Abstract
Acidity in vesicles of macrophages is a general signal that bacteria respond to during infection. Mycobacteria are particularly capable of resisting the acidification in macrophages that engulf the bacteria. In this work, we used label-free quantitative proteomics to study the Mycobacterium smegmatis proteome under acid stress so as to gain an insight into the acidic adaptation in mycobacteria. We quantified 1032 proteins. With a 3-fold change threshold, 20 and 52 proteins were found regulated at false discovery rates of 5% and 14% respectively. We performed a systems analysis based on gene ontology for the global proteome expression profile. We found that the most significant changes induced by the acid stress include a downregulation of transmembrane transporter activity and an upregulation of enzymes involved in fatty acid metabolism. The results suggest that reduced transmembrane transport and increased fatty acid metabolism probably contribute to or associate with acid tolerance in mycobacteria.
Keywords: Mycobacterium smegmatis, acid stress, label-free proteomics, gene ontology, systems biology, membrane transport
Introduction
During bacterial infection, macrophages become activated to result in a series of events specifically designed to induce killing of engulfed microorganisms. These events include the gradual acidification of phagosomes, phagolysosome fusion, induction of reactive oxygen and nitrogen intermediates, and antigen processing [1]. Mycobacterium species, especially Mycobacterium tuberculosis (Mtb), has evolved effective biochemical mechanisms to resist the killing by macrophages. Upon infection, mycobacteria sense and respond to the hostile intraphagosomal environment. The response leads to the synthesis and secretion of critical proteins or lipids to interfere with the host defense. Acidity is a dominant signal that mycobacteria respond to during invasion because the membrane-bound acidic vesicles are the primary weaponry for macrophages to eliminate invading pathogens. On the other hand, there is evidence that vesicles containing live Mtb are not acidic [2, 3], which suggests that Mtb possesses biochemical mechanisms to evade the bactericidal action of the phagolysosome. This ability for Mtb to resist the phagolysosome fusion, however, is not necessarily present during the initial entry into host cells. To the contrary, a majority of bacillus-containing phagosomes fuse with lysosomes within the first a few hours of infection. It was suggested that the fusion of Mtb-containing phagosomes with lysosomes was actually advantageous for the Mtb that infected macrophages [4]. Consistent with the phagolysosomal adaptation, Mtb survived at an in vitro pH of 4.5 in a simple buffer and maintained “intrabacterial pH” [5]. It was proposed that an escape from the phagolysosome played a role in Mtb-phagolysosome interaction [6]. Survival and proliferation was enhanced for bacilli emerging from fused phagolysosomes, and intracellular passage increased the ability of Mtb to avoid phagolysosome fusion in a subsequent infection [7]. Taken together, the evidence suggests that an acid adaptation plays an important role in the interaction of mycobacteria with macrophages.
Mycobacterium smegmatis (Msm), a widely used model mycobacterium system to study mycobacterial biology and to screen anti-tuberculosis drugs [8–11], is non-pathogenic and is known to be eventually cleared from macrophages. It is interesting to note, however, that Msm also possesses the ability to initially halt the acidification of the phagosome within five hours after entry into the murine J774 macrophage [12]. After five hours, the intraphagosomal pH slowly acidifies. With live Msm uptaken by the macrophage, only 20–25% of the phagosomes acidified in the first hour post-infection. This level of phagosome acidification rose only slightly over the next seven hours. Between eight and 24 hours, most phagosomes became acidic to lead to an eventual killing of the bacteria by the 48th hour. In contrast, 80% of the phagosomes containing heat-killed Msm acidified rapidly after one hour. The acidification kinetics with the dead Msm was similar to that seen with latex-bead-containing phagosomes. These results indicate that live Msm initially withstands the acid assault and actively delays the acidification of phagosomes.
In this study, we used an advanced proteomics platform and bioinformatics methods to investigate Msm culture cells under acid stress to gain insight into the acidic adaptation of mycobacteria at the proteome level.
Materials and methods
Cell cultures
Msm strain mc2 155 was obtained from the American Type Culture Collection (ATCC; Rockville, Md). Cells were cultured as described previously [13, 14]. Briefly, we grew a pH 5.0 and a pH 7.0 Msm culture in triplicate to mid-log phase and harvested at OD 0.7. The cultures were grown in 100-ml 7H9 medium under shaking at 37°C in loosely capped 250-ml nephelo culture flasks which had a 19-mm diameter side arm. An OD value was recorded at 600 nm in a Spectronic 20D spectrophotometer (Thermo Fisher Scientific, Waltham, MA). An aliquot of 30 ml was collected from each culture replicate and pelleted at 4000 rpm in a 5810R refrigerated Eppendorf centrifuge for 10 min at 4°C. A [15N]-labeled culture was also grown to log-phase for use as an internal standard to determine false positive rates in protein quantitation [13]. Hereafter, we name the stressed pH 5 culture as S, the reference pH 7 culture as R, and the internal standard culture as IS.
Protein sample preparation
Each cell pellet was resuspended in 100 mM ammonium bicarbonate buffer and lysed by bead beating in the presence of a protease inhibitor cocktail (Pierce, Rockford, IL) [14]. The whole cell lysates were cleared by centrifugation at 13,000 g for 30 min at 4°C. Protein concentrations were quantified with the BCA protein quantitation kit (Pierce, Rockford, IL). The triplicate protein extracts for cultures S and R were pooled respectively. The pooled protein extracts from cultures S and R were mixed respectively with an equal amount of protein extract from the IS culture to generate two protein mixtures i.e., SP (for culture S) and RP (for culture R). One hundred micrograms of proteins from each of SP and RP were separated on a 10% Tris-HCl SDS-PAGE gel (Pierce) and fractionated into 5 fractions. Gel bands were processed for in-gel digestion and peptide extraction as described [13, 14].
LC/MS analysis of peptides
The peptide extracts from the gel bands were submitted for analysis with the nanoLC/LTQ-FTMS system (Thermo Finnigan; San Jose, CA) in the Proteomics and Informatics Services Facility (PISF) in the Research Resources Center at University of Illinois at Chicago supported by the Searle Funds at the Chicago Community Trust. Each peptide extract sample was analyzed with duplicate LC/MS injections as previously described [13].
Specifically, in each injection, 5 μL of peptide extract solution was separated on a 150-mm × 75-μm C18 reverse phase column with a 5% to 35% acetonitrile (v/v) gradient in 0.1% trifluoroacetic acid over 60 min. The LTQ-FTMS was operated in a data-dependent acquisition mode with up to 10 MS/MS spectra acquired following each MS scan. The acquired RAW data files were searched against the National Center for Biotechnology Information (NCBI) database of M. smegmatis strain mc2 155 (downloaded in 2006 with the old locus names) in two separate BioWorks searches. One search corresponded to [14N] labeling and the other to [15N] labeling. The precursor ion tolerance was set to ±1.5 Da. Trypsin was designated as the digestion enzyme with two missed cleavages allowed. Peptide and protein probabilities were calculated by BioWorks. Only peptides with P <.01 were accepted for subsequent quantitation of abundance (Table S1). After peptide and protein identifications, we carried out peptide quantitation using Matlab v7.2 (MathWorks, Natick, MA) and Microsoft Excel based on the previously described methods [13, 15, 16].
Protein quantitation
We quantified the protein abundances with a label-free proteomics approach as described previously [13, 15, 17]. The abundance of a protein was represented by the sum of the extracted ion chromatographic intensities of the peptide charge states detected for that protein. A peptide charge state is a peptide ionized to a specific charge in a mass spectrometer. One peptide could be detected at multiple peptide charge states [16]. We identified >5000 unique peptide charge states (p <.01). Only peptide charge states identified at p <.01 were used for subsequent quantitation of proteins. We only accept the proteins having peptide(s) identified by ≥2 peptide charge states identification events (p <.05).
From protein sample SP, the abundance of a protein was quantified for its abundance in culture S (AS) and its abundance in culture IS (AIS,S). Likewise, from protein sample RP, the abundance of a protein was quantified for its abundance in culture R (AR) and in culture IS (AIS,R). In total, each protein had four abundance values i.e., AS, AR, AIS,S and AIS,R. We used AS and AR to determine the positives i.e., the differentially regulated proteins between cultures S and R. AIS,S and AIS,R represented replicate quantitation of the same proteins from culture IS. Thus, the differentially regulated proteins selected based on a difference between AIS,S and AIS,R were false positives. The false discovery rate was the quotient of false positives over positives as applied previously [13].
Results
Cell culturing
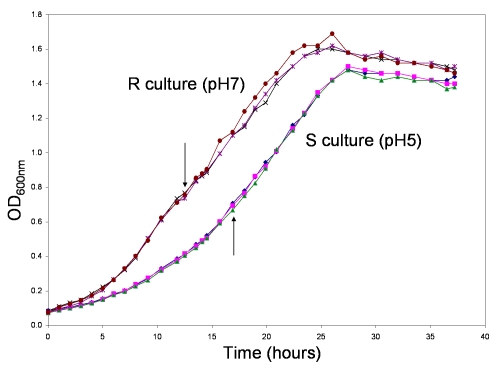
The inoculants for the S and R cultures were pre-grown at pH 5 and pH 7 respectively to late log phase before use. About 2×109 cells were used to inoculate each 100-ml medium to start the growth of the S and R cultures in triplicate (Figure 1). The cells were allowed to grow for approximately three doublings before being harvested at an OD value of about 0.7. This OD value corresponded to about OD 0.4/ml after adjustment for the side arm diameter of the nephelo culture flask. Three doublings are consistent with the typical number of doublings of a bacillus that infects a macrophage to result in a few bacilli in the macrophage [18].
