Abstract
Despite the wealth of evidence supporting the activation of caspases in Alzheimer's disease (AD), chronic administration of a caspase inhibitor has never been tested in any animal model system. The purpose of the current report was to identify a suitable animal model that displays caspase activation and cleavage of critical proteins associated with AD, and secondly, to undertake a pilot study utilizing the novel caspase inhibitor, quinolyl-valyl-O-methylaspartyl-[-2, 6-difluorophenoxy]-methyl ketone (Q-VD-OPh). Analysis of 12 month-old TgCRND8 mice, which represent an early-onset animal model for AD, indicated the activation of caspase-7 as well as the cleavage of tau and the amyloid precursor protein (APP). Having established that TgCRND8 mice represent a suitable model system to target caspases therapeutically, a prophylactic study was initiated utilizing Q-VD-OPh. Three month-old TgCRND8 mice were injected intraperitoneally three times a week for three months with 10 mg/kg Q-VD-OPh and compared to control mice injected with vehicle. Although there was no apparent effect on extracellular Aβ deposition, chronic treatment with Q-VD-OPh did prevent caspase-7 activation and limited the pathological changes associated with tau, including caspase cleavage. These preliminary findings suggest that further studies examining the utility of Q-VD-OPh as a potential therapeutic compound for the treatment of AD are warranted.
Keywords: Caspase, TgCRND8 mice, Alzheimer's disease, Q-VD-OPh, amyloid, tau
Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by extensive neuronal loss leading to cognitive impairment and dementia. AD is diagnosed based upon the extent of senile plaques composed of Aβ and NFTs containing abnormally phosphorylated and truncated tau [1]. Currently, there is strong support for the amyloid cascade hypothesis, and many of the current therapeutic strategies now in clinical trials involve some aspect of modifying Aβ production or clearance [2]. Despite these advances, recent clinical trials targeting Aμ have been disappointing [3]. It is important, therefore, to identify new potential drug targets for the treatment of AD.
An overwhelming body of evidence supports a role for the activation of caspases and cleavage of critical cellular proteins in the human AD brain. Evidence demonstrating the activation of various caspases including caspase 3, 6, 8, and 9, to the cleavage of target proteins such as APP, actin, fodrin, tau and GFAP have supported a role for caspases in promoting the pathology underlying AD (for recent reviews see, [4, 5]). More recently, studies conducted with transgenic AD mice have provided further evidence that caspase activation plays a pivotal role in the initiation and progression of the pathology associated with AD [6–8]. These studies support the notion that administration of caspase inhibitors could be an effective strategy in the treatment of AD and the critical next step is to test directly whether inhibitors of caspases prevents pathology following administration in animal models of AD.
In order to test whether pharmacological inhibition of caspases is a valid approach, it is necessary to have an appropriate AD animal model that exhibits caspase activation as well as the cleavage of critical target proteins. TgCRND8 mice represent an early-onset AD mouse model that exhibit extensive Aβ deposits and neuritic pathology by as early as 3 months [9, 10], an important consideration with chronic treatment of a compound. The goal of the present report was to determine if caspase activation and cleavage of target proteins occurs in TgCRND8 mice and if so, to examine if treatment with the novel caspase inhibitor, Q-VD-OPh prevents any pathology associated with TgCRND8 mice. The results of this study demonstrated the presence of activated caspase-7 and caspase-cleaved fragments of tau in the TgCRND8 brain, which was prevented following chronic administration of Q-VD-OPh. Although further studies are needed, these findings support the utility of Q-VD-OPh as a potential therapeutic compound for the treatment of AD.
Materials and methods
Tissue acquisition
Mice were anesthetized with pentobarbital, perfused with saline, and the brains rapidly removed. Brains were divided into hemispheres and 1 hemisphere sunk in 4% phosphate-buffered paraformaldehyde, while the other hemisphere was snapped frozen at – 50°C in isopentane. Mouse brains were mounted coronally and sectioned serially at 50 μm on a vibratome, and stored for immunohistochemistry.
