Abstract
Background
Fetal growth restriction is associated with metabolic derangements in the newborn, impaired functioning in childhood and chronic diseases in adulthood. Differences between ethnic groups with respect to fetal growth may result in the misclassification of constitutionally small or large babies as having abnormal growth for their gestational age. We have developed intrauterine growth charts based on precise measurements of newborns whose parents were both of European, Chinese or South Asian ethnicity.
Methods
Weight, length and head circumference were measured in 2695 infants born to healthy non-smoking mothers in British Columbia at 37–41 completed weeks of gestation. Gestational age was confirmed by ultrasound before 20 weeks of gestation. Weight was measured by digital scale, length by stadiometer and head circumference by firm plastic tape measures. Means and 95% confidence intervals were compared among newborns grouped by ethnicity and sex. Smoothed graphs were constructed for visual interpretation.
Results
At 40 weeks, infants of European descent (“European” infants) weighed 225.5 g more on average than infants of Chinese descent (“Chinese” infants) (p < 0.001) and 254.6 g more than infants of South Asian descent (“South Asian” infants) (p < 0.001). The mean difference in birth weight between Chinese and South Asian infants (19.1 g) was not statistically significant. The mean length of European infants at 40 weeks of gestation was 0.89 cm greater than that of Chinese infants (p < 0.001). Differences in mean length between European and South Asian babies or between Chinese and South Asian babies was not statistically significant. The mean head circumferance of European babies was 0.50 cm larger than that of Chinese babies at 40 weeks (p < 0.001) but did not differ significantly from that of South Asian babies. South Asian and Chinese babies had similar mean head circumferences at 40 weeks. When differences in mean birth weight, length and head circumference were examined within boys and girls, the observed differences according to ethnicity remained statistically significant.
Conclusion
Important differences in weight, length and head circumferences are reported among babies according to ethnicity. The use of sex- and ethnicity-specific growth charts may prevent the misclassification of newborns as small or large for gestational age.
Introduction
Fetal growth restriction is associated with metabolic derangements in the newborn, impaired functioning in childhood and chronic diseases in adulthood. The evaluation of growth parameters at birth is undertaken to identify newborns who have suffered growth restriction in utero and therefore may have been exposed to adverse conditions with implications for the immediate neonatal period.1 Severely growth-restricted newborns are particularly susceptible to hypothermia, hypoglycemia, polycythemia, blood hyperviscosity and infection in the early neonatal period.2, 3 The perinatal mortality rate of 15 per 1000 births at the 5th birth-weight centile for gestational age rises to 150 per 1000 at the 1st centile.4 Mortality and morbidity rates are still higher among neonates at the 10th centile for birth weight than among neonates whose birth weight is appropriate for their gestational age. Excess mortality is associated in part with an increased risk (30%–60%) of minor and major anomalies.2
The misclassification of a healthy but constitutionally small newborn as “growth restricted” may lead to unnecessary monitoring, including repeated phlebotomy for the measurement of serum glucose levels and other parameters. Anxiety on the part of the parents as well as the unnecessary use of limited nursing and laboratory resources are additional adverse outcomes of the identification of “false positives.” On the other hand, the health risks faced by small babies who are inappropriately classified as having normal growth — “false negatives” — may be overlooked. Intrauterine growth restriction is a marker for maternal health problems such as pre-existing or gestational hypertension, viral infections and poor nutritional status, and for harmful behaviours such as the use of tobacco or illicit drugs. The failure to identify growth-restricted neonates may therefore be a missed opportunity to intervene on behalf of the mother and to help ensure that future pregnancies are healthy.2 Fetal growth restriction has been shown to have a deleterious effect on cognitive function in childhood and adolescence, independent of genetic or major organ system malformations.5, 6 Decreased insulin sensitivity in the preteen years has also been associated with fetal growth restriction.7 Failure to identify growth-restricted newborns may also preclude opportunities to identify and provide early intervention for children at risk for disabilities. The increasing weight of evidence linking reduced fetal growth with adult-onset diabetes and coronary artery disease (adult metabolic syndrome)8 underlines the importance of the intrauterine environment as a determinant of adult health and reinforces the need for precision in the diagnosis of intrauterine growth restriction.
