Abstract
An inverted circadian rhythm of melatonin (MT) likely contributes to the sleep disturbance in patients with Smith-Magenis syndrome (SMS). Plasma MT levels have documented this altered rhythm, but daytime levels of salivary MT has not been determined. Daytime measures of salivary MT might have utility in home/outpatient settings for assessing MT levels in undiagnosed patients with clinical features of SMS. The objective of this study was to determine the utility of daytime salivary MT as a diagnostic test in SMS. Thirty individuals with confirmed SMS [28 with del 17p11.2 and 2 with the retinoic acid induced 1 (RAI1) gene mutation] and five controls were studied. Single or serial daytime salivary MT levels were measured. The mean midday salivary MT level was 79.0 pg/ml in SMS patients, compared with 16.3 pg/ml in controls, with nine patients having values similar to controls. The median MT level in SMS patients was 49.0 pg/ml (first and third quartile values = 15.5 and 106.8 pg/ml). Twenty-six (90%) of 29 patients had at least one MT value > 15.5 pg/ml, including 70 (78%) of 90 samples from patients with del 17p11.2 and one (20%) of five samples from the two patients with the RAI1 mutation. Neither the pattern of medication use nor age had an effect on daytime salivary MT levels. Although most SMS patients had elevated daytime salivary MT levels, multiple sampling appears necessary to distinguish patients with SMS from other conditions.
Keywords: saliva, melatonin, Smith-Magenis syndrome
INTRODUCTION
Smith-Magenis syndrome (SMS) is a multiple congenital anomaly and mental retardation syndrome [Greenberg et al., 1996; Smith et al., 2006] associated with either an interstitial deletion involving chromosome 17p11.2 [including the Retinoic Acid Induced 1 (RAI1) gene] or a mutation in the RAI1 gene [Greenberg et al., 1991; Slager et al., 2003; Smith et al., 1986]. The SMS phenotype includes distinctive craniofacial and skeletal features, infantile hypotonia, expressive language delay, cognitive and mental retardation, stereotypes, neurobehavioral problems and sleep disorder [Smith et al., 2006]. Other variable features include cardiac defects, renal abnormalities, seizures, cleft palate, low immunoglobulin levels, hypercholesterolemia and hypothyroidism [Smith et al., 2006]. SMS occurs in all ethnic groups with an estimated prevalence of 1/25,000 [Colley et al., 1990].
The disordered sleep pattern in SMS is characterized by early sleep onset, difficulty staying asleep, frequent and early waking, decreased sleep time [Smith et al., 1998, 2006] with patient related increases and decreases of REM sleep [Smith et al., 1998]. In order to understand the physiological mechanisms that might contribute to disturbed sleep in SMS, prior studies examined levels of melatonin (MT), a key phase-marker of the human circadian system [Lewy, 2007]. The pineal hormone MT, the hormone of darkness [Reiter, 2003], is normally secreted at night, with a circadian rhythm dependent upon outputs from the suprachiasmatic nucleus [Moore and Klein, 1974]. This hormone has been found to play a part in regulating the sleep/activity rhythm [Zizapel, 2007]. By measuring either plasma MT or urinary 6-sulfatoxymelatonin, the major metabolite of MT, studies in patients with SMS have identified an inversion of the circadian rhythm of MT [De Leersynder et al., 2001; Potocki et al., 2000] such that higher MT secretion occurs during the day than at night. Salivary MT, which correlates well with plasma MT and urinary 6-sulfatoxymelatonin levels [McIntyre et al., 1987; Nowak et al., 1987], has not been measured in SMS patients. Because salivary samples are easy to collect, measuring daytime salivary MT level may have utility in identifying patients with possible SMS. Therefore, in this study, we determined whether daytime salivary MT levels were increased in patients with SMS as in plasma samples. We also assessed the variability of salivary MT levels by sampling at multiple time points. The relations between age and patterns of medication use and daytime MT levels were also determined.
METHODS
Thirty patients enrolled in an IRB-approved SMS natural history study (protocol 01-HG-0109) at the National Institutes of Health (NIH) participated in the study. Five relatives were recruited as controls. Of the 30 patients participating in the study, 28 (93%) had del 17p11.2 and two (7%) had the RAI1 mutation. There were 13 (43%) males and 17 (57%) females. Mean age at the time of their first admission was 9.8 y (4.5 m to 20 y).