Immunohistochemistry
Free-floating 50 μm-thick serial sections were used for immunohistochemical and immunofluorescence studies as previously described [11]. Antibody dilutions were the following: mAb MC-1 (1:500), mAb PHF-1 (1:400), anti beta-amyloid mAb 1560 clone 6E10 (1:400, Chemicon), rabbit active caspase-7 (1:100, Oncogene), rabbit active caspase-3 (1:50, BD Pharmingen), rabbit tau caspase-cleavage product (CCP) (1:100, in house antibody), APPccp (1:100, in house antibody) and mAb TauC3 (1:100, Chemicon). For Aβ visualization, antigen retrieval was accomplished by pre-treating tissue sections in 95% formic acid for 5 minutes followed by three washes in Tris buffered saline. Antigen visualization was determined using ABC complex (ABC Elite immunoperoxidase kit, Vector labs), followed by DAB substrate (Vector Labs). For immunofluorescence co-localization studies, antigen visualization was accomplished using an Alexa fluor 488-labeled tyramide (green, Ex/Em = 495/519) or streptavidin Alexa Fluor 555 (red, Ex/Em = 555/565), both from Invitrogen (Carlsbad, CA). For these studies, brain tissue was collected from 12 month-old TgCRND8 mice (n=6) as well as age-matched NonTg control mice (n=6).
Western blot analysis
Sample preparation was according to Oddo et al., [12]. Western blot analysis was performed utilizing the One-Step™ Advanced Western mouse kit according to manufacturer's instructions (GenScript Corporation, Piscataway, NJ). All samples were analyzed for protein content using the BCA assay (Pierce) to ensure equal protein loading.
Treatment of TgCRND8 mice with Q-VD-OPh
Stock solutions of Q-VD-OPh (SM Biochemicals, Yorba Linda, CA) were prepared in DMSO and diluted in sterile PBS solution prior to injection. A final concentration of 10 mg/kg was chosen based on previous reports indicating neuroprotection at this concentration of Q-VD-OPh [13–15]. Three-month old mice were divided into two groups: control, vehicle (n=3) or treated (n=2). Mice were injected i.p. three times a week with either Q-VD-OPh or vehicle for a total time period of 3 months.
Results
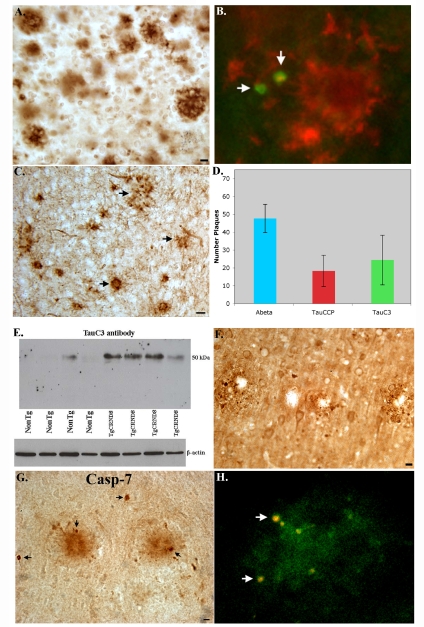
In order to test whether pharmacological inhibition of caspases is a valid approach, it is necessary to have an appropriate AD animal model that exhibits caspase activation as well as the cleavage of critical target proteins. TgCRND8 mice are characterized as a “single” transgenic model containing a double mutant of human APP (KM670/671NL + V717F) under the control of the PrP gene promoter [9]. In addition, TgCRND8 mice represent an early-onset AD mouse model that exhibit extensive Aβ deposits and neuritic pathology by as early as 3 months [9, 10], an important consideration with chronic treatment of a compound. As an initial approach, 12 month-old TgCRND8 mice were examined for the caspase-cleavage of tau using two different site-directed antibodies. Figure 1A displays the typical plaque staining observed in 12 month-old TgCRND8 mice following staining with the mAb 6E10, an anti-Aβ antibody. In Figure 1B, double-label immunofluorescence studies were performed to determine whether caspase-cleaved tau co-localized with Aβ in plaque-rich regions. In this case, experiments were undertaken using the monoclonal antibody to Aβ, 6E10 and the rabbit polyclonal antibody, TauCCP [16]. As shown in Figure 1B (arrows), neuronal cell bodies containing caspase-cleaved tau were found within the vicinity of plaques. It is noteworthy that TauCCP-positive neurons displayed cell bodies that were shrunken and condensed in appeared, characteristic features of cells undergoing apoptosis. We confirmed the results with TauCCP using a similar caspase-cleavage antibody to tau (TauC3) developed by Gamblin et al. [17]. Like the TauCCP antibody, TauC3 labeling was found within plaque-rich regions (Figure 1C).
