Abstract
Background
Although a purported advantage of newer antihypertensive drug classes is a reduced need for laboratory testing, little is known about the frequency of laboratory monitoring of hypertensive patients in clinical practice and whether this differs across drug classes.
Methods
This population-based cohort study used linked administrative databases in Ontario, Canada. All elderly residents of Ontario (age 66 and over) who were newly treated for uncomplicated hypertension between 1994 and 2002 were followed for 24 months or until they were admitted to hospital, died, or were no longer on their initially prescribed monotherapy. We examined the frequency and type of laboratory tests performed while patients were treated with antihypertensive monotherapy.
Results
In a cohort of 164,413 patients, 39% were treated with thiazides and 46% were prescribed "newer" drug classes as initial therapy. At baseline, 96,534 patients (59%) did not have any laboratory testing done, and during 1,701,520 months of monotherapy (mean time on initial agent 10.3 months) only 79,985 (49%) had any tests done. Laboratory testing was significantly less frequent in patients prescribed newer drug classes than thiazides: the adjusted rate ratios for laboratory testing were 0.94 (95% confidence interval [CI] 0.93–0.95) with angiotensin-converting enzyme inhibitors, 0.80 (95% CI 0.79–0.81) with calcium-channel blockers, and 0.79 (95% CI 0.76–0.82) with angiotensin-receptor blockers. However, the absolute increase in testing was small (16 extra electrolyte tests, 6 extra renal function tests, 4 extra glucose tests, and 6 fewer serum cholesterol tests per 100 patients every 6 months), such that the extra laboratory testing observed with thiazides resulted in an additional cost of only C$0.63 per patient every 6 months in comparison with the cost of the newer drug classes.
Conclusion
Laboratory testing in clinical practice was significantly less frequent among patients prescribed newer drug classes than among those prescribed thiazides; however, laboratory monitoring was infrequent in this cohort of elderly patients with hypertension but without comorbidities, and the magnitude of differences between drug classes was small.
Introduction
Thiazide diuretics, angiotensin-converting enzyme (ACE) inhibitors, calcium-channel blockers and angiotensin receptor blockers (hereafter, the latter 3 are referred to as "newer agents") prevent cardiovascular morbidity and mortality in elderly patients with uncomplicated hypertension,1, 2 and the reduction in events is directly related to the reduction in blood pressure.2, 3 Thus, debates over which drug class should be recommended for initial therapy in hypertension frequently revolve around issues of costs, adherence, and tolerability. Although defining the predictors of long-term adherence with antihypertensive agents is an area of active research, differences in tolerability between drug classes are best judged in randomized trials, several of which have reported similar adherence and tolerability with each of the major drug classes.4-7 Thus, cost is increasingly cited as the key factor in choosing between drug classes for initial therapy in patients with uncomplicated hypertension.8
Advocates of the use of thiazides as first-line treatment for elderly hypertensive patients cite their cheaper acquisition costs,9 while opponents maintain that there is less need for (and thus less cost associated with) laboratory testing with newer agents. However, there is little published evidence on the frequency of laboratory monitoring in hypertensive individuals (and none examining differences between drug classes), and without such data one can only speculate as to whether the cheaper acquisition costs of thiazides are offset by increased costs for laboratory monitoring. Indeed, given the paucity of data, attempts to model the economic implications of using thiazides versus newer drug classes have been forced to make assumptions about the frequency of laboratory testing with different drug classes by basing the frequency of testing on what is recommended in clinical practice guidelines.9, 10
Given that randomized trial protocols specify the type and frequency of laboratory tests, and standardize these across treatment arms, none of the randomized trials of antihypertensive agents can be used to answer this question. Thus, a cohort study is the strongest study design to explore antihypertensive prescribing practices and the impact of initial drug choice on subsequent laboratory testing practices.
Methods
Purpose of study
This study was conducted to examine the frequency of laboratory monitoring in patients newly started on antihypertensive therapy who did not have comorbidities or non-blood pressure lowering indications for these drugs; our primary interest was in determining whether the pattern of laboratory monitoring differed according to the drug class that was prescribed as initial monotherapy.
