Abstract
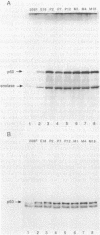
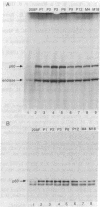
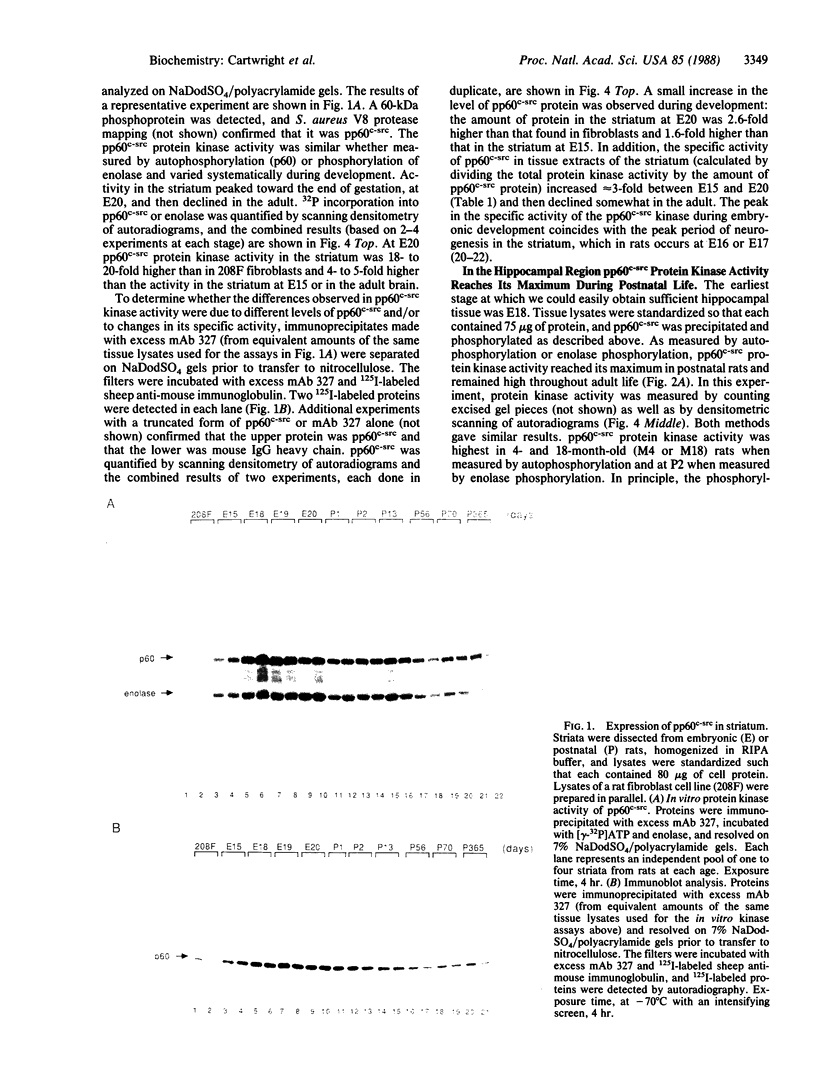
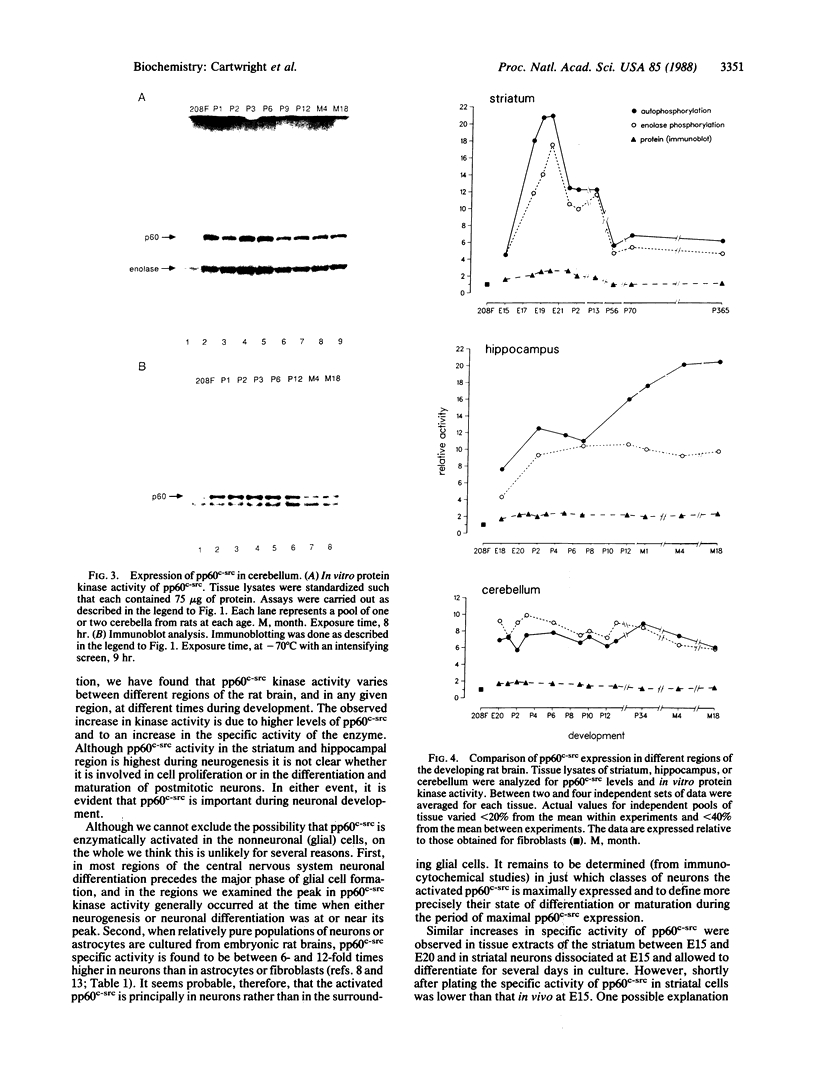
We have studied pp60c-src expression in the striatum, hippocampus, and cerebellum of the developing rat brain. In the striatum, pp60c-src protein kinase activity peaks during embryonic development and then declines in the adult. The peak activity occurs in the striatum on embryonic day 20 (E20) when it is 18- to 20-fold higher than the activity in fibroblasts and 4- to 5-fold higher than the activity in the striatum at E15 or in the adult striatum. In the hippocampal region, pp60c-src activity reaches a maximum shortly after birth but remains high throughout life. On postnatal day 2 (P2) the activity in the hippocampus is 9- to 13-fold higher than the activity in fibroblasts and twice as high as the activity in the hippocampus at E18. In the cerebellum, the kinase activity remains constant from E20 onward and is 6- to 10-fold higher than that observed in fibroblasts. The increase in pp60c-src kinase activity observed during the development of the striatum and hippocampus is due to an increase in the amount of pp60c-src protein and to an increase in the specific activity of the kinase. The increase in specific activity in these regions coincides with the peak periods of neurogenesis and neuronal growth. In the striatum, we have found that the increase in pp60c-src activity also parallels the increase observed in culture as embryonic striatal neurons differentiate. Taken together, our results are consonant with the idea that pp60c-src is the product of a developmentally regulated gene that is important for the differentiation and/or the continuing function of neurons.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemà S., Casalbore P., Agostini E., Tatò F. Differentiation of PC12 phaeochromocytoma cells induced by v-src oncogene. Nature. 1985 Aug 8;316(6028):557–559. doi: 10.1038/316557a0. [DOI] [PubMed] [Google Scholar]
- Altman J., Das G. D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965 Jun;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. 3. Maturation of the components of the granular layer. J Comp Neurol. 1972 Aug;145(4):465–513. doi: 10.1002/cne.901450403. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972 Jul;145(3):353–397. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972 Aug;145(4):399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- Barnekow A., Gessler M. Activation of the pp60c-src kinase during differentiation of monomyelocytic cells in vitro. EMBO J. 1986 Apr;5(4):701–705. doi: 10.1002/j.1460-2075.1986.tb04270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer S. A. Development of the hippocampal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J Comp Neurol. 