Figure 1.
Caspase activation in 12 month-old TgCRND8 mice. A) Representative bright-field micrograph illustrating Aβ deposition in TgCRND8 mice utilizing the 6E10 mAB (1:400). B) Double-label immunofluorescence image with mAb 6E10 to detect extracellular Aβ deposits (red) and a polyclonal TauCCP antibody to detect caspase-cleaved tau (arrows, green). C) Representative image using TauC3 indicated the presence of caspase-cleaved tau within plaque-rich regions. D) Semi-quantitative analysis of the number of extracellular plaques labeled with 6E10 (blue bar), the TauCCP antibody (red bar) and TauC3 (green bar). N=6 different animals ± S.D. E) Western blot analysis of brain extracts probed with the mAb TauC3 (1:500) indicating the presence of caspase-cleaved tau in all four TgCRND8 mice with weak labeling in only one of four age-matched NonTg control mice. Bottom panel represents loading control blot following stripping and reprobing using a rabbit antibody to beta-actin (1:400). Data are representative of three independent experiments. F) Representative IH staining utilizing an antibody (APPccp) that detects caspase-cleaved APP. Staining with APPccp was localized within plaques of the neocortex. G) Representative IH analysis in TgCRND8 mice depicting staining with an antibody to active caspase-7. Caspase-7 labeling was evident in plaques and within neurons in the vicinity of plaques (G, arrows). H) Double-label immunofluorescence overlap image from a representative TgCRND8 mouse showing colocalization of active caspase-7 (green) together with the apoptotic label, propidium iodide (red). Propidium iodide staining was only evident in neuronal cell bodies that were also labeled with active caspase-7 (arrows, H). All scare bars represent 10 μm except for Panel C, which represents 20 μm.
A semi-quantitative analysis indicated that TauCCP and TauC3 labeled less than half the number plaques as were present following staining with mAb 6E10 (Figure 1D). These data indicate that only a subset of plaques actually contain caspase-cleaved tau suggesting that Aβ deposition and caspase activation may not be causally related. Western blot analysis using the TauC3 antibody confirmed the presence of caspase-cleaved tau in 12 month-old TgCRND8 mice (Figure 1E).
Next, we tested whether APP is cleaved by caspases using a site-directed caspase-cleavage product antibody to APP. This antibody, termed APPccp, selectively recognizes the fragment of APP generated by caspase-mediated cleavage of APP following cleavage at position D739 [18]. Evidence for caspase-cleaved APP was evident in TgCRND8 mice, with punctate labeling in plaque regions (Figure 1F). Experiments were also performed to examine the extent of caspase-3 activation in 12 month-old TgCRND8 mice. Surprisingly, we found evidence for caspase-7 activation not caspase-3 within plaque regions of 12 month-old TgCRND8 mice (Figure 1G, arrows). There was no evidence for the activation of caspase-3 using several antibodies to the active domain of the enzyme (data not shown). The identification of caspase-7 as the major executioner caspase activated in TgCRND8 mice is a novel finding and to our knowledge no other human or animal studies have implicated the involvement of this caspase in Alzheimer's disease. To confirm the presence of active caspase-7 in neurons undergoing apoptosis, double-label immunofluorescence experiments were carried out utilizing propidium iodide, a general marker for apoptotic cells. As shown in Figure 1H, strong co-localization of the two markers within shrunken cell bodies was evident in plaque-rich regions of TgCRND8 indicating a causal relationship between the activation of caspase-7 and the execution of apoptosis.
