiol 2005;96(6A): 5G–10G. [DOI] [PubMed]
- 3.Ahmed A, Allman RM, Fonarow GC, Love TE, Zannad F, Dell'italia LJ, et al. Incident heart failure hospitalization and subsequent mortality in chronic heart failure: a propensity-matched study. J Card Fail 2008;14(3):211–8. [DOI] [PMC free article] [PubMed]
- 4.Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007;116(13):1482–7. [DOI] [PubMed]
- 5.Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, et al. Acute heart failure syndromes: current state and framework for future research. Circulation 2005;112(25): 3958–68. [DOI] [PubMed]
- 6.O'Connor CM, Stough WG, Gallup DS, Hasselblad V, Gheorghiade M. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: observations from the IMPACT-HF registry. J Card Fail 2005;11(3):200–5. [DOI] [PubMed]
- 7.Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J 2006;27(22):2725–36. [DOI] [PubMed]
- 8.Fonarow GC, Heywood JT, Heidenreich PA, Lopatin M, Yancy CW; ADHERE Scientific Advisory Committee and Investigators. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2007;153(6): 1021–8. [DOI] [PubMed]
- 9.Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, She L, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA 2006;296(18):2217–26. [DOI] [PubMed]
- 10.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail 2007;13(6):422–30. [DOI] [PubMed]
- 11.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC; ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database [published erratum appears in J Am Coll Cardiol 2006;47(7):1502]. J Am Coll Cardiol 2006;47 (1):76–84. [DOI] [PubMed]
- 12.Opasich C, Rapezzi C, Lucci D, Gorini M, Pozzar F, Zanelli E, et al. Precipitating factors and decision-making processes of short-term worsening heart failure despite “optimal” treatment (from the IN-CHF Registry). Am J Cardiol 2001;88(4):382–7. [DOI] [PubMed]
- 13.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med 2008;168(8):847–54. [DOI] [PubMed]
- 14.Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005;149 (2):209–16. [DOI] [PubMed]
- 15.Nieminen MS, Bohm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J 2005;26(4):384–416. [DOI] [PubMed]
- 16.Cotter G, Moshkovitz Y, Milovanov O, Salah A, Blatt A, Krakover R, et al. Acute heart failure: a novel approach to its pathogenesis and treatment. Eur J Heart Fail 2002;4(3):227–34. [DOI] [PubMed]
- 17.Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA, Mudge GH, Stevenson LW. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 2003;41(10):1797–804. [DOI] [PubMed]
- 18.Heart Failure Society of America. Evaluation and management of patients with acute decompensated heart failure. J Card Fail 2006;12(1):e86–e103. [DOI] [PubMed]
- 19.Peacock WF 4th, Fonarow GC, Emerman CL, Mills RM, Wynne J; ADHERE Scientific Advisory Committee and Investigators; Adhere Study Group. Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE. Cardiology 2007;107(1):44–51. [DOI] [PubMed]
- 20.Maisel AS, Peacock WF, McMullin N, Jessie R, Fonarow GC, Wynne J, Mills RM. Timing of immunoreactive B-type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE (Acute Decompensated Heart Failure National Registry) analysis. J Am Coll Cardiol 2008;52(7):534–40. [DOI] [PubMed]
- 21.Perloff JK, Roberts WC. The mitral apparatus. Functional anatomy of mitral regurgitation. Circulation 1972;46(2):227–39. [DOI] [PubMed]
- 22.Gottlieb SS, Abraham W, Butler J, Forman DE, Loh E, Massie BM, et al. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail 2002;8(3):136–41. [DOI] [PubMed]
- 23.Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O'Connor CM, Califf RM, Adams KF Jr. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail 2007;9 (10):1064–9. [DOI] [PMC free article] [PubMed]
- 24.Howard PA, Dunn MI. Aggressive diuresis for severe heart failure in the elderly. Chest 2001;119(3):807–10. [DOI] [PubMed]
- 25.Dikshit K, Vyden JK, Forrester JS, Chatterjee K, Prakash R, Swan HJ. Renal and extrarenal hemodynamic effects of furosemide in congestive heart failure after acute myocardial infarction. N Engl J Med 1973;288(21):1087–90. [DOI] [PubMed]
- 26.Lopez B, Querejeta R, Gonzalez A, Sanchez E, Larman M, Diez J. Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. J Am Coll Cardiol 2004;43(11):2028–35. [DOI] [PubMed]
- 27.Lopez B, Querejeta R, Gonzalez A, Beaumont J, Larman M, Diez J. Impact of treatment on myocardial lysyl oxidase expression and collagen cross-linking in patients with heart failure. Hypertension 2009;53(2):236–42. [DOI] [PubMed]
- 28.Kasama S, Toyama T, Hatori T, Sumino H, Kumakura H, Takayama Y, et al. Effects of torasemide on cardiac sympathetic nerve activity and left ventricular remodelling in patients with congestive heart failure. Heart 2006;92 (10):1434–40. [DOI] [PMC free article] [PubMed]
- 29.Cosin J, Diez J. Torasemide in chronic heart failure: results of the TORIC study [published erratum appears in Eur J Heart Fail 2002;4(5):667]. Eur J Heart Fail 2002;4(4):507–13. [DOI] [PubMed]
- 30.Morgan DB, Davidson C. Hypokalaemia and diuretics: an analysis of publications. Br Med J 1980;280(6218):905–8. [DOI] [PMC free article] [PubMed]