Variability in fetal growth parameters at birth is known to be associated with the sex of the baby, gestational age at birth and multiplicity.9 Controversy exists as to which growth curves are the most appropriate to use in neonatal assessment, but there is growing consensus that standards must relate to specific geographic areas and must include race/ethnicity in addition to other parameters.2 Most studies reporting morphometric differences at birth according to race/ethnicity have made comparisons between, rather than within, countries.10, 11 In contrast, a Canadian study compared infants of European descent with infants born to immigrant Chinese parents in Montréal between 1978 and 1990 and used an obstetric and neonatal database to compile “risk-free” cohorts.12 In this study, racial origin was identified on the basis of the mother’s name before marriage. The study reported mean birthweight-for-gestational-age increments of 180g or more for infants of European versus Chinese descent, but did not report on length or head circumference. Comparisons of length and head circumference allow symmetrical and asymmetrical growth to be distinguished; this is important to the assessment of the severity and duration of restricted growth in utero.2
In the current study, we developed charts for birth weight, length and head circumference specific to race/ethnicity, sex and gestational age for newborns of European, Chinese and South Asian descent.
Methods
Setting
The study was conducted at BC Women’s Hospital, a primary obstetrical care facility for residents of Vancouver and the surrounding Lower Mainland area of British Columbia. Forty percent of women delivering at BC Women’s Hospital are of East Asian (China, Hong Kong) descent, and 10% are of South Asian (India, Pakistan) descent. During the study period, 2000–2003, orthopedic surgical residents at BC Women’s Hospital undertook routine neonatal screening for congenital dislocation of the hip. The thrice-weekly assembly of all newborns in the nursery for this purpose afforded us an opportunity to undertake precise measurements of length and head circumference.
Sample
Because our objective was to develop standards for normal intrauterine growth, we restricted our sample to newborns of healthy mothers. Newborns were eligible if their mothers had expected dates of confinement (EDC) between 37 and 41 completed weeks of pregnancy (as confirmed by ultrasound measurement of fetal biparietal diameters or crown–rump length obtained before 20 weeks of gestation). We limited our inclusion criteria to 37 to 41 weeks of gestation for two reasons: we had limited access to newborns of younger gestational age; and we assumed that pre-term babies might have been exposed in utero to conditions that affect growth and therefore might not represent a “normal” growth standard.13
Parents of study subjects were (a) both of European descent; (b) both of East Asian descent; or (c) both of South Asian descent. (For convenience, we refer to these groups as “European,” “Chinese” or “South Asian.”) Race/ethnicity was determined by a perusal of (a) the prenatal record, in which ethnicity is recorded, and (b) a sociodemographic information form completed by the obstetrical nurses, which includes a field for race/ethnicity. If these two sources of information were not in agreement, the infant was not included in the study. Infants born to First Nations (Aboriginal) parents were not enrolled, as they constituted only 1% of the population born at BC Women’s Hospital and would therefore not constitute a sample large enough to support precise morphometric estimates.
Infants were excluded if their mothers’ pregnancies were complicated by any conditions that could alter fetal growth, including pre-existing or gestational insulin-dependent diabetes, pre-eclampsia, fetal anomalies, multiple gestation or known or suspected syndromes. Babies born to mothers who disclosed the use of harmful substances (tobacco, alcohol or illicit drugs) during pregnancy, as reported on the prenatal record, were excluded.