Based on these preliminary observations, we hypothesize that caspases are activated in TgCRND8 mice and contribute to the processing of APP and tau. Taken together, these data suggest TgCRND8 mice represent a good model to test whether pharmacologic inhibition of caspases is a feasible approach for preventing the pathology associated with these mice.
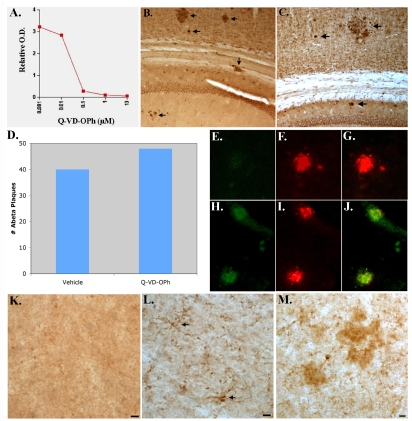
Because caspase-7 appears to be a critical executioner caspase activated in TgCRND8 mice, we assessed whether Q-VD-OPh is capable of inhibiting this caspase in vitro. We confirmed that Q-VD-OPh is a potent inhibitor of caspase-7 with an IC50 of 48 nM utilizing a cell-free assay consisting of human recombinant caspase-7, Q-VD-OPh, and the substrate AMC-DEVD-pNa (Figure 2A). The calculated IC50 of 48 nM for inhibiting caspase-7 by Q-VD-OPh is well in line with known IC50 values (25-400 nM) for other caspases including caspase -1, 3, 8, 9, 10, and 12 (Product sheet, SM Biochemicals).
Figure 2.
The effects of chronic administration of Q-VD-OPh on Aβ deposition, caspase-7 activation, and the caspase-cleavage of tau in TgCRND8 mice. A) Cell-free inhibition of caspase-7 activity by Q-VD-OPh. Data display a concentration-dependent inhibition of caspase-7 cleavage of the substrate AMC-DEVD-pNA in a cell-free system consisting of active human recombinant caspase-7. The calculated IC50 of Q-VD-OPh was determined to be approximately 48 nM. B-D) Three month-old TgCRND8 mice were injected 3 times a week for 3 months with 10 mg/kg of Q-VD-OPh (B) or vehicle (C). Data are representative of 3 independent experiments utilizing the monoclonal antibody to Aβ (clone 6E10, 1:400) and indicate the presence of extracellular Aβ deposits in the cortex and hippocampus of both animals (arrows). Panel D depicts semi-quantitative analysis of the number of Aβ plaques for vehicle or following Q-VD-OPh-treatment. E-J) Double-immunofluorescence images with active caspase-7 in green and 6E10 in red. (E-G) represent animal treated with Q-VD-OPh indicating that while caspase-7 activation was prevented (E), there was no apparent effect on plaque formation (F). Panel G represents the overlap image for Panels E and F. (H-J) Identical to above except panels represent a control animal treated with vehicle. In this case both active caspase-7 (H) and plaque labeling (I) are evident. (K-M) Prevention of the caspase-cleavage of tau following treatment with Q-VD-OPh. (K) Represents an animal treated with Q-VD-OPh, whereas Panels L and M represent vehicle controls following staining with the TauC3 antibody. No apparent staining was observed in the treated animal, while the presence of caspase-cleaved tau was evident in astrocytes (arrows, L) and extracellular plaques (M) in the vehicle controls. Scale bars represent 10 μm.
Having established that TgCRND8 mice represent a suitable model system to target caspases therapeutically, a small pilot study was initiated utilizing Q-VD-OPh. Three-month old mice were divided into two groups: control, vehicle (n=3) or treated (n=2). Mice were injected i.p. three times a week with either Q-VD-OPh (10 mg/kg) or vehicle for a total time period of 3 months. By the end of three months, 2 mice from the control group survived and 1 mouse from Q-VD-OPh-treated survived. Importantly, we do not believe the death associated with the treatment group was drug-related. There were no signs of toxicity as far as animal behavior, weight loss, motor dysfunction, or tumor formation (physical observations). It should be noted that TgCRND8 mice have a propensity for seizure-related deaths and since an equal number from the treatment group and vehicle died, we do not believe these deaths to be associated with Q-VD-OPh per se.