Assembly of cohort
As previously described in detail,11 we cross-linked the Ontario Drug Benefit database with the Ontario Health Insurance Plan (OHIP) physician claims database (which records all fee-for-service billings and the most responsible diagnoses at each visit), the Canadian Institute for Health Information hospitalization database (which records the primary diagnosis and up to 15 secondary diagnoses for all discharges from acute care hospitals), and the Registered Persons Database (which records dates of death or emigration from Ontario) to identify a cohort of all Ontario residents aged 66 years or older who received a new outpatient prescription for an antihypertensive drug between 1 July 1994 and 31 March 2002. These databases record information for all Ontario residents aged 65 and older, and the comprehensiveness and validity of these administrative databases in Ontario has been validated.12
To create a cohort of elderly patients newly treated with antihypertensive medication for presumed hypertension, we excluded any of these patients newly prescribed an antihypertensive drug who had diabetes mellitus (these patients were identified by cross-linking with the Ontario Diabetes Database),13 or an indication for antihypertensive agents other than hypertension (myocardial infarction or angina, heart failure, arrhythmias, renal disease [including nephropathy], stroke or transient ischemic attack, liver disease, esophageal varices, hyperthyroidism, or migraine headaches).
These co-morbid conditions were defined as being present if a patient had (1) a physician billing claim in the 3 years prior to being dispensed the antihypertensive drug, or a hospital discharge code in the 4 years preceding the index antihypertensive prescription for any of these diagnoses; or (2) received a prescription for marker medications for these conditions in the Ontario Drug Benefit database in the year preceding his or her antihypertensive prescription (for example, we used a nitrate prescription as a marker for coronary disease, digoxin and loop diuretics as a marker for heart failure, and propylthiouracil or methimazole for hyperthyroidism).
To identify incident cases of treated hypertension in our cohort, we included only patients aged 66 and over (the Ontario Drug Benefit database starts at age 65, and all patients had to have at least a 12 month wash-out period), and excluded any patients with a claim date for an antihypertensive drug in the 12 months before the start of the study to exclude prevalent hypertensive patients who were obtaining medication refills. Finally, to examine laboratory testing by drug class, we excluded any patients who were started on more than one antihypertensive agent concurrently at the time of initial treatment. We followed our cohort of patients newly treated with antihypertensive drugs for 2 years, or until they died, were admitted to hospital, discontinued the initially prescribed drug class, or had another antihypertensive agent added for concurrent therapy.11
Proportion of patients tested at least once
We linked our cohort to the OHIP database to examine laboratory testing patterns in the 6 months immediately preceding their first antihypertensive prescription (to define baseline testing patterns), and in the first 24 months after the initial antihypertensive dispensation (to define testing patterns during follow-up). The OHIP database includes billing claims data for laboratory tests performed in laboratories outside the hospital setting.14-16 We examined all claims for laboratory monitoring of blood-based tests, and a priori focused specifically on the 4 tests that were recommended for all new hypertensive patients in the Canadian national guidelines for all years of this study — i.e., measurements of serum electrolytes, cholesterol, glucose, and renal function. We defined "electrolyte tests" as a measurement of serum sodium or potassium; "cholesterol tests" as having total cholesterol, low- or high-density lipoprotein cholesterol, and/or triglycerides measured; "glucose test" as having fasting or random blood glucose measured; and "renal function tests" as having serum creatinine or creatinine clearance measured.
We calculated the proportion of patients prescribed each drug class who had each test done at least once while they were on monotherapy. Associations between drug class and whether laboratory tests were done at least once were assessed in logistic regression models comparing thiazides to newer agents with adjustment for patient age, gender, Charlson co-morbidity score,17 and baseline testing patterns (i.e., in the 6 months before the initial antihypertensive prescription). Patients treated with beta-blockers were excluded from this analysis, since beta-blockers are not recommended for initial monotherapy in elderly patients with uncomplicated hypertension, and thus the question of whether patients treated with beta-blockers received more or less testing is moot.8, 18, 19
Frequency and number of tests
To account for varying lengths of time on antihypertensive monotherapy, we calculated the test density for each test per 6 months of monotherapy treatment (defined as the number of tests performed per 100 patients treated with monotherapy with that particular agent for 6 months). To compare results across drug classes, we performed Poisson regression (with censoring at time of hospital admission, death, discontinuation of monotherapy, or switching of drug class) to compare the frequency of testing between drug classes during monotherapy after adjustment for age, gender, co-morbidity, and testing in the 6 months before the initial antihypertensive prescription.
All analyses were conducted using SAS version 8.02 (SAS Institute, Cary, NC).
