Abstract
Aim
To assess serum uric acid (SUA) levels determined on admission as a potential predictor of short-term mortality and long-term survival in acute myocardial infarction (AMI) patients.
Method
Data for this retrospective prognostic study were drawn from the patient database of the Varaždin County General Hospital in Varaždin, Croatia. We included consecutive patients with verified AMI admitted within 48 hours since the symptom onset during the period between January 1, 1996 and December 31, 2001. Long-term survival/mortality data were collected through direct contacts with patients and search of the community death registries. Relative risks (RR) and hazard ratios (HR) by 10 µmol/L increase in SUA were determined using modified Poisson regression with robust error variance and proportional hazard regression, respectively.
Results
A total of 621 patients (age 27-90 years, 64.7% men, 77.5% AMI with ST elevation, SUA 63-993 µmol/L) were included. Higher SUA on admission was independently associated with higher in-hospital mortality (RR, 1.016; 95% confidence interval [CI], 1.001-1.031, P = 0.043) and higher thirty-day mortality (RR, 1.016; 95% CI, 1.003-1.029, P = 0.018). Considered covariates were demographics, pre-index event cardiovascular morbidity and treatment, on-admission serum creatinine, total cholesterol and triglycerides, AMI characteristics, and peak creatine phosphokinase. Higher SUA on admission was also independently associated with poorer long-term survival (ie, higher all-cause mortality) (HR, 1.105; 95% CI, 1.020-1.195, P = 0.010). Considered covariates were demographics, laboratory variables on admission, AMI characteristics, peak creatine phosphokinase, acute complications, and treatment at discharge.
Conclusion
Higher serum uric acid determined on admission is associated with higher in-hospital mortality and thirty-day mortality and poorer long-term survival after AMI.
In humans, uric acid (UA) is the end product of purine catabolism (1). Its serum levels (SUA), governed by the production (liver) and elimination (mainly the kidney) rates, are influenced by genetically determined factors (eg, activity of synthesizing enzymes or renal transporter systems), racial and demographic characteristics (eg, sex, gonadal function in women, obesity), diet (eg, purine-rich foods, fructose, alcohol), habits (eg, SUA is lower in smokers and increases after quitting), morbidity (eg, heart or renal failure, malignancies), and medications (eg, diuretics, cytotoxic agents) (1-4). The role of SUA in cardiovascular and renal diseases has been intensively investigated, although not without controversy (5). On the molecular and cellular level, UA exerts a number of effects of potential interest: it is one of the most important antioxidants in plasma, but at high concentrations it may promote oxidative stress; it may induce endothelial dysfunction and vascular smooth muscle cell proliferation in vitro, platelet aggregation, and microinflammation; increased UA causes tubulointerstitial inflammation, morphological and functional changes in the glomeruli and renal arteriole and increased salt sensitivity (3,5). There is now sufficient evidence to consider increased SUA as an etiological factor in “hyperuricemic hypertension” or “salt-sensitive kindey-dependent hypertension” (3,5). Clinical and epidemiological studies have linked increased SUA to occurrence and outcomes of diabetes mellitus, metabolic syndrome, and chronic renal failure (3,5,6). It has also been suggested as a risk factor for occurrence and a predictor of poorer outcomes in acute stroke (7-9) and a risk factor for occurrence/outcomes in various aspects of cardiovascular morbidity (3,5,6). However, there are also views that SUA is not relevant in the pathophysiology of cardiovascular diseases and that it should be viewed as a secondary side-marker of etiologically relevant processes (3,5).
Acute myocardial infarction (AMI) is the most dramatic manifestation of the coronary artery disease (CAD) (10). High SUA has been indicated as a risk factor for CAD (10) and as an independent prognostic factor of poorer outcomes (occurrence of AMI, fatal AMI, sudden death, all-cause mortality) in patients with verified CAD (11,12). Less is known about SUA as a potential prognostic/risk factor for outcomes in patients affected specifically by AMI. A recent retrospective analysis from Japan (13) observed a univariate association between higher SUA on admission (within 48 hours since the symptom onset) and higher thirty-day mortality (fourth vs first quartile SUA values) in AMI patients. It also reported an independent association between higher SUA and poorer long-term survival (13). Having in mind potential ethnic/racial specificities and cultural differences (eg, diet, alcohol consumption), we aimed to investigate SUA levels determined on admission as a potential predictor of short-term mortality (while accounting for relevant covariates) and long-term survival in a sample of patients of European descent (Caucasians) with verified AMI.