Following treatment, mice were sacrificed and brain sections were examined for Aβ utilizing the mAB 6E10 by immunohistochemistry. Results from immunohistochemistry indicated that the number of Aβ plaques appeared equivalent in the Q-VD-OPh-treated mouse as compared to the vehicle controls (Figure 2B-D). In this case, plaques appeared more diffuse and were not core plaques that we observed in 12 month-old animals. Based on these data, it appears that Q-VD-OPh does not prevent Aβ deposition.
Double-labeling immunofluorescence experiments were performed to assess whether Q-VD-OPh prevented caspase-7 activation. In this case, Aβ was visualized using the 6E10 mAb (red, Figure 2E-J) together with a rabbit active caspase-7 antibody (green, Figure 2E-J). While Q-VD-OPh treatment did not prevent Aβ deposition (Figure 2F), it did appear to limit the extent of caspase-7 activation (Figure 2E) as compared to vehicle controls (Figure 2H).
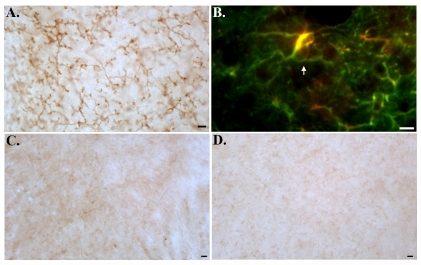
We also examined the extent of caspase-cleaved tau using a specific antibody (TauC3) in TgCRND8 mice following treatment with Q-VD-OPh. Immunohistochemical analysis indicated that despite the apparent lack of effect on Aβ deposition, Q-VD-OPh treatment appeared to attenuate caspase-cleaved tau present within degenerating astrocytes (middle panel, Figure 2L, arrows) and within plaques (Figure 2M). The staining of TauC3 within astrocytes was widespread throughout all brain regions examined and was largely prevented following treatment with Q-VD-OPh. These data suggest that caspase activation and cleavage of tau lie downstream to Aβ production and deposition. Interestingly, we obtained very similar results to TauC3 by utilizing MC-1, an antibody that recognizes an aberrant folded conformational change in tau, which is one of the earliest steps in tau pathology [19, 20]. As depicted in Figure 3A and B, MC-1 immuno-labeling was widespread in vehicle-control animals and appeared to be found predominantly within astrocytes (Figure 3A and B). We confirmed the presence of MC-1 immunolabeling within astrocytes following double-label immunofluorescence experiments using MC-1 and an antibody to GFAP (arrow, Figure 3B). In addition, we were unable to detect any neuronal labeling with MC-1 in vehicle control mice or in 12 month-old TgCRND8 mice for that matter (data not shown). However, we were able to detect extensive labeling of MC-1 within astrocytes in 12 month-old TgCRND8 mice (data not shown). In contrast to vehicle-control animals, there was substantially less staining of MC-1 in the mouse treated with Q-VD-OPh (Figure 3C and D). Taken together, the results presented in Figures 2 and 3 suggest that although caspase inhibition has little effect on Aβ deposition, it does prevent pathological changes to tau.
Figure 3.
Prevention of MC-1 labeling within astrocytes of TgCRND8 mice following chronic treatment with the caspase inhibitor, Q-VD-OPh. (A) Representative bright-field micrograph following labeling with MC-1 (1:500) in vehicle controls. (C and D) indicates the absence of MC-1 labeling in an animal following treatment with Q-VD-OPh. To confirm the presence of MC-1 labeling of astrocytes in vehicle controls, double-label immunofluorescence experiments were carried out utilizing MC-1 (red) and an antibody to GFAP (green). Co-localization of both antibodies was evident within astrocytes (arrow, B). All scale bars represent 10 μm.
