Abstract
This study investigated working memory (WM) consolidation, that is, the time required to create durable WM representations, at different levels of WM load in schizophrenia. Twenty-three schizophrenia spectrum patients and 16 control subjects participated in a change-detection task in which a sample array of 1–3 squares appeared followed by a delay and a test array. An array of pattern masks was inserted into the delay interval—covering the locations of the sample-array squares—100–800 ms after the offset of the sample array. If a durable WM representation is formed prior to mask onset, the mask should not impair performance. The degree of masking at an interval reflects the degree of WM consolidation at that time. Neither group showed masking at set size 1. Unlike controls, patients demonstrated robust masking effects at set size 2. Both groups showed masking at set size 3, but masking effects were larger and longer lasting in patients. These data demonstrate abnormally prolonged WM consolidation in schizophrenia. This impairment may slow the formation of stable representations of the visual environment, impacting everyday visually guided behavior.
Keywords: schizophrenia, working memory, consolidation, change-detection task, encoding
Working memory (WM) impairments have been widely documented in the schizophrenia literature, but the precise nature of the underlying deficit(s) remains unspecified (Gold, Carpenter, Randolph, Goldberg, & Weinberger, 1997; Goldman-Rakic, 1994; Park & Holzman, 1992). The literature does, however, provide important empirical constraints on theories of the impairment. The fact that deficits are observed across a wide variety of stimulus types argues for an amodal level of impairment (Coleman et al., 2002; Fleming et al., 1995; Fleming et al., 1997; Javitt, Strous, Grochowski, Ritter, & Cowan, 1997; see Lee & Park, 2005, for a review; Park & Holzman, 1992). The fact that deficits are apparent at short as well as long retention intervals, with inconsistent evidence of magnified impairment with increasing delay, argues against maintenance being the primary locus of impairment (Javitt et al., 1997; Lencz et al., 2003). Although deficits are magnified at higher levels of WM load (Carter et al., 1998), the fact that deficits may also be observed when subjects are asked to remember only 1–2 highly discriminable targets (Park & Holzman, 1992) suggests that a simple reduction in storage capacity cannot explain the overall pattern of results documented in the literature. Further, the fact that deficits are observed with unspeeded free response and forced choice methods argues against an impairment localized at the level of response selection. We view these as the core findings that any theory of WM impairments in schizophrenia must explain.
It is clearly plausible that multiple independent specific impairments underlie the performance deficits observed in schizophrenia patients in WM tasks. However, an explanation of this broad range of findings based on a single underlying impairment would be far more satisfying and would point toward specific neural mechanisms. One such explanation is that the processes that transform perceptual representations into WM representations are impaired in schizophrenia, resulting in the creation of imprecise and noisy WM representations.1 This hypothesis explains the core findings reviewed above in the following manner. First, evidence from the basic science literature indicates that amodal, central processes are involved in creating WM representations (Jolicoeur, 1999; Jolicoeur & Dell’Acqua, 1998), consistent with the finding of WM deficits across modalities in schizophrenia. Second, noisier representations may lead to impaired performance even when the number of items being remembered is within the subject’s storage capacity. However, the impact of noisy representations on performance may be amplified as memory load increases. Finally, the effects of inaccurate or noisy WM encoding would be expected to influence performance regardless of the nature of the responses required by the task (assuming that the task is sufficiently sensitive). While this hypothesis is speculative, an impairment of WM encoding processes may provide an integrative account of many of the deficits documented in the schizophrenia WM literature. The goal of the present study was to specifically test the hypothesis that the process of transforming perceptual representations into WM representations—called WM consolidation—is impaired in patients with schizophrenia.
It should be noted that there have been prior hints of prolonged WM consolidation in the schizophrenia literature. Some studies have equated baseline WM performance in schizophrenia patients and healthy controls by increasing exposure time, allowing more time for consolidation, and these studies have found that patients require far longer than controls to reach criterion levels of performance (Hartman, Steketee, Silva, Lanning, & McCann, 2003; Tek et al., 2002). Knight and colleagues (1985) previously showed prolonged vulnerability to pattern masks (random arrays of shapes) in a subgroup of schizophrenia patients and suggested that patients have a deficit in consolidating information in WM. Studies using attentional blink paradigms have demonstrated that patients require longer intervals between two target stimuli in order to accurately report the occurrence of the second target, suggestive of prolonged encoding times for the first target stimulus (Cheung, Chen, Chen, Woo, & Yee, 2002; Li et al., 2002; Wynn, Breitmeyer, Nuechterlein, & Green, 2006). Related evidence has been produced from studies using rapid serial visual presentation (RSVP) tasks, for which patients have been observed to remain susceptible to disruption for longer than controls (Park & Hooker, 1998). Although these results are consistent with the idea of slowed WM consolidation, the attentional blink and RSVP tasks also involve aspects of task switching that make it impossible to use these paradigms to measure the speed of WM consolidation (see Vogel, Woodman, & Luck, 2006, for an extensive discussion). Thus, although findings from other paradigms are consistent with impaired WM consolidation, these findings are only suggestive.
We recently reported empirical evidence of a deficit in the rate at which patients with schizophrenia are able to perform WM consolidation using a masking paradigm that was originally developed by Vogel et al. (2006). In this experiment (Fuller, Luck, McMahon, & Gold, 2005), observers were presented with a sample array of three colored squares for 100 ms, which was followed after a 1000-ms retention interval by a test array (see Figure 1a for an example of a similar paradigm). The test array was identical to the sample array on half the trials, and on the other half, the color of one square changed. Observers were asked to make an un-speeded same-different judgment. On some trials, the delay interval was unfilled, allowing 1000 ms of uninterrupted processing time. On other trials, an array of pattern masks was presented at an interval varying from 17–483 ms following the offset of the sample array. These masks were intended to overwrite the perceptual representation of the sample array before this representation could be transformed into a more durable, distraction-resistant WM representation. If the mask array is presented before the WM representation is formed, performance of the change-detection task should be poor; if the mask array is presented after a durable WM representation has been formed, the mask should not interfere with change-detection performance. By varying the interval between the sample array and the mask, it is possible to estimate the time course of the consolidation process: consolidation is complete when masked performance equals no-mask performance.
