Abstract
Spinal p38 MAP kinase plays a key role in chronic pain behavior. However, clinical development of p38 inhibitors has been hindered by significant toxicity. To evaluate alternative strategies of p38 regulation, we determined if known upstream activators of p38 (MKK3 and MKK6), are involved in development and maintenance of pain and spinal p38 phosphorylation. Acute pain behaviors were not altered in MKK3 or MKK6 deficient mice. The phase 2 formalin response was delayed in MKK3−/− mice, but unchanged in magnitude, while the response remained normal in MKK6−/− mice. More striking, late formalin allodynia (3 to 18 days post-injection) was prominent in wild type and MKK6−/− mice, but was delayed for several days in MKK3−/− mice. In wild type, but not MKK3−/− mice, intraplantar formalin elicited increases in ipsilateral spinal MKK3/6 phosphorylation acutely and again at 9 days post injection. Phosphorylation of MKK3/6 correlated with phase 2 formalin behavior. Wild type and MKK3−/− mice both expressed increases in spinal phosphorylated p38, however in WT mice this response began several days earlier, and was of higher magnitude and duration than in MKK3−/− mice. This phosphorylation correlated with the late allodynia. Phosphorylated MKK3/6 was detected only in astrocytes, given that P-p38 is usually not seen in astrocytes this argues for astrocytic release of soluble mediators that affect p38 phosphorylation in microglia. Taking these data together, MKK3, but not MKK6, is necessary for normal development of chronic pain behavior and phosphorylation of spinal p38.
Keywords: MKK6, neuropathic pain, inflammation
Peripheral inflammation, nerve injury and intraplantar formalin cause chronic pain behavior and increased phosphorylated p38 (P-p38) mitogen activated (MAP) kinase in spinal cord dorsal horn (Jin et al., 2003; Schafers et al., 2003a; Svensson et al., 2003b; Tsuda et al., 2004; Zhuang et al., 2006; Ito et al., 2007). Spinal pretreatment with a p38 antagonist reduces pain behavior in these animal models (Schafers et al., 2003b; Svensson et al., 2003b). At least two p38 isoforms, p38α and p38β, are expressed in spinal cord. Selective p38β, but not p38a knockdown with spinal administration of antisense oligonucleotides reduces the phase 2, but not phase 1 response after intraplantar formalin injection and blocks pain behavior induced by spinal administration of algesic substances (Svensson et al., 2005). Thus, spinal p38 activation, most likely p38β, contributes to chronic pain behavior.
The principle upstream activators of p38, especially after exposure to pro-inflammatory cytokines, are the dual kinases, MAP kinase kinase (MKK)3 and MKK6 (Inoue et al., 2005; Zarubin and Han, 2005; Inoue et al., 2006), although in some cases MKK4 is also upstream of p38 activation (Kang et al., 2006) and TAB-1 activation of p38α has been demonstrated independent of MKKs in a yeast two-hybrid screen (Ge et al., 2002). Phosphorylation of MKK3 activates p38α, while P-MKK6 activates both α and ß isoforms (Enslen et al., 1998). However, these data are derived from either in vitro or in vivo somatic tissues and the role of these specific MKKs in neural tissue (spinal cord or brain) or in the generation of nociception or pain is unknown.
Non-neural elements (microglia and astrocytes) play major roles in the generation and maintenance of chronic pain. Astrocytes contain unique transporter molecules that regulate synaptic glutamate levels and therefore neuronal excitability. They also synthesize and secrete a variety of agents such as cytokines.
In the present study, we determined whether MKK3 and MKK6 are necessary for the expression of either acute pain behavior or maintained pain behavior that results from spinal sensitization. Behavioral experiments show that MKK3, but not MKK6, deficiency delays both the phase 2 formalin response and the late allodynia that is present for weeks following intraplantar formalin, while having no effects on acute measures of pain. In parallel, Western blots demonstrate that genetic deletion of MKK3 delays and reduces spinal p38 phosphorylation over the same period. Interestingly, P-MKK3/6 was detected only in astrocytes. Taken together, these data suggest that MKK3 is an indirect upstream activator of p38 in the nervous system, but that other pathways likely contribute to p38 phosphorylation and spinal sensitization.
Experimental Procedures
Animals
Mice (20-30 gms, male) were gang housed on a 12/12hr light dark cycle and fed food and water ad libitum. Knock out (KO) mice for MKK3 and MKK6 were originally generated at Yale University, New Haven, CT (Lu et al., 1999; Tanaka et al., 2002) where they were backcrossed onto a C57Bl/6 line obtained from Charles River Labs. MKK3−/− and MKK6−/− mice were bred at UCSD and genotyped according to standard protocols available at the Jackson Labs web site: http//www.jax.org/imr/supp_proto.html. Although younger MKK3−/− and MKK6−/− mice were smaller than WT, at the time of the experiment no differences in general behavior or appearance were noted among the three different genotypes. All animal protocols were approved by the Institutional Animal Care Committee of the University of California, San Diego.
Behavioral testing
Acute pain
All test devices were adapted to mice and testing was carried out in a dedicated mouse room. Mice were acclimated for a minimum of two h (one h in the room and one h in the behavioral device) for two days prior to the test day and then again prior to testing on the day of the experiment. Acute thermal escape latencies were measured by focusing a radiant heat source on the plantar hindpaw surface in a modified Hargreaves device (Dirig et al., 1997). Mechanical 50% probability withdrawal thresholds of the ventral hindpaw were assessed using a modified version (buckling forces of von Frey filaments from 0.2-2.0 gm) of the up-down method (Chaplan et al., 1994). For both acute thermal and mechanical assays, each mouse was tested three times/day on both hindpaws at 15 min intervals. This was performed on two days. Thus, acute thresholds for each mouse are the averages of six responses of each paw.