Figure 1.
Growth curves of the S and R cultures in triplicate. The arrows indicate the timepoints to harvest the culture samples for proteomic analysis.
The intraphagosomal pH decreases to <5.0 after uptake of dead mycobacteria or latex particles due to the fusion of phagosomes with lysosomes. The fused phagolysosome has been shown to have a pH ranging from 4.7 to 5.0 in mouse peritoneal macrophages and baby hamster kidney cells [19]. Typically, Mtb maintains the intraphagosomal pH between 6.2 and 6.6 [20]. When the mycobacterium-containing macrophages are activated by interferon-γ, the intraphagosomal pH could decrease to 5.2, at which point the activated macrophages become bacteriostatic or bactericidal. It was observed that most of the bacilli infecting macrophages were initially within fused vacuoles [6].
Thus, the pH of 5.0 that we chose in this study represents the low end of the pH that a mycobacterium could possibly encounter in a macrophage early in an infection [20]. In a previous study, we studied the immediate response of Msm to an acid shock with a protein turnover approach [21]. In this study, we examined the adaptive acid stress response of Msm in the cultures shown in Figure 1.
Protein quantitation
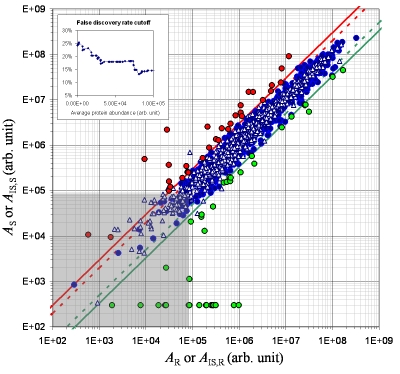
We quantified 1032 proteins (the list is available from the Author upon request). Each protein had four abundance values i.e., AS, AR, AIS,S and AIS,R (see Methods). Figure 2 shows the overlay of two scatter plots based on these four protein abundance values for the 1032 proteins. One plots AS versus AR. The other plots AIS,S versus AIS,R. The ratio of AS versus AR was used to select the proteins regulated in the acid stressed culture S versus the reference culture R. The ratio of AIS,S versus AIS,R was used to determine false positives due to the variations introduced during sample handling and data analyses.
Figure 2.
Selection of differentially expressed proteins from the 1032 detected proteins. Two scatter plots are overlaid. One corresponds to the AS/AR ratio (circular colored markers) and the other to the AIS,S/AIS,R ratio (the triangular white markers). A red line indicates the upper threshold of 3-fold (solid) or 2-fold (dashed) change. A green line indicates the lower threshold of 3-fold (solid) or 2-fold (dashed) change. The red, blue, and green circular markers respectively represent the upregulated, unchanged, and downregulated proteins in the S (pH 5) vs the R (pH 7) culture. The insert at the upper left corner indicates the relationship between the false discovery rate and the average protein abundance in the low-abundance region as indicated with the grey shade.
Differentially regulated proteins
We determined the Msm proteins that were differentially expressed between the acid stressed culture S and the reference culture R. From the 1032 proteins, 70 unlabeled proteins were found regulated between the pH 5 and pH 7 growth conditions at a 3-fold change threshold. But 17 labeled internal standard proteins were also found “regulated” at the same threshold. The presence of the 17 false positives resulted in a false discovery rate of 24% which we considered too high for subsequent biological interpretation. If we used a 2-fold change threshold, the false discovery rate would be 40% even though the total number of differentially regulated proteins would increase to 162. Thus, we empirically chose the 3-fold change cutoff to select differentially regulated proteins.
As shown in the insert graph in Figure 2, there was an inverse relationship between the false discovery rate and the protein abundance level. Based on this relationship, we reduced the false discovery rate by applying an additional threshold of protein abundance level. Above an average protein abundance level of approximately 1×105, the false discovery rate decreased to 14%. This 14% false discovery rate cutoff resulted in 52 differentialy regulated unlabeled proteins (Table 1). Further incorporation of additional lower-abundance proteins resulted in a significant increase of false discovery rate. Thus, we chose the 14% false discovery rate cut-off as a compromise between the acceptable error rate and the number of selected differentially regulated proteins. Sufficient numbers of differentially regulated proteins affords a statistical test in a system-based analysis. To interpret individual differentially regulated proteins, a ≤5% false discovery rate would be necessary. At a false discovery rate of 5%, 20 proteins were found regulated. These 20 high-confidence differentially regulated proteins are indicated in Table 1.
Table 1.
The 52 differentially regulated proteins selected at 14% false discovery rate. The 20 proteins shown in bold belong to the high-confidence list of differentially regulated proteins selected at 5% false discovery rate.
| Locus | Protein description | PCS_IDs | AR | AS | AIS,R | AIS,S | AS/AR |
|---|---|---|---|---|---|---|---|
| (Upregulated proteins) | |||||||
| MSMEG_0614 | Methyltransferase | 2 | 3.58E+05 | 1.29E+06 | 1.79E+05 | 3.20E+05 | 3.61a |
| MSMEG_0969 | Glutamate-1-semialdehyde-2,1-aminomutase (hemL) | 8 | 3.22E+06 | 9.92E+06 | 1.72E+06 | 4.57E+06 | 3.08a |
| MSMEG_1037 | Alcohol dehydrogenase, zinc-containing | 61 | 1.16E+07 | 9.09E+07 | 5.86E+06 | 8.82E+06 | 7.82a |
| MSMEG_1475 | Conserved hypothetical protein | 4 | 6.54E+05 | 2.99E+06 | 6.56E+05 | 6.59E+05 | 4.57a |
| MSMEG_1903 | Caib-baif family | 6 | 2.26E+05 | 8.85E+05 | 9.29E+04 | 6.94E+04 | 3.91a |
| MSMEG_2669 | Hydrolase | 46 | 5.03E+06 | 2.10E+07 | 4.72E+06 | 5.22E+06 | 4.17a |
| MSMEG_2956 | NAD-dependent epimerase-dehydratase family protein | 47 | 1.59E+06 | 1.49E+07 | 6.24E+06 | 7.72E+06 | 9.37ab |
| MSMEG_3932 | 14 kDa antigen | 2 | 2.09E+05 | 2.56E+06 | 5.07E+04 | 4.75E+04 | 12.2 |
| MSMEG_3945 | Universal stress protein family | 14 | 1.08E+06 | 5.22E+06 | 4.01E+05 | 3.21E+05 | 4.83a |
| MSMEG_3950 | Universal stress protein family | 20 | 7.81E+06 | 2.41E+07 | 1.30E+06 | 1.43E+06 | 3.09a |
| MSMEG_4381 | Amidase | 2 | 8.13E+04 | 2.50E+05 | 3.19E+05 | 5.63E+05 | 3.08a |
| MSMEG_4971 | Oxidoreductase | 2 | 1.64E+05 | 5.19E+05 | 1.36E+06 | 9.37E+05 | 3.16a |
| MSMEG_5136 | Helix-turn-helix motif | 8 | 8.36E+05 | 4.53E+06 | 4.69E+04 | 6.80E+04 | 5.42a |
| MSMEG_5164 | Zinc-binding alcohol dehydrogenase family protein | 6 | 3.17E+05 | 1.35E+06 | 3.48E+06 | 8.77E+06 | 4.27a |
| MSMEG_5243 | Helix-turn-helix motif | 4 | 1.14E+06 | 4.35E+06 | 2.33E+05 | 2.07E+05 | 3.82 |
| MSMEG_5245 | Universal stress protein family | 8 | 1.08E+06 | 7.58E+06 | 3.06E+05 | 4.46E+05 | 7.02a |
| MSMEG_5246 | Conserved hypothetical protein | 22 | 1.98E+06 | 1.49E+07 | 2.94E+06 | 2.11E+06 | 7.53a |
| MSMEG_5285 | Phospholipase, patatin family | 14 | 5.80E+05 | 2.26E+06 | 1.42E+05 | 2.21E+05 | 3.90a |
| MSMEG_5419 | Putative lipoprotein | 66 | 4.80E+06 | 1.56E+07 | 3.26E+06 | 3.81E+06 | 3.24 |
| MSMEG_5739 | Putative long-chain fatty-acid–CoA ligase | 16 | 4.23E+05 | 2.36E+06 | 3.81E+05 | 5.32E+05 | 5.57ab |
| MSMEG_6454 | Conserved hypothetical protein | 56 | 8.18E+06 | 4.89E+07 | 3.58E+06 | 3.41E+06 | 5.98a |
| (Down-regulated proteins) | |||||||
| MSMEG_0131 | AMP-binding enzyme, putative | 2 | 9.20E+04 | 2.08E+04 | 9.30E+04 | 7.72E+04 | 0.23a |
| MSMEG_0317 | Conserved hypothetical protein | 4 | 1.87E+06 | 5.74E+05 | 8.30E+05 | 4.95E+05 | 0.31 |
| MSMEG_0394 | Hypothetical protein | 27 | 1.07E+06 | 2.53E+05 | 6.73E+05 | 4.81E+05 | 0.24 |
| MSMEG_0987 | Hypothetical protein | 2 | 7.68E+05 | 3.00E+02 | 1.45E+06 | 1.49E+06 | 0.00 |
| MSMEG_1052 | Hypothetical protein | 12 | 1.81E+05 | 1.30E+04 | 3.36E+05 | 3.90E+05 | 0.07ab |
| MSMEG_1445 | 30S ribosomal protein S17 | 2 | 1.94E+05 | 3.00E+02 | 4.34E+04 | 5.69E+04 | 0.00a |
