Abstract
Monosodium glutamate (MSG), a common flavor enhancer in various canned food and stereotypically associated with food in Chinese restaurants, has been claimed and tested to have side effects including headache and dizziness. However, the mechanism behind MSG-induced headache was not clear. Using dissociated mouse neuronal culture and cell injury assays, we determined whether incubation of neurons with clinically relevant concentrations of MSG induces cell swelling or death, and whether any measure can be taken to prevent or reduce MSG effects. We demonstrated that (1) Treatment with MSG induces a dose-dependent swelling and death of mature neurons (12-14 days in culture) with little effect on young immature neurons (<1 week in culture). The threshold concentration of MSG for neuronal injury is 3 µM; (2) MSG only injures neurons with little effect on glial cells; (3) Boiling MSG does not affect its toxicity but the addition of Vitamin C provides significant protection against MSG toxicity; (4) Pretreatment of neurons with a low dose of MSG reduces subsequent injury by a large dose of MSG. Together, our studies suggest that the side effect of MSG may be mediated, at least in part, by its toxic effect on brain neurons. Pre-exposure to low doses of MSG or the use of Vitamin C may prevent or reduce the side effects of MSG.
Keywords: Monosodium glutamate, headache, neuron, injury, tolerance
Introduction
Monosodium glutamate (MSG), a common flavor enhancer found in various canned food and stereotypically associated with food in Chinese restaurants [1, 2], has been claimed and tested to have various side effects including headache and dizziness [3–5]. A double-blind, placebo-controlled, randomized study has confirmed the link between MSG usage and these various symptoms [4]. Case studies were also presented in which the elimination of all food sources of MSG resulted in decreased headache frequency [3].
However, the mechanism underlying MSG induced side effects such as headache has not been clear. In addition, there appeared to be some controversy over the MSG mediated headaches. For example, there was no report that children experience headaches after MSG intake. Also, MSG is commonly used among Chinese people, but it does not seem to be a problem among this population.
Therefore, the purpose of this study was to find some explanation for MSG-induced headaches and to explore possible ways to prevent or reduce the MSG-induced side effects. Using dissociated mouse neuronal culture and cell injury assays, we questioned whether the treatment of neurons with clinically relevant concentrations of MSG induces cell swelling or death which might partially explain its side effect. We demonstrate that (1) Treatment with MSG induces a dose-dependent swelling and death of mature neurons (12-14 days in culture) with little effect on young immature neurons (<1 week in culture); (2) MSG only injures neurons with little effect on glial cells; (3) Boiling MSG does not affect its toxicity but the addition of Vitamin C provides significant protection against MSG toxicity; (4) Pretreatment of neurons with a low, non-injurious dose of MSG reduces subsequent injury by a large dose of MSG. In conclusion, our studies suggest that MSG side effects may be partially mediated by its toxic effect on brain neurons. There are certain ways that one might either prevent or reduce the side effect of MSG.
Materials and methods
Primary cortical neuronal cultures
The method for culturing mouse cortical neurons was essentially the same as the technique described previously [6;7]. The use of mice for neuronal cell culture was approved by the Institutional Animal Care and Use Committee of Legacy Clinical Research and Technology Center. Briefly, time-pregnant (E16) Swiss mice were anaesthetized with halothane followed by decapitation. Brains were rapidly removed from fetuses and placed in ice-cold Ca2+/Mg2+-free Dulbecco PBS solution. Cerebral cortices were dissected and enzyme digested with 0.05% trypsin-EDTA for 10 min at 37°C, followed by trituration with fire-polished glass pipettes, and plated on poly-L-ornithine-coated 24-well plates or individual culture dishes (35 mm in diameter), at a density of 2 × 105 per well or 1 × 106 cells per dish. Neurons were cultured with Neurobasal medium supplemented with B27 and maintained at 37°C in a humidified 5% CO2 atmosphere incubator. Cultures were fed twice a week and used for the studies 6 ∼ 14 days after plating.