Results
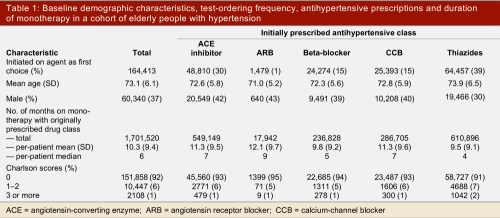
For the period July 1994 to March 2002 we identified 164,413 people over age 66 years who were newly started on monotherapy with an antihypertensive drug, and who did not have co-morbidities or indications other than hypertension for that medication. Their mean age was 73 years (standard deviation [SD] 6 years). In this cohort, 60,340 (37%) were male, and the first-line antihypertensives prescribed were thiazides (39%), ACE inhibitors (30%), calcium-channel blockers (15%), beta-blockers (15%), and angiotensin receptor blockers (1%)(Table 1). The duration of monotherapy with the initially prescribed antihypertensive drug ranged from a maximum of 24 months in 41,886 patients (25%) to less than 6 months in 81,002 patients (49%). The mean duration of monotherapy with the initially prescribed agent was 10.3 months.
Table 1.
Baseline demographic characteristics, test-ordering frequency, antihypertensive prescriptions and duration of monotherapy in a cohort of elderly people with hypertension
Proportion of patients having tests done at least once
Before their initial antihypertensive prescription, patients prescribed newer agents were more likely to have had laboratory tests done than patients who were prescribed thiazides (Figure 1, all p <0.0001). It should be noted, however, that 96,534 patients (59%) did not have any laboratory testing done in the 6 months before their initial antihypertensive prescription. After being prescribed antihypertensive therapy, 79,985 of the cohort patients (49%) had at least 1 laboratory test done at any point during follow-up (50% of those who had testing done before their initial prescription, and 48% of those who did not have testing done before their initial prescription).
Figure 1.
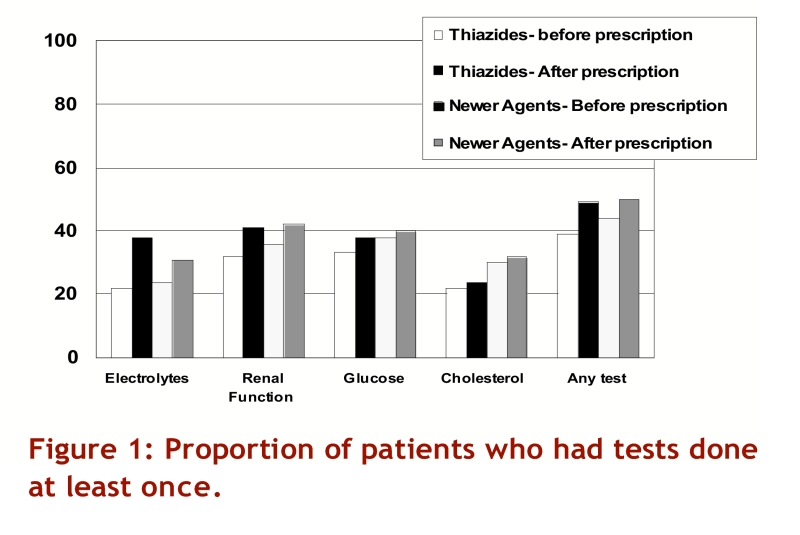
Proportion of patients who had tests done at least once
In comparison with patients prescribed newer agents, a greater proportion of patients prescribed thiazides had their serum electrolytes measured at least once during follow-up (38% v. 31%, odds ratio [OR] 1.38, 95% confidence interval [CI] 1.35–1.41, after adjusting for age, gender, co-morbidity, and baseline testing), but fewer thiazide-treated patients had any monitoring of their renal function (41% v. 42%, adjusted OR 0.95, 95% CI 0.93–0.97), serum glucose (38% v. 40%, adjusted OR 0.90, 95% CI 0.88–0.92), or cholesterol (24% v. 32%, adjusted OR 0.72, 95% CI 0.70–0.74)(Figure 1). As a result, the number of patients who had at least one laboratory test performed while on monotherapy with their initial antihypertensive prescription did not differ between thiazides and newer agents (49% v. 50%, adjusted OR 0.98, 95% CI 0.96–1.01).
Frequency and number of tests
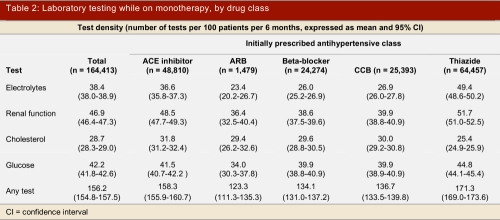
During 1,701,520 months of monotherapy in this cohort of patients, the most frequently performed laboratory tests were renal function tests (77,052 every 6 months), serum glucose (69,393 every 6 months), serum electrolytes (63,193 every 6 months), and cholesterol measurements (47,115 every 6 months). Patients treated with thiazides had more measurements of renal function, serum electrolytes, or glucose during follow-up than patients prescribed newer agents, but had less monitoring of serum cholesterol levels (Table 2). These associations were statistically significant on multivariate Poisson regression analyses adjusting for age, gender, co-morbidity, and baseline testing patterns (Table 3).