Formalin-phase 1 and 2 responses and prolonged allodynia
An automated motion detector system (Yaksh et al., 2001) was used to quantify flinching behavior induced by a single 20 μl injection of 2.5% formalin solution into the dorsal surface of the left hind paw. Flinches were counted in 1 min bins for the first hour after injection. Male WT and knockout mice were tested in the same session. Mechanical thresholds were measured on the ventral surface of the injected paw, as it was performed for basal testing, on days 3, 7, 9, 14 and 18 post-formalin injection to assess long-term allodynia. A separate group of WT mice were not injected with any substance, but were repeatedly tested as a comparison to control for handling and learning.
Western Blots
Mice were deeply anesthetized with isoflurane and the spinal cord hydroextruded and dissected on ice. The left lumbar enlargement was frozen on dry ice and stored at −70° C until processing. Frozen spinal tissue was ground and then homogenized in chilled lysis buffer (50mM Tris pH 7.4, 150mM NaCl, 1mM EDTA pH 8, 0.5% Triton X-100 containing protease and phosphatase inhibitors (Sigma Chemicals, St. Louis, MO)). After centrifugation (22,000×g for 15 min at 4°C, protein concentrations in the extracts were determined using the DC protein assay reagent (Bio-Rad, Hercules CA). Lysates were fractionated on Tris-glycine-buffered 10% SDS-PAGE and transferred to nitrocellulose membrane (Perkin-Elmer Life Sciences Inc, Boston MA). Membranes were blocked with Tris buffered saline and 0.1% Tween 20 (TBS-T) containing 5% nonfat milk for 1 hr at room temperature followed by incubation with primary antibody (P-p38, P-MKK3/6 (anti-rabbit, 1:1000, Cell Signalling), MKK3 (anti-rabbit, 1:1000, Santa Cruz), MKK6 (anti - rabbit, 1:1000, Stressgen), GAPDH (anti-mouse, 1:1000, Santa Cruz) or β-actin (1:5000, Sigma) at 4° C overnight. The loading was normalized with β-actin or GAPDH. After washing with TBS-T, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody (1:200) for two hs at room temperature. Immunoreactive protein was detected with chemi-luminescence and autoradiography (Perkin-Elmer, Boston, MA) and analyzed using National Institutes of Health (NIH) Image (version 1.63, NIH, Bethesda MD).
Immunohistochemistry
Mice were deeply anesthetized with isoflurane and transcardially perfused with room temperature heparinized 0.9% saline containing phosphatase inhibitors (Sigma Chemicals, St. Louis, MO) followed by chilled 4% paraformaldahyde in 0.1 M phosphate buffer. Spinal cords were removed and post-fixed in perfusate for 6 hs and transferred, for cryoprotection at 4°C, first to 20% sucrose for 12-24 hs and then to 30% sucrose until they sank. Within five days, the fixed lumbar enlargements were snap frozen and transverse sections (30 μm) from L4-5 were cut on a freezing microtome. Free floating sections were single or double stained with antibodies to MKK3 (1:250; rabbit anti-human sc961, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) MKK6 (1:250; rabbit anti-human KAP-MA014, Stressgen) or P-MKK3/6 (1:250; rabbit anti-human 9231, Cell Signaling). Individual antibodies for P-MKK3 and M-MKK6 are not currently available, thus we measured phosphorylation of MKK3 and MKK6 together. Cell markers Ox-42 (1:100; Biosource International) or 1ba-1 (1:1000; Wako, Osaka, Japan) for microglia, glial fibrillary acidic protein (GFAP) (1:1000; Chemicon, Temecula, CA) for astrocytes and NeuN for neurons (1:1000; Chemicon) confirmed the cellular location of the enzymes. At least three random sections were taken from each level and reported results were observed in a minimum of three animals under each condition. Binding sites were visualized with secondary antibody conjugated Alexa-594 (1:250; Molecular Probes, Eugene, OR) or Alexa-488 (1:250, Molecular Probes). The tyramide signal amplification florescence procedure (TSA, Perkin Elmer, Boston, MA) was used in visualizing MKK6 and Ox-42. Non-specific staining was determined by exclusion of primary antibodies. Staining for P-p38 (1:100; rabbit anti-human, Cell Signaling) was performed identically except that we used thinner sections (10 μm), which were slide mounted rather than free floating. Ipsilateral and contralateral images were captured with a fluorescence microscope (Olympus, Melville, NY) at 10-40X. To confirm antibody co-localization, confocal images were acquired with a Leica TCS SP2 confocal system; single optical sections of 0.3-0.4 μm thickness were taken and images processed with Adobe Photoshop software.
Statistics
A minimum of six mice/group were used for all behavioral analysis. Values are expressed as mean ± SEM. Statistics were performed on the 50% probability withdrawal threshold at multiple timepoints using ANOVA for repeated measures (behavior late allodynia-days 3-18); unpaired t-tests were used to compare areas under the curve (formalin phase 1 and 2) and the acute behavioral data. Protein levels in Western blots were measured in 3-5 animals/group. ANOVA was used to compare phospho-protein levels at different time points within the same strain, levels from different strains at the same timepoint were compared using unpaired t-tests. p≤ 0.05 was considered to be significant.