Patients were evaluated at the Hatfield Clinical Research Center of the National Institutes of Health (Bethesda, MD). Medication use (in particular, the use of exogenous MT and β-blockers) was recorded. Five patients (17%) were on no medications. Thirteen (40%) of 30 patients gave a history of current or past MT use, including three (10%) patients on the combination of bedtime MT and a daytime β-blocker. Of the 13 patients who had used MT, two patients discontinued MT for two weeks including one who also stopped the daytime β-blocker and three patients discontinued MT for 36 hours before the salivary sampling. Seven patients took MT (1.5 to 10 mg) the night before salivary sampling. Six (20%) patients were on an atypical antipsychotic medication (risperidone, quetiapine and aripiprazole). Eight (27%) patients were on anticonvulsants (topiramate, lamotrigene, carbamazepine, valproic acid and zonisamide) either for their seizure disorders or for the mood stabilizing action. Other medications were used in four (13%) patients. Neither of the two patients with the RAI1 mutation was on MT; both were on psychotropic agents and one was on an anticonvulsant.
Salivary samples were collected between 0730h and 1730h during 41 admissions using a collection device as described previously [Wolff et al. 1997]. Light levels at the time of saliva collection were 700–800 lux. In 12 patients, a single sample was obtained between 1100h and 1200h because prior studies in SMS indicated peak plasma MT at this time [Cornelissen et al., 2003]; two samples were replicates from two visits. In addition, to document the variability of daytime salivary MT levels, eighteen patients had serial sampling (2 to 5 samples) between 0730h and 1730h. Core body temperature was measured prior to serial MT sampling. Nine patients had samplings on two separate occasions and two patients had samplings on three separate admissions.
Salivary MT concentrations were measured by a radioimmunoassay using an antiserum described by Rollag and Niswender [1976]. The assay was conducted according to a previously described procedure [Brainard et al., 2001].
All results were expressed as the mean ± SEM. Student’s t test was used to determine differences between groups and ANOVA with post-hoc testing was used for comparisons within multiple groups. The chi-square test was used to analyze for differences between groups for categorical variables. Linear regression (correlation) was used to assess the association between age and MT levels. A P-value < 0.05 was considered significant.
RESULTS
Salivary MT levels in SMS subjects versus relative controls
A total of 106 salivary MT samples were collected from patients with SMS and controls. Two samples were excluded, one due to insufficient sample, and a second one due to food contamination; one patient (with only one sample) was consequently dropped from the analysis. Of the samples analyzed, 95 were from patients with SMS and nine samples from relative controls.
Salivary MT levels were analyzed in samples collected between 1030 and 1330 h of the first NIH patient admission (Fig 1) to determine the diagnostic utility of midday sampling. Mean daytime salivary MT level in SMS patients was 79.0 ± 18.9 pg/ml (n=29) compared with 16.3 ± 1.6 pg/ml (n=5) (p=0.18) in controls. The mean salivary MT level in the four patients whose relatives volunteered as controls was 112.8 ± 56.4 pg/ml. Median salivary MT level in SMS patients was 49 pg/ml with the first quartile = 15.5 pg/ml and third quartile = 106.8 pg/ml. Twenty six (90%) of 29 patients had at least one value > 15.5 pg/ml, with 70 (78%) of 90 samples (range 2.5 to 694 pg/ml) from patients with del 17p11.2 and only 1 (20%) of 5 samples (range 1 to24.5 pg/ml) from the two patients with the RAI1 mutation.
Figure 1.
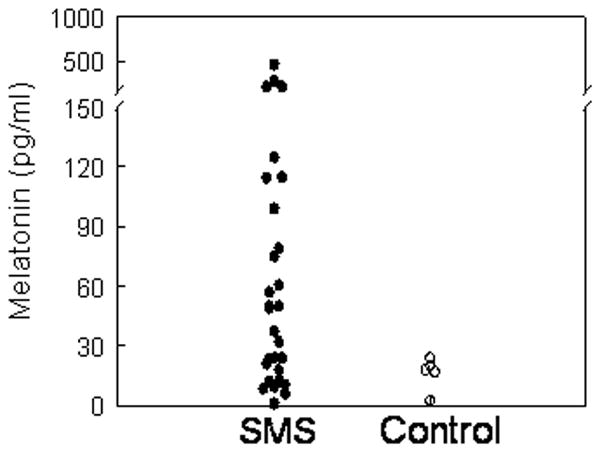
Midday sampling of daytime salivary MT levels in 29 patients with SMS and 5 relative controls.