Table 2.
Laboratory testing while on monotherapy, by drug class
Table 3.
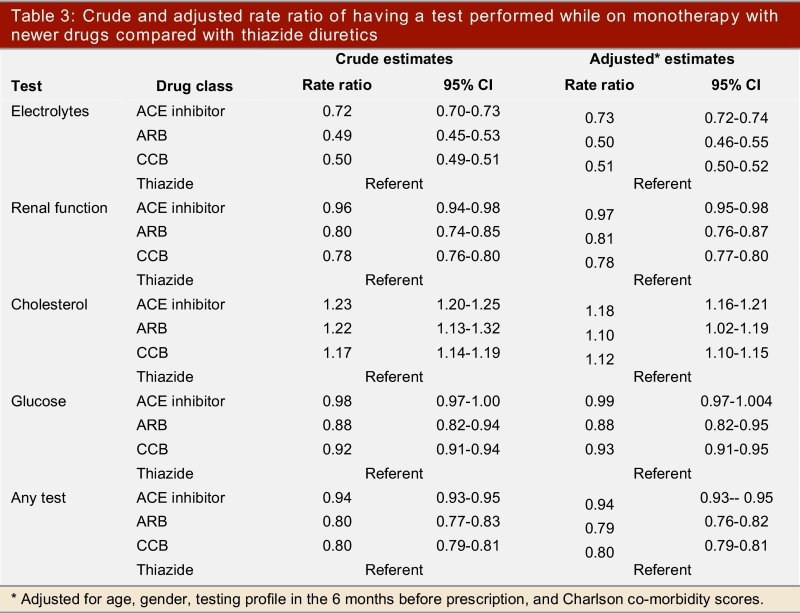
Crude and adjusted rate ratio of having a test performed while on monotherapy with newer drugs compared with thiazide diuretics
Magnitude of differences
Patients treated with thiazide agents had 16 extra electrolyte tests, 6 extra renal function tests, and 4 extra serum glucose tests (but 6 fewer serum cholesterol tests) per 100 patients every 6 months compared with patients prescribed newer agents. The direct cost implications of the laboratory testing profiles we observed in this population-based cohort are, on a per-patient basis and per 6 months, C$11.88 for thiazides and C$11.25 for those treated with the newer drugs. In other words, when considering laboratory testing costs only, the choice of a thiazide for initial monotherapy in our cohort of elderly patients with uncomplicated hypertension resulted in an extra C$0.63 in laboratory testing costs every 6 months.
Discussion
The frequency of laboratory testing in elderly patients newly treated with antihypertensive agents was lower than anticipated, given that the tests we focused on are all recommended to be done as part of the initial workup for newly diagnosed hypertensive patients.8, 20, 21 At baseline, 59% of our cohort did not have any laboratory testing done before initiation of antihypertensive therapy and, over a mean follow-up of 10.3 months, less than half had any laboratory testing done (and almost two-thirds did not have their serum electrolytes or renal function monitored even once). Patients prescribed thiazides were significantly more likely to have their serum electrolytes, glucose, and renal function monitored than patients prescribed any of the newer drug classes, even after adjustment for age, gender, co-morbidities and baseline testing before the initial prescription. However, the magnitude of the increase in laboratory testing frequency was small, and the additional costs per patient of C$0.63 per 6 months are substantially less than the acquisition costs for a 6-month supply of ACE inhibitors (ranging between C$126.79 and C$242.28 in the Ontario Drug Benefit Plan, depending on the particular agent and dose prescribed), angiotensin-receptor blockers (C$214.90 to C$230.74), or calcium-channel blockers (C$90.16 to C$437.93) compared with thiazides ($14.13 for a 6-month supply of 25 mg daily hydrochlorothiazide).