Results
Behavior
Normal acute pain behavior in MKK3−/− and MKK6−/− mice
Thermal and mechanical stimuli
Mean thermal latency and mechanical withdrawal threshold for MKK3−/− and MKK6−/− mice did not differ from those of WT mice (Table I). Also, no differences were seen between right and left paws (data not shown). Thus, escape responses to acute nociceptive stimulation was unaffected by either MKK3 or MKK6 deficiency.
Table 1.
Thermal Latencies
| WT | 11.59s ± 0.23 | N=23 |
| MKK3−/− | 10.96s ± 0.39 | N=16 |
| Mechanical Thresholds | |||||||
| Experiment 1 | Experiment 2 | ||||||
| WT | 1.63g ± 0.05 | N=28 | WT | 2.00g ± 0.00 | N=8 | ||
| MKK3−/− | 1.54g ± 0.07 | N=21 | MKK6−/− | 2.00g ± 0.00 | N=7 | ||
Two grams was the upper limit for 50% probability mechanical withdrawal threshold.
Formalin response phase 1
The phase 1 response (1 to 9 min post injection) is the acute behavioral response to intraplantar formalin and, to a great extent, reflects the degree of primary afferent fiber activity (Puig and Sorkin, 1996). Total number of phase 1 flinches in the WT mice (N=16) was 341±18 compared to 336±28 in the MKK3−/− mice (N=16) and the curves are superimposable (Figure 1A). In different test sessions, WT (N=6) and MKK6−/− (N=6) mice emitted 473±21 and 424± 40 flinches, respectively (p≥0.05). Thus, the phase 1 formalin response was unaffected by either MKK3 or MKK6 deficiency.
Figure 1.
Influence of MKK3 and MKK6 on the response to intraplantar formalin A. Wild type and knockout mice were tested simultaneously for their phase 1 and 2 response to formalin injected into the paw. Data were collected in 1 min bins. Top panel indicates mean ± SEM for WT and MKK3−/− mice. Both strains had equivalent responses during phase 1 (0-9 min). Overall, the responses during phase 2 were also equivalent when flinches were summed from 10-60 min, however, there was a significant time shift in the response pattern. Bottom panel shows mean ± SEM for WT and MKK6−/− mice. Responses were similar for both phase 1 and 2 post-formalin. B. Mechanical withdrawal thresholds were measured prior to formalin injection (day 0) and on days 3, 7, 9, 14 and 18 post-formalin. Wild type and MKK6 −/− mice developed allodynia by day 7 post-formalin compared to their pre-formalin responses (*= p≤ 0.01). In contrast, formalin injected MKK3−/− mice exhibited a delayed decrease in threshold. ^= p≤0.05 compared to WT, N=6-8
Formalin response phase 2
Phase 2 (10 to 60 min) of the formalin test is, in part, the product of spinal facilitation of afferent drive (Haley et al., 1990) and is frequently used as a measure of spinal sensitization. It has been used as a tool to screen drugs candidates for treatment of chronic pain. Wild type mice emitted a total of 1306±118 responses while MKK3−/− mice emitted 1352±133 responses during this period. Although there was no significant difference in the total number of phase 2 flinches between the groups (p≥ 0.5), closer examination of the time course showed that the phase 2 responses of the MKK3−/− mice was delayed by several min (Figure 1A, top panel). Separate analysis of the number of responses emitted in the 10-22 and 30-42 min periods indicated differences between the groups. Note that the N in these experiments was 16 for each group. Experiments were performed twice to determine the robustness of this delay. The delay was observed in both groups and the data combined. This same delay was evident in separate experiments when female wild type and MKK3 −/− mice were tested (data not shown). During a separate set of experiments in which WT and MKK6−/− mice were tested simultaneously, WT male mice emitted a total of 1305±92 flinches during phase 2 compared to 1423±118 flinches for the MKK6 −/− mice (Figure 1A, bottom panel). There was no genotype-related difference in magnitude of the total phase 2 response between these two groups and no shift in time course was observed.
Decreased formalin-induced late allodynia in MKK3−/− mice
Formalin injection into the dorsal surface of the paw in rats results in a prolonged allodynia, a withdrawal response to innocuous stimuli, on the plantar surface of the paw that lasts for weeks. This chronic pain behavior results from spinal cord sensitization, including microglial activation (Fu et al., 1999; Fu et al., 2000). Prior to formalin injection, 50% probability withdrawal threshold of the left (injected) paw of this group of WT mice was 1.86 g± 0.04. Thresholds of the MKK3−/− mice were similar (1.81 g± 0.08). Following formalin injection into the dorsal surface of the paw, mechanical withdrawal thresholds of the WT mice decreased (i.e., increased allodynia) over the next several days and the difference was significant on post-injection days 7-18 (P≤ 0.001; falling approximately 55% to a nadir of 0.84g. ±0.19 by day 18 (Figure 1B). Thus, mice, like rats, develop a sustained late allodynia following intraplantar formalin injection (Fu et al., 2001). More importantly, allodynia was significantly delayed in the MKK3−/− mice. Thresholds remained normal through post injection day 9 and were decreased only on days 14 and 18 (Figure 1B). Thus, although maximal allodynia achieved was the same for both groups, the MKK3−/− animals took a week longer to develop the sensitization. We tested WT and MKK6−/− mice in a second experiment. Following formalin injection, mean thresholds fell precipitously and equivalently for both strains. On day 9, withdrawal thresholds were 1.08g ± 0.13 for the WT mice and 0.81g± 0.19 for the MKK6−/− mice (p≤ 0.001, compared to basal; p≥ 0.05 between WT and MKK6−/−). When a separate group of WT mice were not injected with formalin and simply retested at the same time points (time control), threshold never decreased more than 10% from baseline at any time point (data not shown).