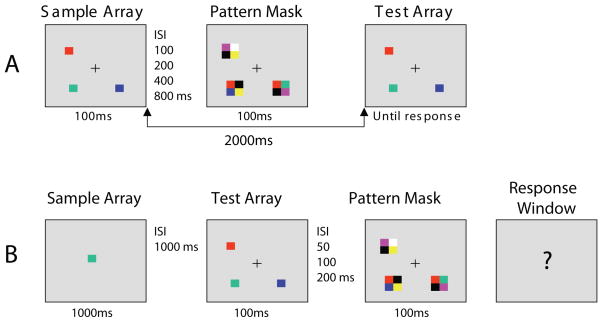
Figure 1.
(A) Example of the stimuli used in the working memory task. Subjects indicated by button press whether the squares in the sample and test array were identical or one item changed in color. (B) Example of the stimuli used in the perceptual control task. When the response window appeared (question mark), subjects indicated by button press whether or not the square in the sample array was present in the test array.
Control subjects reached their no-mask performance within 250 ms, indicating that WM consolidation was complete by this time. In contrast, patients with schizophrenia failed to reach their no-mask performance by 483 ms, the longest interval tested. This result indicates a marked prolongation of consolidation. Although exaggerated perceptual-level masking effects have been frequently documented in schizophrenia (Green & Nuechterlein, 1999; Green, Nuechterlein, Breitmeyer, & Mintz, 1999; Rassovsky, Green, Nuechterlein, Breitmeyer, & Mintz, 2005), there are several reasons to believe that the effects we observed do not reflect an effect of the masks on the formation of the perceptual representations. First, masking was observed at intervals that were more than twice as great as those that lead to conventional backward masking. Second, the original study of Vogel et al. (2006) demonstrated that masks in this paradigm do not influence the formation of perceptual representations. Third, we included a carefully matched control condition to show that perceptual masking does not occur at the delay intervals for which patients showed the largest consolidation impairments. In this control condition, observers searched for a prespecified target color in a test array of three colored rectangles. This array was presented for 100 ms, just like the sample array in the main experiment, and it was followed by masks using the same intervals as in the WM paradigm. Although significant perceptual masking was observed with a 17-ms delay, no masking was observed at longer delay intervals. Thus, the patient deficits observed in the WM paradigm at longer delays were not caused by perceptual masking, but instead reflect a slowing of the consolidation process. This prolonged consolidation time suggests that WM representations remain abnormally vulnerable to the impact of external distraction. The representations may also be vulnerable to internally generated “noise,” leading to reduced storage capacity even in the absence of external distractors.
The present study was designed to replicate our prior evidence of prolonged WM consolidation and extend that evidence in two important ways. First, in our prior study, patients continued to demonstrate masking with three item arrays for the longest interval tested, 483 ms. Although the entire pattern of results was consistent with a slowing of the consolidation process, it was difficult to rule out the possibility that consolidation was simply ineffective rather than slowed. The present study included an 800-ms masking interval to determine the outer temporal limits of masking vulnerability. Second, WM consolidation is load-sensitive: the time required to consolidate an array of items increases substantially as the number of items in the array increases. In normal subjects, arrays of 1–2 items can be consolidated in a short amount of time, with more gradual consolidation for arrays of 3–4 items. The present study tested set sizes 1, 2, and 3 to assess interactions between memory load and masking interval. If consolidation is slower in patients than in control subjects, then masking should be evident at smaller set sizes in patients than in control subjects. For example, consolidation in healthy individuals may be complete for a set size of only two items at the shortest masking intervals that can easily be tested, leading to no effect of masking at this set size. If consolidation is slowed in patients, however, the masks may disrupt the representation before it has been consolidated even with a set size of only two items, leading to significant masking effects in these individuals. By testing multiple set sizes and a broader range of masking intervals, we sought to buttress our previous evidence that the process of forming WM representations is impaired in schizophrenia, consistent with the broader hypothesis that impairments in this process may underlie many of the performance deficits observed in schizophrenia patients in WM tasks.
Method
Participants
Twenty-three patients meeting DSM–IV criteria for schizophrenia (14 undifferentiated, 7 paranoid) or schizoaffective disorder (2) and 16 healthy controls participated in the study. The patients were clinically stable outpatients who had been receiving the same medication at the same dose for at least eight weeks. Nineteen patients were receiving second-generation antipsychotics, and four were receiving first generation antipsychotics. Patient diagnosis was determined using a best estimates approach that combined information from previous medical records, collateral informants (when available), and the results of the Structured Clinical Interview for DSM–IV Diagnosis. Diagnosis was established at a consensus conference including clinical staff that worked with the patient, chaired by a senior research psychiatrist or by one of the authors (JG).
Seventeen healthy control subjects were recruited from the community by newspaper advertisements, wall notices, and word of mouth. All controls were screened using the complete Structured Clinical Interview for DSM–IV Axis I Disorders (SCID I; First, Spitzer, Miriam, & Williams, 1997a) and the complete Structured Clinical Interview for DSM–IV Personality Disorders (SCID II; First, Spitzer, Miriam, & Williams, 1997b). One volunteer revealed a history of psychiatric treatment after completing the study and was therefore excluded from analysis, leaving a total of 16 healthy subjects. These subjects were free of a current or past history of major psychiatric illness, including depressive or psychotic disorders, as well as personality disorders or mental retardation and denied a family history of psychotic disorders in first-degree relatives. All subjects (patients and controls) were free of a DSM–IV diagnosis of substance abuse or dependence within the last 12 months and other medical or neurological disorders that might interfere with test performance. All subjects were between 18 and 55 years of age.