Patients and methods
This retrospective prognostic analysis used data from the patient database of the Varaždin County General Hospital in Varaždin, Croatia and was approved by the institution’s Ethics Committee.
Patients
Candidates for inclusion were acute coronary syndrome patients admitted to Varaždin County General Hospital between January 1, 1996 and December 31, 2001 meeting the following criteria: a) to be Caucasians of European descent; b) to have verified AMI (clinical symptoms, typical electrocardiographic [ECG] findings on a standard 12-lead ECG [new ST-T changes, new left bundle branch block or development of pathological Q waves], elevated cardiac biochemical markers [creatin phosphokinase, CPK-MB fraction] with or without typical echocardiographic findings [typical new regional wall motion abnormality]); c) to have been admitted and laboratory evaluated within 48 hours from the symptom onset. During the analyzed period (1996-2001), troponin levels were not used as a diagnostic aid. All source data were (re)evaluated by the same investigator (S.C.). Patients who were dead on admission were not included. Of the 943 patients alive on admission, 265 were excluded since AMI could not be verified, 48 due to late referral, and 9 due to the late laboratory evaluation. This left 621 patients for the analysis.
Standardized in-house procedure for AMI
All patients with acute coronary syndromes were handled according to a standardized algorithm. On-admission, laboratory evaluation always included serum uric acid (μmol/L), serum creatinine (Cr, μmol/L), CPK levels (U/L), total cholesterol (mmol/L), and triglycerides (mmol/L). They were admitted to the Intensive Care unit (ICU) and were all given antiplatelet treatment, except where contraindicated. In cases of verified AMI with ST elevation, and when indicated, fibrinolytic treatment with streptokinase was commenced. During the observed period (1996-2001), primary percutaneous interventions were not employed at the institution – the method was introduced in 2005 as a part of the National Program for Primary Percutaneous Interventions. During the ICU stay, all patients were continuously ECG-monitored, whereas oxygenation and other interventions were performed as needed. Stabilized patients were transferred to the Department of Cardiology until discharge.
Clinical and laboratory measurements
Standard 12-lead ECG recordings were made using various models of Siemens (Erlangen, Germany) ECG apparatuses. Integrated biochemical analyzers of the Abbott-Architect series (Abbott Park, IL, USA) were used for laboratory measurements of serum uric acid, creatine phosphokinase activity, serum creatinine, total cholesterol, and triglycerides.
Outcomes
We defined two primary outcomes related to short-term mortality (in-hospital and thirty-day) and one secondary outcome related to long-term survival (long-term all-cause mortality). In-hospital mortality was defined as a proportion of patients who died during the hospital stay initiated due to the onset of AMI. Thirty-day mortality was defined as a proportion of patients who died within 30 days since the symptom onset, either during the initial hospital stay or after discharge. In the case of out-of-the hospital deaths, death dates were verified from death certificates. Since mortality rate during the first 30 days after the index event in AMI patients is by far higher than during any other subsequent 30-day period, only patients surviving the first 30 days were considered for the analysis of long-term survival/all-cause mortality, and day 30 post index event was considered as “time 0” for determination of “time-to-event.” All patients were contacted by phone or in writing. When it was confirmed that they were alive, this date was used to calculate “censored time.” If no confirmation was received, community death registers were searched to verify the date of death and calculate the “failure time.” For patients who did not respond to our attempts to contact them and for whom death date could not be determined through a search of the community death register, the last documented date of a control visit was used to determine “censored time.”