Regulation of kinase activation post formalin
Because spinal p38 activation contributes to spinal sensitization and chronic pain behavior, we evaluated MKK3/6 and p38 phosphorylation in the three mouse genotypes following formalin injection using immunoblotting of the ipsilateral lumbar enlargement.
Basal levels
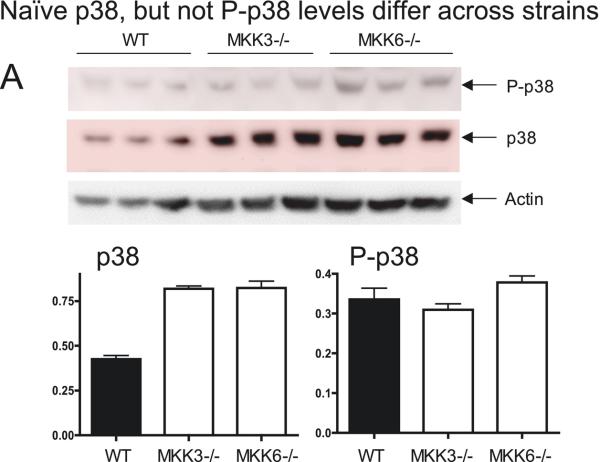
In spinal cord of WT mice, basal levels of MKK3, MKK6 and p38 were detectable, as were low levels of P-MKK3/6 and P-p38 (Figure 2, 3 and 4). When we ran blots from the different mouse strains in the same experiment (Figure 2), we observed that greater amounts of p38 (normalized to β-actin) in both MKK3−/−and MKK6−/− mice, compared to WT mice (P≤0.001). These results contrast with earlier work (Lu et al., 1999; Tanaka et al., 2002) demonstrating normal levels of p38 in spleen and kidneys of MKK3−/− and MKK6−/− mice. This might indicate tissue specific differences in p38 synthesis and compensatory regulatory pathways in neural tissue compared with somatic tissues. Despite these varying amounts of substrate, levels of P-p38 did not differ among the groups due to a smaller percentage of the p38 in the MKK3−/− and MKK6−/− mice being phosphorylated. Given the absence of one enzyme, all of the P-MKK3/6 consisted of P-MKK6 for the MKK3−/− mice and P-MKK3 for the MKK6−/− mice. There was no difference in basal levels of P-MKK3/6 between WT and MKK3−/− mice although there was a tendency for P-MKK3/6 to be lower in the MKK3−/− animals (data not shown).
Figure 2.
Samples of ipsilateral lumbar spinal cord from wild type, MKK3−/− and MKK6−/− naïve mice were immunoblotted on the same gel for direct comparison of p38 and P-p38 levels. All samples were normalized to β-actin and the y-axes on the histograms are in arbitrary units. In naïve animals, samples from WT mice contained less p38 than either MKK3−/− or MKK6−/− mice (p≤0.001). However, examination of P-p38 revealed no difference among any of the three strains. N=3
Figure 3.
Short term changes in phosphorylation of spinal MKK 3/6 and p38 in response to formalin injection in the paw dorsum. Representative Western blots for WT (left and MKK3−/− mice (right) are shown on the top half of the figure and graphs comparing mean responses for each kinase are illustrated on the bottom. *p ≤ 0.05 compared to the protein level in the uninjected animal, ^ p ≤ 0.05 compared to the protein level in the WT animals at the same timepoint N=3-5
Figure 4.

Formalin induced Long term changes in phosphorylation of spinal cord MKK 3/6 and p38 in response to formalin injection in the paw dorsum. Representative immunoblots for WT (left) and MKK3−/− mice (right) are shown in A and B. The MKK3 band is missing in blots from the MKK3−/− animals in panel B, confirming protein deletion. Composite graphs comparing mean responses over time for each kinase are illustrated on the bottom in panel C. P-MKK3/6 levels were unchanged on day 3, 7 and 14 compared to naïve in both strains and levels did not differ between the strains. More importantly, P-p38 levels were significantly elevated in WT spinal cords on day 3, 7 and 14 compared to MKK3−/−. * p ≤ 0.05 compared to the protein level in the uninjected animal; ^ p ≤ 0.05 compared to the protein level in the WT animals at the same timepoint. N=3-7
Formalin injection induced changes
In the first hour following formalin injection, spinal P-MKK3/6 expression in WT mice (N=4) exhibited a biphasic response with levels peaking first at 10-15 and then at 30-60 min (Figure 3, bottom right). The elevations in P-MKK3/6 preceded and then continued throughout most of the phase 2 behavioral response. During the same time periods, spinal levels of P-MKK3/6 did not change in the MKK3−/− animals. P-p38, however, did not change in the spinal cords of either strain with after injection (Figure 3, bottom left). Representative gels for WT mice (left) and MKK3−/− mice (right) are shown.