Demographic features are shown in Table 1. The patient group scored significantly lower than the control group on the Repeatable Battery for Assessment of Neuropsychological Status (RBANS; Randolph, 1998; p < .05), but the two groups did not differ significantly in age, race, sex, Wide Range Achievement Test (3rd Edition; Wilkinson, 1993) scores, years of education, or father’s education (p > .5). We therefore conclude that the differences in RBANS score reflect the cognitive consequences of schizophrenia rather than differences in the demographics of the two groups.
Table 1.
Demographic Features of Sample
| Control group (n =16) | Patients (n = 23) | |
|---|---|---|
| Age | 40.2 (10.26) | 43.6 (8.7) |
| Years of education | 13.9 (1.5) | 12.9 (2.6) |
| Father’s years of education* | 13.0 (4.3) | 13.7 (3.7) |
| RBANS total score+ | 89.9 (7.2) | 76.3 (13.5)*** |
| WRAT 3rd edition† | 95.6 (13.6) | 92.1 (18.1) |
| Sex | ||
| Male | 9 (56%) | 19 (83%) |
| Female | 7 (44%) | 4 (17%) |
| Race | ||
| African American | 8 (50%) | 9 (39%) |
| Asian | 1 (6%) | 0 |
| Hispanic | 1 (6%) | 0 |
| White | 6 (38%) | 13 (57%) |
| Other | 0 | 1 (4%) |
Note. RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; WRAT = Wide Range Achievement Test, 3rd Edition. Standard deviations are provided in parentheses.
Data missing for 1 patient.
Data missing for 1 patient and 3 controls.
Data missing for 2 patients and 2 controls.
p < .05.
Stimuli
Stimuli were presented on a computer monitor with a gray background and a continuously visible central fixation point at a nominal viewing distance of 70 cm. Each sample array consisted of 1, 2, or 3 colored squares placed randomly around a notional circle with a radius of 2.93° of visual angle (see Figure 1). When three squares were presented, they were separated from each other by 120° of polar angle around the circle. When two squares were presented, they were separated by 120° of polar angle around the circle in one direction and 240° in the other. Each square subtended 0.78 × 0.78° of visual angle, and the color of each square was randomly selected without replacement from a set of highly discriminable colors: red, white, black, blue, violet, green, and yellow. The test array was identical to the sample array, except that on 50% of the trials the color of one square changed to a new color that had not been present at any location in the target array. Each mask array contained one mask item at the location of each item in the sample array. Each mask item consisted of four colored squares, each of which subtended 0.78 × 0.78° of visual angle, joined to make a larger square centered over the location of the previously seen target square (see Figure 1). The four colors were chosen randomly without replacement from the same set of colors used for the sample and test arrays with the constraint that a given mask did not contain the color of the sample-array or test-array item at the same location.
Procedure
After hearing a detailed description of the study, each subject gave written informed consent to participate. The University of Maryland Institutional Review Board approved the study. Each subject performed one trial block of the WM task without any masks, three trial blocks of the WM task with masks, and one trial block of the control task. The order of blocks was counterbalanced with the constraint that the three WM blocks with masks were always presented consecutively. One block of 20 practice trials preceded each condition and was repeated if necessary.
Working Memory Condition
Each trial began with a delay of 1500 ms followed by a 100-ms presentation of the sample array, which contained 1, 2, or 3 colored squares. After a delay period of 100, 200, 400, or 800 ms, during which only a fixation cross was visible, an array of 1, 2, or 3 masks appeared for 100 ms, with one mask centered on the location of each previously presented sample square. Thus, the sample-to-mask interstimulus interval ranged from 100–800 ms, but the stimulus onset asynchrony (SOA) ranged from 200–900 ms. The delay between sample array offset and test array was 2000 ms, irrespective of the masking interval. The test array remained visible until the subject responded. The target array was identical to the test array on 50% of the trials (same trials), and one item changed in color on 50% of the trials (different trials). Participants made an unspeeded same/different button press on a labeled response box. The three set sizes (1, 2, 3) and four masking intervals (100, 200, 400, 800 ms) were randomly intermixed in three blocks of 120 trials each. There were 30 trials at each set size for each masking interval.
No-mask trials were presented in a separate block. There were 90 trials, 30 at each set size. These trials were identical to the mask trials except that no masks were presented. The interval between the target array and test array allowed for 2000 ms of uninterrupted consolidation and maintenance time.
Perceptual Control Condition
The perceptual control condition was designed to have the same perceptual requirements as the WM task without requiring WM consolidation. This condition required subjects to search an array of three masked items for a predefined target color, which was cued at the beginning of the trial. This task was identical to the WM condition except as follows. The cue (a single colored square at the center of the screen) appeared for 1000 ms at the beginning of the trial. This was followed by a 1000-ms delay and then a 100-ms target array consisting of three colored squares, which was masked after a delay of 50, 100, or 200 ms. A response screen then appeared, in which the fixation cross became a question mark, and participants made an unspeeded button press to indicate whether or not the target color had been present in the test array. The target color was present in 50% of the trials. The three array-to-mask intervals were mixed in one block of 90 trials with 30 trials at each interval.
Statistical Methods
The major dependent variable was A′, a measure of sensitivity (similar to d′) that is widely used in signal detection experiments. To compute A′, the hit rate (H) was first quantified as the proportion of correct responses for trials in which the target and test arrays were identical, and the false alarm rate (F) was quantified as the proportion of incorrect responses in which the two test stimuli were different.2 A′ scores were calculated using the formulae: A′ = 0.5 + (H − F) (1 + H − F)/4 H (1 − F), when H = F, and A′ = 0.5 + (F − H) (1 + F − H)/4 F(1 − H), when F > H (Stanislaw & Todorov, 1999, Equation 2). Extreme values of H or F were adjusted by replacing zero values with 0.5/n and values of 1.0 with (n − 0.5)/n, where n is the number of trials; this prevents values of zero from appearing in the denominator of the A′ equation.