Statistical analysis
Due to data skewness, all continuous variables are summarized as medians (ranges). In-hospital and thirty-day mortality were analyzed using modified Poisson regression with robust error variance to obtain adjusted incidence ratios, ie, relative risk (RR) rather than odds ratio since data were collected prospectively and event rates were relatively high (≥10%) (14). “Time-to-event” data were analyzed by fitting proportional hazard regression models with the exact method for ties. On-admission SUA levels as a dependent variable were analyzed using multiple linear regression. Residuals were normally distributed. We used SAS for Windows software, version 9.1.3. (SAS Inc., Cary, NC, USA) licensed to Zagreb University School of Medicine.
Results
Patients’ characteristics
Patient characteristics are summarized in Table 1. Cardiovascular treatments utilized before the index event included angiotensin converting enzyme inhibitors (25.3%), calcium channel blockers (21.7%), nitrates (21.6%), diuretics (14.7%), antiplatelets (13.9%), digitalis (8.5%), beta blockers (5.8%), and statins (2.7%). Patients were admitted between 0.5 and 48 hours after the symptom onset and SUA values on admission varied between 63 and 993 µmol/L. In a multivariate analysis (Table 2), higher SUA levels were associated with higher serum creatinine, male sex, history of heart failure, and history of cerebrovascular insult. Smokers had lower SUA than non-smokers (Table 2).
Table 1.
Patient characteristics and short-term mortality (n = 621)
| Characteristic | Data† |
|---|---|
| Men | 402 (64.7) |
| Time: symptom onset to admission (hours) | 6 (0.5-48) |
| STEMI/NSTEMI | 481 (77.5)/140 |
| Right bundle branch block | 22 (3.5) |
| Left bundle branch block | 20 (3.2) |
| Age (years) | 65 (27-91) |
| Current smokers | 208 (33.5) |
| Prior acute myocardial infarction | 94 (15.1) |
| Prior cerebrovascular insult | 57 (9.2) |
| Prior angina | 126 (20.3) |
| Prior by-pass, stent or PTCA | 9 (1.4) |
| Prior hypertension | 459 (73.9) |
| Prior symptomatic heart failure | 61 (9.8) |
| Diabetes mellitus | 129 (20.8) |
| Serum uric acid on admission (µmol/L) | 315 (63-993) |
| Serum creatinine on admission (µmol/L) | 105 (43-804) |
| Serum triglyceride on admission (mmol/L) | 1.24 (0.22–16.9) |
| Serum total cholesterol on admission (mmol/L) | 5.34 (1.6 –11.3) |
| Peak creatine phosphokinase (U/L) | 771 (0-9350) |
| Fibrinolytics after index event | 48 (7.7) |
| In-hospital mortality | 62 (9.98) |
| Thirty-day mortality | 77 (12.4) |
*Abbreviations: STEMI – myocardial infarction with ST elevation; NSTEMI – myocardial infarction without ST elevation; PTCA – percutaneous transluminal coronary angioplasty.
†Data are counts (%) or medians (ranges).
Table 2.
Multivariate analysis of serum uric acid on admission (n = 621)*
| Variables | Estimate (95% CI)† | P |
|---|---|---|
| Serum creatinine | 0.77 (0.61-0.94) | <0.001 |
| Male sex | 28.4 (8.59-48.2) | 0.005 |
| Prior symptomatic heart failure | 73.1 (42.4-104) | <0.001 |
| Prior cerebrovascular insult | 41.0 (9.77-72.3) | 0.010 |
| Current smoker | -26.9 (-47.3 to -6.54) | 0.009 |
*Linear regression analysis, using stepwise selection procedure with P < 0.05 to enter and P < 0.10 to stay in the model was employed. Potential independents: demographic and pre-index event medical history data and laboratory variables determined on admission.
†Estimates are given with 95% confidence intervals (CI).