Three days post-formalin injection, spinal P-MKK3/6 had returned to basal for the WT mice and remained at this level through day 7 (Figure 4). P-MKK3/6 expression was transiently increased in WT animals on day 9 (p≤ 0.01; Figure 4C) and returned once more to basal levels by day 14. In contrast, P-p38 was increased in the spinal cord of WT mice on days 3-14 after formalin injection (p≤0.0001; N=3-6; Figures 4). The increase and plateau in P-p38 slightly preceded development of sensitization to light touch observed in the behavioral experiments and remained elevated through at least day 14. On days 7 and 9, spinal P-p38 in MKK3−/− mice was also elevated as a function of time after formalin (p≤ 0.0003); levels were significantly higher in WT compared with MKK3−/− tissue on all post injection days except day 9 (p≤ 0.02). P-p38 in MKK3−/− animals returned to baseline and were no longer elevated in comparison to naïve MKK3−/− animals by day 14 and were lower than those seen in day 14 WT animals. Thus, lack of MKK3 not only resulted in a delay of the injury-induced increase in P-p38, it also reduced the magnitude and duration of the response.
Immunohistochemistry
In naïve animals, there were low levels of P-MKK3/6 like-immunoreactivity in the lateral superficial dorsal horn (Figure 5A). One h after intraplantar formalin, we observed increased P-MKK3/6 staining across the superficial dorsal horn and in the overlying white matter. Scattered staining was also apparent in cells of the deeper laminae, predominantly IV and V (Figure 5B). At all time points examined, P-MKK3/6 staining was detected only in astrocytes as indicated by co-localization with GFAP (Figure 5 C-F). Although MKK6 and MKK3 staining was plentiful in neurons (data not shown), the phosphorylated proteins were not observed in NeuN staining cells (see Figure 6A). Immunoreactivity for MKK3 and MKK6 (not shown) as well as for P-MKK3/6 (Figure 6B) was below the limit of detection in microglia. In contrast, P-p38 was not observed in astrocytes (Figure 7), even at 7 days post formalin injection when immunoblotting results indicated that P-p38 was maximal.
Figure 5.
Formalin induces short-term increases in P-MKK3/6 in astrocytes. A. In dorsal horn from naïve mice, there was very little staining for P-MKK3/6 (green). One h post-formalin (B-F), there is a clear increase in P-MKK3/6 staining. C. GFAP, stained red, co-localizes with P-MKK3/6 stained green. The low power merged image shows yellow co-localization concentrated in the superficial dorsal horn and to a lesser extent in the deeper laminae. Higher power of superficial dorsal horn within the box shows images stained for P-MKK3/6 (D), GFAP (E) and the merged image in F which indicates that P-MKK3/6 is localized to the astrocytes. Not all astrocytes show yellow indicating that some do not contain P=MKK3/6. Scare bar in A = 100 μm, in D = 25 μm
Figure 6.
Seven days post-formalin P-MKK3/6 is detected only in astrocytes. A. P-MKK3/6, stained green, is not seen in neurons (Neu N is stained red) however the red structures have the morphology of astrocytes (co-staining not shown). The edge of the superficial dorsal horn is seen in the upper right corner and shows that the higher concentration of P-MKK3/6 is found within Laminae I and II. In panel B, OX-42 (microglial marker) is stained red and P-MKK3/6 in green. Lack of co-staining (yellow) indicates there is no phosphorylation of MKK3 or MKK6 in microglia. Scale bars = 40
Figure 7.
Seven days post-formalin P-p38 is not seen in astrocytes. A and D In dorsal horn there was staining for P-p38 (green), B and E show immunostaining for GFAP. Merging of the pictures in C and F indicate a lack of co-localization. Scale bars = 25 μm
Discussion
These studies explored whether the kinases that regulate p38 play contribute to pain behavior in mice. Our major findings are: 1) MKK3 and MKK6 do not participate in acute pain; 2) MKK3, but not MKK6, participates in the initiation of formalin phase 2 behavior and the subsequent prolonged allodynia and its phosphorylation correlates with phase 2 activity; 3) lack of P-MKK3 reduces and delays spinal p38 phosphorylation post-injury; and 4) P-MKK3/6 in mice is expressed in astrocytes.
Activation of spinal p38 plays a major role in central sensitization and pain behavior in a number of chronic pain models ranging from animal models of arthritis to neuropathic pain (Jin et al., 2003; Boyle et al., 2006). Our data confirm this and indicate that delay of spinal P-p38 level increases leads to an equivalent delay in development of late allodynia. While several studies have dissected and defined the mechanisms by which p38 activation occurs in non-neural tissues (Enslen et al., 1998; Tanaka et al., 2002; Brancho et al., 2003; Chabaud-Riou and Firestein, 2004), few have examined these pathways in the nervous system (Jeon et al., 2000; Shimokawara et al., 2002) and none has addressed the role of the upstream kinases in spinal sensitization and pain. Several upstream kinases, especially MKK3 and MKK6, activate p38 directly in the periphery, but the particular upstream activator can vary with cell type and stimulus. Following stimulation with pro-inflammatory cytokines, MKK3 selectively activates p38α and γ and MKK6 activates the p38 isoforms α, β2 and γ (Enslen et al., 1998; Hammaker et al., 2003; Inoue et al., 2005). Under other stimulus conditions, such as exposure to ultraviolet light, MKK4 (Brancho et al., 2003) and TAB1 (Ge et al., 2002) can also activate p38. Understanding the molecular pathways leading to p38 activation in the spinal cord following peripheral injury or high frequency activity in peripheral C fibers could identify new, more specific therapeutic targets for different types of pain.