| MSMEG_1605 | Phosphate transport system regulatory protein PhoU (phoU) | 2 | 8.48E+04 | 1.13E+03 | 9.13E+03 | 4.25E+03 | 0.01 |
| MSMEG_1680 | Conserved hypothetical protein | 20 | 8.20E+06 | 2.46E+06 | 3.89E+06 | 4.36E+06 | 0.30 |
| MSMEG_1681 | Endoribonuclease L-PSP superfamily | 156 | 2.57E+07 | 7.57E+06 | 1.90E+07 | 2.68E+07 | 0.29 |
| MSMEG_1682 | Flavin-containing monooxygenase FMO | 62 | 2.66E+07 | 7.76E+06 | 1.12E+06 | 1.34E+06 | 0.29ab |
| MSMEG_1832 | Conserved hypothetical protein | 2 | 3.14E+05 | 3.00E+02 | 4.89E+05 | 4.74E+05 | 0.00 |
| MSMEG_1951 | Conserved domain protein | 14 | 4.67E+05 | 1.50E+05 | 1.29E+06 | 1.41E+06 | 0.32 |
| MSMEG_2116 | PTS system, glucose-specific IIBC component | 6 | 1.56E+05 | 2.98E+04 | 2.63E+05 | 1.72E+05 | 0.19ab |
| MSMEG_2789 | Acetyltransferase, GNAT family | 2 | 1.39E+05 | 3.00E+02 | 1.45E+05 | 1.56E+05 | 0.00a |
| MSMEG_2942 | Glyoxalase-bleomycin resistance protein-dioxygenase superfamily protein | 2 | 2.70E+05 | 3.00E+02 | 1.84E+06 | 1.98E+06 | 0.00ab |
| MSMEG_3962 | Lactate 2-monooxygenase | 64 | 3.15E+07 | 5.44E+06 | 2.00E+07 | 2.37E+07 | 0.17ab |
| MSMEG_4075 | CoA-binding protein | 2 | 1.54E+05 | 2.65E+04 | 5.62E+05 | 3.66E+05 | 0.17a |
| MSMEG_4298 | 3-Methyl-2-oxobutanoate hydroxymethyltransferase (panB) | 53 | 1.69E+08 | 4.48E+07 | 2.11E+06 | 1.36E+06 | 0.27a |
| MSMEG_4476 | Hypothetical protein | 2 | 2.80E+05 | 3.00E+02 | 8.88E+04 | 2.04E+05 | 0.00 |
| MSMEG_4935 | ATP synthase F1, epsilon subunit (atpC) | 41 | 1.33E+07 | 4.26E+06 | 4.53E+06 | 3.98E+06 | 0.32ab |
| MSMEG_4936 | ATP synthase F1, beta subunit (atpD) | 190 | 1.01E+08 | 3.19E+07 | 4.31E+07 | 4.67E+07 | 0.31ab |
| MSMEG_5006 | Phosphohistidine phosphatase | 2 | 8.29E+04 | 3.00E+02 | 3.33E+04 | 3.91E+04 | 0.00 |
| MSMEG_5022 | Flavin-containing monooxygenase FMO | 12 | 5.38E+05 | 1.55E+05 | 8.05E+05 | 6.37E+05 | 0.29ab |
| MSMEG_5694 | Conserved hypothetical protein | 2 | 2.52E+05 | 3.00E+02 | 1.82E+06 | 1.28E+06 | 0.00 |
| MSMEG_5703 | Molybdenum cofactor biosynthesis protein C (moaC) | 2 | 9.80E+05 | 3.00E+02 | 7.42E+05 | 1.16E+06 | 0.00a |
| MSMEG_5835 | Fumarate reductase-succinate dehydrogenase flavoprotein | 2 | 6.58E+05 | 1.92E+05 | 1.70E+05 | 5.88E+04 | 0.29a |
| MSMEG_6075 | 2C-methyl-D-erythritol 2,4-cyclodiphosphate synthase (ispF) | 2 | 8.50E+04 | 3.00E+02 | 1.74E+05 | 2.78E+05 | 0.00a |
| MSMEG_6159 | Conserved domain protein | 10 | 6.17E+05 | 2.01E+05 | 4.21E+05 | 1.10E+05 | 0.33a |
| MSMEG_6210 | Conserved hypothetical protein | 2 | 2.75E+05 | 4.46E+04 | 9.40E+05 | 1.13E+06 | 0.16 |
| MSMEG_6457 | Oxidoreductase molybdopterin binding domain, putative | 2 | 6.40E+05 | 1.61E+05 | 4.07E+05 | 3.96E+05 | 0.25a |
| MSMEG_6913 | Putative transcriptional regulatory protein | 2 | 1.99E+05 | 3.00E+02 | 1.19E+05 | 1.67E+05 | 0.00a |
Annotated in GO.
Present in the unique enriched GO terms (Table 2). PCS_IDs - the number of times peptides from a protein were identified by MS/MS scan.
Thus, we identified 20, 52, and 70 differentially regulated proteins at estimated false discovery rates of 5%, 14%, and 24% respectively. For the systems analyses in the following, we used the 52 differentially regulated proteins selected at the 14% false discovery rate.
Interactions among the differentially regulated proteins
We first examined the interaction among the 52 differentially regulated proteins based on the evidence from the String database [22]. The purpose was to identify functionally related proteins that might be co-regulated under the acid stress condition. We identified two protein clusters with >2 proteins (Figure 3). One cluster was upregulated and the other downregulated. All of these six proteins were among the 20 high-confidence differentially regulated proteins selected at a false discovery rate of 5% (Table 1).
Figure 3.
The two protein clusters identified among the 52 differentially regulated proteins. Red and green indicate up- and down-regulation respectively. The numbers on the edges indicate the interaction confidence scores.
The three proteins in the upregulated protein cluster i.e., MSMEG_5243, MSMEG_5245, and MSMEG_5246 belong to a multi-gene locus encoding the DevR response regulator in Msm [23]. MSMEG_5245 is annotated as a universal stress protein (USP). It has a conserved USP domain and shares 50% similarity and 34% identity with Rv3134c that is the first gene in the Mtb devR/devS operon [24]. In Msm, downstream of MSMEG_5245 is MSMEG_5244 that shares 94% similarity and 85% identity with the Mtb devR. MSMEG_5244 is the only devR homologue in Msm. MSMEG_5244 was detected but not found to be regulated by the thresholds applied in this study. It was shown to be a stationary-phase regulator required for the adaptation of Msm to oxygen-starvation and resistance to heat stress [23].
MSMEG_5245 responded to an oxygen-starvation condition in a devR-dependant manner, but did not respond to a carbon-starvation condition or a UV stress [23]. An inhibition of the Msm aerobic respiration, however, did not induce the DevR regulon [25]. The inhibition of aerobic respiration under hypoxic conditions or nitric oxide induction did not result in an increase in the DevS kinase activity [11]. Although it was shown that the DevSR two-component system has a regulatory role during hypoxia-induced dormancy in Mtb, Msm, and BCG [26], the direct linkage of the DevSR two-component system to the functional state of the respiratory electron transport chain remains to be determined. In this study, MSMEG_5245 was upregulated in response to the acid stress along with another two UPS proteins i.e., MSMEG_3950 and MSMEG_3945 (Table 1). The result in this study suggests that the Msm DevSR two-component system also responds to an acid stress in addition to heat stress and oxygen starvation [23].
There are three proteins in the downregulated protein cluster including MSMEG_1680-1682. MSMEG_1680 is a conserved hypothetical protein. MSMEG_1681 is an endoribonuclease L-PSP superfamily protein that belongs to a widely distributed family of YER057c/YjgF/UK114 proteins of unknown function [27]. Members of the YER057c/YjgF/UK114 family of proteins are conserved among all domains of life. In bacteria, endoribonuclease L-PSP superfamily proteins were suggested to be potentially involved in the transformation or transport of small molecules, such as antibiotics [28, 29]. A rat endoribonuclease L-PSP superfamily protein was shown to inhibit protein translation at the initiation but not at the elongation stage [30]. The conservation of this protein in all domains of life suggests its fundamental function in a cell. MSMEG_1682 is a flavin-containing monooxygenase which is a complementary enzyme system to the cytochrome P450 family of enzymes and oxygenates several soft, highly polarizable nucleophilic heteroatom-containing chemicals and drugs [31].
Gene ontology analysis
To assess the major biological themes perturbed by the acid stress in Msm, we performed a gene ontology (GO) analysis for the 52 differentially regulated proteins (Table 1).
The GO project provides controlled vocabularies for the description of the biological process, molecular function, and cellular component in a cell [32]. For the 1032 quantified Msm proteins, 835 were annotated in the GO annotation for Msm [33]. The GO annotations for these 835 Msm proteins were extracted using the BiNGO plug-in in Cytoscape [34]. The extracted GO annotations for these 835 proteins were used as the custom annotation file to determine the enriched GO terms for the 52 differentially regulated proteins (Table 1). We searched for enriched GO terms in the GO hierarchy consisting of the three branches including biological_process, molecular_function, and cellular_component.