- 31.Brilla CG, Matsubara LS, Weber KT. Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J Mol Cell Cardiol 1993; 25(5):563–75. [DOI] [PubMed]
- 32.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341 (10):709–17. [DOI] [PubMed]
- 33.Gottlieb SS, Robinson S, Krichten CM, Fisher ML. Renal response to indomethacin in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 1992;70(9):890–3. [DOI] [PubMed]
- 34.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, et al. Influence of beta-blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure: findings from the OPTIMIZE-HF program. J Am Coll Cardiol 2008;52(3):190–9. [DOI] [PubMed]
- 35.Salvador DR, Rey NR, Ramos GC, Punzalan FE. Continuous infusion versus bolus injection of loop diuretics in congestive heart failure. Cochrane Database Syst Rev 2004;(1): CD003178. [DOI] [PubMed]
- 36.Kiyingi A, Field MJ, Pawsey CC, Yiannikas J, Lawrence JR, Arter WJ. Metolazone in treatment of severe refractory congestive cardiac failure. Lancet 1990;335(8680):29–31. [DOI] [PubMed]
- 37.Bart BA, Boyle A, Bank AJ, Anand I, Olivari MT, Kraemer M, et al. Ultrafiltration versus usual care for hospitalized patients with heart failure: the Relief for Acutely Fluid-Overloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) trial. J Am Coll Cardiol 2005;46(11):2043–6. [DOI] [PubMed]
- 38.Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure [published erratum appears in J Am Coll Cardiol 2007;49(10):1136]. J Am Coll Cardiol 2007;49(6):675–83. [DOI] [PubMed]
- 39.Liang KV, Hiniker AR, Williams AW, Karon BL, Greene EL, Redfield MM. Use of a novel ultrafiltration device as a treatment strategy for diuretic resistant, refractory heart failure: initial clinical experience in a single center. J Card Fail 2006; 12(9):707–14. [DOI] [PubMed]
- 40.Rogers HL, Marshall J, Bock J, Dowling TC, Feller E, Robinson S, Gottlieb SS. A randomized, controlled trial of the renal effects of ultrafiltration as compared to furosemide in patients with acute decompensated heart failure. J Card Fail 2008;14 (1):1–5. [DOI] [PubMed]
- 41.Cuffe MS, Califf RM, Adams KF Jr, Benza R, Bourge R, Colucci WS, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA 2002;287(12):1541–7. [DOI] [PubMed]
- 42.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 2006;355(20):2099–112. [DOI] [PubMed]
- 43.Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007;297(12):1319–31. [DOI] [PubMed]
- 44.Finley JJ 4th, Konstam MA, Udelson JE. Arginine vasopressin antagonists for the treatment of heart failure and hyponatremia [published erratum appears in Circulation 2008;119 (21):e552]. Circulation 2008;118(4):410–21. [DOI] [PubMed]
- 45.Goldsmith SR, Elkayam U, Haught WH, Barve A, He W. Efficacy and safety of the vasopressin V1A/V2-receptor antagonist conivaptan in acute decompensated heart failure: a dose-ranging pilot study. J Card Fail 2008;14(8):641–7. [DOI] [PubMed]
- 46.Conrad KP, Novak J. Emerging role of relaxin in renal and cardiovascular function. Am J Physiol Regul Integr Comp Physiol 2004;287(2):R250-61. [DOI] [PubMed]
- 47.Dschietzig T, Teichman S, Unemori E, Wood S, Boehmer J, Richter C, et al. Intravenous recombinant human relaxin in compensated heart failure: a safety, tolerability, and pharmacodynamic trial. J Card Fail 2009;15(3):182–90. [DOI] [PubMed]
- 48.Teerlink JR, Metra M, Felker GM, Ponikowski P, Voors AA, Weatherley BD, et al. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet 2009;373(9673):1429–39. [DOI] [PubMed]
- 49.Edlund A, Ohlsen H, Sollevi A. Renal effects of local infusion of adenosine in man. Clin Sci (Lond) 1994;87(2):143–9. [DOI] [PubMed]
- 50.Marraccini P, Fedele S, Marzilli M, Orsini E, Dukic G, Serasini L, L'Abbate A. Adenosine-induced renal vasoconstriction in man. Cardiovasc Res 1996;32(5):949–53. [PubMed]
- 51.deGoma EM, Vagelos RH, Fowler MB, Ashley EA. Emerging therapies for the management of decompensated heart failure: from bench to bedside. J Am Coll Cardiol 2006;48(12):2397–409. [DOI] [PubMed]
- 52.Gottlieb SS, Brater DC, Thomas I, Havranek E, Bourge R, Goldman S, et al. BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy [published erratum appears in Circulation 2002;106(13):1743]. Circulation 2002; 105(11):1348–53. [DOI] [PubMed]
- 53.Cotter G, Dittrich HC, Weatherley BD, Bloomfield DM, O'Connor CM, Metra M, Massie BM. The PROTECT pilot study: a randomized, placebo-controlled, dose-finding study of the adenosine A1 receptor antagonist rolofylline in patients with acute heart failure and renal impairment. J Card Fail 2008;14(8):631–40. [DOI] [PubMed]
- 54.Mitrovic V, Seferovic PM, Simeunovic D, Ristic AD, Miric M, Moiseyev VS, et al. Haemodynamic and clinical effects of ularitide in decompensated heart failure. Eur Heart J 2006; 27(23):2823–32. [DOI] [PubMed]
- 55.Luss H, Mitrovic V, Seferovic PM, Simeunovic D, Ristic AD, Moiseyev VS, et al. Renal effects of ularitide in patients with decompensated heart failure. Am Heart J 2008;155(6):1012.e1-8. [DOI] [PubMed]
- 56.Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet 2002;360(9328):196–202. [DOI] [PubMed]
- 57.Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA 2007;297(17):1883–91. [DOI] [PubMed]