Our studies utilized the formalin model, which results in several phases of pain behavior. Phase 1, in the first 9 min of the model, is a measure of early acute pain while phase 2 (10-60 min post injection) followed by a late allodynia (>1 day) model different aspects of spinal sensitization and glial activation (Haley et al., 1990; Puig and Sorkin, 1996; Fu et al., 2000). By examining MKK3 and MKK6 deficient mice, we determined if these two upstream kinases regulate distinct components of pain behavior and spinal p38 activation. The most striking and unanticipated results related to differing functions of MKK3 and MKK6 in the regulation of spinal p38 and late allodynia. While deletion of MKK3 resulted in a modest, but statistically significant, delay of the formalin phase 2 response, the more impressive finding was a delay of several days in the development of the late allodynia. MKK6 deficiency, in contrast, had no effect on phase 2 or the development of allodynia during the first 9 days following injury. This observation was surprising because MKK6 is considered to be a more selective upstream regulator of p38β (Enslen et al., 1998), and selective knockdown of p38β or its genetic deletion, diminishes the phase 2 formalin response in rats (Svensson et al., 2005) and inflammation-induced pain behavior in mice (Martin et al., 2005), respectively. Loss of late pain behavior was associated with delayed and diminished P-p38 levels in the ipsilateral spinal cord. Interestingly, in both the WT and the MKK3−/− mice, increased P-p38 levels always temporally preceded the pain behavior. There was a strong correlation between expression of P-p38 and the developing pain behavior. Our observations raise the possibility that one can potentially suppress or diminish chronic pain without ablating the entire p38 pathway, thereby minimizing side effects and suppression of host defense.
While MKK3 regulates chronic pain behavior, there was no obvious effect of kinase deficiency in acute pain models. Neither MKK3 nor MKK6 contribute to acute pain transmission nor behavior as measured by thermal escape latencies, mechanical withdrawal thresholds or phase 1 formalin responses. These results were consistent with previous studies showing that pharmacological blockade of p38 activation does not alter behavioral measures of acute pain or field potentials evoked by tetanic C-fiber stimulation (Svensson et al., 2003b; Liu et al., 2007).
The effects of p38 or specific microglial inhibition have not been investigated on chronic allodynia occurring post-formalin injection. However, pre- and post-treatment with lidocaine in this model indicates that spinal sensitization associated with the phase 2 response is necessary for the expression of long-term allodynia (Fu et al., 2000). It is therefore especially surprising that MKK3−/− animals, which exhibit no decrement in the magnitude of phase 2, have such a change in the long-term allodynia. Some aspects of decreased chronic pain behavior could be due to deletion of MKK3 in other parts of the nervous system. However, reduction of formalin-evoked early phosphorylation of MKK3 and/or MKK6 due to genetic deletion of MKK3 is associated with a delayed and reduced formalin-induced increase in spinal P-p38. It seems likely that this latter alteration in spinal P-p38 is responsible, at least in part, for the delay in pain behavior. Although is is possible that MKK might contribute to pain through other p38 independent pathways.
Dorsal horn P-p38 in a number of pain models in rats is found predominantly in microglia (Svensson et al., 2003a; Tsuda et al., 2004; Boyle et al., 2006). An exception is seen in spinal cord slices incubated in αβmeATP, these exhibit co-localization of P-p38 with astrocytes rather than microglia (Ikeda et al., 2007). Direct activation of p38 by an upstream kinase would require co-localization of that kinase with P-p38. Thus, it was surprising that we could not detect P-MKK3/6 in the microglia it is possible that P-MKK3/6 levels in microglia were below the limits of detection. Alternatively, although P-p38 is not reported in spinal astrocytes in pain models, immunostaining has been for the most part performed in rats. There is one report examining mouse dorsal horn indicating that P-p38 is seen in astrocytes after spinal nerve ligation (Xu et al., 2007). Another study observed that under extreme conditions such as during very late stages of a model of amyotrophic lateral sclerosis in transgenic superoxide dismutase (SOD)-1 mutant mice, ventral horn hypertrophic astrocytes begin to express P-p38 (Tortarolo et al., 2003). This P-p38 expression was not seen in the early or mid stages of the disease. Despite these findings, we did not observe P-p38 staining in dorsal horn astrocytes following formalin injection, even at times when P-p38 expression was substantially elevated. Co-localization of p38 and MKKs in identified spinal cord cells has not been demonstrated, e.g., P-MKK3/6 is not found in ventral horn microglia or astrocytes in SOD-1 mutant mice despite the presence of P-p38 (Veglianese et al., 2006) (Tortarolo et al., 2003). Our data indicate that likely circuitry includes an indirect linkage from P-MKK3 activation in astrocytes to p38 activation in microglia. Certainly, astrocytes contribute to persistent pain states (DeLeo et al., 2000; Hashizume et al., 2000) and release mediators which are known to effect microglia. Alternatively, other upstream kinases like MKK4 and ASK-1 in microglia might be involved.
Despite lack of co-localization of P-MKK3/6 and P-p38, congenital lack of MKK3 definitely altered the time course and magnitude of p38 phosphorylation in response to a noxious, tissue damaging stimulus. Importantly, some level of p38 phosphorylation occurred in these transgenic animals prior to their development of pain behavior. It is unknown if either p38 phosphorylation or pain behavior would occur if the loss or blockade of MKK3 were imposed later in development such as in a conditional knock out or following drug administration.