1980 Mar 1;190(1):87–114. doi: 10.1002/cne.901900107. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Cotton P. C., Queral A. E., Barrett J. N., Nonner D., Keane R. W. Neurones express high levels of a structurally modified, activated form of pp60c-src. Nature. 1985 Aug 8;316(6028):554–557. doi: 10.1038/316554a0. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Lustig A., Messer A. Changes in the pattern of expression of pp60c-src in cerebellar mutants of mice. J Neurosci Res. 1987;18(4):532–538. doi: 10.1002/jnr.490180405. [DOI] [PubMed] [Google Scholar]
- Brugge J., Cotton P., Lustig A., Yonemoto W., Lipsich L., Coussens P., Barrett J. N., Nonner D., Keane R. W. Characterization of the altered form of the c-src gene product in neuronal cells. Genes Dev. 1987 May;1(3):287–296. doi: 10.1101/gad.1.3.287. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., Hutchinson M. A., Eckhart W. Structural and functional modification of pp60c-src associated with polyoma middle tumor antigen from infected or transformed cells. Mol Cell Biol. 1985 Oct;5(10):2647–2652. doi: 10.1128/mcb.5.10.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Kaplan P. L., Cooper J. A., Hunter T., Eckhart W. Altered sites of tyrosine phosphorylation in pp60c-src associated with polyomavirus middle tumor antigen. Mol Cell Biol. 1986 May;6(5):1562–1570. doi: 10.1128/mcb.6.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Simantov R., Kaplan P. L., Hunter T., Eckhart W. Alterations in pp60c-src accompany differentiation of neurons from rat embryo striatum. Mol Cell Biol. 1987 May;7(5):1830–1840. doi: 10.1128/mcb.7.5.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cooper J. A., Esch F. S., Taylor S. S., Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984 Jun 25;259(12):7835–7841. [PubMed] [Google Scholar]
- Cotton P. C., Brugge J. S. Neural tissues express high levels of the cellular src gene product pp60c-src. Mol Cell Biol. 1983 Jun;3(6):1157–1162. doi: 10.1128/mcb.3.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Fentress J. C., Stanfield B. B., Cowan W. M. Observation on the development of the striatum in mice and rats. Anat Embryol (Berl) 1981;163(3):275–298. doi: 10.1007/BF00315705. [DOI] [PubMed] [Google Scholar]
- Fults D. W., Towle A. C., Lauder J. M., Maness P. F. pp60c-src in the developing cerebellum. Mol Cell Biol. 1985 Jan;5(1):27–32. doi: 10.1128/mcb.5.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee C. E., Griffin J., Sastre L., Miller L. J., Springer T. A., Piwnica-Worms H., Roberts T. M. Differentiation of myeloid cells is accompanied by increased levels of pp60c-src protein and kinase activity. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5131–5135. doi: 10.1073/pnas.83.14.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. A tail of two src's: mutatis mutandis. Cell. 1987 Apr 10;49(1):1–4. doi: 10.1016/0092-8674(87)90745-8. [DOI] [PubMed] [Google Scholar]