Cell injury assay - LDH measurement
Lactate dehydrogenase (LDH) is a ubiquitous protein present in all cells. It is rapidly released into cell culture medium upon damage of the cell membrane and the concentration of LDH released is proportional to the number of cells damaged [8, 9]. LDH released in the culture medium was measured using the LDH assay kit (Roche Molecular Biochemicals). 50 µl samples of cell culture medium were collected in a 96-well plate and mixed with 50 µl reaction solution from the LDH kit. Absorbance at OD490 nm was measured 30 minutes after the reaction. To calculate a relative percentage of cell injury in each well, the maximal releasable LDH was also obtained in each well by a 15 min incubation with 0.5% triton-X100 at the end of each experiment.
Alive/dead staining
For fluorescein-diacetate (FDA) and propidium iodide (PI) staining of live and dead cells, neurons were incubated in medium containing FDA (5 µM) and PI (2 µM) for 30 min followed by three washes with dye-free physiological solution. Alive (FDA-positive) and dead (PI-positive) cells were viewed and counted on a microscope (Nikon) equipped with epifluorescence at 580/630 nm excitation/emission for PI, and 500/550 nm for FDA. Images were collected using a digital camera (Nikon Coolpix 5000).
Data analysis
All data were expressed as mean ± SE. Student's t-test and ANOVA were used for statistical analysis where appropriate. The criterion of significance was set at p<0.05.
Results
Dose-dependent injury of mature neurons by MSG (3 - 300 µM)
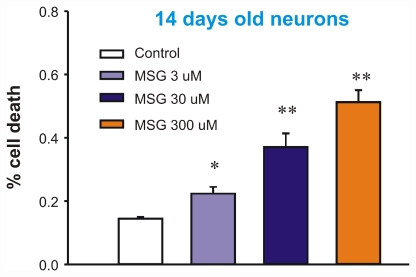
We first determined whether treatment of mature neurons with MSG can cause cell swelling or injury. At 14 days after plating, mouse cortical neurons cultured in 24-well plates were incubated with different concentration of MSG (Manufactured by Ajinomoto Food Ingredients LLC, Chicago, IL). Cell injury was analyzed by LDH release 24h after the incubation. As shown in Figure 1, incubation of neurons with MSG caused a dose-dependent increase in LDH release, indicating neuronal injury. At 3, 30, or 300 µM MSG, the percentage of cell death was approximately 25%, ∼40%, or 50% respectively (n=8). Similar to LDH data, live/dead staining with fluorescein diacetate/propidium iodide also showed a dose-dependent cell damage by MSG (Figure 2).
Figure 1.
Dose-dependent injury of mature neurons by MSG (3 - 300 µM). At day 14, cultured mouse cortical neurons in 24 wells were treated with different concentrations of MSG for 12h. 50 µl sample of culture medium was withdrawn from each well for LDH measurement. Cells in each well were then lysed with 0.5% Triton X-100. This treatment gives total LDH. Percentage of cell death is calculated by dividing the LDH value by the total value in each well. n = 8, *p<0.05, **p<0.01 compared to control.
Figure 2.
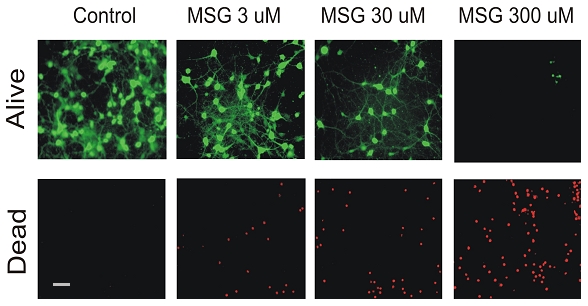
Analyze MSG damage of neurons by alive and dead staining. Cultured mouse cortical neurons were treated with different concentrations of MSG for 12h. Alive cells were stained with fluorescein diacetate. Dead cell were stained with propidium iodide. Increasing MSG doses decreased total number of live neurons (green) but increased total number of dead neurons (red). Same experiments were repeated 3 times. Scale bar: 40 µm.