Among the MT samples in the first quartile, possible effects of the RAI1 mutation and age were evaluated with the following results: A) One patient with the RAI1 mutation had an MT value of 1 pg/ml, B) the youngest patients, ages 4.5 m and 14 m, contributed samples with MT values of 6 and 9.5 pg/ml, respectively; when the first patient returned for the follow-up visit at age 17 m, MT had increased to 71.5 pg/ml; the second young patient had two follow-up visits at ages 23 m and 27 m with MT values of 43 and 82 pg/ml, respectively.
Variability of daytime salivary MT levels
To determine the variability of daytime salivary MT levels, MT data from the nine subjects with del 17p11.2 who had undergone sampling for at least five time points were analyzed. Three individuals had a visually identifiable sharp peak (Fig 2A), while the other six patients did not appear to have a discernible peak (Fig. 2B). Of interest, in one set of twins, there was an obvious daytime peak at 1515 h in the SMS patient; the unaffected twin (Fig. 2C) had very low daytime levels. Because the patient had traveled from the mountain time zone, the peak daytime salivary MT level would occur at 1315 h instead of 1515 h. There was only a minimal variation of body temperature with no demonstrated relationship with the fluctuating daytime salivary MT level (data not shown).
Figure 2.
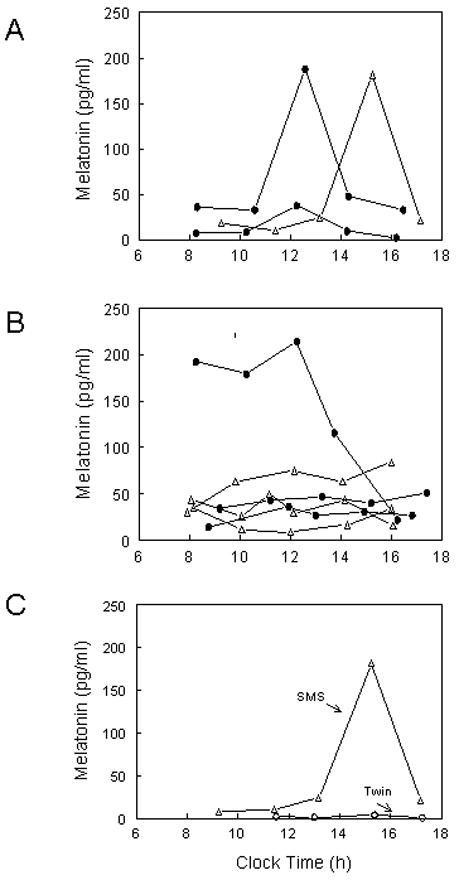
Serial daytime salivary MT levels in SMS patients. A. Three patients with a sharp daytime peak and B. Six patients with no discernible peak. (Δ) depicts patients who have stopped MT at least 36 h prior to sampling and (●) depicts patients not on MT. C. Daytime salivary MT levels in a set of twins. Elevated daytime MT levels with a sharp peak were noted in the SMS patient (Δ) while the unaffected twin (○) had very low daytime levels
Effects of age and medication use on salivary MT levels
In our SMS patients, between 16 m and 20 y (Fig. 3), age did not have an effect on peak daytime salivary MT levels in all 29 patients or in the 21 patients not on MT or on whom MT was withheld for 36 h before sampling. Although the mean age of patients taking MT as a sleep aid (10.6 ± 1.3) did not differ from the non-MT user (8.8 ± 1.6), only one of the ten patients under age 6 was on MT compared with 12 of 19 patients over age 6 taking MT (χ2= 7.49, P<0.01). MT users tended to have higher peak daytime salivary MT levels even when they were on β-blockers (Fig. 4). When MT was discontinued at least 2 weeks or withheld 36 h before the sleep study, daytime MT levels were similar to those not on MT (Fig. 4).