SUA and short-term mortality
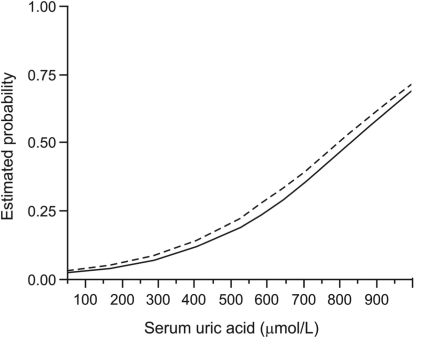
In-hospital mortality was 9.98% and thirty-day mortality was 12.4% (Table 1). There was a clear-cut univariate association between higher SUA levels and higher in-hospital and thirty-day mortality (Figure 1). For multivariate analysis, a regression model was first built for each outcome using a stepwise procedure and then variables associated with higher SUA (depicted in Table 2) and AMI type were forced into the models, if not already included. With all the adjustments, higher SUA levels were independently associated with both higher in-hospital mortality (RR by 10 units, 1.016; 95% CI, 1.001-1.031, P = 0.043) and higher thirty-day mortality (RR by 10 units, 1.016; 95% CI, 1.003-1.029, P = 0.018) (Table 3).
Figure 1.
Univariate association between serum uric acid levels on admission and in-hospital and thirty-day mortality in acute myocardial infarction patients (N = 621). Estimated probabilities are from logit models with serum uric acid as a single continuous independent. Relative risk (RR) per 10 units change in uric acid levels with 95% confidence intervals (CI) is from modified Poisson regression with robust error variance with serum uric acid as a single independent. Solid line – in-hospital mortality (RR, 1.038; 95% CI, 1.026-1.051); dashed line – thirty-day mortality (RR, 1.035; 95% CI, 1.024-1.047).
Table 3.
Multivariate analysis of in-hospital and thirty-day mortality in acute myocardial infarction patients (n = 621)*
| Independent variables |
Relative risk (95% confidence interval)† |
|||
|---|---|---|---|---|
| in-hospital mortality | P | thirty-day mortality | P | |
| Serum UA (10 units) | 1.016 (1.001-1.031) | 0.043 | 1.016 (1.003-1.029) | 0.018 |
| Male sex | 0.568 (0.346-0.933) | 0.026 | 0.646 (0.427-0.978) | 0.038 |
| Age | 1.040 (1.020-1.061) | <0.001 | 1.043 (1.025-1.061) | <0.001 |
| Prior CVI | 2.016 (1.173-3.465) | 0.011 | 2.309 (1.515-3.520) | <0.001 |
| Prior hypertension | 0.564 (0.324-0.981 | 0.043 | 0.622 (0.389-0.994) | 0.047 |
| Peak CPK (10 units) | 1.001 (1.000-1.003) | 0.096 | 1.001 (1.000-1.002) | 0.068 |
| RBBB | 3.029 (1.322-6.941) | 0.009 | — | — |
| Prior AMI | 2.200 (1.256-3.852) | 0.006 | — | — |
| Serum Cr (10 units)‡ | 1.025 (1.001-1.049) | 0.045 | 1.019 (0.995-1.044) | 0.118 |
| Prior heart failure§ | 1.305 (0.687-2.479) | 0.416 | 1.523 (0.911-2.544) | 0.108 |
| STEMI§ | 1.433 (0.765-2.685) | 0.262 | 1.267 (0.781-2.155) | 0.316 |
*Abbreviations: UA – uric acid; CVI – cerebrovascular insult; CPK – creatine phosphokinase; RBBB – right bundle branch block; AMI – acute myocardial infarction; Cr – creatinine; STEMI – myocardial infarction with ST elevation.
†Statistics: modified Poisson regression with robust error variance. A model was first built for each outcome using a stepwise selection procedure (P < 0.05 to enter, P < 0.10 to stay in the model) and then variables associated with higher serum uric acid on admission (depicted in Table 2) and infarction type were forced into the model, if not already included. Potential independents (depicted in Table 1): demographics, pre-index event medical history and treatment, laboratory variables determined on admission, myocardial infarction characteristics.
‡Forced into the model explaining variability of thirty-day mortality.
§Forced into both models.