- Hutchinson M. A., Hunter T., Eckhart W. Characterization of T antigens in polyoma-infected and transformed cells. Cell. 1978 Sep;15(1):65–77. doi: 10.1016/0092-8674(78)90083-1. [DOI] [PubMed] [Google Scholar]
- Levy B. T., Sorge L. K., Meymandi A., Maness P. F. pp60c-src Kinase is in chick and human embryonic tissues. Dev Biol. 1984 Jul;104(1):9–17. doi: 10.1016/0012-1606(84)90031-9. [DOI] [PubMed] [Google Scholar]
- Levy J. B., Dorai T., Wang L. H., Brugge J. S. The structurally distinct form of pp60c-src detected in neuronal cells is encoded by a unique c-src mRNA. Mol Cell Biol. 1987 Nov;7(11):4142–4145. doi: 10.1128/mcb.7.11.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S. A., Brugge J. S., Levine J. M. Induction of altered c-src product during neural differentiation of embryonal carcinoma cells. Science. 1986 Nov 14;234(4778):873–876. doi: 10.1126/science.3095923. [DOI] [PubMed] [Google Scholar]
- MIALE I. L., SIDMAN R. L. An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp Neurol. 1961 Oct;4:277–296. doi: 10.1016/0014-4886(61)90055-3. [DOI] [PubMed] [Google Scholar]
- Maness P. F., Sorge L. K., Fults D. W. An early developmental phase of pp60c-src expression in the neural ectoderm. Dev Biol. 1986 Sep;117(1):83–89. doi: 10.1016/0012-1606(86)90350-7. [DOI] [PubMed] [Google Scholar]
- Martinez R., Mathey-Prevot B., Bernards A., Baltimore D. Neuronal pp60c-src contains a six-amino acid insertion relative to its non-neuronal counterpart. Science. 1987 Jul 24;237(4813):411–415. doi: 10.1126/science.2440106. [DOI] [PubMed] [Google Scholar]
- Rosen N., Bolen J. B., Schwartz A. M., Cohen P., DeSeau V., Israel M. A. Analysis of pp60c-src protein kinase activity in human tumor cell lines and tissues. J Biol Chem. 1986 Oct 15;261(29):13754–13759. [PubMed] [Google Scholar]
- Schlessinger A. R., Cowan W. M., Gottlieb D. I. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol. 1975 Jan 15;159(2):149–175. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- Sefton B. M. The viral tyrosine protein kinases. Curr Top Microbiol Immunol. 1986;123:39–72. doi: 10.1007/978-3-642-70810-7_3. [DOI] [PubMed] [Google Scholar]
- Simon M. A., Drees B., Kornberg T., Bishop J. M. The nucleotide sequence and the tissue-specific expression of Drosophila c-src. Cell. 1985 Oct;42(3):831–840. doi: 10.1016/0092-8674(85)90279-x. [DOI] [PubMed] [Google Scholar]
- Sorge L. K., Levy B. T., Maness P. F. pp60c-src is developmentally regulated in the neural retina. Cell. 1984 Feb;36(2):249–257. doi: 10.1016/0092-8674(84)90218-6. [DOI] [PubMed] [Google Scholar]
- Wiestler O. D., Walter G. Developmental expression of two forms of pp60c-src in mouse brain. Mol Cell Biol. 1988 Jan;8(1):502–504. doi: 10.1128/mcb.8.1.502. [DOI] [PMC free article] [PubMed] [Google Scholar]