Figure 3.
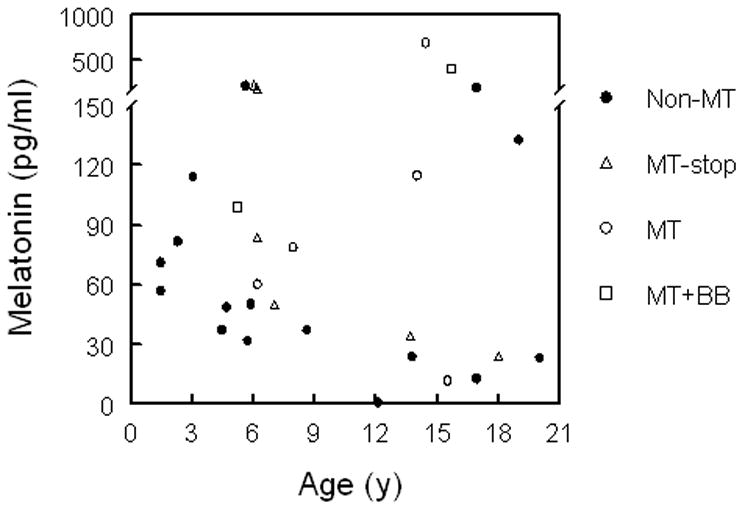
Peak daytime salivary MT levels in patients with SMS according to age. There is no correlation between MT levels and age, R=0.14 by regression analysis, P-value=0.46 (n=29) and R=−0.17, P-value=0.44 (patients not on MT or those that stopped MT for at least 36 h, n=22). BB=β-blocker
Figure 4.
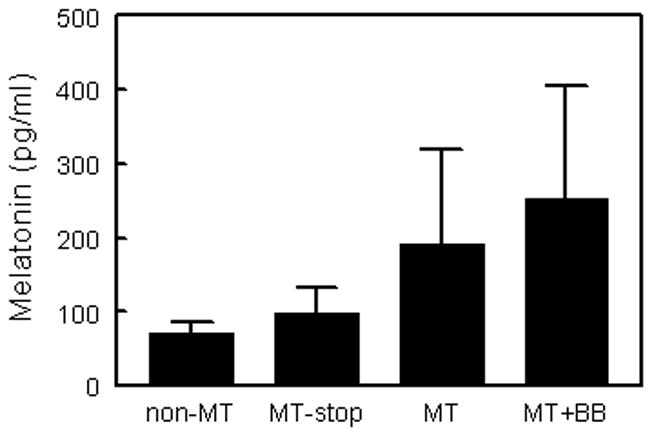
Mean peak daytime salivary MT levels in patients with SMS according to MT use. Non-MT (patients not on MT, n=16), MT-stop (patients who had stopped MT for at least 36 h, n=6), MT (current MT users, n=5) and MT+BB (patients on both MT and a β-blocker, n=2). For the 5 patients who took MT the evening before sampling, R=0.6 between MT dose and salivary MT level (p=0.1).
Analysis of the effects of medication use on salivary MT levels did not reveal a difference in peak daytime MT levels between patients who were medication-free compared with those who were on at least one medication (Table 1). Neither anticonvulsants nor atypical antipsychotic medications had an effect on peak daytime salivary MT levels. Of the two patients on the combination of nighttime MT and a daytime β-blocker, their peak MT levels were 99 and 405 pg/ml.
Table I.
Effects of anticonvulsant and atypical antipsychotic medications on peak daytime salivary MT levels
| Daytime MT levels (pg/ml) | |||
|---|---|---|---|
| Type of Medication | Drug (−) | Drug (+) | P-value |
| Any medication | 69.1 ± 33.4 (n=5) | 118.6 ± 31.0 (n=24) | 0.49 |
| Anticonvulsant | 126.6 ± 33.8 (n=7) | 58.1 ± 27.0 (n=22) | 0.26 |
| Atypical antipsychotic | 112.2 ± 32.8 (n=6) | 101.9 ± 38.7 (n=23) | 0.88 |
DISCUSSION
Our study represents the first report of salivary MT levels in patients with SMS. By using salivary MT to characterize daytime MT secretion, our study confirms elevated daytime MT production in the majority of patients with SMS, similar to previous reports that measured plasma MT and urinary 6-sulfatoxymelatonin [De Leersynder et al., 2001; Potocki et al., 2000].