Gestational age
Gestational age at birth for each newborn was derived from the EDC, calculated on the basis of both early ultrasound and the date of the last menstrual period (LMP). Obstetrical wheels use Nagle’s rule to align dates of LMP with the corresponding EDC. In view of the fact that comparisons of palm-sized wheels have revealed differences in calibration of weekly intervals,14 we used a large-scale obstetrical wheel to estimate EDC from LMP. To determine EDC based on ultrasound evaluations, original ultrasound reports were reviewed for all participants and EDCs were recalculated. In the event of a disparity between the two methods, the initial estimate by LMP of gestational age at birth was adjusted to that derived by ultrasound if the discrepancy was ≥ 6 days at 7–12 weeks, ≥ 7 days at 13–14 weeks, ≥ 8 days at 15–20 weeks, and ≥ 14 days at 20–24 weeks gestation, according to the method devised by Synnes and colleagues.15
Morphometry was completed on a total of 2695 eligible newborns. After recalculation of gestational age at birth on the basis of original ultrasound reports and LMP, 28.5% of the gestational ages were changed (n = 765). Adjustments ranged from 1 to 3 weeks. The reasons for adjustments to the gestational age included: errors in calculation of EDC based on LMP, when the use of LMP was appropriate (8.2%, n = 221); failure to adjust EDC according to ultrasound as per Synnes and colleagues (6.3%, n = 166); incorrect calculation of the EDC from the ultrasound report (2.1%, n = 57); inappropriate correction of EDC according to ultrasound as per Synnes and colleagues (6.0%, n = 161); and an inability, based on the information given, to reconcile the discrepancy between EDC calculated in the infant’s chart and that calculated by the authors, in which case the latter was used (5.9%, n = 160).
Morphometry
Measurement of growth parameters was completed within 48 hours of birth. Length was measured using a stadiometer, a hard plastic platform with a vertical headboard against which the crown of the baby’s head was placed. With diapers loosened to permit free movement of the legs, the legs were held flat (knees down) and a movable footboard was pressed gently against the balls of the feet, which were held perpendicular to the legs. The measurement from the crown to the soles of the feet was taken using the centimetre scale on the stadiometer. The head circumference was measured using a firm heavy-weight plastic tape. Paper tapes were judged to be inadequate in view of the observed variability in printed measurement intervals, and metal tape measures were not used in view of the risk of lacerations. The plastic tape was checked throughout the study against an identical but unused tape to ensure that stretching had not taken place. All measurements were made by an experienced pediatric nurse assisted by a medical student. Inter-rater reliability between the nurse and the student was within 0.1 cm for both head circumference and length. Birth weight was documented in the birth record immediately after birth, when the baby had been dried but before breastfeeding. Two digital scales in the delivery suite were calibrated using a standard weight to ensure accuracy and comparability.
Statistical analysis
Means and 95% confidence intervals were graphed to compare differences between newborns grouped first by ethnicity and then by sex. We present data using means and standard deviations to reflect biological variability as opposed to percentiles, which are essentially a mathematical cut-off. Plots of crude data suggested moderate nonlinearity; therefore, smoothing of sample means and standard deviations was undertaken, using a locally weighted least-square regression (using S-PLUS, Version 6.1: Mathsoft Inc.).16 Smoothing could not be done for South Asian newborns at 260–263 days of gestational age because of insufficient data points. To achieve smooth projection for this interval, point (mean) and interval estimates of weight, head circumference, and length were derived by simple incremental projection: y(t) = y(t–1)+(y(t–1)–y(t–2))*P, where P is a scale parameter given between 0.5 and 1, based on the data.
The influence of sociodemographic confounders on differences between groups was assessed in a multivariate linear regression. A variable was adjusted for if its inclusion in the model changed the estimates for the odds ratio by 10% or more.17
Results
Morphometry was completed on 2695 newborns: 1195 born to European parents; 975 born to Chinese parents; and 525 born to South Asian parents (Table 1). At gestational ages of 37 to 41 completed weeks, Chinese and South Asian infants had significantly lower mean birth weights than European infants but did not differ from each other in this respect (Figure 1). The mean differences in birth weight among babies of differing ethnicities were similar to, or greater than, the differences between sexes at a given gestational age. At 40 weeks, European babies weighed 225.5 g more on average than Chinese babies (p < 0.001) and 254.6 g more than South Asian babies, (p < 0.001). The mean difference between boys and girls overall at 40 weeks was 90.9 g (p < 0.001). The mean difference in birth weight between Chinese and South Asian babies (19.1 g) was not statistically significant.
Table 1.

Characteristics of mothers by ethnicity, n (%)
Figure 1.
Birth weight, length and head circumference distribution by gestational age and ethnicity (blue = European; green = Chinese; gold = South Asian). Values for weight are in grams; values for length and head circumference are in centimetres. CI = Confidence interval.