The distribution of antihypertensive drug classes prescribed in our cohort of elderly patients, and the fact that only one-quarter remained on initially prescribed monotherapy for the entire 2-year follow-up period in our study, are consistent with recently published data from other locales.22-25 Our finding that many patients started on antihypertensive therapy do not have guideline-recommended laboratory testing done also confirms the results of previous studies conducted in other settings, including a chart-based audit conducted in one Canadian health region.26-29 In addition to reporting on a larger and population-based sample from a different locale, we have extended these earlier cross-sectional studies by reporting longitudinally on a wider variety of tests in patients treated with all the major antihypertensive drug classes, and with adjustment for age, gender, co-morbidity, and previous testing patterns. The lower degree of laboratory monitoring we found compared with these earlier studies is not unexpected, since our cohort explicitly excluded patients with diabetes or cardiac co-morbidities and patients treated with multiple antihypertensive agents.27, 30
Study limitations
Although our study includes complete information on prescribing and laboratory testing for a large, representative, and population-based sample of all adults aged 66 and over newly treated with antihypertensive agents without co-morbidities or indications other than hypertension, and included 100% follow-up in Canada’s largest province, there are some limitations. Many of these do not affect the validity of our findings. For example, although the use of antihypertensive prescriptions without other indications to define cases of hypertension is a relatively specific marker for the condition, it will miss those patients who have not been prescribed therapy. Yet, data for 1999–2002 from the National Health and Nutrition Examination Survey (NHANES) in the United States, and from the National Public Health and Community Health Surveys in Canada for 2000–2003 have shown that over 80% of elderly people with recognized hypertension are prescribed antihypertensive therapy.31, 32
Second, our study was limited to elderly people; however, the prevalence of hypertension increases with age, and more than half of all elderly people are hypertensive.33
Third, in creating our cohort we excluded patients with diabetes or cardiac co-morbidities, and any patients treated with more than one antihypertensive agent; thus our cohort was undoubtedly a relatively healthy group compared with many elderly hypertensive populations. As a result, the testing patterns we observed likely underestimate true testing rates in elderly people with hypertension, as physicians are likely to more closely follow laboratory parameters in their sicker patients. However, restricting our cohort to healthy patients enhances our ability to attribute differences in testing patterns between drug classes to those agents rather than to differing patterns of co-morbidities in patients prescribed particular drug classes.
Fourth, just as we have data only on patients who fill their antihypertensive prescriptions, our laboratory test data are also affected by patient compliance. To the extent that some patients do not follow through with recommended testing, our observed testing rates likely underestimate physician test-ordering behaviour.
Fifth, a formal cost-effectiveness (or cost-minimization) analysis would require incorporation of the costs induced by testing (such as additional diagnoses and physician visits, clinical events, and indirect costs), which we did not capture; however, our data do permit estimation of the direct costs for the testing patterns we observed.
Sixth, we examined testing only within the first 24 months after a new antihypertensive prescription. Previous studies have demonstrated that if testing is done at all, it is usually done within the first month of a new prescription.28 Indeed, we did find that testing frequency was highest in the first 90 days after prescription and declined subsequently.
Some study limitations, however, could affect the interpretation of our findings, and are unavoidable given the datasets at our disposal. For example, we did not have access to detailed clinical information such as blood pressure levels or co-morbidities that are not coded in administrative databases; and the reasons why physicians chose to prescribe one drug over another are not recorded in administrative data. Although this does not directly affect our question of interest (whether laboratory testing patterns differ across antihypertensive drug classes), it does prevent the classification of the "appropriateness" of the observed testing patterns. As co-morbidities may influence the choice of antihypertensive agent and the frequency of laboratory monitoring, the influence of these unmeasured confounders on our findings are unknown. However, as thiazide-treated patients were older and had higher Charlson scores, it is not unreasonable to speculate that these unmeasured confounders would bias toward higher test frequency in thiazide-treated patients. So, although the magnitude of this bias is impossible to ascertain in this data set, it is reassuring that the direction of this bias actually serves to strengthen our conclusion that the use of thiazides did not induce a marked increase in laboratory testing.
Second, we censored follow-up at the time patients were switched from antihypertensive monotherapy, admitted to hospital, or died, and calculated test densities with each drug class to adjust for varying lengths of follow-up. Thus, while randomized trials suggest little difference between drug classes in these outcomes (and randomized trials would be the most appropriate design to detect such differences), it should be acknowledged that any differential censoring (if, for example, one drug class caused excess hospital admissions compared with the other agents) could have influenced our results by spuriously reducing the apparent association between that drug class and laboratory testing (or spuriously increasing the apparent association if hospital admission were preceded by an increasing pattern of testing).
Third, the OHIP database records only those tests done at community (i.e., not hospital-based) laboratories and those paid from OHIP funds. However, our study focused only on outpatients, and 76% of all outpatient laboratory tests in Ontario are captured in these databases.14, 15 It seems unlikely that patients treated with one particular drug class would be more (or less) likely to get their tests done in a hospital laboratory or a private laboratory versus a publicly funded community laboratory.