Time-dependent injury of neurons by MSG treatment
We next determined whether MSG induced cell injury is time-dependent. Mature neurons were treated with a 30 µM dose of MSG. The change in cell morphology was monitored at different time points following the incubation with MSG. As shown in Figure 3, 30 minutes after the MSG treatment, a clear swelling of neurons was observed. After 2h of MSG treatment, severe swelling of neurons was seen. 12h after MSG treatment, neurons were completely lysed. Thus, neuronal damage by MSG is time dependent.
Figure 3.
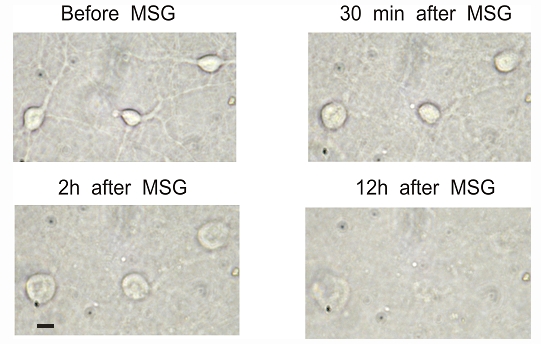
Time-dependent injury of neuronal cells by 30 µM MSG. Cultured mouse cortical neurons in 35 mm dish were continuously monitored after treatment with 30 µM MSG. Neurons started to swell even after 30 minutes of MSG treatment. The swelling got worse at 2h. 12h after MSG treatment, neurons were lysed. Scale bar: 15 µm.
MSG has less effect on immature neurons
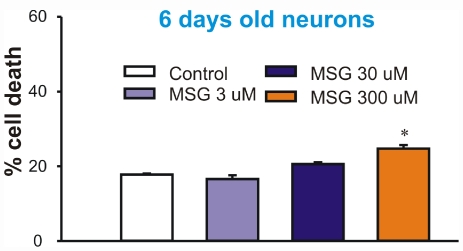
After testing the effect of MSG on the mature neurons, we examined whether MSG has similar effects on young immature neurons (cultured for less than 7 days). To our surprise, immature neurons were very resistant to MSG injury. Incubation of these neurons with 3 or 30 µM MSG for 24h did not induce any injury, while incubation with 300 µM MSG only induced a slight increase of LDH release (Figure 4). This result suggests that the young and immature neurons are less sensitive to the MSG damage as compared to mature neurons. This result might explain why young children are less affected by MSG intake.
Figure 4.
Younger neurons (6-7 days old) were not sensitive to MSG damage. At day 6-7, cultured mouse cortical neurons in 24 wells were treated with different concentrations of MSG for 12h. 50 µl sample of medium was withdrawn from each well for LDH measurement. Cells were then lysed with 0.5% Triton X-100. This treatment gives total LDH. Percentage of cell death is calculated by dividing the LDH value by the total value in each well. n = 8.
MSG does not damage glial cells
We next examined whether MSG can cause injury to glial cells, another major cell type in the brain. Individual dishes containing cultured glial cells were treated with 0, 3, 30, or 300 µM of MSG for 24h. As shown in Figure 5, treatment of glial cells with MSG, at concentrations as high as 300 µM, did not cause clear damage. Thus, MSG-induced cell injury is neuron specific.
Figure 5.
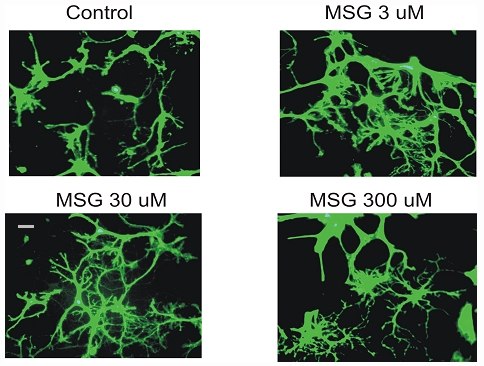
MSG does not damage glial cells. Individual dishes containing cultured glial cells were treated with 0, 3, 30, or 300 µM MSG for 24h. Cells were then labeled with fluorescein diacetate. Same experiments were repeated 3 times. MSG did not cause significant damage to glial cells even at 300 µM.