Discussion
Numerous studies have implicated the activation of caspases as well as the cleavage of critical proteins associated with the pathology in AD [5]. Caspases are indispensable for the execution of apoptosis, being responsible for the phenotypic characteristics of apoptosis following the cleavage of critical cellular proteins [21]. In AD, however, the aberrant activation of caspases may not only contribute to the neurodegeneration associated with this disease, but may also promote the underlying pathology including the facilitation of NFT formation. Thus, caspase activation and cleavage of tau may be a proximal event in NFT formation [16, 17]. Based upon the available data, caspases represent potential therapeutic targets for the treatment of AD [4]. Precedent for caspase inhibition in other neurodegenerative disorders has been demonstrated. For example, in a study by Li et al., the authors used the pan-caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp fluoromethyl-ketone (Z-VAD-fmk) to treat transgenic mice expressing mutant human SOD1, a model of amyotrophic lateral sclerosis (ALS) [22]. In this case, administration of Z-VAD-fmk delayed disease onset and mortality in ALS mice [22]. Caspase inhibition also delayed disease progression and death in a mouse model of Huntington's disease [23], and increased the survival of dopaminergic neurons in a rat model of Parkinson's disease [24]. An important caveat to these studies is that they all involved acute treatment with Z-VAD (<14 days) and therefore, whether such a strategy would be effective in a chronic disease such as AD is unknown.
The goal of the present study was to examine the feasibility of chronically treating TgCRND8 mice with a novel caspase inhibitor, Q-VD-OPh. Q-VD-OPh has been shown to be a potent and selective caspase inhibitor [25, 26]. Improvements over Z-VAD-fmk include potency, stability, and cell permeability [25]. In addition, Q-VD-OPh has demonstrated efficacy in affording CNS neuroprotection in animal models of Parkinson's, Huntington's disease and stroke [14, 27]. Moreover, this compound is not toxic to cells even at extremely high concentrations and is systemically active [26]. Therefore, Q-VD-OPh would seem to be an ideal candidate to test whether pharmacological inhibition of caspases in vivo can prevent the pathology associated with AD.
In order to test whether pharmacological inhibition of caspases is a valid approach, it is necessary to have an appropriate AD animal model that exhibits caspase activation as well as the cleavage of critical target proteins. Experiments in 12 month-old TgCRND8 mice confirmed that caspase activation and cleavage of target proteins occurs in this animal model of AD. In this regard, evidence for the activation of caspase-7 and cleavage of tau as well as APP was demonstrated to occur particularly in plaque-rich regions. Following validation that TgCRND8 mice represent a suitable model system to target caspases therapeutically, a small pilot study was initiated employing Q-VD-OPh. In this case, a prophylactic approach was taken by treating young TgCRND8 mice beginning at 3 months of age. Treatment with Q-VD-OPh consisted of 3 injections per week systemically for three months. Importantly, no visible adverse effects were noted following treatment with Q-VD-OPh, in particular tumor formation. Pathological examination of brain regions revealed that chronic treatment with Q-VD-OPh did not prevent Aβ deposition, but prevented caspase activation. These results are in line with previous studies in postmortem human AD and animal model studies indicating that caspase activation most likely lies downstream of Aβ formation [5]. In addition, Q-VD-OPh limited pathological changes to tau including cleavage and early conformational changes. The data concerning TauC3 and MC-1 were intriguing: staining in vehicle controls indicated labeling predominantly within reactive astrocytes and not within neuronal populations. In support of these findings is a recent study by Reyes et al., who demonstrated robust tau nitration within reactive astrocytes of the AD brain [28]. Moreover, these authors showed that a subset of nitrated-positive astrocytes also labeled with the antibody Alz-50, a very similar antibody to MC-1 that recognizes a conformation-dependent tau epitope [28]. This suggests that in AD, the same factors that may influence tau alterations in neurons may also affect tau expressed in glial cells. The potential consequence of astrocytes containing alterations of tau in young TgCRND8 mice is unknown and will require further investigation.