Fourth, we have neither examined nor adjusted for any potential changes in laboratory testing patterns over the 8-year duration of this study. Although the question of whether laboratory utilization in Ontario has changed over the past decade is an interesting one, it would be best answered with a larger data set and controlling for ordering/prescribing physician, system-level factors (particularly since health care restructuring was operant at that time), as well as patient-level variables.
Fifth, it is not possible with administrative claims databases to definitely attribute causation (i.e., that conducting the observed tests was specifically due to the therapy prescribed), but merely association (i.e., that certain test ordering patterns are associated with particular drug classes).
Finally, because we are unable to link the prescribing physician with the laboratory and drug benefit databases, we cannot adjust for the prescribing physician as a possible confounder of the association between antihypertensives and test frequency. Although the fact that there are over 28,000 physicians practising in Ontario implies that the impact of a handful of physicians is likely to be negligible on our results, future studies should investigate whether clinicians who frequently order laboratory tests differ in their prescribing behaviours from those that order fewer tests.
It must be recognized that our study is a description of current patterns of practice as they relate to testing; whether or not frequency of "appropriate" testing actually improves hypertension-related health outcomes is not something that has yet been answered in the literature, nor was our study designed to address this issue. Indeed, none of the plethora of large randomized trials of antihypertensive agents published in the past decade is able to answer this question because many trial participants withdrew from therapy and the majority of trial patients were treated with multiple drugs. Both of these facts make it difficult to attribute laboratory abnormalities, and the timing of their detection, to the initially allocated drug(s). As a result, current recommendations for the frequency with which to monitor laboratory tests in patients prescribed antihypertensive therapies are largely subjective and are based on expert opinion with little empirical evidence to support either appropriateness or cost-effectiveness. There is clearly a need for better research to inform future recommendations for laboratory monitoring based on the frequency, severity and timing of abnormalities seen when particular therapies are used in clinical practice.
In conclusion, we found that in a cohort of elderly patients newly treated with antihypertensive drugs and without non-blood pressure lowering indications or cardiac co-morbidities, laboratory testing before and after their first prescription was infrequent. Although those initially treated with thiazides had laboratory tests performed more frequently, the magnitude of the increase was small. As a result, although the costs for laboratory testing in thiazide-treated patients were higher than for those patients treated with newer agents (C$0.63 per 6 months), when balanced against their known efficacy1 and the substantially higher acquisition costs for ACE-inhibitors, angiotensin-receptor blockers or calcium-channel blockers, the use of thiazides remains an attractive economic option for the treatment of uncomplicated hypertension. Our study refutes the claim that the use of thiazide diuretics for hypertension treatment results in marked additional laboratory monitoring costs, and thus provides support for those who advocate the use of thiazide diuretics as first-line agents for the treatment of uncomplicated hypertension.
Acknowledgments
This research was supported by a grant-in-aid from the Heart and Stroke Foundation of Ontario (grant #NA 5459). The Foundation was not involved in the design or conduct of the study, collection of the data, analysis or interpretation, or the preparation, review or approval of the manuscript. KT is supported by a Canadian Institutes of Health Research (CIHR) Short-Term Clinician Investigator Award. FM and SM are supported by Alberta Heritage Foundation for Medical Research Population Health Scholar Awards and by CIHR New Investigator Awards, and FM also holds the University of Alberta/Merck Frosst/Aventis Chair in Patient Health Management.
Biographies
Dr. McAlister is an Associate Professor in the Division of General Internal Medicine at the University of Alberta.
Dr. Majumdar is an Associate Professor in the Division of General Internal Medicine at the University of Alberta.
Dr. Tu is an Assistant Professor in the Department of Family Medicine at the University of Toronto.
Dr. Padwal is an Assistant Professor in the Division of General Internal Medicine at the University of Alberta.
Zhongliang Chen is a data analyst at ICES.
Dr. Campbell is a Professor in the Division of General Internal Medicine and the Department of Clinical Pharmacology at the University of Calgary.
Footnotes
Competing interests: None declared.