A different picture emerged from the comparisons of birth length. Overall, European babies were significantly longer than Chinese babies. South Asian babies’ mean lengths were between those of European and Chinese babies and not significantly different from either (Figure 2). The mean difference in birth length between European and Chinese babies at 40 weeks of gestation was 0.89 cm (p < 0.001). Mean length did not differ significantly between European and South Asian babies (the mean length of European babies at 40 weeks was 0.20 cm greater) or between Chinese and South Asian babies (the mean difference was 0.60 cm). The mean difference in length between sexes at 40 weeks was 0.68 cm (p < 0.001).
Figure 2.
Values for birth weight, length and head circumference at term: European girls. Mean (solid line) +/- 2 standard deviations (dotted lines).
More variation according to ethnicity emerged from comparisons of head circumference. Head circumferences for Chinese and South Asian babies were similar at the same gestation, and both groups tended to have smaller head circumferences than European babies (Figure 3). At 40 weeks, European babies had larger head circumferences, with a mean difference of 0.50 cm compared with Chinese babies at 40 weeks (p < 0.001), but not compared with South Asian babies, with a mean difference of 0.22 cm (p = 0.11). South Asian babies had slightly larger head circumferences at 40 weeks compared with Chinese babies, with a mean difference of 0.27 cm, but this difference was not statistically significant (p = 0.06). The mean difference in head circumference at 40 weeks by sex was 0.89 cm (p < 0.001). Differences in head circumference by sex were significantly different at all gestational ages.
Figure 3.
Values for birth weight, length and head circumference at term: European boys. Mean (solid line) +/- 2 standard deviations (dotted lines).
Distributions are presented separately for boys and girls in Table 2. When differences in birth weight, length and head circumference were examined within boys and girls, the observed differences according to ethnicity remained statistically significant. In multivariate models predicting birth weight, length and head circumference, sociodemographic variables and parity were not statistically significant when ethnicity was included in the model. Smoothed curves for weight, length and head circumference by sex and ethnicity are presented in Figure 2–Figure 7.
Table 2.
Mean (and standard deviation) for birth weight, length and head circumference by sex, gestational age at birth and ethnicity
Figure 7.
Values for birth weight, length and head circumference at term: South Asian boys. Mean (solid line) +/- 2 standard deviations (dotted lines).
Figure 4.
Values for birth weight, length and head circumference at term: Chinese girls. Mean (solid line) +/- 2 standard deviations (dotted lines).
Figure 5.
Values for birth weight, length and head circumference at term: Chinese boys. Mean (solid line) +/- 2 standard deviations (dotted lines).
Figure 6.
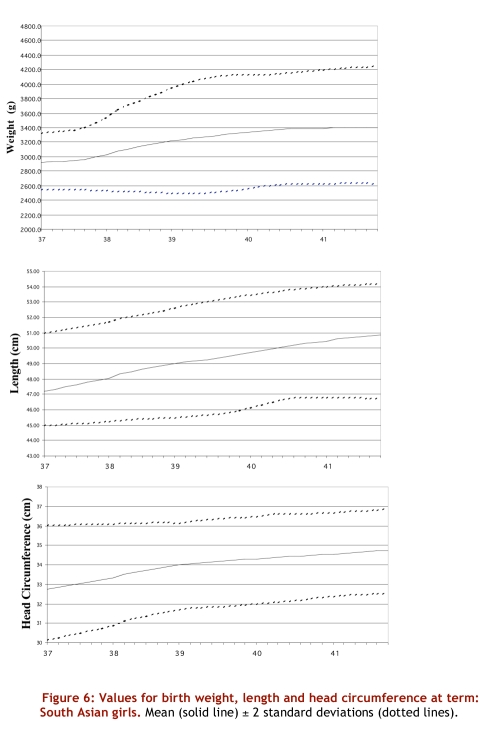
Values for birth weight, length and head circumference at term: South Asian girls. Mean (solid line) +/- 2 standard deviations (dotted lines).