The data were analyzed with the generalized estimating equations (GEE) method for repeated measures ANOVA (Lang & Zeger, 1986), as implemented in SAS® PROC MIXED (Cary, 2006), with five levels of masking (no mask, 100 msec, 200 msec, 400 msec, and 800 msec) × 3 levels of WM load (set size = 1, 2, or 3) within each subject, and the two groups as a between-subjects factor. The GEE method allows testing repeated measures hypotheses with a working model for the within-subject covariance matrix, while not requiring as extremely conservative a correction for model misspecification as the Geisser-Greenhouse procedure. The GEE method also allows some missing within-subject data (e.g., when subjects do not complete a full testing sequence due to fatigue or technical malfunction).
The primary analysis questions related to how the effects of group and WM load in the no mask condition were modified by onset of masking at different delays. To address these question, we used a cell mean parametrization of the overall ANOVA model, where Yijkl = Xijkl μjkl + eijkl, where Yijkl is the observation for the i-th individual in group j at set size k and masking condition l, Xijkl = 1 for an observation from group j at set size k and masking condition l, and = 0 otherwise, μjkl is the mean for group j at set size k and masking condition l, and eijkl is a random error term, with the errors correlated within subject. With this parametrization, ANOVA hypotheses about how group, set size, and masking onset delay modify the effect of masking can be formulated in terms of contrasts of differences between appropriately chosen subsets of the 30 cell means. As a simple example, if μ111 is the mean for the no mask condition in Group 1 at set size 1, the hypothesis that the effect of masking depends on the delay interval in Group 1 at set size 1 can be tested by an F test of the null hypothesis that the three contrasts (μ111 −μ112) − (μ111 −μ113), (μ111 −μ112) − (μ111 −μ114), and (μ111 −μ112) − (μ111 −μ115) are all equal to zero. In a similar fashion, one can build other contrasts to examine ANOVA contrasts of main effects of group, delay interval, set size, and interactions of group × set size, set size × delay interval, and group × set size × delay interval, which look at how these factors or combinations of factors modify the basic effect of masking versus the no masked condition. By analyzing the effect of all factors in terms of the masking difference score (mean mask performance minus mean no-mask performance), we may directly estimate impact of decreased consolidation time on accuracy under varying conditions.
To reduce Type I error rates while performing these ANOVA tests, they were done in hierarchical fashion, first testing to see if the 3-way group × set size × delay interval interaction was significant. Given that the overall interaction was significant, we then performed post hoc tests at each level of set size to determine whether the group × delay interval interaction was significant. If that interaction was significant, we tested group differences at individual levels of delay interval. If it was not significant, we tested for a main effect of group. An additional set of post hoc analyses was performed from the overall ANOVA model using contrasts among the means for the no mask condition to examine group × set size interactions when masking is not present.
Effect sizes for healthy control-patient differences under the no-mask condition and in the masking difference scores are presented in Table 3. They were calculated using the GEE estimates of the A′ difference between controls and patients at each combination of set size and masking delay divided by a pooled estimate of the within-cell standard deviation. The pooled estimate of the standard deviation was estimated as the square root of the sum of the within- and between-subjects variance components of error from a repeated measures model with fixed effects for group, set size, condition (no mask and four masked conditions), and their interactions.
Table 3.
A′ Analysis: Interactions Between Group, Set Size, and Masking Condition
| HC |
SC |
HC-SC Differences |
||||||
|---|---|---|---|---|---|---|---|---|
| Mask onset | Mean | SE | Mean | SE | Diff | SE(Diff) | p-value | Effect Size† |
| Set Size = 1 | ||||||||
| No mask | 0.984 | 0.008 | 0.961 | 0.010 | 0.022 | 0.013 | 0.084 | 0.282 |
| 100 ms - no mask | −0.008 | 0.005 | −0.011 | 0.019 | 0.005 | 0.019 | 0.810 | 0.064 |
| 200 ms - no mask | −0.002 | 0.005 | −0.018 | 0.017 | 0.016 | 0.018 | 0.370 | 0.205 |
| 400 ms - no mask | −0.004 | 0.002 | 0.000 | 0.012 | 0.003 | 0.013 | 0.800 | 0.039 |
| 800 ms - no mask | −0.007 | 0.007 | 0.001 | 0.015 | 0.006 | 0.016 | 0.710 | 0.077 |
| Set Size = 2 | ||||||||
| No mask | 0.979 | 0.008 | 0.965 | 0.01 | 0.014 | 0.013 | 0.290 | 0.180 |
| 100 ms - no mask | −0.017 | 0.010 | −0.064* | 0.016 | 0.047 | 0.019 | 0.014 | 0.603 |
| 200 ms - no mask | −0.004 | 0.007 | −0.037* | 0.012 | 0.035 | 0.014 | 0.015 | 0.449 |
| 400 ms - no mask | −0.005 | 0.005 | −0.044* | 0.016 | 0.039 | 0.017 | 0.023 | 0.501 |
| 800 ms - no mask | 0.003 | 0.009 | −0.018* | 0.007 | 0.022 | 0.012 | 0.072 | 0.282 |
| Set Size = 3 | ||||||||
| No mask | 0.960 | 0.012 | 0.917 | 0.017 | 0.042 | 0.022 | 0.055 | 0.539 |
| 100 ms - no mask | −0.079* | 0.014 | −0.081* | 0.018 | 0.001 | 0.023 | 0.970 | 0.013 |
| 200 ms - no mask | −0.045* | 0.013 | −0.099* | 0.023 | 0.055 | 0.027 | 0.043 | 0.706 |
| 400 ms - no mask | −0.030* | 0.015 | −0.051* | 0.023 | 0.021 | 0.027 | 0.450 | 0.270 |
| 800 ms - no mask | −0.012 | 0.013 | −0.024 | 0.019 | 0.013 | 0.024 | 0.580 | 0.167 |
Note. p < .05 for within group difference from no mask condition.