Boiling does not reduce damaging effect of MSG
Next, we explored possible ways of preventing or reducing the MSG damage of neurons. We first determined whether boiling MSG for 10 min reduces its toxicity. 14 days old cultured neurons were treated with boiled MSG (30 µM) for 12h. As shown in Figure 6, treatment of neurons with boiled MSG produced a similar injury of neurons as non-boiled MSG (Figure 6). Therefore, boiling MSG is not sufficient to reduce its damaging effect.
Figure 6.
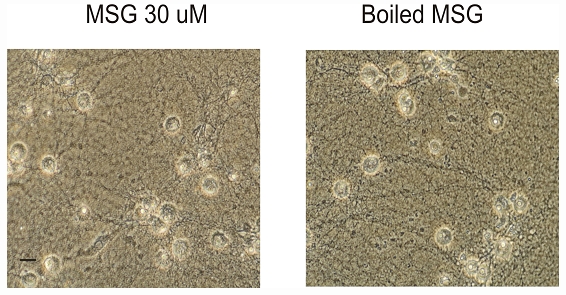
Boiling MSG does not reduce its toxic effect. At day 14, cultured mouse cortical neurons in 35 mm culture dishes were treated with 30 µM non-boiled MSG or boiled MSG for 12h. Treatment of neurons with boiled MSG produced the same damage as non-boiled MSG. The same experiments were repeated 3 times. Scale bar: 20 µm.
Vitamin C reduces MSG damage of neurons
Ascorbic acid or Vitamin C is an important endogenous neuroprotectant in the brain [10;11]. It has been show to be neuroprotective in various conditions. Therefore, we tested whether the addition of Vitamin C has any effect on MSG-induced damage of neurons. 1 mM vitamin C was added to the culture medium during MSG treatment. As shown in Figure 7A and B, treating neurons with vitamin C provided a clear protection against MSG damage (n=3 experiments).
Figure 7.
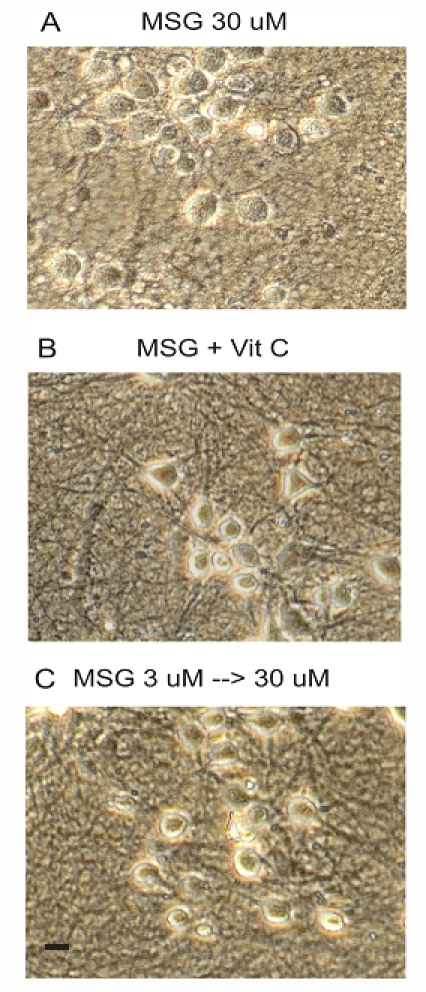
Treatment with Vitamin C or pretreatment with low dose of MSG reduced MSG damage of mature neurons. A. Neurons treated with 30 µM MSG alone for 24h. B. Neurons treated with 30 µM MSG + 1 mM Vitamin C for 24h. C. Neurons were treated with 3 µM MSG for 12h followed by 30 µM MSG for 24h. Scale bar: 20 µm.