References
- 1.Psaty Bruce M, Lumley Thomas, Furberg Curt D, Schellenbaum Gina, Pahor Marco, Alderman Michael H, Weiss Noel S. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003 May 21;289(19):2534–2544. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 2.Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively designed overviews of randomised trials. Lancet. 2003;362:1527–35. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 3.Staessen Jan A, Wang Ji-Guang, Thijs Lutgarde. Cardiovascular prevention and blood pressure reduction: a quantitative overview updated until 1 March 2003. J Hypertens. 2003 Jun;21(6):1055–1076. doi: 10.1097/01.hjh.0000059044.65882.db. [DOI] [PubMed] [Google Scholar]
- 4.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs. diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288(23):2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 5.Neaton JD, Grimm RH, Prineas RJ. Treatment of Mild Hypertension Study. Final results. JAMA. 1993;270:7113–24. [PubMed] [Google Scholar]
- 6.Materson B J, Reda D J, Cushman W C, Massie B M, Freis E D, Kochar M S, Hamburger R J, Fye C, Lakshman R, Gottdiener J. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med. 1993 Apr 1;328(13):914–921. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]
- 7.Hansson L, Lindholm L H, Ekbom T, Dahlöf B, Lanke J, Scherstén B, Wester P O, Hedner T, de Faire U. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet. 1999 Nov 20;354(9192):1751–1756. doi: 10.1016/S0140-6736(99)10327-1. [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Clinical Excellence/British Hypertension Society NICE Clinical Guideline No. 34. Hypertension: Management of hypertension in adults in primary care. 2006. http://www.nice.org.uk/CG034.
- 9.Fischer Michael A, Avorn Jerry. Economic implications of evidence-based prescribing for hypertension: can better care cost less? JAMA. 2004 Apr 21;291(15):1850–1856. doi: 10.1001/jama.291.15.1850. [DOI] [PubMed] [Google Scholar]
- 10.Stafilas Panagiotis C, Sarafidis Panteleimon A, Lasaridis Anastasios N, Aletras Vassilios H, Niakas Dimitris A. An economic evaluation of the 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of mild-to-moderate hypertension in Greece. Am J Hypertens. 2005 Sep;18(9 Pt 1):1233–40; discussion 1241-2. doi: 10.1016/j.amjhyper.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Tu Karen, Campbell Norman R C, Duong-Hua Minh, McAlister Finlay A. Hypertension management in the elderly has improved: Ontario prescribing trends, 1994 to 2002. Hypertension. 2005 Jun;45(6):1113–1118. doi: 10.1161/01.HYP.0000164573.01177.95. [DOI] [PubMed] [Google Scholar]
- 12.Williams JI, Young W. 1996. A summary of studies on the quality of health care administrative databases in Canada; pp. 339–347. http://www.ices.on.ca/file/Practice2-appendix.pdf. [Google Scholar]
- 13.Hux Janet E, Ivis Frank, Flintoft Virginia, Bica Adina. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002 Mar;25(3):512–516. doi: 10.2337/diacare.25.3.512. [DOI] [PubMed] [Google Scholar]
- 14.1995. Salient features of the laboratory industry in Ontario, 1992–93. [Google Scholar]
- 15.van Walraven C, Chan B. Effect of population-based interventions on laboratory utilization: a time-series analysis. JAMA. 1998;280:2028–33. doi: 10.1001/jama.280.23.2028. [DOI] [PubMed] [Google Scholar]
- 16.van Walraven C, Raymond M. Population-based study of repeat laboratory testing. Clin Chemistry. 2003;49:1997–2005. doi: 10.1373/clinchem.2003.021220. [DOI] [PubMed] [Google Scholar]
- 17.Charlson M E, Pompei P, Ales K L, MacKenzie C R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Khan Nadia, McAlister Finlay A. Re-examining the efficacy of beta-blockers for the treatment of hypertension: a meta-analysis. CMAJ. 2006 Jun 6;174(12):1737–1742. doi: 10.1503/cmaj.060110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messerli F H, Grossman E, Goldbourt U. Are beta-blockers efficacious as first-line therapy for hypertension in the elderly? A systematic review. JAMA. 1998 Jun 17;279(23):1903–1907. doi: 10.1001/jama.279.23.1903. [DOI] [PubMed] [Google Scholar]