Among the 27 individuals with del17p11.2, only two patients had peak daytime values in the first quartile, levels similar to those obtained from the relative controls. It is possible that these patients could have elevated daytime levels if the frequency of sampling was increased; alternatively perhaps not all patients with SMS have elevated daytime MT levels. In the original series reported by Potocki et al. [2000], one patient did not have an inverted MT rhythm and in a recent report, a second SMS patient with an atypical deletion was found to have a normal MT rhythm [Boudreau et al., 2009]. As for the time of sampling, a single midday salivary MT level performed between 1030h and 1330h would appear to be beneficial in identifying the inverted MT rhythm because the majority of patients have elevated daytime MT levels during this time interval. However, the current data suggest that very young patients may not have an elevated daytime MT level when sampling is performed during this time interval. The possible impact of time zone changes on MT level also needs to be considered in the assessment. Although urinary 6-sulfatoxymelatonin excretion significantly correlated in siblings [Griefahn et al., 2003], none of the four SMS patients whose relatives were recruited as controls had low midday salivary MT levels.
With regard to the peak of daytime salivary MT levels, only 3 of 9 patients with 5 or 6 samplings between 0800h and 1800h had a sharp peak. Among those with a sharp peak, after correction for time zone changes in one patient, peak MT level occurred around 1230h, which appears later than the shift of 9 h in the nocturnal peak in plasma MT level reported previously [Cornelissen et al., 2003]. This could be secondary to infrequent sampling, travel through time zones, medication use, plasma vs salivary measurement or inaccuracy of the estimated peak reported previously due to a small sample size [Cornelissen et al., 2003].
In this study, heart rate, sleep, activity, arousal and mental status were not monitored in patients who had 5 to 6 samplings for 10 hours as the primary purpose of the study was to determine the diagnostic utility of daytime salivary MT level. However, body temperature was monitored and there was no relationship between body temperature and the fluctuating MT levels during this sampling interval. In a previous study, the circadian pattern of cortisol, growth hormone and prolactin secretion was found to be preserved in SMS patients [De Leersnyder et al., 2001], and no inversion of the body temperature rhythm was observed in a preliminary study (Duncan et al.,2004, Am. Soc. Human Genet. Abs. No. 814). Because a transient increase in MT level could occur with exercise [Buxton et al., 2003], it will be of interest to determine whether circulating catecholamine levels rather than neurally released norepinephrine could control the peak in MT in SMS patients. This possibility can be evaluated by measuring salivary catecholamine metabolites in SMS patients.
Salivary MT levels could potentially be influenced by age [Brzezinski, 1997; Kennaway et al., 1992; Waldhause et al., 1984]. In infancy, MT secretion starts during the third or fourth months of life [Kennaway et al., 1992]. Melatonin level increases rapidly, peaking at ages 1 to 3 y, then declines to a plateau that persists throughout early adulthood [Kennaway et al., 1992; Waldhauser et al., 1984]. In our study, the youngest patients (ages 4.5 m and 14 m) had low daytime salivary MT levels and these levels were much higher when they were measured 9 to 13 m later. However, in contrast to the age-related decline in nighttime MT levels [Waldhauser et al., 1984], we found no relationship between daytime salivary MT levels and age among our patients. Again, the lack of this relationship could be attributed to those factors listed above.
Our study indicates increasing use of MT in patients over age 6. Whereas only one patient under age 6 was taking MT, over 60% of patients over age 6 were on MT. The use of MT could have two potential effects in our patients, the promotion of sleep onset and maintenance, and the phase shifting of circadian rhythm [Lewy et al., 1992; Zhdanova et al., 2001]. Because MT is commonly used as a sleep aid, its use suggests more disrupted sleep and an attempt to improve sleep quality. The dose of MT available over-the-counter and used by these patients could increase the daytime level of salivary MT as reported in a previous study [Zhdanova et al., 2001]. Although the correlation was not significant in this study, we cannot exclude the possibility that exogenous MT use may contribute to an increase in daytime salivary MT levels in some patients in view of the small sample size.