Discussion
We report, for the first time, sex- and ethnicity-specific parameters for weight, length, and head circumference for infants born at 37–41 completed weeks of gestation. These standards assume optimal growth and can be used to detect clinically relevant deviations from the normal range and thus help to identify newborns at risk for the adverse health outcomes associated with abnormal intrauterine growth. These standards are to be distinguished from population-based references, which include all infants with morbidities and must be interpreted more broadly. Our model for this study, the first Canadian “gold standard” developed by Usher and McLean in 1969, used precise measurement techniques and was restricted to infants of mothers who had experienced healthy pregnancies.18 Our charts differ from references currently in use19 in that they are derived from a healthy population, that is, infants of mothers who had experienced healthy pregnancies and who did not use alcohol, tobacco or illicit drugs. In addition, we used precise measurement techniques, including recalculation of EDC from ultrasonography. Measurements at birth conducted without stadiometers or standardized techniques, as documented routinely in health records, have been shown to have a 5% relative error in measurement of infant length.20
Our data indicate that variation in growth by ethnicity is as great as that measured on the basis of sex. Infants who are not assessed using standards derived from their specific ethnic group may be misclassified as small or large for gestational age at birth. A British study conducted at three hospitals with a geographically defined catchment area excluded babies of mothers with known maternal or fetal morbidities and without confirmation of EDC by early (24-week) ultrasound.21 For the years 1986–91, mean birth weights for newborns of non-smoking English/European, Afro-Caribbean and Indian/Pakistani mothers were presented for both sexes combined. The authors did not report how the racial groups were defined. Mean birth weights at 40 weeks for South Asian babies (3334 g) were lower than our values for boys (3452 g) and girls (3376 g). This difference may reflect secular increases in birth weight.22, 23 A US study comparing birth weights of babies born to parents of Chinese descent with those born to parents of European descent in the period 1980–87 reported mean differences of 200 g at 40–41 weeks of gestation, a relative difference of 6% of the total birth weight. This study, derived from birth records, combined data for both boys and girls.24 Another US study using data from the same period reported differences in the range of 5%–6% between Chinese and European newborns at term.25
Reasons for differing intrauterine growth according to race/ethnicity among apparently healthy populations are relatively unexplored. In Canada, Wen and colleagues reported more rapid fetal growth early in the third trimester but slower growth near and after term among Chinese versus European infants.12 Lower mean birth weights were attributed to differences in fetal growth rate rather than to gestational duration after adjustment for maternal determinants of fetal growth. Our data support this explanation. Careful examination of Figure 1 indicates a flatter slope for increase in weight by gestational week for babies of Chinese versus European descent. The ponderal index ([weight (g) / length3 (cm)] x 100) is a measure of growth restriction that identifies “wasting” or “thin” babies. The mean ponderal index for European (2.84, standard deviation [SD] 0.33) versus Chinese babies (2.82, SD 0.40) was not different (p = 0.50) after adjustment for gestational age. In contrast, the ponderal index for European versus South Asian babies in our data (2.68, SD 0.46) was significantly different after adjustment for gestational age (p < 0.001). This suggests that growth of South Asian babies in our setting may be restricted by factors not accounted for in our sampling (e.g., nutritional status of the mother). Fetal growth among South Asian women in Canada has not been studied in detail but may be influenced by dietary or other factors.