Our findings have implications related to target selection and drug design for novel anti-nociceptive therapies. A variety of p38 inhibitors have been tested in patients with inflammatory diseases, and thus far toxicity has interfered with drug development. It is not clear whether these problems, such as hepatoxicity, are compound specific or mechanism based, but this problem has led us to examine alternative strategies that interfere with p38 signaling. By looking upstream of p38, we hoped to identify a kinase that interfered with a subset of key anti-nociceptive functions while permitting p38 activation by other stimuli. MKK3 might be the target of choice, because it can regulate immune complex-mediated inflammation in peripheral somatic tissue, but has no effect on some Toll-like receptor functions (Inoue et al., 2006). We found that MKK3 likely exerts this effect by alterations in spinal levels of P-p38. Studies involving deletion of MKK3 in adult animals will have to be performed to determine if pharmacological targeting of MKK3 will lead to prolonged analgesic and anti-inflammatory effects with fewer side effects than total p38 inhibition.
Table II.
| Relative Levels of Kinases in Naïve Mice | ||
|---|---|---|
| P-p38 | P-MKK3/6 | |
| WT | 0.55±0.01 | 0.54±0.03 |
| MKK3−/− | 0.28±0.01* | 0.67±0.04** |
Values are in relative units and have been normalized to GAPDH
indicates p ≤ 0.001 compared to WT
indicates p ≤ 0.05 compared to WT
Acknowledgements:
This work was supported by National Institutes of Health Grant NS048563 (LSS and GSF) and 5R01AI070555 (GSF). We thank Dr. Camilla Svensson for many valuable discussions concerning the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Boyle DL, Jones TL, Hammaker D, Svensson CI, Rosengren S, Albani S, Sorkin L, Firestein GS. Regulation of peripheral inflammation by spinal p38 MAP kinase in rats. PLoS Med. 2006;3:e338. doi: 10.1371/journal.pmed.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancho D, Tanaka N, Jaeschke A, Ventura JJ, Kelkar N, Tanaka Y, Kyuuma M, Takeshita T, Flavell RA, Davis RJ. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003;17:1969–1978. doi: 10.1101/gad.1107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabaud-Riou M, Firestein GS. Expression and activation of mitogen-activated protein kinase kinases-3 and -6 in rheumatoid arthritis. Am J Pathol. 2004;164:177–184. doi: 10.1016/S0002-9440(10)63108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Rutkowski MD, Stalder AK, Campbell IL. Transgenic expression of TNF by astrocytes increases mechanical allodynia in a mouse neuropathy model. Neuroreport. 2000;11:599–602. doi: 10.1097/00001756-200002280-00033. [DOI] [PubMed] [Google Scholar]
- Dirig DM, Salami A, Rathbun ML, Ozaki GT, Yaksh TL. Characterization of variables defining hindpaw withdrawal latency evoked by radiant thermal stimuli. Journal of Neuroscience Methods. 1997;76:183–191. doi: 10.1016/s0165-0270(97)00097-6. [DOI] [PubMed] [Google Scholar]
- Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J Biol Chem. 1998;273:1741–1748. doi: 10.1074/jbc.273.3.1741. [DOI] [PubMed] [Google Scholar]
- Fu KY, Light AR, Maixner W. Relationship between nociceptor activity, peripheral edema, spinal microglial activation and long-term hyperalgesia induced by formalin. Neuroscience. 2000;101:1127–1135. doi: 10.1016/s0306-4522(00)00376-6. [DOI] [PubMed] [Google Scholar]
- Fu KY, Light AR, Maixner W. Long-lasting inflammation and long-term hyperalgesia after subcutaneous formalin injection into the rat hindpaw. J Pain. 2001;2:2–11. doi: 10.1054/jpai.2001.9804. [DOI] [PubMed] [Google Scholar]
- Fu KY, Light AR, Matsushima GK, Maixner W. Microglial reactions after subcutaneous formalin injection into the rat hind paw. Brain Res. 1999;825:59–67. doi: 10.1016/s0006-8993(99)01186-5. [DOI] [PubMed] [Google Scholar]
- Ge B, Gram H, Di Padova F, Huang B, New L, Ulevitch RJ, Luo Y, Han J. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295:1291–1294. doi: 10.1126/science.1067289. [DOI] [PubMed] [Google Scholar]
- Haley JE, Sullivan AF, Dickenson AH. Evidence for spinal N-methyl-D-aspartate receptor involvement in prolonged chemical nociception in the rat. Brain Research. 1990;518:218–226. doi: 10.1016/0006-8993(90)90975-h. [DOI] [PubMed] [Google Scholar]
- Hammaker D, Sweeney S, Firestein GS. Signal transduction networks in rheumatoid arthritis. Ann Rheum Dis. 2003;62(Suppl 2):ii86–89. doi: 10.1136/ard.62.suppl_2.ii86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume H, DeLeo JA, Colburn RW, Weinstein JN. Spinal glial activation and cytokine expression after lumbar root injury in the rat. Spine. 2000;25:1206–1217. doi: 10.1097/00007632-200005150-00003. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Tsuda M, Inoue K, Murase K. Long-term potentiation of neuronal excitation by neuron-glia interactions in the rat spinal dorsal horn. Eur J Neurosci. 2007;25:1297–1306. doi: 10.1111/j.1460-9568.2007.05386.x. [DOI] [PubMed] [Google Scholar]