A surprising observation is that the two patients who were on the combination of nighttime MT and a daytime β-blocker had elevated daytime MT levels of 99 and 405pg/ml, respectively. Potentially, this could reflect the use of exogenous MT, since β-blockers should suppress endogenous MT secretion in SMS patients [De Leersnyder et al., 2001; Mayeda et al., 1998]. However, a low dose (12.5 mg of atenolol) may have been insufficient to suppress MT in one patient. Although the use of other medications could also have an effect on daytime salivary MT [von Bahr et al., 2000], neither anticonvulsants nor atypical antipsychotic medications, the two class of medications commonly used in our patients, had an influence on the peak daytime salivary MT levels.
In this report, daytime MT levels were determined in two SMS patients with previously characterized RAI1 mutation [Slager et al., 2003]. Their daytime MT levels were similar to those obtained from the relative controls and lower than those from patients with del 17p11.2. Neither one of these two patients used MT as a sleep aid. Whereas one patient (age 20) had daytime salivary MT values of 2 and 23.5 pg/ml and self-reported sleep abnormalities, the second patient (age 12) had three daytime salivary MT values of 1 pg/ml between 1115 h and 1600 h and elevated stage 1 sleep as suggested by a clinical polysomnographic study at age 14 performed at the NIH. Although these observations could suggest a more normal circadian rhythm of MT in some patients with the RAI1 mutation, an inversion of the urinary excretion of 6-sulfatoxymelatonin was recently reported in two patients (ages 11 and 27 y) with the RAI1 mutation in a preliminary study (Deng et al., 2007, Am. Soc. Human Genet. Abs. No. 636). Both patients had self-reported sleep disturbance. However, the older patient had a normal polysomnographic study whereas the younger patient had decreased sleep time, multiple nocturnal awakenings and abnormal sleep stage disturbance. This subgroup of patients warrants further investigation to better characterize their MT rhythm and relationship to sleep abnormalities.
The current data suggest some utility of daytime salivary MT for identifying individuals with possible SMS. In general, salivary MT level is 30% of plasma level because 70% of MT is bound to protein [Kennaway and Voultsios, 1998]. Salivary MT measurement is likely more representative of the biologically relevant level because only free hormone level is measured. Moreover, good correlations exist between salivary and plasma MT and salivary MT and urinary 6-sulfatoxymelatonin levels [McIntyre et al., 1987; Nowak et al., 1987]. Because plasma sampling is painful and collecting serial urine samples from children with SMS is challenging due to incontinence [Smith et al., 2006], there is an obvious advantage with salivary sampling.
With regard to the utility of a midday salivary MT measurement as a diagnostic test in SMS, using a MT cut-off of 25 pg/ml (the highest MT value in the control subjects was 24 pg/ml), elevated daytime MT level was found in 20 of 29 (69%) SMS patients. With additional data from sampling at multiple time points, two more patients (22 of 29, 76%) were found to have salivary MT levels above this cutoff. Excluding patients who had taken exogenous MT the evening before the day of sampling, 16 of 21 patients (76%) had daytime salivary MT values above the cut-off. Because MT levels peak at 1 to 3 years, daytime salivary MT measurements may be of limited utility in the very young patients.
In summary, elevated daytime salivary MT levels were observed in the majority of SMS patients with del17p11.2, contrasting to the two patients with the RAI1 mutation. The utility of a midday salivary MT measurement appears insufficient to distinguish patients with SMS from other conditions because many SMS patients had MT levels similar to healthy controls during this time interval. Multiple sampling at additional time points may increase the sensitivity of a SMS salivary MT test.
Acknowledgments
This research was supported by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health and a NIH Clinical Center Bench-to-Bedside award to ACMS.