We compared our findings with a recently published Canadian sex- but not ethnicity-specific reference for birth weight for gestational age based on birth certificate data. Mean birth weights in our distribution at 40 weeks of gestation are 74 g larger on average for European boys, 161 g larger for European girls and 100 g smaller for babies of Chinese and South Asian descent, reflecting the differences in the direction expected for each ethnic group.19 Another British Columbia reference for birth weights measured from 1981 through 1990 for babies of European, Chinese and South Asian descent reports values that are comparable or slightly smaller than ours. Again, this is likely due to secular trends, and because they are reported for both sexes combined they have limited utility.26
Our study is limited by our inability to report growth standards for babies of less than 37 weeks of gestation. Investigators extending our work should be encouraged to identify a population of newborns for whom prematurity appears to be unrelated to factors that affect fetal growth. We are also limited by small numbers in categories formed by stratification according to sex and ethnicity. It must be understood, however, that the homogeneity of our sample combined with accurate measurement has improved precision. The dispersion around the mean in our categories of birth weight at two standard deviations is similar to that at the 5th and 95th percentiles in the new Canadian distribution based on all live births.19
We did not evaluate maternal age as a confounder in the relationship between ethnicity and growth parameters, but maternal age is not generally acknowledged to be an important predictor of morphometric parameters.27 Further, we had no reason to believe that age was distributed differently among ethnic groups. Although maternal height, weight and body mass index did differ between groups, we did not attempt to control for these factors, which may, as inherited traits, be causally related to differences between ethnic groups. Instead, we attempted to control for environmental factors by recruiting only healthy non-smoking mothers and assessing the impact of parity and socio-economic status on our findings. We acknowledge that our data do not allow us to evaluate intergenerational transmission of socio-economic factors that are known to influence differences between racial and ethnic groups.28,29
Our charts may be used by clinicians to diagnose individual newborns as small or large for gestational age at birth and to determine the need for close observation and follow-up. Our data should not be interpreted as “explaining” the determinants of restricted fetal growth, since race/ethnicity may be proxy indicators of diet, activity, stress, education, access to health care and other factors.30,31 One group has reported interactions between race and maternal variables such as education, marital status and access to prenatal care in a study of determinants of gestational-age-specific birth weight.25 Ongoing efforts to elucidate the determinants of fetal growth among racial/ethnic groups should include evaluation of these parameters. Conversely, there may be intrinsic determinants of fetal growth. The current study provides contemporary charts that may prove most useful for accurate classification of babies of European, Chinese or South Asian descent as growth restricted or large for gestational age.
Contributors
Drs. Thiessen, Klein and Whitfield assisted Dr. Janssen with the design of the study and in the interpretation of data. Dr. Whitfield also directed the data analysis along with Dr. Janssen. Dr. Ying MacNab undertook the smoothing of data and the production of the graphs. Ms. Cullis-Kuhl is a pediatric nurse who collected all of the data. All of the co-authors played an active role in writing and revising the manuscript and have read and agree with the final submitted version.
Acknowledgments
We would like to thank Maartje van Elst, medical student, University of Maastrich, Netherlands, for assistance with newborn measurement.
Biographies
Patricia A. Janssen, PhD, is a perinatal epidemiologist and is Associate Professor, Department of Health Care and Epidemiology, University of British Columbia, and Co-Director of the Interdisciplinary Women’s Reproductive Health Research Training Program at the Child and Family Research Institute in Vancouver, BC.
Paul Thiessen, MD, FRCPC, is Clinical Professor, Department of Pediatrics, University of British Columbia, and Medical Director of Newborn Care at BC Women’s Hospital, Vancouver, BC.
Michael C. Klein, MD, FRCPC, is Emeritus Professor of Family Practice and Pediatrics at the University of British Columbia, Vancouver, BC.
Michael F. Whitfield, MD, FRCPC, is Professor, Department of Pediatrics, University of British Columbia, and Associate Director of the Neonatal Follow-up Program at BC Children’s Hospital, Vancouver, BC.
Ying C. MacNab, PhD, is a biostatistician at the Centre for Applied Health Research at the Child and Family Research Institute, and Associate Professor, Department of Health Care and Epidemiology, University of British Columbia, Vancouver, BC.
Sue C. Cullis-Kuhl, RN, RSCN, SCM, is a pediatric nurse at BC Children’s Hospital, Vancouver, BC.
Footnotes
Competing interests: None declared.
Funding Source: After peer review, funding for this study was provided by the British Columbia Children’s Hospital Foundation. The Foundation did not contribute to the study design, collection, analysis or interpretation of data, nor to the composition of this manuscript. Ethical approval was granted by the University of British Columbia Clinical Ethics Board and the BC Women’s Hospital Research Review Committee.