- Inoue T, Boyle DL, Corr M, Hammaker D, Davis RJ, Flavell RA, Firestein GS. Mitogen-activated protein kinase kinase 3 is a pivotal pathway regulating p38 activation in inflammatory arthritis. Proc Natl Acad Sci U S A. 2006;103:5484–5489. doi: 10.1073/pnas.0509188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Hammaker D, Boyle DL, Firestein GS. Regulation of p38 MAPK by MAPK kinases 3 and 6 in fibroblast-like synoviocytes. J Immunol. 2005;174:4301–4306. doi: 10.4049/jimmunol.174.7.4301. [DOI] [PubMed] [Google Scholar]
- Ito T, Ohtori S, Inoue G, Koshi T, Doya H, Ozawa T, Saito T, Moriya H, Takahashi K. Glial phosphorylated p38 MAP kinase mediates pain in a rat model of lumbar disc herniation and induces motor dysfunction in a rat model of lumbar spinal canal stenosis. Spine. 2007;32:159–167. doi: 10.1097/01.brs.0000251437.10545.e9. [DOI] [PubMed] [Google Scholar]
- Jeon SH, Kim YS, Bae CD, Park JB. Activation of JNK and p38 in rat hippocampus after kainic acid induced seizure. Exp Mol Med. 2000;32:227–230. doi: 10.1038/emm.2000.37. [DOI] [PubMed] [Google Scholar]
- Jin S-X, Z-Y Z, Woolf C, Ji R. p38 mitogen-activated protein kinase Is activated after a spinal nerve ligation in spinal Cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YJ, Seit-Nebi A, Davis RJ, Han J. Multiple activation mechanisms of p38alpha mitogen-activated protein kinase. J Biol Chem. 2006;281:26225–26234. doi: 10.1074/jbc.M606800200. [DOI] [PubMed] [Google Scholar]
- Liu YL, Zhou LJ, Hu NW, Xu JT, Wu CY, Zhang T, Li YY, Liu XG. Tumor necrosis factor-alpha induces long-term potentiation of C-fiber evoked field potentials in spinal dorsal horn in rats with nerve injury: the role of NF-kappa B, JNK and p38 MAPK. Neuropharmacology. 2007;52:708–715. doi: 10.1016/j.neuropharm.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Lu HT, Yang DD, Wysk M, Gatti E, Mellman I, Davis RJ, Flavell RA. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. Embo J. 1999;18:1845–1857. doi: 10.1093/emboj/18.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WJ, Murphy B, Parsons J, Cupo S, Zaller D, O'Keefe S. p38b contributes to injury-induced nociceptive, but not inflammatory responses in mice. Neurosci Abs. 2005 [Google Scholar]
- Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Schafers M, Sorkin LS, Geis C, Shubayev VI. Spinal nerve ligation induces transient upregulation of tumor necrosis factor receptors 1 and 2 in injured and adjacent uninjured dorsal root ganglia in the rat. Neurosci Lett. 2003a;347:179–182. doi: 10.1016/s0304-3940(03)00695-5. [DOI] [PubMed] [Google Scholar]
- Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003b;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawara T, Yamada E, Masui K, Mishima K, Enomoto Y, Inoue K, Sakaki T, Ichijima K. Changes in expression of p38 mitogen-activated protein kinase in the dorsal motor nucleus of the vagus nerve and hypoglossal nucleus after axotomy in adult rats. Neuropathology. 2002;22:261–268. doi: 10.1046/j.1440-1789.2002.00463.x. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Fitzsimmons B, Azizi S, Powell HC, Hua XY, Yaksh TL. Spinal p38beta isoform mediates tissue injury-induced hyperalgesia and spinal sensitization. J Neurochem. 2005;92:1508–1520. doi: 10.1111/j.1471-4159.2004.02996.x. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Hua XY, Protter AA, Powell HC, Yaksh TL. Spinal p38 MAP kinase is necessary for NMDA-induced spinal PGE(2) release and thermal hyperalgesia. Neuroreport. 2003a;14:1153–1157. doi: 10.1097/00001756-200306110-00010. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003b;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Kamanaka M, Enslen H, Dong C, Wysk M, Davis RJ, Flavell RA. Differential involvement of p38 mitogen-activated protein kinase kinases MKK3 and MKK6 in T-cell apoptosis. EMBO Rep. 2002;3:785–791. doi: 10.1093/embo-reports/kvf153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortarolo M, Veglianese P, Calvaresi N, Botturi A, Rossi C, Giorgini A, Migheli A, Bendotti C. Persistent activation of p38 mitogen-activated protein kinase in a mouse model of familial amyotrophic lateral sclerosis correlates with disease progression. Mol Cell Neurosci. 2003;23:180–192. doi: 10.1016/s1044-7431(03)00022-8. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- Veglianese P, Lo Coco D, Bao Cutrona M, Magnoni R, Pennacchini D, Pozzi B, Gowing G, Julien JP, Tortarolo M, Bendotti C. Activation of the p38MAPK cascade is associated with upregulation of TNF alpha receptors in the spinal motor neurons of mouse models of familial ALS. Mol Cell Neurosci. 2006;31:218–231. doi: 10.1016/j.mcn.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Xu M, Bruchas MR, Ippolito DL, Gendron L, Chavkin C. Sciatic nerve ligation-induced proliferation of spinal cord astrocytes is mediated by kappa opioid activation of p38 mitogen-activated protein kinase. J Neurosci. 2007;27:2570–2581. doi: 10.1523/JNEUROSCI.3728-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Ozaki G, McCumber D, Rathbun M, Svensson C, Malkmus S, Yaksh MC. An automated flinch detecting system for use in the formalin nociceptive bioassay. J Appl Physiol. 2001;90:2386–2402. doi: 10.1152/jappl.2001.90.6.2386. [DOI] [PubMed] [Google Scholar]
- Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun. 2006 doi: 10.1016/j.bbi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]