Selection of enriched GO terms
For the 52 differentially regulated proteins, 37 were annotated in the GO and formed a 132-node network when analyzed with Cytoscape (data not shown). Of the 132 nodes (GO terms), 75 were enriched with differentially regulated proteins based on a hypergeometric test (p<.05) [34].
A close examination of the 75 enriched GO terms indicates that there was significant interdependence and redundancy among them. Many enriched GO terms had the same set of differentially regulated proteins. Such redundancy was mostly because we had a relatively small number of differentially regulated proteins for the enrichment test.
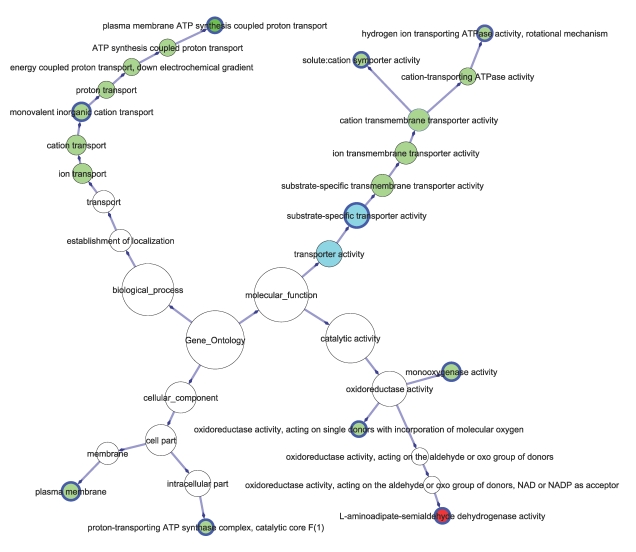
We were interested in the enriched GO terms that had their unique sets of proteins, or so-called unique enriched GO terms. If multiple enriched GO terms shared the same set of proteins and were in parent-child relationship along one branch of the GO hierarchy tree [35], we chose the one at the end of the branch as the unique enriched GO terms, as illustrated in the simplified GO network shown in Figure 4. In Figure 4, we only retained the unique enriched GO terms and their parents with ≥2 proteins. The unique enriched GO terms are indicated with a thicker border in Figure 4.
Figure 4.
Enriched GO terms for the 52 differentially regulated proteins. The colored nodes are enriched GO terms. The nodes with a thicker border are unique enriched GO terms (see text). The size of a node is proportional to the number of proteins that it contains. A red node contains only upregulated proteins, and a green node contains only downregulated ones. A cyan node contains both up- and down-regulated proteins.
We identified 10 unique enriched GO terms (Table 2). Other enriched GO terms were the consequence of the enrichment of one or more of these 10 child terms. For example, although the last seven GO terms were enriched in the biological_process branch, the GO term plasma membrane ATP synthesis coupled proton transport was the first one to have MSMEG_4935 and MSMEG_4936 (Table 2). Another three GO terms upstream of it contained the same two proteins. They did not contribute to new information. Thus, they were not included in Table 2. Further up along the biological_process branch was the GO term monovalent inorganic cation transport that had an additional protein MSMEG_1052. Because this GO term included an additional protein different from those in its child terms, this GO term was included in Table 2. Another nine GO terms in Table 2 were selected based on the same principle.
Table 2.
Enriched unique GO terms for the 52 differentially regulated proteins.a
| GO_ID | p | x | n | GO term description | Proteins in test set |
|---|---|---|---|---|---|
| 45261 | 0.006 | 2 | 3 | Proton-transporting ATP synthase complex, catalytic core F(1) | MSMEG_4935; MSMEG_4936 |
| 5886 | 0.008 | 3 | 10 | Plasma membrane | MSMEG_4935; MSMEG_2116; MSMEG_4936 |
| 15672 | 0.006 | 3 | 9 | Monovalent inorganic cation transport | MSMEG_4935; MSMEG_1052; MSMEG_4936 |
| 42777 | 0.006 | 2 | 3 | Plasma membrane ATP synthesis coupled proton transport | MSMEG_4935; MSMEG_4936 |
| 16701 | 0.01 | 2 | 4 | Oxidoreductase activity, acting on single donors with incorporation of molecular oxygen | MSMEG_2942; MSMEG_3962 |
| 4043 | 0.01 | 2 | 4 | L-Aminoadipate-semialdehyde dehydrogenase activity | MSMEG_2956; MSMEG_5739 |
| 4497 | 0.01 | 3 | 12 | Monooxygenase activity | MSMEG_1682; MSMEG_5022; MSMEG_3962 |
| 22892 | 0.001 | 6 | 30 | Substrate-specific transporter activity | MSMEG_4935; MSMEG_1052; MSMEG_2116; MSMEG_4936; MSMEG_2956; MSMEG_5739 |
| 46961 | 0.006 | 2 | 3 | Hydrogen ion transporting ATPase activity, rotational mechanism | MSMEG_4935; MSMEG_4936 |
| 15294 | 0.01 | 2 | 4 | Solute:cation symporter activity | MSMEG_1052; MSMEG_2116 |
p – the p-value of a hypergeometric test performed with BiNGO in Cytoscape.
x – the number of differentially regulated proteins in the test set of a GO term.
n – the number of detected proteins in a GO term.
In Figure 4, the GO term L-aminoadipate-semialdehyde dehydrogenase activity contains only upregulated proteins. Another two GO terms i.e., substrate-specific transporter activity and transporter activity contain both up- and downregulated proteins. All other enriched GO terms contain only downregulated proteins. Substrate-specific transporter activity contains two upregulated and four downregulated proteins (Table 2). The enrichment of transporter activity is the direct result of the enrichment of its child term substrate-specific transporter activity.
Cellular component
The GO hierarchy branch cellular_component contains nine differentially regulated proteins (Figure 4). The enriched GO term proton-transporting ATP synthase complex, catalytic core F(1) contains AtpC (MEMEG_4935) and AtpD (MSMEG_4936). AtpC and AtpD are the subunits of the F1 motor. The F1 motor produces ATP in the presence of a proton gradient. It also participates in the regulation of intrabacterial pH homeostasis. The enriched GO term plasma membrane contains MSMEG_2116 in addition to AtpC and AtpD (Table 2). MSMEG_2116 is a glucose-specific IIBC component of the phosphoenolpyruvate (PEP): carbohydrate phosphotransferase system (PTS). It catalyzes the phosphorylation of N-acetyl-D-glucosamine (GlcNAc) to N-acetyl-D-glucosamine-6-phosphate (GlcNAc-6-p). The PEP:PTS system is involved in the transport of a large number of carbohydrates, in chemotaxis towards these carbon sources, and in the regulation of a number of other metabolic pathways [36]. The PTS catalyzes the uptake of carbohydrates and their conversion into their respective phosphoesters during transport. The result here suggests that the acidic growth condition affects the substrate uptake and energy production, consistent with the slower exponential phase growth of the S culture (Figure 1). It remains to be determined whether the reduced substrate uptake results in lower ATP synthesis or the lower ATP synthesis results in reduced energy-dependent substrate uptake. We also cannot exclude that these two processes are separately regulated by the acid stress and that one is not necessarily the consequence of the other.
Biological process
The GO hierarchy branch biological_process contains 29 differentially regulated proteins (Figure 4). The enriched GO term plasma membrane ATP synthesis coupled proton transport contains AtpC and AtpD. Again, the downregulation of this GO term suggests that ATP synthesis could be reduced under the acid stress. The GO term monovalent inorganic cation transport contains AtpC, AtpD, and MSMEG_1052. MSMEG_1052 is a hypothetical protein. The abundance of this hypothetical protein was downregulated by 14-fold (Table 1), suggesting that it has a role in the acid stress response. Indeed, the results in Table 2 indicate that MSMEG_1052 was also annotated in several other enriched GO terms in the molecular_function ontology including substrate-specific transporter activity, solute:cation symporter activity, and active transmembrane transporter activity. These results support a role of MSMEG_1052 in transporter activity. Interestingly, the String database [22] annotates MSMEG_1052 as an amino acid carrier protein and shows that it interacts with an alanine racemase (MSMEG_1575), a sodium/proline symporter (MSMEG_5303), a hexapeptide transferase family protein (MSMEG_1055), and an uncharacterized putative protein (MSMEG_1053). The protein interaction evidence suggests that MSMEG_1052 might be involved in the transport of amino acids. In this study, the down-regulation of MSMEG_1052 suggests a reduced amino acid transport in acid stressed Msm cells.
Molecular function
The GO hierarchy branch molecular_function contains 32 proteins (Figure 4). The enriched GO term L-aminoadipate-semialdehyde dehydrogenase activity contains two upregulated proteins i.e., MSMEG_5739 and MSMEG_2956. Annotated as an NAD-dependent epimerase-dehydratase family protein (Table 2), MSMEG_2956 also shares 55% identity and 72% similarity with MSMEG_5739 which is annotated as a putative long-chain fatty-acid-CoA ligase. Long-chain fatty-aid CoA ligases catalyze the bioactivation of fatty acids to form acyl-CoA thioesters. Acyl-CoA thioesters are used as substrates for subsequent metabolic pathways. The long-chain ligases exist as a super family of membrane proteins. They play key roles in both fatty acid activation and xenobiotic acyl-CoA formation [37].
The upregulation of MSMEG_5739 and MSMEG_2956 suggests that Msm increased the metabolism of fatty acids under the acid stress. A recent work by Deb et al. showed that Mtb accumulated triacylglycerol and wax ester under multiple stresses including acidity [38]. Thus, the upregulation of MSMEG_5739 and MSMEG_2956 in Msm under the acid stress implies that the cells likely increased anabolism of fatty acids. Accumulation of triacylglycerol and wax ester under stress could in turn result in a decrease in the cell wall/membrane permeability.