Effect size calculation: ([HC Mean−SC Mean]/pooled standard deviation).
Results
Perceptual Control Task
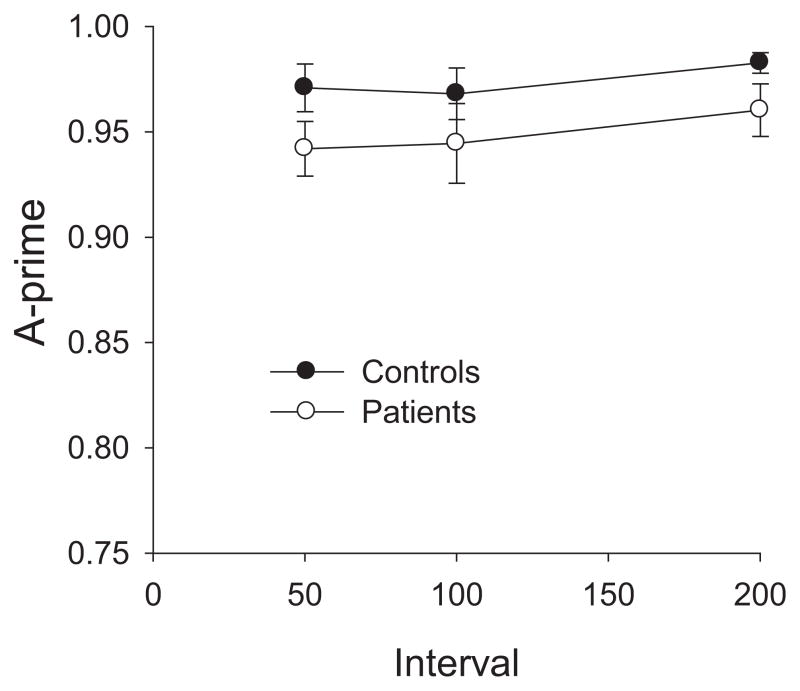
The purpose of this perceptual control condition was to confirm that each masking interval in the WM task was free from perceptual masking effects. As seen in Figure 2, although patients were somewhat less accurate than controls overall, the manipulation of target-mask interval had very little effect in either group. In an ANOVA with factors of Group and SOA, there was no significant main effect of Group (p = .14), SOA (p = .12), or Group × SOA interaction (p = .92). Thus, the two groups were not differentially affected by perceptual (backward) masking. This is not a surprising finding, given that the interval between target onset and mask onset was 150 ms, longer than the intervals typically tested in backward masking experiments (Green et al., 1999; Rassovsky et al., 2005; Rund, Landro, & Orbeck, 1993; Schechter, Butler, Silipo, Zemon, & Javitt, 2003). Because no substantial masking was observed with a 50-ms mask delay, we can be confident that no perceptual masking was present in the WM condition, in which the minimum delay was 100 ms.
Figure 2.
The results of the perceptual control task are presented as A′ values. Controls (filled circles) and Patients (empty circles) did not differ from each other in levels of perceptual (backward) masking. Error bars represent standard error of the mean.
Working Memory Task
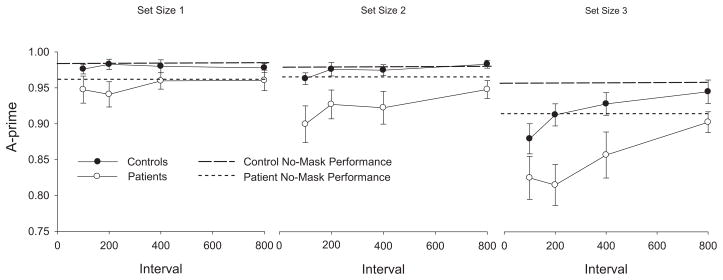
The mean A′ values from the WM task are displayed in Figure 3, which shows no-mask performance as a broken line. Healthy controls performed at ceiling levels across masking intervals at set size 1, showed only a hint of masking effects at the shortest interval tested at set size 2, and showed substantial masking effects at the shortest SOAs at set size 3. This is the same pattern that was observed previously in healthy young adults by Vogel et al. (2006). In contrast, patients showed a hint of masking at the shortest intervals at set size 1, clear masking at the three shortest intervals at set size 2, and marked masking effects through the 400-ms delay at set size 3. Thus, the patients exhibited masking at smaller set sizes and at longer masking intervals than did the control subjects.
Figure 3.
The results of the working memory task. Each group’s no mask performance for each set size is shown as a dotted line. Patients’ performance (empty circles) differed from their no-mask performance at set size 2 and 3 whereas control subjects’ performance (filled circles) differed from their no-mask performance at set size 3 only. Error bars represent standard error of the mean.
In the overall ANOVA model (see Table 2), the three way group × set size × condition (unmasked vs. each level of masked delay) contrast was statistically significant, (F(8, 516) = 2.19, p = .027. Accordingly, post hoc tests were performed examining group × condition interactions at each level of set size.
Table 2.