In summary, there are numerous neurodegenerative disorders in which caspases specifically and apoptosis in general are known to play a role including AD. However, despite the wealth of evidence supporting the activation of this family of proteases in these disorders, chronic administration of a caspase inhibitor has never been tested in any animal or model system. We now show that TgCRND8 mice appear to be an excellent animal model to examine the role of caspases, displaying caspase activation and cleavage of target proteins including tau. Further, the results of the small pilot study with the novel caspase inhibitor, Q-VD-OPh, represent the first time whereby chronic administration of a caspase inhibitor was undertaken. While Q-VD-OPh did not prevent Aβ deposition, it did limit the extent of caspase activation and pathological changes to tau in plaque regions and within reactive astrocytes. Although additional studies are warranted with Q-VD-OPh using a larger data set and also examining whether chronic treatment improves memory deficits in TgCRND8 mice, results from this pilot study support the feasibility of caspases as drug targets for the treatment of AD.
Acknowledgments
Funded by NIH/NCRR grant #P20RR016454 and a grant from the American Health Assistance Foundation (AHAF) to T.T.R. This work was also supported by a gracious donation from the KO AD Foundation (Boise, ID) to T.T.R. The authors also acknowledge Dr. Peter Davies (Albert Einstein College of Medicine, Bronx, NY) for providing both the MC-1 and PHF-1 antibodies used in this study.
References
- 1.Golde TE, Dickson D, Hutton M. Filling the gaps in the abeta cascade hypothesis of Alzheimer's disease. Curr Alzheimer Res. 2006;3:421–430. doi: 10.2174/156720506779025189. [DOI] [PubMed] [Google Scholar]
- 2.Christensen DD. Alzheimer's disease: progress in the development of anti-amyloid disease-modifying therapies. CNS Spectr. 2007;12:113–116. 119–123. doi: 10.1017/s1092852900020629. [DOI] [PubMed] [Google Scholar]
- 3.Frisoni GB, Delacourte A. Neuroimaging outcomes in clinical trials in Alzheimer's disease. J Nutr Health Aging. 2009;13:209–212. doi: 10.1007/s12603-009-0060-7. [DOI] [PubMed] [Google Scholar]
- 4.Rohn TT, Head E. Caspases as therapeutic targets in Alzheimer's disease: is it time to “cut” to the chase? Int J Clin Exp Pathol. 2009;2:108–118. [PMC free article] [PubMed] [Google Scholar]
- 5.Rohn TT, Head E. Caspase activation in Alzheimer's disease: early to rise and late to bed. Rev Neurosci. 2008;19:383–393. doi: 10.1515/revneuro.2008.19.6.383. [DOI] [PubMed] [Google Scholar]
- 6.Rohn TT, Vyas V, Hernandez-Estrada T, Nichol KE, Christie LA, Head E. Lack of pathology in a triple transgenic mouse model of Alzheimer's disease after overexpression of the anti-apoptotic protein Bcl-2. J Neurosci. 2008;28:3051–3059. doi: 10.1523/JNEUROSCI.5620-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spires-Jones TL, de Calignon A, Matsui T, Zehr C, Pitstick R, Wu HY, Osetek JD, Jones PB, Bacskai BJ, Feany MB, Carlson GA, Ashe KH, Lewis J, Hyman BT. In vivo imaging reveals dissociation between caspase activation and acute neuronal death in tangle-bearing neurons. J Neurosci. 2008;28:862–867. doi: 10.1523/JNEUROSCI.3072-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galvan V, Gorostiza OF, Banwait S, Ataie M, Logvinova AV, Sitaraman S, Carlson E, Sagi SA, Chevallier N, Jin K, Greenberg DA, Bredesen DE. Reversal of Alzheimer's-like pathology and behavior in human APP transgenic mice by mutation of Asp664. Proc Natl Acad Sci U S A. 2006;103:7130–7135. doi: 10.1073/pnas.0509695103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, George-Hyslop PS, Westaway D. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. Biol Chem. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- 10.Bellucci A, Rosi MC, Grossi C, Fiorentini A, Luccarini I, Casamenti F. Abnormal processing of tau in the brain of aged TgCRND8 mice. Neurobiol Dis. 2007;27:328–338. doi: 10.1016/j.nbd.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Rohn TT, Rissman RA, Davis MC, Kim Y-E, Cotman C, Head E. Caspase-9 Activation and caspase cleavage of tau in the Alzheimer's disease brain. Neurobiol Dis. 2002;11:341–354. doi: 10.1006/nbdi.2002.0549. [DOI] [PubMed] [Google Scholar]