In the microarray study by Fisher et al. [39], the Mtb homologues of MSMEG_5739 and MSMEG_2956 were not upregulated by the acid shock in that experiment. Meanwhile, although the nonribosomal peptide synthetases/polyketide synthases were found most significantly induced in the acid shocked Mtb cells in the microarray study by Fisher et al. [39], the two detected proteins of their Msm homologues MSMEG_0408 and MSMEG_6392 were not regulated in this study. The duration of acid shock in the study by Fisher et al. was short. Thus, the acid shock condition in Fisher et al.'s study was somewhat different from the acid-adapted growth condition in this study. In addition, given the moderate correlation of microarray and proteomic data [40], a discrepancy between microarray and proteomic data should not be regarded purely as an experimental variation. This kind of discrepancy warrants a proteomic study to measure the abundance of a protein that is the combined result of transcription, translation, and other post-translational regulations such as degradation or turnover [41].
On the other hand, fadD9 (Rv2590) and nrp (Rv0101), which are two Mtb homologues of MSMEG_2956, were upregulated in Mtb that infected activated murine bone marrow macrophages [42]. A dozen of other Mtb genes homologous to the upregulated Msm protein in Table 1 were upregulated in the bacilli that infected activated macrophages, as will be discussed later.
Two downregulated proteins i.e., MSMEG_2942 and MSMEG_3962 are within the enriched GO term oxidoreductase activity, acting on single donors with incorporation of molecular oxygen. MSMEG_2942 is a glyox-alase/bleomycin resistance protein/dioxygenase superfamily protein. Glyoxalase I catalyzes the first step of the glyoxal pathway to convert methylglyoxal and reduced glutathione to S-lactoylglutathione. S-lactoylglutathione is then converted by glyoxalase II to lactic acid [43, 44]. The glyoxalase system is believed to have evolved to detoxify reactive 2-oxoaldehydes which include mainly methylglyoxal. Methylglyoxal is formed endogenously as a by-product of the triosephosphate isomerase reaction in glycolysis [45]. MSMEG_3962 is a lactate 2-monooxygenase that catalyzes the oxidation of sodium lactate by dioxygen to pyruvate. Pyruvate is then decarboxylated to acetate. The down-regulation of MSMEG_2942 and MSMEG_3962 could be due to the reduced transport of glucose into the cell under the acid stress, which in turn led to a lower glycolysis activity.
The enriched GO term monooxygenase activity contains three downregulated proteins including MSMEG_1682, MSMEG_5022, and MSMEG_3962. MSMEG_1682 and MSMEG_5022 share 30% identity and 48% similarity. Both are flavin-containing monooxygenases that metabolize many clinically important xenobiotic compounds as well as endogenous substrates as part of a discrete physiological process [46]. MSMEG_5022 shares 27% identity and 37% similarity with the Mtb monooxygenase EthA. The EthA transcript level determined by microarray analysis showed a down-regulation after four, 24, and 96 hours of starvation in a nutrient-starvation model of Mtb persistence [47]. EthA is responsible for the oxidative activation of the second-line anti-tubercular prodrugs ethionamide and thiacetazone [48].
Under the enriched GO term substrate-specific transporter activity, the unique enriched child term hydrogen ion transporting ATPase activity, rotational mechanism contains AtpC and AtpD. The unique enriched GO term solute:cation symporter activity contains MSMEG_1052 and MSMEG_2116. As described before, MSMEG_2116 is a glucose-specific IIBC component of the PEP:PTS system. The PEP:PTS system is involved in transport and phosphorylation of a large number of carbohydrates, in chemotaxis towards these carbon sources, and in regulation of a number of other metabolic pathways [36]. The result again suggests that the acidic growth condition affected the substrate uptake that requires energy. MSMEG_1052 is a probable amino acid carrier protein and is predicted to have a sodium:amino acid symporter activity based on its GO annotation [49]. A sodium:amino acid symporter catalyzes the transfer of an amino acid solute located outside a membrane, to enter with Na+ into the cytoplasm. MSMEG_1052 contains the domain of the sodium:alanine symporter family [50]. The acidic growth condition appeared to repress the sodium:alanine transport, or amino acid transport in general.
Intraphagosomal regulation of Mtb genes homologous to the differentially regulated Msm proteins
To assess the state of the biochemical environment in phagosomes harboring Mtb, Schnappinger et al. captured the transcriptional responses of Mtb in macrophages before and after immunologic activation [42]. We examined whether the Mtb genes homologous to the Msm differentially regulated proteins in Table 1 responded to the intraphagosomal environment. In Table 3, we compiled a list of the Mtb genes with homology to the Msm genes encoding the differentially regulated Msm proteins shown in Table 1. The matched genes between Mtb and Msm were selected with the multi-genome homology comparison tool from the J. Craig Venter Institute (www.jcvi.org). We arbitrarily set the gene similarity threshold at 60%. As shown in Table 3, 20 Mtb genes are homologous to 12 upregulated Msm proteins, and 15 Mtb genes are homologous to 14 downregulated Msm proteins. In Table 3, we incorporated the 3-time-point mRNA relative expression ratios for the 35 Mtb genes in both naïve and activated murine wild-type bone marrow macrophages from Table S1 entitled “Regulation of All Analyzed Genes” in [42]. The Mtb genes were regarded differentially expressed when the mRNA relative expression ratio had a >2-fold change. The mRNA relative expression ratio was calculated as the mRNA abundance in intraphagosomal Mtb relative to that in Mtb from a log-phase 7H9 broth culture [41].
Table 3.
Regulation of the homologous Mtb genes in intraphagosomal bacilli. These Mtb genes are homologues to the differentially regulated Msm proteins shown in Table 1. The mRNA relative abundances were determined for the bacilli that infected naïve and activated murine bone marrow macrophages for 4, 24, and 48 hours respectively [42].
| Mtb gene | Mtb gene description | In naïve macrophages | In activated macrophages | Homologous Msm protein |
Similarity (%) |
||||
|---|---|---|---|---|---|---|---|---|---|
| 4 hr | 24 hr | 48 hr | 4 hr | 24 hr | 48 hr | ||||
| (Mtb genes matched to the genes of the upregulated Msm proteins in Table 1) | |||||||||
| Rv3127 | hypothetical protein | 1.3 | 1.7 | 1.9 | 1.7 | 21.3 | 7.5 | MSMEG_5246 | 60.00 |
| acg | hypothetical protein | 0.9 | 1.3 | 1.6 | 1.3 | 19.7 | 9.6 | MSMEG_5246 | 65.24 |
| acr | 14 KD antigen | 0.6 | 1.2 | 1.4 | 0.8 | 17.1 | 11.6 | MSMEG_3932 | 78.32 |
| Rv3129 | hypothetical protein | 1.1 | 0.6 | 1.3 | 1.3 | 11.8 | 7.8 | MSMEG_5243 | 76.59 |
| TB31.7 | hypothetical protein | 1.1 | 1.4 | 1.4 | 1.1 | 9.2 | 5.2 | MSMEG_3945 | 60.49 |
| Rv2005c | hypothetical protein | 0.9 | 1.0 | 1.2 | 1.1 | 6.7 | 2.5 | MSMEG_3950 | 65.52 |
| Rv0893c | hypothetical protein | 1.4 | 1.6 | 1.8 | 2.6 | 2.6 | 2.5 | MSMEG_0614 | 71.19 |
| Rv2026c | hypothetical protein | 1.6 | 1.5 | 1.7 | 1.8 | 2.4 | 2.2 | MSMEG_3945 | 60.99 |
| Rv0725c | hypothetical protein | 1.7 | 2.0 | 2.3 | 2.1 | 2.4 | 2.6 | MSMEG_0614 | 61.36 |
| Rv3767c | hypothetical protein | 2.3 | 2.0 | 1.7 | 2.4 | 2.2 | 2.0 | MSMEG_0614 | 60.60 |
| fadD9 | acyl-CoA synthetase | 2.4 | 2.2 | 1.8 | 2.4 | 2.2 | 2.0 | MSMEG_2956 | 82.08 |
| nrp | peptide synthetase | 1.3 | 1.6 | 1.6 | 1.5 | 2.1 | 1.7 | MSMEG_2956 | 73.58 |
| Rv1062 | hypothetical protein | 1.2 | 1.4 | 1.8 | 1.5 | 2.1 | 1.8 | MSMEG_5285 | 72.93 |
| Rv3787c | hypothetical protein | 1.4 | 1.3 | 1.3 | 1.6 | 1.7 | 1.7 | MSMEG_0614 | 62.79 |
| Rv2781c | hypothetical protein | 1.1 | 1.6 | 1.7 | 1.2 | 1.4 | 2.3 | MSMEG_4971 | 60.42 |
| Rv1889c | hypothetical protein | 1.4 | 1.3 | 1.3 | 1.5 | 1.4 | 1.4 | MSMEG_0614 | 62.39 |
| Rv0281 | hypothetical protein | 0.7 | 1.2 | 1.7 | 0.7 | 1.3 | 1.7 | MSMEG_0614 | 74.08 |
| hemL | glutamate-1-semialdehyde 2,1-aminomutase | 1.0 | 0.9 | 1.1 | 1.2 | 1.1 | 1.1 | MSMEG_0969 | 85.42 |
| Rv2765 | hypothetical protein | 1.0 | 0.8 | 0.9 | 1.0 | 0.9 | 0.9 | MSMEG_2669 | 73.57 |
| adhC | alcohol dehydrogenase | 0.9 | 0.8 | 0.8 | 0.8 | 0.7 | 0.7 | MSMEG_1037 | 74.06 |
| (Mtb genes matched to the genes of the downregulated Msm proteins in Table 1) | |||||||||
| cspA | cold shock protein | 0.5 | 0.6 | 0.4 | 0.4 | 0.3 | 0.3 | MSMEG_6159 | 98.50 |
| atpD | ATP synthase beta chain | 0.6 | 0.4 | 0.4 | 0.5 | 0.3 | 0.3 | MSMEG_4936 | 98.50 |
| Rv0227c | hypothetical protein | 0.6 | 0.5 | 0.4 | 0.5 | 0.3 | 0.3 | MSMEG_0317 | 75.74 |