SUA and long-term survival (all-cause mortality)
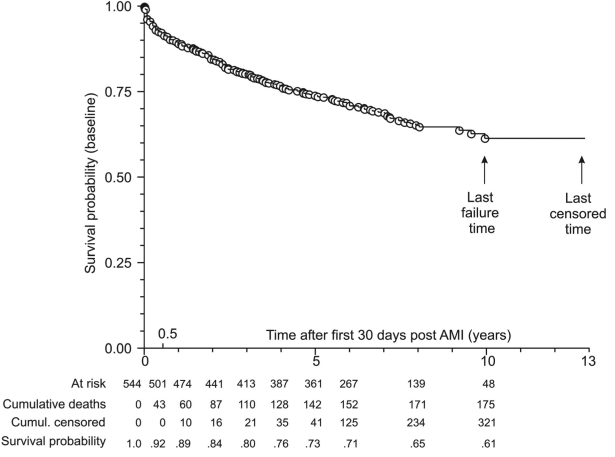
Characteristics of patients who survived the first 30 days after AMI and were evaluated for long-term survival (n = 544) are summarized in Table 4. The shortest observed censored time was 207 days (after day 30 post-index event) and the longest one was 13 years. A total of 175 (32.2%) deaths were observed, the first one 3 days after day 30 post-index event and the last one 10 years later. There was a clear-cut univariate association between higher SUA on admission and poorer long-term survival (higher all-cause mortality) (Figure 2). Multivariate analysis indicated a significant independent association of higher SUA and poorer survival (HR by 10 units, 1.105; 95% CI, 1.020-1.195, P = 0.010), which was conditional on age (significant SUA/age interaction) (Table 5). This is also illustrated by separate multivariate analyses in patients younger than the median age (age 27-63 years, n = 274) and patients older than the median age (>63-90 years, n = 270) (Figure 3): higher SUA was independently associated with poorer survival in the former (HR by 10 units, 1.030; 95% CI, 1.009-1.051, P = 0.021) but not in the latter (HR by 10 units, 1.004; 95% CI, 0.991-1.017, P = 0.575).
Table 4.
Patients surviving the first 30 d after acute myocardial infarction and considered for long-term survival analysis (n = 544)*
| Characteristic | Data† |
|---|---|
| Men | 367 (67.5) |
| STEMI/NSTEMI | 418 (76.8)/126 |
| Age (years) | 63 (27-90) |
| Serum uric acid on admission (µmol/L) | 312 (63-827) |
| Serum creatinine on admission (µmol/L) | 104 (43-804) |
| Serum triglyceride on admission (mmol/L) | 1.26 (0.22-16.9) |
| Serum total cholesterol on admission (mmol/L) | 5.44 (1.67-11.3) |
| Diabetes mellitus | 109 (20.0) |
| Hypertension | 412 (75.7) |
| History of cerebrovascular insult | 40 (7.35) |
| In-hospital MACE: | |
| symptomatic heart failure | 149 (27.4) |
| ventricular tachycardia/fibrillation | 147 (27.0) |
| atrial fibrillation/undulation | 52 (9.6) |
| re-infarction or angina | 33 (6.1) |
| atrio-ventricular block | 26 (4.8) |
| Treatment at discharge: | |
| antiplatelets | 445 (81.8) |
| nitrates | 440 (80.9) |
| ACE inhibitors | 342 (62.9) |
| diuretics | 234 (43.0) |
| beta blockers | 141 (25.9) |
| statins | 84 (15.4) |
| calcium channel blockers | 69 (12.7) |
| digitalis | 39 (7.2) |
| Observed period after day 30 post index event (days) | |
| censored observations | 2525 (207-4715) |
| deaths | 730 (3-3615) |
| all-cause mortality during the observed period | 175 (32.2) |
*Abbreviations: STEMI/NTSEMI – myocardial infarction with/ without ST elevation; MACE – major adverse cardiac events; ACE – angiotensin converting enzyme.
†Data are presented as counts (%) or medians (ranges).
Figure 2.
Univariate association between serum uric acid (SUA) levels on admission and long-term survival (after 30 days post index event) in acute myocardial infarction (AMI) patients (N = 544). Survival probability was estimated in a proportional hazard regression model with SUA on admission as an independent variable. Hazard ratio (by 10 units) of SUA, 1.027; 95% confidence interval, 1.015-1.039.