- 20.Hemmelgarn Brenda R, McAllister Finlay A, Myers Martin G, McKay Donald W, Bolli Peter, Abbott Carl, Schiffrin Ernesto L, Grover Steven, Honos George, Lebel Marcel, Mann Karen, Wilson Thomas, Penner Brian, Tremblay Guy, Tobe Sheldon W, Feldman Ross D. The 2005 Canadian Hypertension Education Program recommendations for the management of hypertension: part 1- blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol. 2005 Jun;21(8):645–656. [PubMed] [Google Scholar]
- 21.Chobanian Aram V, Bakris George L, Black Henry R, Cushman William C, Green Lee A, Izzo Joseph L, Jones Daniel W, Materson Barry J, Oparil Suzanne, Wright Jackson T, Roccella Edward J. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003 May 21;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 22.Gu Qiuping, Paulose-Ram Ryne, Dillon Charles, Burt Vicki. Antihypertensive medication use among US adults with hypertension. Circulation. 2006 Jan 17;113(2):213–221. doi: 10.1161/CIRCULATIONAHA.105.542290. [DOI] [PubMed] [Google Scholar]
- 23.Monane M, Bohn R L, Gurwitz J H, Glynn R J, Levin R, Avorn J. The effects of initial drug choice and comorbidity on antihypertensive therapy compliance: results from a population-based study in the elderly. Am J Hypertens. 1997 Jul;10(7 Pt 1):697–704. doi: 10.1016/S0895-7061(97)00056-3. [DOI] [PubMed] [Google Scholar]
- 24.Bourgault C, Rainville B, Suissa S. Antihypertensive drug therapy in Saskatchewan: patterns of use and determinants in hypertension. Arch Intern Med. 2001;161(15):1873–1879. doi: 10.1001/archinte.161.15.1873. [DOI] [PubMed] [Google Scholar]
- 25.Cushman WC, Ford CE, Cutler JA. Success and predictors of blood pressure control in diverse North American settings: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) J Clin Hypertens. 2002;4:393–404. doi: 10.1111/j.1524-6175.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- 26.Hurley Judith S, Roberts Melissa, Solberg Leif I, Gunter Margaret J, Nelson Winnie W, Young Linda, Frost Floyd J. Laboratory safety monitoring of chronic medications in ambulatory care settings. J Gen Intern Med. 2005 Apr;20(4):331–333. doi: 10.1111/j.1525-1497.2005.40182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raebel Marsha A, McClure David L, Simon Steven R, Chan K Arnold, Feldstein Adrianne, Andrade Susan E, Lafata Jennifer Elston, Roblin Douglas, Davis Robert L, Gunter Margaret J, Platt Richard. Laboratory monitoring of potassium and creatinine in ambulatory patients receiving angiotensin converting enzyme inhibitors and angiotensin receptor blockers. Pharmacoepidemiol Drug Saf. 2007 Jan;16(1):55–64. doi: 10.1002/pds.1217. [DOI] [PubMed] [Google Scholar]
- 28.Raebel Marsha A, Lyons Ella E, Andrade Susan E, Chan K Arnold, Chester Elizabeth A, Davis Robert L, Ellis Jennifer L, Feldstein Adrianne, Gunter Margaret J, Lafata Jennifer Elston, Long Charron L, Magid David J, Selby Joseph V, Simon Steven R, Platt Richard. Laboratory monitoring of drugs at initiation of therapy in ambulatory care. J Gen Intern Med. 2005 Dec;20(12):1120–1126. doi: 10.1111/j.1525-1497.2005.0257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAlister F A, Teo K K, Lewanczuk R Z, Wells G, Montague T J. Contemporary practice patterns in the management of newly diagnosed hypertension. CMAJ. 1997 Jul 1;157(1):23–30. [PMC free article] [PubMed] [Google Scholar]
- 30.Simon Steven R, Andrade Susan E, Ellis Jennifer L, Nelson Winnie W, Gurwitz Jerry H, Lafata Jennifer Elston, Davis Robert L, Feldstein Adrianne, Raebel Marsha A. Baseline laboratory monitoring of cardiovascular medications in elderly health maintenance organization enrollees. J Am Geriatr Soc. 2005 Dec;53(12):2165–2169. doi: 10.1111/j.1532-5415.2005.00498.x. [DOI] [PubMed] [Google Scholar]
- 31.Onysko Jay, Maxwell Colleen, Eliasziw Michael, Zhang Jenny X, Johansen Helen, Campbell Norm R C. Large increases in hypertension diagnosis and treatment in Canada after a healthcare professional education program. Hypertension. 2006 Nov;48(5):853–860. doi: 10.1161/01.HYP.0000242335.32890.c6. [DOI] [PubMed] [Google Scholar]
- 32.Hajjar Ihab, Kotchen Theodore A. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. JAMA. 2003 Jul 9;290(2):199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 33.Fields Larry E, Burt Vicki L, Cutler Jeffery A, Hughes Jeffrey, Roccella Edward J, Sorlie Paul. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004 Oct;44(4):398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]