| fadD5 | probable fatty-acid CoA ligase | 0.5 | 0.5 | 0.5 | 0.6 | 0.5 | 0.5 | MSMEG_0131 | 84.58 |
| panB | 3-methyl-2-oxobutanoate hydroxymethyltransferase | 1.0 | 1.2 | 1.0 | 0.9 | 0.7 | 0.7 | MSMEG_4298 | 83.27 |
| rpsQ | 30s ribosomal protein s17 | 1.0 | 1.0 | 0.8 | 0.8 | 0.7 | 0.7 | MSMEG_1445 | 90.72 |
| moaC2 | probable MoaC-2 protein involved in molybdopterin synthesis | 0.9 | 1.1 | 1.1 | 0.8 | 0.7 | 0.8 | MSMEG_5703 | 71.89 |
| phoY1 | probable phosphate transport system regulatory protein | 0.9 | 0.8 | 0.7 | 0.9 | 0.8 | 0.7 | MSMEG_1605 | 69.40 |
| Rv0042c | hypothetical protein | 0.9 | 0.9 | 0.8 | 0.9 | 0.9 | 0.7 | MSMEG_6913 | 65.03 |
| Rv3259 | hypothetical protein | 1.1 | 0.9 | 1.0 | 1.0 | 1.1 | 1.0 | MSMEG_1832 | 92.08 |
| phoY2 | probable phosphate transport system regulatory protein | 1.0 | 1.4 | 1.2 | 1.0 | 1.2 | 1.1 | MSMEG_1605 | 76.05 |
| Rv0875c | hypothetical protein | 0.8 | 1.1 | 1.4 | 0.9 | 1.6 | 1.5 | MSMEG_5694 | 74.05 |
| ispF | hypothetical protein | 1.4 | 1.6 | 2.1 | 1.6 | 1.7 | 2.3 | MSMEG_6075 | 69.53 |
| Rv0785 | hypothetical protein | 1.2 | 1.7 | 1.9 | 1.4 | 2.2 | 1.8 | MSMEG_5835 | 82.95 |
| Rv2669 | hypothetical protein | 1.3 | 1.5 | 1.6 | 1.4 | 2.3 | 1.7 | MSMEG_2789 | 63.80 |
It is interesting to note that 14 out of the 20 Mtb genes homologous to the upregulated Msm proteins were upregulated in at least one time point in either or both of the naïve and activated macrophages. None of these 20 Mtb genes were downregulated at any timepoint in the infected macrophages. Meanwhile, 12 out of the 15 Mtb genes homologous to the downregulated Msm proteins were either downregulated (four) or unchanged (eight) in the macrophages (Table 3).
Discussion
In this study, we have utilized the advanced nanoLC/LTQ-FTMS proteomics system and bioinformatics methods to investigate the response of Msm to acid stress. Acid stress has an implication in the interaction of mycobacteria with macrophages. Msm is a fast-growing mycobacterium whose adaptive response to hypoxia and nitric oxide exposure is similar to that of Mtb [51]. Although microarray has produced a significant amount of transcriptional expression information [42, 52], proteomics allows the study of final gene products in action and their interaction with the microenvironment they are in [53]. The label-free quantitative proteomics system allowed us to unbiasedly investigate the response of Msm to an acidic growth condition. We also incorporated a labeled internal standard sample to estimate and control the false discovery rate when the number of analysis replicates were limited [13].
Out of the 1032 detected proteins, the acid stress induced 21 proteins (Table 1). It is noteworthy that 14 of the 20 Mtb genes homologous to these upregulated Msm proteins were most significantly induced at 24 hour after Mtb infected the activated macrophage (Table 3). For convenience, the 20 Mtb genes homologous to the upregulated Msm proteins are called “the 20 homologous Mtb genes” hereafter. Oxidative stress introduced with the addition of hydrogen peroxide to the Mtb 7H9 broth culture did not induce these 20 homologous Mtb genes except for fadD9 (Rv2590) which is a homologue of MSMEG_2956 [42]. The lack of an effect of hydrogen peroxide on 19 of the 20 homologous Mtb genes suggests that oxidative stress was not be the only factor to induce 14 of the 20 homologous Mtb genes. The presence of palmitic acid in the Mtb 7H9 broth culture induced none of these 20 homologous Mtb genes, excluding that fatty acids in macrophages would induce 14 of the 20 homologous Mtb genes in activated macrophages. Interestingly, only three of the 20 homologous Mtb genes were marginally induced in naïve macrophages (Table 3). It appears that the induction of the 20 homologous Mtb genes was specific to activated macrophages with a peak response at 24 hours post infection. Macrophage activation leads to phagosome maturation and acidification. These results suggest that acid stress response could play a role in Mtb pathogenesis.
One of the mycobacterial virulence factors, the alpha-crystallin-like protein (acr; Rv2031c and MSMEG_3932), was most significantly induced both in the intraphagosomal Mtb in activated macrophages and in the acid stressed Msm cells in 7H9 culture (Tables 1 & 3). The alpha-crystallin-like protein, or the 14-kDa antigen, was the third most significantly induced gene (by 17-fold) among the 20 homologous Mtb genes, and is the most significantly induced protein (by 12-fold) among the 21 upregulated Msm proteins. This result indicates that acid stress could induce some of the mycobacterial virulence factors and further supports that acidic adaptation involved in Mtb pathogenesis.
We performed a gene ontology analysis for the 52 differentially regulated proteins (Table 1) to assess their enrichment among the detected proteins with the bioinformatics software Cytoscape [35]. The Gene Ontology (GO) project provides controlled vocabularies for the description of biological process, molecular function, and cellular component in a cell [32]. The GO terms can be used as attributes of proteins to facilitate uniform queries. Although GO does not necessarily represent the best functional categorization of genes for all occasions because redundancy and interdependence exist in the GO network, we choose to perform GO analysis of the 52 differentially regulated proteins in this study for several reasons. First, we try not to rely only on the one-gene-one-genotype paradigm to interpret the roles of the differentially regulated proteins in the acid stressed Msm cells. Second, GO categorizes proteins into sets represented by the GO terms. These protein sets allow more rigorous statistical analysis to improve confidence in a biological conclusion. For example, the presence of multiple differentially regulated proteins in a biological process indicates that the biological process is more likely to be differentially regulated than when only a single differentially regulated protein is present in that biological process. Third, GO provides a platform to evaluate the influence of the acid stress from three complementary ontologies i.e., biological process, cellular component, and molecular function. Such a sub-proteomic analysis is useful when a particular ontology needs to be analyzed, for example, to gain information of enrichment of proteins in specific organelles [17, 54] or sub-cellular localization [55]. Thus, a GO analysis provides a system-based approach to interpret large scale proteomics data [56], focuses on the behavior of protein sets, and thus bears a higher statistical confidence in a biological conclusion compared to an analysis of individual proteins alone.
The gene ontology analysis revealed that two major biological processes were affected by the acid stress. One is membrane transport activity and the other is fatty acid metabolism. In a DNA microarray analysis of the global transcriptional response of Mtb to a low pH under in vitro conditions, Fisher et al. identified 81 genes differentially expressed at >1.5-fold in a pH 5.5 versus pH 6.9 growth medium [39]. Many of those genes involved in fatty acid metabolism, consistent with our finding that proteins involved in fatty acid metabolism were upregulated in the acid stressed Msm cells.
Upon infection, mycobacteria sense the intraphagosomal environment and synthesize critical proteins to interfere with the host defense. Although Msm is non-pathogenic, it possesses the ability to initially delay the acidification of the phagosome at the early stage of entry into macrophages [12, 57]. This ability of Msm to delay acidification of phagosomes is similar to that of Mtb. Although the ability of Mtb to resist phagolysosome fusion is a hallmark in its pathogenesis, Mtb can survive in acidified compartments. Mtb infection of freshly isolated human alveolar macrophages revealed that the majority of phagosomes containing intact bacteria were in an intimate contact with lysosomes [58]. ‘Turning on’ the phagolysosome fusion or ‘reversing’ the usual nonfusion pattern in normal mouse peritoneal macrophages did not influence the outcome of infection with virulent Mtb. Lysosome contents manifestly failed to exercise an antibacterial effect on Mtb [59]. The failure of the lysosome contents to kill Mtb after its adaptation to an intracellular environment suggests that an initial acid stress response prepares the mycobacterium for subsequent survival in the hostile intracellular environment. Indeed, it was suggested that the fusion of Mtb-containing phagosomes with lysosomes at the initial stage of infection was actually advantageous for the survival of the Mtb cells in the macrophage [4]. It seems quite likely that the bacteria have adapted to be able to live within a phagolysosome in activated macrophages [60–62].