Table 5.
Multivariate analysis of time to death (all-cause) in 544 acute myocardial infarction patients who survived the first 30 d after the index event*
| Independent variables | Hazard ratio (95% confidence interval) | P |
|---|---|---|
| Serum uric acid on admission (10 units) | 1.105 (1.020, 1.195) | 0.010 |
| Serum creatinine on admission (10 units) | 1.041 (1.010, 1.061) | 0.003 |
| Symptomatic heart failure at discharge | 1.767 (1.260, 2.478) | 0.001 |
| Age | 1.099 (1.054, 1.145) | <0.001 |
| Use of digitalis after discharge | 1.704 (1.081, 2.685) | 0.022 |
| Serum uric acid/age† | 0.999 (0.998, 1.000) | 0.017 |
*Proportional hazard regression with exact method for ties. Stepwise procedure with P < 0.05 to enter and P < 0.10 to stay in the model was employed. Potential independents (Table 4): demographic data, laboratory variables determined on admission, myocardial infarction characteristics and acute complications, treatment at discharge.
†Interaction term.
Figure 3.
Multivariate analysis of time to death (all-cause) in acute myocardial infarction (AMI) patients who survived the first 30 days after the index event and were either younger (age 27-63 years) or older (>63 years) than the median age. A separate proportional hazard regression model was built for each subgroup. Serum uric acid (SUA) levels were included in both models, whereas other effects were selected using a stepwise procedure (P < 0.05 to enter, P < 0.10 to stay in the model). Potential independents were the same as in analysis shown in Table 5. Open circles – age 27-63 years, n = 274, 46 deaths. Adjusted HR for SUA (10 units), 1.030; confidence interval, 1.009-1.051, P = 0.021 (symptomatic heart failure at discharge, post-AMI ventricular tachycardia or fibrillation, and use of digitalis after discharge). Closed circles – age >63 years, n = 270, 129 deaths. Adjusted HR for SUA (10 units); confidence interval, 0.991-1.017, P = 0.575 (serum creatinine on admission, symptomatic heart failure at discharge, use of beta blockers, and use of nitrates after discharge).
Discussion
Our study demonstrated that higher serum uric acid determined on admission is associated with higher in-hospital mortality and thirty-day mortality and poorer long-term survival after AMI.
The (wide) range of SUA levels determined on admission in the current cohort was practically identical to that observed in Japanese AMI patients (13) (all within 48 hours since the symptom onset) and the observed cross-sectional associations were expected considering previous knowledge (1,3,4,13). Currently, SUA is generally not considered a relevant factor in the assessment of risk/prediction of outcomes in AMI patients (3,15). The main finding of the present study in a sample of Caucasians of European descent is that higher SUA determined on admission (within 48 hours since the symptom onset) predicts higher in-hospital and thirty-day mortality after AMI. The results are in line with those in Japanese patients (13) and add to the observation that the relationship is independent, ie, not confounded by other factors influencing mortality and/or actual SUA levels. This is particularly interesting regarding serum creatinine because a) higher serum creatinine on admission is a well-accepted predictor of mortality after AMI (15) and b) just like others (13), we found an independent cross-sectional association between higher serum creatinine and higher SUA. The latter observation appears logical since both UA and creatinine are eliminated by the kidney. The present results suggest that in AMI patients, like in non-AMI CAD patients (11), the “risk” associated with SUA is separated from the “risk” attributable to serum creatinine, indicating renal function. Moreover, considering the adjusted RRs of in-hospital and thirty-day mortality (Table 3), risk increase associated with 10-unit (µmol/L) increase in SUA is not much different from that associated with 10-unit (µmol/L) increase in serum creatinine. Consequently, it appears reasonable to suggest that risk stratification systems for AMI patients, like GRACE (15), could benefit from inclusion of SUA in addition to serum creatinine (and other elements), particularly as it is a cheap marker that is readily, quickly, and reliably obtainable. By saying that, we do not neglect the fact that the present study, just as the Japanese one (13), suffers from limitations inherent to its retrospective nature, primarily susceptibility to bias (selection, treatment, assessment). However, we believe that the characteristics of the analyzed setting (AMI is an acute serious condition that is “handled” in standardized ways in ICUs where patients are continuously and closely followed-up and data are collected prospectively) and clear and simple inclusion and evaluation criteria that we used minimized potential biases. Therefore, we consider that the present estimates have achieved a reasonable level of internal validity. However, their generalizability is limited by the fact that they refer to AMI patients treated exclusively conservatively in the late 1990s and that, therefore, the established relationships might not be fully applicable to up-to-date settings with treatment strategies combining novel pharmacological and invasive procedures. Other effects on short-term mortality are difficult to interpret since they were not the primary objective of the study: the goal was not to find the best set of explanatory variables or to evaluate all possible effects, but to test the hypotheses about SUA. Consequently, covariate selection was guided by previous knowledge and by statistical significance within the current sample. Non-inclusion or “insignificance” of a particular variable in the present models does not mean that it might not, in general, have an impact on outcomes after AMI, particularly if the effect is small or associated with a relatively infrequent patient/disease-related characteristic. Bundle branch blocks (BBB) are illustrative in this respect. In the present sample, only 22 (3.5%) patients had right BBB (RBBB) (either at presentation or during the hospital stay) and 20 (3.2%) had left BBB (LBBB). The former was independently associated with higher in-hospital mortality, whereas the latter was not. A meticulous analysis of 17 073 AMI patients (16) found a similar prevalence of LBBB and RBBB but the absolute numbers were almost 20 times higher. Newly developed LBBB was associated with higher 30-day mortality, whereas independent association was found for both “presenting” and newly developed RBBB (conditional on AMI localization) (16). Such a detailed analysis of BBB impact (or any other factor besides SUA) on AMI outcomes was beyond the scope of the present study. Hence, our results do not “eliminate” LBBB as irrelevant in general. At the same time, lack of an independent effect of LBBB does not compromise the conclusion about the independent effect of SUA. The same principle applies to the analysis of the secondary outcome, which confirmed observations from the Japanese study (13) about higher SUA as an independent predictor of higher long-term all-cause mortality after AMI. It should be noted, however, that SUA varies over time and is susceptible to various influences. Hence, it should be preferably treated as a time-varying covariate (which is almost never the case), and “predictivity” based on a single-point measurement might not be too informative. In the present cohort, the “effect” of SUA was independent of but conditional on age, ie, was apparent in “younger” patients but not in the “older” ones. We argue that this is due to inherently much higher hazard rates in the latter subgroup. Looking at the entire cohort, however, the “effect” of SUA was but small: the reported HR by 10 units increase corresponded to an HR of around 1.65 by 50 units increase and this is comparable to an HR by 5 years increase in age (around 1.60) or to that for “symptomatic heart failure at discharge” (around 1.77). Heart failure is a known prognostic factor of poor long-term outcomes after AMI with a “dose-dependent” effect (more severe the condition [eg, higher the Killip’s class], poorer the survival) (13) but we could not “grade” it. However, the effect of a more severe failure might have been aliased by “use of digitalis at discharge;” digitalis is typically used in more severe patients, but per se has no impact on long-term survival (17), and 36/46 of the present “30-day survivors” who were prescribed with digitalis at discharge were diagnosed with symptomatic heart failure at the same time. Finally, from the today’s perspective (15), the use of beta-blockers in long-term treatment of “30-day survivors” in the present cohort was seemingly lower than “optimal” (25.9% were prescribed with beta-blockers at discharge). We have no explanation for this observation, which apparently reflects the practice from 10-13 years ago. Underuse of beta-blockers may affect long-term survival (12), but we find it unlikely that this fact biased the observations related to SUA – present observations are fully in line with those from a more recent Japanese cohort (13).