Pretreatment of neurons with a low dose of MSG reduces the injury of neurons by subsequent high dose of MSG
It has been widely accepted that a stimulus or stressor capable of causing injury to a tissue or organ may, when applied close to the threshold of damage, activate endogenous protective mechanisms which can potentially reduce the damaging effect of subsequent, more severe stimuli [12]. For example, a sub-threshold ischemic insult applied to the brain has been shown to activate certain cellular pathways that can help to reduce damage caused by subsequent long ischemic episodes, a phenomenon known as ischemic preconditioning or ischemic tolerance [13]. For this reason, we determined whether a pretreatment of mature neurons with a low dose of MSG can establish tolerance against injury of neurons by subsequent high doses of MSG. We selected 3 µM MSG since it did not produce much injury. Neurons were first treated with 3 µM MSG for 12h, followed by treatment with 30 µM MSG for 24h. As shown in Figure 7A and C, pre-treating neurons with 3 µM MSG for 12h reduced the subsequent neuronal injury by 30 µM MSG (n=3 experiments). This data suggests that taking a low and non-damaging dose of MSG may prevent injury of neurons by subsequent high doses of MSG.
Discussion
Since the report of “Chinese Restaurant Syndrome” in 1968, various studies have confirmed the link between MSG intake and its side effects including headaches [4, 5]. In those studies, an oral dose of MSG at 3g or higher reproduced restaurant syndrome within 30 min [5]. A direct intravenous dose of 50 mg also produced similar symptoms. Although the MSG concentration in the blood is difficult to predict with oral doses of MSG, a direct intravenous injection of 50 mg MSG is expected to yield a blood concentration of 53 µM (based on the total blood volume of 5 litters). Although the blood brain barrier (BBB) has low permeability to MSG, the presence of high affinity glutamate transporters located at the BBB capillary luminal membrane could facilitate the uptake of MSG into the brain. Assuming 10% of the blood concentration can be reached in the cerebral spinal fluid, it would yield ∼5 µM MSG in the brain which is above the threshold concentration of MSG for causing neuronal injury in our studies. Thus, the concentrations of MSG used in our studies were clinically relevant. Our finding that neuronal swelling takes place at ∼30 min after MSG treatment is also consistent with the time course of MSG-induced headache in clinical studies.
Using neuronal culture technique and cell injury assay, we studied the effect of MSG on mouse cortical neurons, a commonly used in vitro preparation for cell injury studies. We demonstrated that incubation with MSG, at clinical relevant concentrations, induced swelling and injury of mature neurons. This finding may partially explain the headache induced by MSG intake.
In contrast to mature neurons, immature neurons are very resistant to MSG damage. For example, incubation of immature neurons with 30 µM MSG, a concentration sufficient to induce substantial injury of mature neurons, did not induce clear injury of immature neurons. This finding might explain why young children, whose brain neurons are not fully mature, do not experience headaches after MSG intake.
We showed that boiling MSG is not sufficient to reduce its toxicity. However, taking vitamin C is effective in reducing MSG-induced neuronal injury. More interestingly, we demonstrated that pretreatment of neurons with a low dose of MSG can make neurons tolerant to subsequent high doses of MSG. Although the exact mechanism underlying this tolerance remains to be determined, such knowledge may give us an idea of how to take preventative measures for MSG-induced headaches.
The reason why young neurons are not damaged by MSG is not clear. Since young neurons are not fully developed, they may be missing some receptors that are required for MSG binding or downstream signaling pathway(s) required for MSG toxicity. The finding that pre-exposure of a low dose of MSG can generate tolerance may explain why Chinese populations do not experience headaches after MSG intake. It is likely that they have already established tolerance to MSG due to previous exposures to low doses of MSG when they were young. In the present study, we only examined the tolerance 12h after pre-exposure to MSG. In future studies, it would be interesting to determine whether the tolerance can last for days or longer.
The results of the “Boiled MSG” revealed that no preventative measures can be made by over-cooking of MSG-containing food. We had hoped that boiling might be able to destroy the structure of MSG thus reducing or eliminate its damaging effects. However, boiling MSG for 10 minutes did not reduce any damaging effects at all. Future studies may consider boiling MSG for a much longer time (e.g. 1h).