Thus, mycobacteria possibly have at least two sets of adaptive mechanisms [61]. One is to restrict the phagolysosome fusion early in infection. The other is to adapt to phagolysosome fusion within the activated macrophages of granulomas later in infection. In a prior study, we showed that Msm could readily adapt to an acid shock by significantly readjusting its proteome to resume growth at an acidic condition [21]. The results from this study suggest that reduced membrane transport could be part of the acidic adaptation process. Inadvertently, Purdy et al. recently showed that decreased outer membrane permeability protects Msm from killing by the bactericidal action of ubiquitin-derived peptides and macrophages [10].
The induction of fatty acid metabolism genes or proteins by acid stress might contribute to the thickening of the cell wall to protect the cells or to the storage of an energy source when the cells transit to a slower growth or non-replicating state under the stress. It is not clear, however, whether the upregulated fatty acid metabolism proteins could also relate to accelerated fatty acid catabolism under acid stress, which probably requires a metabolite analysis to confirm.
Conclusion
We found that the most significant changes induced by a low pH include a down-regulation of proteins involved in transmembrane transporter activity and an upregulation of enzymes involved in fatty acid metabolism based on a GO analysis. While an inspection of other differentially regulated proteins individually could also lead to conclusions about the involvement of those proteins in the acid stress response, the GO enrichment analyses reduce the possibility that some proteins may appear to be involved in the stress response merely out of chance. The functions of many Msm proteins are annotated based on homology and not yet functionally confirmed [63]. There is also a possibility that the upregulation of some proteins is due to regulon overlap or pathway crosstalk instead of a function specific to the particular stress [64]. Thus, before further functional analysis can be carried out for many proteins, we use the GO analysis approach to distill the major changes that occurred in the acid stressed Msm cells. The GO analysis should lead to a more reliable conclusion than an interpretation of individual proteins alone.
On the other hand, GO might not capture all of the significant changes. For example, a protein interaction network analysis revealed the upregulation of a 3-protein cluster including MSMEG_5243, MSMEG_5245, and MSMEG_5246, which are related to the DevSR two-component system. Thus, an alternative gene interaction network analysis could complement the GO analysis [65]. Although further analysis of the role of the Msm DevSR two-component system in acid stress is out of the scope of this study, the upregulation of these three proteins implies that the Msm DevSR two-component system probably responds to a broader range of stresses in addition to heat, hypoxia, and NO [11, 23].
Acknowledgement
We thank Giovanni Lostumbo and Prahlad Rao for proofreading the manuscript.
References
- 1.Hestvik AL, Hmama Z, Av-Gay Y. Mycobacterial manipulation of the host cell. FEMS Microbiol Rev. 2005;29:1041–1050. doi: 10.1016/j.femsre.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Crowle AJ, Dahl R, Ross E, May MH. Evidence that vesicles containing living, virulent Mycobacterium tuberculosis or Mycobacterium avium in cultured human macrophages are not acidic. Infect Immun. 1991;59:1823–1831. doi: 10.1128/iai.59.5.1823-1831.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, Allen RD, Gluck SL, Heuser J, Russell DG. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 4.Brown CA, Draper P, Hart PD. Mycobacteria and lysosomes: a paradox. Nature. 1969;221:658–660. doi: 10.1038/221658a0. [DOI] [PubMed] [Google Scholar]
- 5.Vandal OH, Pierini LM, Schnappinger D, Nathan CF, Ehrt S. A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat Med. 2008;14:849–854. doi: 10.1038/nmXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonough KA, Kress Y, Bloom BR. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect Immun. 1993;61:2763–2773. doi: 10.1128/iai.61.7.2763-2773.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonough KA, Florczyk MA, Kress Y. Intracellular passage within macrophages affects the trafficking of virulent tubercle bacilli upon reinfection of other macrophages in a serum-dependent manner. Tuber Lung Dis. 2000;80:259–271. doi: 10.1054/tuld.2000.0268. [DOI] [PubMed] [Google Scholar]
- 8.Singh AK, Reyrat JM. Laboratory maintenance of Mycobacterium smegmatis. Curr Protoc Microbiol. 2009. Chapter 10: Unit10C 11. [DOI] [PubMed]
- 9.Jhamb SS, Singh PP. A short-term model for preliminary screening of potential anti-tubercular compounds. Scand J Infect Dis. 2009:1–4. doi: 10.3109/00365540903214314. [DOI] [PubMed] [Google Scholar]
- 10.Purdy GE, Niederweis M, Russell DG. Decreased outer membrane permeability protects mycobacteria from killing by ubiquitin-derived peptides. Mol Microbiol. 2009;73:844–857. doi: 10.1111/j.1365-2958.2009.06801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JM, Cho HY, Cho HJ, Ko IJ, Park SW, Baik HS, Oh JH, Eom CY, Kim YM, Kang BS, Oh JI. O2- and NO-sensing mechanism through the DevSR two-component system in Mycobacterium smegmatis. J Bacteriol. 2008;190:6795–6804. doi: 10.1128/JB.00401-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anes E, Peyron P, Staali L, Jordao L, Gutierrez MG, Kress H, Hagedorn M, Maridonneau-Parini I, Skinner MA, Wildeman AG, Kalamidas SA, Kuehnel M, Griffiths G. Dynamic life and death interactions between Mycobacterium smegmatis and J774 macrophages. Cell Microbiol. 2006;8:939–960. doi: 10.1111/j.1462-5822.2005.00675.x. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Roxas BA. An assessment of false discovery rates and statistical significance in label-free quantitative proteomics with combined filters. BMC Bioinformatics. 2009;10:43. doi: 10.1186/1471-2105-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roxas BA, Li Q. Significance analysis of microarray for relative quantitation of LC/MS data in proteomics. BMC Bioinformatics. 2008;9:187. doi: 10.1186/1471-2105-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao PK, Rodriguez GM, Smith I, Li Q. Protein dynamics in iron-starved Mycobacterium tuberculosis revealed by turnover and abundance measurement using hybrid-linear ion trap-fourier transform mass spectrometry. Anal Chem. 2008;80:6860–6869. doi: 10.1021/ac800288t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreev VP, Li L, Cao L, Gu Y, Rejtar T, Wu SL, Karger BL. A new algorithm using cross-assignment for label-free quantitation with LC-LTQ-FT MS. J Proteome Res. 2007;6:2186–2194. doi: 10.1021/pr0606880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao PK, Singh CR, Jagannath C, Li Q. A systems biology approach to study the phagosomal proteome modulated by mycobacterial infections. Int J Clin Exp Med. 2009;2:233–247. [PMC free article] [PubMed] [Google Scholar]
- 18.Katti MK, Dai G, Armitige LY, Rivera Marrero C, Daniel S, Singh CR, Lindsey DR, Dhandayuthapani S, Hunter RL, Jagannath C. The Delta fbpA mutant derived from Mycobacterium tuberculosis H37Rv has an enhanced susceptibility to intracellular antimicrobial oxidative mechanisms, undergoes limited phagosome maturation and activates macrophages and dendritic cells. Cell Microbiol. 2008;10:1286–1303. doi: 10.1111/j.1462-5822.2008.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geisow MJ, D'Arcy Hart P, Young MR. Temporal changes of lysosome and phagosome pH during phagolysosome formation in macrophages: studies by fluorescence spectroscopy. J Cell Biol. 1981;89:645–652. doi: 10.1083/jcb.89.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pethe K, Swenson DL, Alonso S, Anderson J, Wang C, Russell DG. Isolation of Mycobacterium tuberculosis mutants defective in the arrest of phagosome maturation. Proc Natl Acad Sci U S A. 2004;101:13642–13647. doi: 10.1073/pnas.0401657101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao PK, Roxas BA, Li Q. Determination of global protein turnover in stressed mycobacterium cells using hybrid-linear ion trap-fourier transform mass spectrometry. Anal Chem. 2008;80:396–406. doi: 10.1021/ac701690d. [DOI] [PubMed] [Google Scholar]
- 22. http://string.embl.de/
- 23.O'Toole R, Smeulders MJ, Blokpoel MC, Kay EJ, Lougheed K, Williams HD. A two-component regulator of universal stress protein expression and adaptation to oxygen starvation in Mycobacterium smegmatis. J Bacteriol. 2003;185:1543–1554. doi: 10.1128/JB.185.5.1543-1554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roback P, Beard J, Baumann D, Gille C, Henry K, Krohn S, Wiste H, Voskuil MI, Rainville C, Rutherford R. A predicted operon map for Mycobacterium tuberculosis. Nucleic Acids Res. 2007;35:5085–5095. doi: 10.1093/nar/gkm518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsoso LG, Kana BD, Crellin PK, Lea-Smith DJ, Pelosi A, Powell D, Dawes SS, Rubin H, Coppel RL, Mizrahi V. Function of the cytochrome bc1-aa3 branch of the respiratory network in mycobacteria and network adaptation occurring in response to its disruption. J Bacteriol. 2005;187:6300–6308. doi: 10.1128/JB.187.18.6300-6308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayuri, Bagchi G, Das TK, Tyagi JS. Molecular analysis of the dormancy response in Mycobacterium smegmatis: expression analysis of genes encoding the DevR-DevS two-component system, Rv3134c and chaperone alpha-crystallin homologues. FEMS Microbiol Lett. 2002;211:231–237. doi: 10.1111/j.1574-6968.2002.tb11230.x. [DOI] [PubMed] [Google Scholar]