References
- 1.Kramer M S, Platt R, Yang H, McNamara H, Usher R H. Are all growth-restricted newborns created equal(ly)? Pediatrics. 1999 Mar;103(3):599–602. doi: 10.1542/peds.103.3.599. [DOI] [PubMed] [Google Scholar]
- 2.Creasy R, Resnick R, Iams J, editors. Maternal fetal medicine. 5th ed. 2004. Maternal-Fetal Medicine: Principles and Practice. [Google Scholar]
- 3.Brodsky Dara, Christou Helen. Current concepts in intrauterine growth restriction. J Intensive Care Med. 2004;19(6):307–319. doi: 10.1177/0885066604269663. [DOI] [PubMed] [Google Scholar]
- 4.Manning F. Intrauterine growth retardation. In Fetal medicine: principles and practice. 1995. Intrauterine growth retardation; p. 312. [Google Scholar]
- 5.Low J A, Handley-Derry M H, Burke S O, Peters R D, Pater E A, Killen H L, Derrick E J. Association of intrauterine fetal growth retardation and learning deficits at age 9 to 11 years. Am J Obstet Gynecol. 1992 Dec;167(6):1499–1505. doi: 10.1016/0002-9378(92)91727-r. [DOI] [PubMed] [Google Scholar]
- 6.Indredavik M, Vik T, Heyerdahl S. Psychiatric symptoms and disorders in adolescents with low birthweight. Child Care Health Dev. 2005;31(1):121–125. doi: 10.1111/j.1365-2214.2005.00500_1.x. [DOI] [Google Scholar]
- 7.Veening Margreet A, Van Weissenbruch Mirjam M, Delemarre-Van De Waal Henriette A. Glucose tolerance, insulin sensitivity, and insulin secretion in children born small for gestational age. J Clin Endocrinol Metab. 2002 Oct;87(10):4657–4661. doi: 10.1210/jc.2001-011940. [DOI] [PubMed] [Google Scholar]
- 8.Gillman M W, Rich-Edwards J W. The fetal origin of adult disease: from sceptic to convert. Paediatr Perinat Epidemiol. 2000 Jul;14(3):192–193. doi: 10.1046/j.1365-3016.2000.00265.x. [DOI] [PubMed] [Google Scholar]
- 9.Stein ZA, Susser M. Intrauterine growth retardation: epidemiological issues and public health significance. Semin Perinatol. 1984;8(1):5–14. [PubMed] [Google Scholar]
- 10.Cheng M C, Chew P C, Ratnam S S. Birth weight distribution of Singapore Chinese, Malay and Indian infants from 34 weeks to 42 weeks gestation. J Obstet Gynaecol Br Commonw. 1972 Feb;79(2):149–153. doi: 10.1111/j.1471-0528.1972.tb15770.x. [DOI] [PubMed] [Google Scholar]
- 11.Lin C C, Emanuel I. A comparison of American and Chinese intrauterine growth standards. Are American babies really smaller? Am J Epidemiol. 1972 May;95(5):418–430. doi: 10.1093/oxfordjournals.aje.a121409. [DOI] [PubMed] [Google Scholar]
- 12.Wen S W, Kramer M S, Usher R H. Comparison of birth weight distributions between Chinese and Caucasian infants. Am J Epidemiol. 1995 Jun 15;141(12):1177–1187. doi: 10.1093/oxfordjournals.aje.a117391. [DOI] [PubMed] [Google Scholar]
- 13.Joseph K S, Kramer M S, Allen A C, Cyr M, Fair M, Ohlsson A, Wen S W. Gestational age- and birthweight-specific declines in infant mortality in Canada, 1985-94. Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. Paediatr Perinat Epidemiol. 2000 Oct;14(4):332–339. doi: 10.1046/j.1365-3016.2000.00266.x. [DOI] [PubMed] [Google Scholar]
- 14.Grzybowski S, Nout R, Kirkham M. Maternity care calendar wheel. Improved obstetric wheel developed in British Columbia. Can Fam Physician. 1999 Mar;45:661–666. [PMC free article] [PubMed] [Google Scholar]
- 15.Synnes A R, Ling E W, Whitfield M F, Mackinnon M, Lopes L, Wong G, Effer S B. Perinatal outcomes of a large cohort of extremely low gestational age infants (twenty-three to twenty-eight completed weeks of gestation). J Pediatr. 1994 Dec;125(6 Pt 1):952–960. doi: 10.1016/S0022-3476(05)82015-3. [DOI] [PubMed] [Google Scholar]