Glutamate is an endogenous neurotransmitter required for a variety of physiological functions of neurons [14]. Increased release of endogenous glutamate has been suggested to play an important role in neuronal injury associated with a number of neurological disorders [14, 15]. Previous studies have also shown that incubation of neurons with high concentrations of exogenously applied glutamate (e.g. 500 µM) rapidly induces neuronal cell death [16]. We show here that with a threshold concentration of as low as 3 µM, MSG can induce detectable neuronal injury if incubated for a prolonged period of time. Further studies may determine whether cell injury induced by low doses of MSG share other characteristics with previously reported glutamate toxicity, e.g. reduced by removal of the extracellular Ca2+ [16] and blockade by glutamate antagonists.
In conclusion, our studies suggest that the headaches caused by MSG intake may be related to its injurious effect on brain neurons. Although the brain does not register pain because of the lack of nociceptors, the increase of intracranial pressure due to cell swelling is well known to cause headaches. There are some possible ways, e.g. taking Vitamin C or pre-exposure to a low dose of MSG, that may either prevent or reduce the side effects of MSG.
References
- 1.Morselli PL, Garattini S. Monosodium glutamate and the Chinese restaurant syndrome. Nature. 1970;227((5258)):611–612. doi: 10.1038/227611a0. [DOI] [PubMed] [Google Scholar]
- 2.Schaumburg H. Chinese-restaurant syndrome. N Engl J Med. 1968;278((20)):1122. doi: 10.1056/nejm196805162782014. [DOI] [PubMed] [Google Scholar]
- 3.Scopp AL. MSG and hydrolyzed vegetable protein induced headache: review and case studies. Headache. 1991;31((2)):107–110. doi: 10.1111/j.1526-4610.1991.hed3102107.x. [DOI] [PubMed] [Google Scholar]
- 4.Yang WH, Drouin MA, Herbert M, Mao Y, Karsh J. The monosodium glutamate symptom complex: assessment in a double-blind, placebo-controlled, randomized study. J Allergy Clin Immunol. 1997;99((6 Pt 1)):757–762. doi: 10.1016/s0091-6749(97)80008-5. [DOI] [PubMed] [Google Scholar]
- 5.Schaumburg HH, Byck R, Gerstl R, Mashman JH. Monosodium L-glutamate: its pharmacology and role in the Chinese restaurant syndrome. Science. 1969;163((869)):826–828. doi: 10.1126/science.163.3869.826. [DOI] [PubMed] [Google Scholar]
- 6.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable Acid-sensing ion channels. Cell. 2004;118((6)):687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Chu XP, Wemmie JA, Wang WZ, Zhu XM, Saugstad JA, Price MP, Simon RP, Xiong ZG. Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J Neurosci. 2004;24((40)):8678–8689. doi: 10.1523/JNEUROSCI.2844-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukhin AG, Ivanova SA, Allen JW, Faden AI. Mechanical injury to neuronal/glial cultures in microplates: role of NMDA receptors and pH in secondary neuronal cell death. J Neurosci Res. 1998;51((6)):748–758. doi: 10.1002/(SICI)1097-4547(19980315)51:6<748::AID-JNR8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 9.Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987;20((1)):83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- 10.Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23((5)):209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- 11.Grunewald RA. Ascorbic acid in the brain. Brain Res Brain Res Rev. 1993;18((1)):123–133. doi: 10.1016/0165-0173(93)90010-w. [DOI] [PubMed] [Google Scholar]
- 12.Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26((5)):248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 13.Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, Meller R, Rosenzweig HL, Tobar E, Shaw TE, Chu X, Simon RP. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362((9389)):1028–1037. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- 14.Greenamyre JT. The role of glutamate in neurotransmission and in neurologic disease. Arch Neurol. 1986;43((10)):1058–1063. doi: 10.1001/archneur.1986.00520100062016. [DOI] [PubMed] [Google Scholar]
- 15.Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic–ischemic brain damage. Ann Neurol. 1986;19((2)):105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- 16.Choi DW. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett. 1985;58((3)):293–297. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]