References
- Boudreau EA, Johnson KP, Jackman A, Blancato J, Huizing M, Bendavid C, Jones M, Chandrasekharappa SC, Lewy AJ, Smith ACM, Magenis E. Review of disrupted sleep pattern in Smith-Magenis syndrome and normal melatonin secretion in a patient with an atypical interstitial 17p11.2 deletion. Am J Med Genet Part A. 2009;149A:1382–1391. doi: 10.1002/ajmg.a.32846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton OM, Lee CW, L’Hermite-Baleriaux M, Turek FW, Van Cauter E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am J Physiol. 2003;284:R714–724. doi: 10.1152/ajpregu.00355.2002. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186–195. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- Colley AF, Laversha MA, Vooullaire Le, Rogers JG. Five cases demonstrating the distinctive behavioural features of chromosome deletion 17(p11.2p11.2) (Smith Magenis syndrome) J Pediatr Child Health. 1990;26:17–21. doi: 10.1111/j.1440-1754.1990.tb02372.x. [DOI] [PubMed] [Google Scholar]
- Cornelissen G, Halberg F, Tarquini R, Perfetto F, Salti R, Laffi G, Otsuka K. Point and interval estimations of circadian melatonin ecphasia in Smith-Magenis syndrome. Biomed Pharmacother. 2003;57:31s–34s. doi: 10.1016/j.biopha.2003.08.004. [DOI] [PubMed] [Google Scholar]
- De Leersynder H, De Blois MC, Claustrat B, Romana S, Albrecht U, Von Kleist-Retzow JC, Delobel B, Viot G, Lyonnet S, Vekemans M, Munnich A. Inversion of the circadian rhythm of melatonin in the Smith-Magenis syndrome. J Pediatr. 2001;139:111–116. doi: 10.1067/mpd.2001.115018. [DOI] [PubMed] [Google Scholar]
- De Leersnyder H, de Blois MC, Vekemans M, Sidi D, Villain E, Kindermans C, Munnich A. β1-adrenergic antagonists improve sleep and behavioural disturbance in a circadian disorder, Smith-Magenis syndrome. J Med Genet. 2001;38:586–590. doi: 10.1136/jmg.38.9.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griefahn B, Bröde P, Remer T, Blaszkewicz M. Excretion of 6-hydroxymelatonin sulfate (6-OHMS) in siblings during childhood and adolescence. Neuroendocrinology. 2003;78:241–243. doi: 10.1159/000074444. [DOI] [PubMed] [Google Scholar]
- Greenberg F, Lewis RA, Potocki L, Glaze D, Parke J, Killian J, Murphy MA, Williamson D, Brown E, Dutton R, McCluggage C, Friedman E, Sulek M, Lupski JR. Multidisciplinary clinical study of Smith-Magenis syndrome (deletion 17p11.2) Am J Med Genet. 1996;62:247–254. doi: 10.1002/(SICI)1096-8628(19960329)62:3<247::AID-AJMG9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Greenberg F, Guzzeta V, Montes de Oca-Luna, Magenis E, Smith AC, Richter SF, Kondo I, Dobyns WB, Patel PI, Lupski JR. Molecular analysis of the Smith-Magenis syndrome: A possible contiguous-gene syndrome associated with del(17)(p11.2) Am J Med Genet. 1991;49:1207–1218. [PMC free article] [PubMed] [Google Scholar]
- Kennaway DJ, Stamp GE, Goble FC. Development of melatonin production in infants and the impact of prematurity. J Clin Endocrinol Metab. 1992;75:367–369. doi: 10.1210/jcem.75.2.1639937. [DOI] [PubMed] [Google Scholar]
- Kennaway DJ, Voultsios A. Circadian rhythm of free melatonin in human plasma. J Clin Endocrinol Metab. 1998;83:1013–1015. doi: 10.1210/jcem.83.3.4636. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Ahmed S, Jackson JM, Sack RL. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9:380–392. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- Lewy AJ. Melatonin and human chronobiology. Cold Spring Harb Symp Quant Biol. 2007;72:623–636. doi: 10.1101/sqb.2007.72.055. [DOI] [PubMed] [Google Scholar]
- Mayeda A, Mannon S, Hofstetter J, Adkins M, Baker R, Hu K, Nurnberger J., Jr Effects of indirect light and propranolol on melatonin levels in normal human subjects. Psychiatry Res. 1998;81:9–17. doi: 10.1016/s0165-1781(98)00069-9. [DOI] [PubMed] [Google Scholar]