A′ Analysis of Variance for Group, Set Size, and Masking Condition
| Effect | F | ndf | ddf | p-value |
|---|---|---|---|---|
| ANOVA for no masked condition | ||||
| Group | 3.77 | 1 | 516 | 0.053 |
| Set size | 10.23 | 2 | 516 | 0.000 |
| Group × set size | 2.03 | 2 | 516 | 0.132 |
| ANOVA for no masked versus masked conditions | ||||
| Group × set size × no mask versus masked SOA | 2.19 | 8 | 516 | 0.027 |
| Post hoc ANOVAs by set size: | ||||
| Set size = 1 | ||||
| Group diff in mask effect (avg over SOA) | 0.03 | 1 | 516 | 0.855 |
| SOA difference in mask effect (avg over group) | 1.91 | 3 | 516 | 0.128 |
| Group × SOA | 3.03 | 3 | 516 | 0.029 |
| Set size = 2 | ||||
| Group diff in mask effect (avg over SOA) | 8.88 | 1 | 516 | 0.003 |
| SOA difference in mask effect (avg over group) | 5.37 | 3 | 516 | 0.001 |
| Group × SOA | 0.84 | 3 | 516 | 0.471 |
| Set size = 3 | ||||
| Group diff in mask effect (avg over SOA) | 1.36 | 1 | 516 | 0.244 |
| SOA difference in mask effect (avg over group) | 7.47 | 3 | 516 | 0.000 |
| Group × SOA | 1.76 | 3 | 516 | 0.155 |
At set size 1, the overall masking effect did not differ between groups (p = .86), and there was no significant overall masking effect for either group (minimum p = .23). In addition, the masking difference scores did not differ significantly between groups for any of the delay intervals (minimum p > .37). Thus, both groups were able to consolidate a single item for WM storage within the 100 ms interval prior to the mask.
At set size 2, the overall masking effect was significantly different between groups (p = .003). The overall masking effect was not significant for the control group when analyzed separately (p = .47), suggesting that they were able to fully consolidate two items before the appearance of the 100-ms mask. The overall masking effect was highly significant in the patient group (p < .001), indicating that the average A′ under masked conditions was significantly reduced compared to no-mask performance. Follow-up tests in patients comparing the no-mask condition with each of the masking delay intervals indicated that the masking was significant at each masked interval (maximum p < .01). Further, there was no significant × masking delay interaction (p = .47), indicating that the magnitude of the patient-control difference in masking difference scores was relatively constant across delay intervals. We note that the masking difference scores were significantly greater in patients than in controls for the 100, 200, and 400 ms intervals (maximum p = .03), with a trend at the 800 ms interval (p = .07).
At set size 3, the overall masking effect did not differ between groups (p = .24); the overall masking effect was significant in both controls (p < .001) and patients (p < .001). Moreover, both groups showed statistically significant reductions in A′ (p < .05) at all mask intervals except 800 ms (minimum p = .22) when compared to their own no-mask baseline condition. While the masking difference scores were significantly greater in patients than in controls at 200 ms (p = .04), but not at other masking intervals (p = .97 at 100 ms, p = .45 at 400 ms, p = .58 at 800 ms, as seen in Table 3), this could be a Type I error given that there was no significant group × masking delay interaction (p = .16).
To summarize, no masking effects were present for either group at set size 1; masking effects were present only for the patients at set size 2; and masking effects were present for both groups at set size 3. As in our previous study, there is a suggestion that the masking function had a different shape for patients than for controls at set size 3, with a rapid improvement in performance between 100 and 200 ms in control subjects but not in patients, although this was not statistically significant.
Discussion
The results of this study replicate the findings of Fuller et al. (2005) by providing evidence that patients with schizophrenia require longer than control subjects to fully consolidate items into visual WM. The current results extend the previous findings in several ways. First, the current study investigated the effects of varying set size on consolidation. We found that patients with schizophrenia, similar to controls, were able to fully consolidate a single item into WM with a masking interval as short as 100 ms, the shortest interval tested. At set size 2, controls were able to complete consolidation within this 100-ms masking interval and showed no significant masking at any interval. In patients, however, statistically significant masking effects were evident from the 100-ms interval through the 800-ms interval, and the masking effect was significantly larger in patients than in controls through the 400-ms interval (with a marginally significant difference at the 800-ms interval). Thus, patients may take many times longer than controls to create a durable WM representation of two items.
At set size 3, the pattern of results was quite to similar to those observed by Fuller et al. (2005), although slightly different masking delays were used in the two studies. Across the two studies, both patients and control subjects showed significant masking at the shortest intervals tested at set size 3. In addition, the performance of control subjects in both studies improved between a delay of 100 or 133 ms and a delay of 200 or 250 ms, whereas patient performance in both studies showed little substantial improvement until 367 or 400 ms. As a result, the main difference between groups in the degree of masking was observed at a masking interval of 200 or 250 ms. Thus, the two studies together provide replicable evidence of impaired consolidation at set size 3.
It is important to consider whether consolidation is actually slowed in schizophrenia or whether it is simply ineffective, such that WM representations are never as fully consolidated in patients as in control subjects. The data from this experiment, combined with our earlier study, suggest that both formulations may be correct. Arguing in favor of consolidation slowing is that fact that patient performance improves as a function of increasing masking delay intervals at set size 2 and 3. That is, the impact of the mask on patient performance clearly varies as a function of time, with patients demonstrating more severe and prolonged vulnerability to masks than seen in controls. If patients were simply more prone to the impact of distraction, there would be no reason for this problem to show such clear time dependence. Indeed, our results do suggest that patients are more prone to the impact of distractors, but this vulnerability varies as a function of how much uninterrupted processing time is available to consolidate working memory representations. If consolidation is slowed, why do patients show no reliable masking effects at set size 1? As seen here, and in the prior work of Vogel, vulnerability to masking varies as a function of working memory load. Single items are consolidated very rapidly, perhaps because as new onset objects they attract attention in a nearly automatic fashion, and it would be nearly impossible to demonstrate working memory masking with single items without testing at such short durations that perceptual level backward masking might contaminate performance. Thus, the fact that single items are consolidated normally in patients does not in any way contradict the claim that consolidation is slowed in schizophrenia: consolidation slowing should be expected with displays that include more than one item, the pattern documented here. This evidence of masking cannot be explained by a difference in baseline performance levels across set sizes 1 and 2, because accuracy for no-mask trials was virtually identical for these two set sizes. Thus, the two studies together provide excellent evidence for a slowing of consolidation.