- 27.Sinha S, Rappu P, Lange SC, Mantsala P, Zalkin H, Smith JL. Crystal structure of Bacillus subtilis YabJ, a purine regulatory protein and member of the highly conserved YjgF family. Proc Natl Acad Sci U S A. 1999;96:13074–13079. doi: 10.1073/pnas.96.23.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dietrich LE, Teal TK, Price-Whelan A, Newman DK. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science. 2008;321:1203–1206. doi: 10.1126/science.1160619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cundliffe E. How antibiotic-producing organisms avoid suicide. Annu Rev Microbiol. 1989;43:207–233. doi: 10.1146/annurev.mi.43.100189.001231. [DOI] [PubMed] [Google Scholar]
- 30.Morishita R, Kawagoshi A, Sawasaki T, Madin K, Ogasawara T, Oka T, Endo Y. Ribonuclease activity of rat liver perchloric acid-soluble protein, a potent inhibitor of protein synthesis. J Biol Chem. 1999;274:20688–20692. doi: 10.1074/jbc.274.29.20688. [DOI] [PubMed] [Google Scholar]
- 31.Cashman JR. Role of flavin-containing monooxygenase in drug development. Expert Opin Drug Metab Toxicol. 2008;4:1507–1521. doi: 10.1517/17425250802522188. [DOI] [PubMed] [Google Scholar]
- 32.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. ftp://ftp.ebi.ac.uk/pub/databases/GO/goa/
- 34.Maere S, Heymans K, Kuiper M. BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics. 2005;21:3448–3449. doi: 10.1093/bioinformatics/bti551. [DOI] [PubMed] [Google Scholar]
- 35. http://www.cytoscape.org/
- 36.Postma PW, Lengeler JW, Jacobson GR. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kights KM. Long-Chain-Fatty-Acid CoA Ligases: The Key to Fatty Acid Activation, Formation of Xenobiotic Acyl-CoA Thioesters and Lipophilic Xenobiotic Conjugates. Current Medicinal Chemistry - Immunology, Endocrine & Metabolic Agents. 2003;3:235–244. [Google Scholar]
- 38.Deb C, Lee CM, Dubey VS, Daniel J, Abomoelak B, Sirakova TD, Pawar S, Rogers L, Kolattukudy PE. A novel in vitro multiple-stress dormancy model for Mycobacterium tuberculosis generates a lipid-loaded, drug-tolerant, dormant pathogen. PLoS One. 2009;4:e6077. doi: 10.1371/journal.pone.0006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher MA, Plikaytis BB, Shinnick TM. Microarray analysis of the Mycobacterium tuberculosis transcriptional response to the acidic conditions found in phagosomes. J Bacteriol. 2002;184:4025–4032. doi: 10.1128/JB.184.14.4025-4032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia Q, Hendrickson EL, Zhang Y, Wang T, Taub F, Moore BC, Porat I, Whitman WB, Hackett M, Leigh JA. Quantitative proteomics of the archaeon Methanococcus maripaludis validated by microarray analysis and real time PCR. Mol Cell Proteomics. 2006;5:868–881. doi: 10.1074/mcp.M500369-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Q. Advances in protein turnover analysis at the global level and biological insights. Mass Spectrom Rev. 2009. Sep 15 [Epub ahead of print] [DOI] [PubMed]
- 42.Schnappinger D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, Monahan IM, Dolganov G, Efron B, Butcher PD, Nathan C, Schoolnik GK. Transcriptional Adaptation of Mycobacterium tuberculosis within Macrophages: Insights into the Phagosomal Environment. J Exp Med. 2003;198:693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gillespie E. Effects of S-lactoylglutathione and inhibitors of glyoxalase I on histamine release from human leukocytes. Nature. 1979;277:135–137. doi: 10.1038/277135a0. [DOI] [PubMed] [Google Scholar]
- 44.Kim NS, Umezawa Y, Ohmura S, Kato S. Human glyoxalase I. cDNA cloning, expression, and sequence similarity to glyoxalase I from Pseudomonas putida. J Biol Chem. 1993;268:11217–11221. [PubMed] [Google Scholar]
- 45.Thornalley PJ. Glyoxalase I--structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans. 2003;31:1343–1348. doi: 10.1042/bst0311343. [DOI] [PubMed] [Google Scholar]
- 46.Hao da C, Chen SL, Mu J, Xiao PG. Molecular phylogeny, long-term evolution, and functional divergence of flavin-containing monooxygenases. Genetica. 2009;137:173–187. doi: 10.1007/s10709-009-9382-y. [DOI] [PubMed] [Google Scholar]
- 47.Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol. 2002;43:717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 48.Qian L, Ortiz de Montellano PR. Oxidative activation of thiacetazone by the Mycobacterium tuberculosis flavin monooxygenase EtaA and human FMO1 and FMO3. Chem Res Toxicol. 2006;19:443–449. doi: 10.1021/tx050328b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. http://www.geneontology.org/
- 50. http://www.ncbi.nlm.nih.gov/Structure/index.shtml.
- 51.Dick T, Lee BH, Murugasu-Oei B. Oxygen depletion induced dormancy in Mycobacterium smegmatis. FEMS Microbiol Lett. 1998;163:159–164. doi: 10.1111/j.1574-6968.1998.tb13040.x. [DOI] [PubMed] [Google Scholar]
- 52.Hampshire T, Soneji S, Bacon J, James BW, Hinds J, Laing K, Stabler RA, Marsh PD, Butcher PD. Stationary phase gene expression of Mycobacterium tuberculosis following a progressive nutrient depletion: a model for persistent organisms? Tuberculosis (Edinb) 2004;84:228–238. doi: 10.1016/j.tube.2003.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nissom PM, Sanny A, Kok YJ, Hiang YT, Chuah SH, Shing TK, Lee YY, Wong KT, Hu WS, Sim MY, Philp R. Transcriptome and proteome profiling to understanding the biology of high productivity CHO cells. Mol Biotechnol. 2006;34:125–140. doi: 10.1385/mb:34:2:125. [DOI] [PubMed] [Google Scholar]
- 54.Trost M, English L, Lemieux S, Courcelles M, Desjardins M, Thibault P. The phagosomal proteome in interferon-gamma-activated macrophages. Immunity. 2009;30:143–154. doi: 10.1016/j.immuni.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Dumas E, Desvaux M, Chambon C, Hebraud M. Insight into the core and variant exoproteomes of Listeria monocytogenes species by comparative subproteomic analysis. Proteomics. 2009;9:3136–3155. doi: 10.1002/pmic.200800765. [DOI] [PubMed] [Google Scholar]
- 56.Feltrin E, Campanaro S, Diehl AD, Ehler E, Faulkner G, Fordham J, Gardin C, Harris M, Hill D, Knoell R, Laveder P, Mittempergher L, Nori A, Reggiani C, Sorrentino V, Volpe P, Zara I, Valle G, Deegan Nee Clark J. Muscle Research and Gene Ontology: New standards for improved data integration. BMC Med Genomics. 2009;2:6. doi: 10.1186/1755-8794-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuehnel MP, Goethe R, Habermann A, Mueller E, Rohde M, Griffiths G, Valentin-Weigand P. Characterization of the intracellular survival of Mycobacterium avium ssp. paratuberculosis: phagosomal pH and fusogenicity in J774 macrophages compared with other mycobacteria. Cell Microbiol. 2001;3:551–566. doi: 10.1046/j.1462-5822.2001.00139.x. [DOI] [PubMed] [Google Scholar]
- 58.Borelli V, Vita F, Soranzo MR, Banfi E, Zabucchi G. Ultrastructure of the interaction between mycobacterium tuberculosis- H37Rv-containing phagosomes and the lysosomal compartment in human alveolar macrophages. Exp Mol Pathol. 2002;73:128–134. doi: 10.1006/exmp.2002.2452. [DOI] [PubMed] [Google Scholar]
- 59.Armstrong JA, Hart PD. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975;142:1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan K, Knaak T, Satkamp L, Humbert O, Falkow S, Ramakrishnan L. Complex pattern of Mycobacterium marinum gene expression during long-term granulomatous infection. Proc Natl Acad Sci U S A. 2002;99:3920–3925. doi: 10.1073/pnas.002024599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cosma CL, Sherman DR, Ramakrishnan L. The secret lives of the pathogenic mycobacteria. Annu Rev Microbiol. 2003;57:641–676. doi: 10.1146/annurev.micro.57.030502.091033. [DOI] [PubMed] [Google Scholar]
- 62.Ramakrishnan L, Federspiel NA, Falkow S. Granuloma-specific expression of Mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science. 2000;288:1436–1439. doi: 10.1126/science.288.5470.1436. [DOI] [PubMed] [Google Scholar]
- 63.Wang R, Prince JT, Marcotte EM. Mass spectrometry of the M. smegmatis proteome: protein expression levels correlate with function, operons, and codon bias. Genome Res. 2005;15:1118–1126. doi: 10.1101/gr.3994105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pomposiello PJ, Bennik MH, Demple B. Genome-wide transcriptional profiling of the Escherichia coli responses to superoxide stress and sodium salicylate. J Bacteriol. 2001;183:3890–3902. doi: 10.1128/JB.183.13.3890-3902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Balazsi G, Heath AP, Shi L, Gennaro ML. The temporal response of the Mycobacterium tuberculosis gene regulatory network during growth arrest. Mol Syst Biol. 2008;4:225. doi: 10.1038/msb.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]