- 12.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 13.Renolleau S, Fau S, Goyenvalle C, Joly LM, Chauvier D, Jacotot E, Mariani J, Charriaut-Marlangue C. Specific caspase inhibitor Q-VD-OPh prevents neonatal stroke in P7 rat: a role for gender. J Neurochem. 2007;100:1062–1071. doi: 10.1111/j.1471-4159.2006.04269.x. [DOI] [PubMed] [Google Scholar]
- 14.Yang L, Sugama S, Mischak RP, Kiaei M, Bizat N, Brouillet E, Joh TH, Beal MF. A novel systemically active caspase inhibitor attenuates the toxicities of MPTP, malonate, and 3NP in vivo. Neurobiol Dis. 2004;17:250–259. doi: 10.1016/j.nbd.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Zhu C, Wang X, Huang Z, Qiu L, Xu F, Vahsen N, Nilsson M, Eriksson PS, Hagberg H, Culmsee C, Plesnila N, Kroemer G, Blomgren K. Apoptosis-inducing factor is a major contributor to neuronal loss induced by neonatal cerebral hypoxia-ischemia. Cell Death Differ. 2007;14:775–784. doi: 10.1038/sj.cdd.4402053. [DOI] [PubMed] [Google Scholar]
- 16.Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, Cotman CW. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest. 2004;114:121–130. doi: 10.1172/JCI20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci U S A. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumasaka DK, Galvan V, Head E, Rohn TT. Caspase cleavage of the amyloid precursor protein is prevented after overexpression of Bcl-2 in a triple transgenic mouse model of Alzheimer's disease. Int J Physiol Pathophysiol Pharmacol. 2009;1:48–56. [PMC free article] [PubMed] [Google Scholar]
- 19.Jicha GA, Bowser R, Kazam IG, Davies P. Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J Neurosci Res. 1997;48:128–132. doi: 10.1002/(sici)1097-4547(19970415)48:2<128::aid-jnr5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Weaver CL, Espinoza M, Kress Y, Davies P. Conformational change as one of the earliest alterations of tau in Alzheimer's disease. Neu-robiol Aging. 2000;21:719–727. doi: 10.1016/s0197-4580(00)00157-3. [DOI] [PubMed] [Google Scholar]
- 21.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee JP, Przedborski S, Friedlander RM. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model [see comments] Science. 2000;288:335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- 23.Ona VO, Li M, Vonsattel JP, Andrews LJ, Khan SQ, Chung WM, Frey AS, Menon AS, Li XJ, Stieg PE, Yuan J, Penney JB, Young AB, Cha JH, Friedlander RM. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature. 1999;399:263–267. doi: 10.1038/20446. [DOI] [PubMed] [Google Scholar]
- 24.Schierle GS, Hansson O, Leist M, Nicotera P, Widner H, Brundin P. Caspase inhibition reduces apoptosis and increases survival of nigral transplants. Nat Med. 1999;5:97–100. doi: 10.1038/4785. [DOI] [PubMed] [Google Scholar]
- 25.Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis. 2003;8:345–352. doi: 10.1023/a:1024116916932. [DOI] [PubMed] [Google Scholar]
- 26.Chauvier D, Ankri S, Charriaut-Marlangue C, Casimir R, Jacotot E. Broad-spectrum cas-pase inhibitors: from myth to reality? Cell Death Differ. 2007;14:387–391. doi: 10.1038/sj.cdd.4402044. [DOI] [PubMed] [Google Scholar]
- 27.Braun JS, Prass K, Dirnagl U, Meisel A, Meisel C. Protection from brain damage and bacterial infection in murine stroke by the novel caspase-inhibitor Q-VD-OPH. Exp Neurol. 2007;206:183–191. doi: 10.1016/j.expneurol.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 28.Reyes JF, Reynolds MR, Horowitz PM, Fu Y, Guillozet-Bongaarts AL, Berry R, Binder LI. A possible link between astrocyte activation and tau nitration in Alzheimer's disease. Neurobiol Dis. 2008;31:198–208. doi: 10.1016/j.nbd.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]