However, slowing may not be the whole story. Masks impaired patient performance, but not control performance, even out to a masking interval of 800 ms at set size 2, suggesting that the patients were unable to fully consolidate the items. Our previous study also found significant masking for set size 3 at a delay interval of 483 ms in patients with no sign of masking in control subjects. However, the data from set size 3 in the present study are less clear, with both groups showing significant masking at 400 ms and no significant masking at 800 ms. The lack of a difference between groups at set size 3 is, of course, a null result and is therefore less interpretable than the significant differences observed at set size 2 in the present study and at set size 3 in our prior study. Thus, the combination of the results across the two studies strongly suggests that the slowing of consolidation in patients is accompanied by a reduced effectiveness of the consolidation process, such that patient performance remains vulnerable to the impact of distractors.
Is it possible that impairments in other processes could yield this pattern of findings? It is known that patients with schizophrenia have reduced working memory capacity, and we have argued that consolidation speed varies as a function of load. In the current experiment, a working memory capacity limitation in patients would be expected to differentially impact masking performance at set size 3, where there were trend level differences in no mask performance. Instead, between group differences in masking are most apparent at set size 2 where the groups performed similarly in the no-mask baseline condition. Alternatively, is it possible to invoke perceptual deficits to explain the pattern of findings? This does not appear to be plausible given results in the perceptual control condition where patients were able to search three item displays to identify a target item in the face of masking intervals that disrupt consolidation. Further, in an earlier experiment using array sizes of six items, we found no differences in patient performance between 100ms and 500ms target array exposures, suggesting that perceptual limitations are unlikely to be implicated (Gold, Wilk, McMahon, Buchanan, & Luck, 2003). Thus it seems unlikely that either working memory capacity or perceptual deficits can account for the pattern of results documented here and in our earlier study.
We cannot directly rule out the possibility that the patient deficits are secondary to the impact of antipsychotic medications, although this seems unlikely. Antipsychotic medications have minimal effects on perceptual level masking and on measures that tax WM capacity (Butler, Harkavy-Friedman, Amador, & Gorman, 1996; Daban et al., 2005; Green et al., 1999; Lee & Park, 2005). Further, deficits in these processes have been documented in asymptomatic first-degree relatives who are not taking antipsychotics (Conklin, Curtis, Katsanis, & Iacono, 2000; Green, Nuecherlein, & Breitmeyer, 1997; Green, Nuechterlein, Breitmeyer, & Mintz, 2006; Myles-Worsley & Park, 2002; Park, Holzman, & Goldman-Rakic, 1995). While relevant, the evidence from these other types of tasks may not generalize to consolidation, and we are unaware of any direct experimental evidence on this issue. There is conflicting evidence on whether antipsychotic medication worsens (McGurk et al., 2005; Reilly, Harris, Keshavan, & Sweeney, 2006) or improves (McGurk et al., 2005) spatial WM. However, these effects depend on whether patients were drug naïve or using conventional drugs at baseline and additional controlled clinical trial evidence is necessary to fully evaluate the role of medication effects. Furthermore, it is unclear if this finding would extend to the consolidation process of object WM.
In summary, patients with schizophrenia demonstrate marked impairment in WM consolidation, with a clear slowing of consolidation accompanied by a likely impairment in the degree of consolidation that can be accomplished. This impairment in consolidation may play a causal role in the wide range of WM deficits that have been documented in schizophrenia. Moreover, the need to rapidly form stable, distraction-resistant visual representations may be of fundamental importance outside the laboratory. Healthy individuals typically make approximately three saccadic eye movements per second as they view natural scenes, and each saccade causes a large sensory transient as the visual input shifts rapidly across the retina. This sensory transient is a highly effective masking stimulus, which overwrites any perceptual representations that have not been consolidated into stable WM representations (Irwin, 1991). A slowing of the consolidation process would necessarily lead to either a slowing in the rate of saccades or a reduced ability to integrate visual information across saccades. Previous studies have shown that schizophrenia patients exhibit a reduced rate of saccades during the viewing of natural images (Kojima et al., 1992; Matsushima et al., 1992, 1998; Ryu, Morita, Shoji, Waseda, & Maeda, 2001), and the present finding of slowed consolidation may be the explanation of this slowed rate of eye movements. Patients may also exhibit poorer integration of information across saccades while viewing scenes, but this has yet to be tested.
Acknowledgments
This study was made possible by Grant MH65034 from the National Institute of Mental Health; by the University of Maryland School of Medicine Maryland Psychiatric Research Center Advanced Center for Intervention and Services Research Grant MH068580 from the National Institute of Mental Health; and by Grant M01RR-16500 from the General Clinical Research Centers Program of the National Center of Research Resources, National Institutes of Health.
Footnotes
For an interesting mathematical modeling analysis supporting the view that slowing of early processing contributes to some aspects of WM impairment see Neufeld, Vollick, Carter, Boksman, and Jette (2002).
We analyzed the false alarm rates and found no significant differences between the groups. In a repeated measures ANOVA on false alarm rates, the main effect of Group, F(1, 36) = 0.15, p = .7, was not significant nor was the Group × Set size, F(2, 67) = 0.54, p = .5; Group × Interval, F(2, 61) = 2.33, p = .11; or Group × Set size × Interval, F(2, 80) = 0.41, p = .6, interactions (Huyn-Feldt corrections).