- McIntyre IM, Norman TR, Burrows GD, Armstrong SM. Melatonin rhythm in human plasma and saliva. J Pineal Res. 1987;4:177–183. doi: 10.1111/j.1600-079x.1987.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Moore RY, Klein DC. Visual pathways and the central control of a circadian rhythm in pineal serotonin N-acetyltransferase activity. Brain Res. 1974;71:17–33. doi: 10.1016/0006-8993(74)90188-7. [DOI] [PubMed] [Google Scholar]
- Nowak R, McMillen JC, Redman J, Short RV. The correlation between serum and salivary melatonin concentrations and urinary 6-hydroxymelatonin sulphate excretion rates: two non-invasive techniques for monitoring human circadian rhythmicity. Clin Endocrinol. 1987;27:445–452. doi: 10.1111/j.1365-2265.1987.tb01172.x. [DOI] [PubMed] [Google Scholar]
- Potocki L, Glaze D, Tan DX, Park SS, Kashork CD, Shaffer LG, Reiter RJ, Lupski JR. Circadian rhythm abnormalities of melatonin in Smith-Magenis syndrome. J Med Genet. 2000;37:428–433. doi: 10.1136/jmg.37.6.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ. Melatonin: clinical relevance. Best Pract Res Clin Endocrinol Metab. 2003;17:273–285. doi: 10.1016/s1521-690x(03)00016-2. [DOI] [PubMed] [Google Scholar]
- Rollag MD, Niswender Radioimmunoassay of serum concentrations of melatonin in sheep exposed to different lighting regimens. Endocrinology. 1976;98:482–489. doi: 10.1210/endo-98-2-482. [DOI] [PubMed] [Google Scholar]
- Slager RE, Newton TL, Vlangos CN, Finucane B, Elsea SH. Mutations in RAI1 associated with Smith-Magenis syndrome. Nat Genet. 2003;3:466–468. doi: 10.1038/ng1126. [DOI] [PubMed] [Google Scholar]
- Smith AC, McGavran L, Robinson J, Waldstein G, Macfarlane J, Zonona J, Reiss J, Lahr M, Allen L, Magenis E. Interstitial deletion of (17) (p11.2p11.2) in nine patients. Am J Med Genet. 1986;24:393–414. doi: 10.1002/ajmg.1320240303. [DOI] [PubMed] [Google Scholar]
- Smith AC, Dykens E, Greenberg F. Sleep disturbance in Smith-Magenis syndrome (del 17p11.2) Am J Med Genet. 1998;81:186–191. [PubMed] [Google Scholar]
- Smith ACM, Allanson JE, Elsea SH, Finucane BM, Haas-Givler B, Gropman A, Johnson KP, Lupski JR, Magenis E, Potocki L, Solomon B. GeneReviews at GeneTests: Medical Genetics Information Resource (data-base online) University of Washington; Seattle: 2006. Smith-Magenis Syndrome. Available at http://www.genetests.org. [Google Scholar]
- von Bahr C, Ursing C, Yasui N, Tybring G, Bertilsson L, Rojdmark S. Fluvoxamine but not citalopram increases serum melatonin in healthy subjects - an indication that cytochrome P450 CYP1A2 and CYP2C19 hydroxylate melatonin. Eur J Clin Pharmacol. 2000;56:123–127. doi: 10.1007/s002280050729. [DOI] [PubMed] [Google Scholar]
- Waldhauser F, Weiszenbacher G, Frisch H, Zeitlhuber U, Waldhauser M, Wurtman RJ. Fall in nocturnal serum melatonin during prepuberty and pubescence. Lancet. 1984;1:362–365. doi: 10.1016/s0140-6736(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Wolff A, Begleiter A, Moskona D. A novel system of human submandibular/sublingual saliva collection. J Dent Res. 1997;76:1782–1786. doi: 10.1177/00220345970760111001. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV, Wurtman RJ, Regan MM, Taylor JA, Shi JP, Leclair OU. Melatonin treatment for age-related insomnia. J Clin Endocrinol Metab. 2001;86:4727–4730. doi: 10.1210/jcem.86.10.7901. [DOI] [PubMed] [Google Scholar]
- Zizapel N. Sleep and sleep disturbances: biological basis and clinical implications. Cell Mol Life Sci. 2007;64:1174–1186. doi: 10.1007/s00018-007-6529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