To conclude, higher SUA determined on admission (within 48 hours since the symptom onset) in a cohort of patients from Croatia was independently associated with higher short-term mortality and poorer long-term survival after AMI. The findings are in agreement with the observations in Japanese patients (13) and document relevance of on-admission SUA for risk stratification in AMI patients.
Acknowledgment
This study received no funding and authors have no conflict of interest to declare.
References
- 1.Coleman LA, Roubenoff R. Gout. In: Caballero B, Allen L, Prentice A, editors. Encyclopedia of human nutrition, 2nd edition. Oxford (UK): Elsevier; 2005; p. 419-23. [Google Scholar]
- 2.Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008;40:437–42. doi: 10.1038/ng.106. [DOI] [PubMed] [Google Scholar]
- 3.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–21. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomita M, Mizuno S, Yokota K. Increased levels of serum uric acid among ex-smokers. J Epidemiol. 2008;18:132–4. doi: 10.2188/jea.JE2006332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–90. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 6.Gagliardi AC, Miname MH, Santos RD. Uric acid: A marker of increased cardiovascular risk. Atherosclerosis. 2009;202:11–7. doi: 10.1016/j.atherosclerosis.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Milionis HJ, Kalantzi KJ, Goudevenos JA, Seferiadis K, Mikhailidis DP, Elisaf MS. Serum uric acid levels and risk for acute ischaemic non-embolic stroke in elderly subjects. J Intern Med. 2005;258:435–41. doi: 10.1111/j.1365-2796.2005.01565.x. [DOI] [PubMed] [Google Scholar]
- 8.Weir CJ, Muir SW, Walters MR, Lees KR. Serum urate as an independent predictor of poor outcome and future vascular events after acute stroke. Stroke. 2003;34:1951–6. doi: 10.1161/01.STR.0000081983.34771.D2. [DOI] [PubMed] [Google Scholar]
- 9.Karagiannis A, Mikhailidis DP, Tziomalos K, Sileli M, Savvatianos S, Kakafika A, et al. Serum uric acid as an independent predictor of early death after acute stroke. Circ J. 2007;71:1120–7. doi: 10.1253/circj.71.1120. [DOI] [PubMed] [Google Scholar]
- 10.Allison TG. Coronary heart disease epidemiology. In: Murphy JG, Lloyd MA, editors. Mayo clinic cardiology. 3rd edition. Rochester (MN): Mayo Clinic Scientific Press; 2007. p. 687-93. [Google Scholar]
- 11.Brodov Y, Chouraqui P, Goldenberg I, Boyko V, Mandelzweig L, Behar S. Serum uric acid for risk stratification of patients with coronary artery disease. Cardiology. 2009;114:300–5. doi: 10.1159/000239860. [DOI] [PubMed] [Google Scholar]
- 12.Dunkelgrun M, Welten GM, Goei D, Winkel TA, Schouten O, van Domburg RT, et al. Association between serum uric acid and perioperative and late cardiovascular outcome in patients with suspected or definite coronary artery disease undergoing elective vascular surgery. Am J Cardiol. 2008;102:797–801. doi: 10.1016/j.amjcard.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Kojima S, Sakamoto T, Ishihara M, Kimura K, Miyazaki S, Yamagishi M, et al. Prognostic usefulness of serum uric acid after acute myocardial infarction (the Japanese Acute Coronary Syndrome Study). Am J Cardiol. 2005;96:489–95. doi: 10.1016/j.amjcard.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 15.White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372:570–84. doi: 10.1016/S0140-6736(08)61237-4. [DOI] [PubMed] [Google Scholar]
- 16.Wong CK, Stewart RA, Gao W, French JK, Raffel C, White HD. Prognostic differences between different types of bundle branch block during the early phase of acute myocardial infarction: insights from the Hirulog and Early Reperfusion or Occlusion (HERO)-2 trial. Eur Heart J. 2006;27:21–8. doi: 10.1093/eurheartj/ehi622. [DOI] [PubMed] [Google Scholar]
- 17.Task Force for Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of European Society of Cardiology. Dickstein K, Cohen, Solal A, Filippatos G, McMurray JJ, Ponikowski P, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29:2388–442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]