- 16.Everitt B. Analyzing medical data using S-PLUS. 2001. Analyzing medical data using S-PLUS. [Google Scholar]
- 17.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993 Dec 1;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 18.Usher R, McLean F. Intrauterine growth of live-born Caucasian infants at sea level: standards obtained from measurements in 7 dimensions of infants born between 25 and 44 weeks of gestation. J Pediatr. 1969 Jun;74(6):901–910. doi: 10.1016/s0022-3476(69)80224-6. [DOI] [PubMed] [Google Scholar]
- 19.Kramer M, Platt R, Wen S. A new and improved population-based Canadian reference for birthweight for gestational age. Pediatrics; 2001;108(2):e35. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- 20.Blidner I N, McClemont S, Anderson G D, Sinclair J C. Size-at-birth standards for an urban Canadian population. Can Med Assoc J. 1984 Jan 15;130(2):133–140. [PMC free article] [PubMed] [Google Scholar]
- 21.Wilcox M, Gardosi J, Mongelli M, Ray C, Johnson I. Birth weight from pregnancies dated by ultrasonography in a multicultural British population. BMJ. 1993 Sep 4;307(6904):588–591. doi: 10.1136/bmj.307.6904.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer M, Morin I, Yang H. Why are babies getting bigger? Temporal trends in fetal growth and its determinants. J Pediatr. 2002;141(4):538–42. doi: 10.1067/mpd.2002.128029. [DOI] [PubMed] [Google Scholar]
- 23.Wen SW, Kramer Michael S, Platt Robert, Demissie Kitaw, Joseph K S, Liu Shiliang, Sauve Reg. Secular trends of fetal growth in Canada, 1981 to 1997. Paediatr Perinat Epidemiol. 2003 Oct;17(4):347–354. doi: 10.1046/j.1365-3016.2003.00513.x. [DOI] [PubMed] [Google Scholar]
- 24.Yip R, Li Z, Chong W H. Race and birth weight: the Chinese example. Pediatrics. 1991 May;87(5):688–693. [PubMed] [Google Scholar]
- 25.Wang X, Guyer B, Paige D M. Differences in gestational age-specific birthweight among Chinese, Japanese and white Americans. Int J Epidemiol. 1994 Feb;23(1):119–128. doi: 10.1093/ije/23.1.119. [DOI] [PubMed] [Google Scholar]
- 26.Kierans W, Kramer M, Wilkins R. Charting birth outcome in British Columbia: determinants of optimal health and ultimate risk. 2003. Charting birth outcome in British Columbia: determinants of optimal health and ultimate risk. http://www.vs.gov.bc.ca/stats/pdf/ChartingBirthOutcomeReport.pdf. [Google Scholar]
- 27.Gardosi Jason. Customized fetal growth standards: rationale and clinical application. Semin Perinatol. 2004 Feb;28(1):33–40. doi: 10.1053/j.semperi.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Conley D, Bennett N G. Race and the inheritance of low birth weight. Soc Biol. 2000;47(1-2):77–93. doi: 10.1080/19485565.2000.9989011. [DOI] [PubMed] [Google Scholar]
- 29.Emanuel I, Kimpo C, Moceri V. The association of maternal growth and socio-economic measures with infant birthweight in four ethnic groups. Int J Epidemiol. 2004;33(6):1249–1251. doi: 10.1093/ije/dyh269. [DOI] [PubMed] [Google Scholar]
- 30.Vangen S, Stoltenberg C, Skjaerven R. The heavier the better? Birthweight and perinatal mortality in different ethnic groups. Int J Epidemiol. 2002;31 doi: 10.1093/ije/31.3.654. [DOI] [PubMed] [Google Scholar]
- 31.Alexander G R, de Caunes F, Hulsey T C, Tompkins M E, Allen M. Ethnic variation in postnatal assessments of gestational age: a reappraisal. Paediatr Perinat Epidemiol. 1992 Oct;6(4):423–433. doi: 10.1111/j.1365-3016.1992.tb00786.x. [DOI] [PubMed] [Google Scholar]