Contributor Information
Rebecca L. Fuller, Maryland Psychiatric Research Center, University of Maryland School of Medicine
Steven J. Luck, Department of Psychology, University of Iowa, and Department of Psychology and Center for Mind & Brain, University of California
Elsie L. Braun, Department of Psychology, University of Iowa
Benjamin M. Robinson, Maryland Psychiatric Research Center, University of Maryland School of Medicine
Robert P. McMahon, Maryland Psychiatric Research Center, University of Maryland School of Medicine
James M. Gold, Maryland Psychiatric Research Center, University of Maryland School of Medicine
References
- Butler PD, Harkavy-Friedman JM, Amador XF, Gorman JM. Backward masking in schizophrenia: Relationship to medication status, neuropsychological functioning, and dopamine metabolism. Biological Psychiatry. 1996;40(4):295–298. doi: 10.1016/0006-3223(96)00007-8. [DOI] [PubMed] [Google Scholar]
- Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. American Journal of Psychiatry. 1998;155(9):1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- Cary NC. The SAS/STAT User’s Guide. Cary, NC: SAS Institute Inc; 2006. [Google Scholar]
- Cheung V, Chen EY, Chen RY, Woo MF, Yee BK. A comparison between schizophrenia patients and healthy controls on the expression of attentional blink in a rapid serial visual presentation (RSVP) paradigm. Schizophrenia Bulletin. 2002;28(3):443–458. doi: 10.1093/oxfordjournals.schbul.a006952. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Cook S, Matthysse S, Barnard J, Lo Y, Levy DL, et al. Spatial and object working memory impairments in schizophrenia patients: A Bayesian item-response theory analysis. Journal of Abnormal Psychology. 2002;111(3):425–435. doi: 10.1037//0021-843x.111.3.425. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Curtis CE, Katsanis J, Iacono WG. Verbal working memory impairment in schizophrenia patients and their first-degree relatives: Evidence from the digit span task. American Journal of Psychiatry. 2000;157(2):275–277. doi: 10.1176/appi.ajp.157.2.275. [DOI] [PubMed] [Google Scholar]
- Daban C, Amado I, Bourdel MC, Loo H, Olie JP, Poirier MF, et al. Cognitive dysfunctions in medicated and unmedicated patients with recent-onset schizophrenia. Journal of Psychiatric Research. 2005;39(4):391–398. doi: 10.1016/j.jpsychires.2004.09.001. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM–IV Axis I Disorders (SCID-I) Washington, DC: American Psychiatric Press, Inc; 1997a. [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM–IV Personality Disorders (SCID-II) Washington, DC: American Psychiatric Press, Inc; 1997b. [Google Scholar]
- Fleming K, Goldberg T, Binks S, Randolph C, Gold JM, Weinberger DR. Visuospatial working memory in patients with schizophrenia. Biological Psychiatry. 1997;41(1):43–49. doi: 10.1016/s0006-3223(96)00263-6. [DOI] [PubMed] [Google Scholar]
- Fleming K, Goldberg T, Gold J, Weinberger D. Verbal working memory dysfunction in schizophrenia: Use of a Brown-Peterson paradigm. Psychiatry Research. 1995;56:155–161. doi: 10.1016/0165-1781(95)02589-3. [DOI] [PubMed] [Google Scholar]
- Fuller RL, Luck SJ, McMahon RP, Gold JM. Working memory consolidation is abnormally slow in schizophrenia. Journal of Abnormal Psychology. 2005;114(2):279–290. doi: 10.1037/0021-843X.114.2.279. [DOI] [PubMed] [Google Scholar]
- Gold J, Carpenter C, Randolph C, Goldberg T, Weinberger D. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Archives of General Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- Gold JM, Wilk CM, McMahon RP, Buchanan RW, Luck SJ. Working memory for visual features and conjunctions in schizophrenia. Journal of Abnormal Psychology. 2003;112(1):61–71. [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. Journal of Neuropsychiatry and Clinical Neurosciences. 1994;6(4):348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH. Backward masking performance as an indicator of vulnerability to schizophrenia. Acta Psychiatrica Scandinavica Suppl. 1999;395:34–40. doi: 10.1111/j.1600-0447.1999.tb05981.x. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuecherlein KH, Breitmeyer B. Backward masking performance in unaffected siblings of schizophrenic patients: Evidence for a vulnerability indicator. Archives of General Psychiatry. 1997;54(5):465–472. doi: 10.1001/archpsyc.1997.01830170091012. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Backward masking in unmedicated schizophrenic patients in psychotic remission: Possible reflection of aberrant cortical oscillation. American Journal of Psychiatry. 1999;156(9):1367–1373. doi: 10.1176/ajp.156.9.1367. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Forward and backward visual masking in unaffected siblings of schizophrenic patients. Biological Psychiatry. 2006;59(5):446–451. doi: 10.1016/j.biopsych.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Hartman M, Steketee MC, Silva S, Lanning K, McCann H. Working memory and schizophrenia: Evidence for slowed encoding. Schizophrenia Research. 2003;59(2–3):99–113. doi: 10.1016/s0920-9964(01)00366-8. [DOI] [PubMed] [Google Scholar]
- Irwin DE. Information integration across saccadic eye movements. Cognitive Psychology. 1991;23(3):420–456. doi: 10.1016/0010-0285(91)90015-g. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Strous RD, Grochowski S, Ritter W, Cowan N. Impaired precision, but normal retention, of auditory sensory (“echoic”) memory information in schizophrenia. Journal of Abnormal Psychology. 1997;106(2):315–324. doi: 10.1037//0021-843x.106.2.315. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P. Restricted attentional capacity between sensory modalities. Psychonomic Bulletin & Review. 1999;6:87–92. doi: 10.3758/bf03210813. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P, Dell’Acqua R. The demonstration of short-term consolidation. Cognitive Psychology. 1998;36(2):138–202. doi: 10.1006/cogp.1998.0684. [DOI] [PubMed] [Google Scholar]
- Knight RA, Elliott DS, Freedman EG. Short-term visual memory in schizophrenics. Journal of Abnormal Psychology. 1985;94(4):427–442. doi: 10.1037//0021-843x.94.4.427. [DOI] [PubMed] [Google Scholar]
- Kojima T, Matsushima E, Ando K, Ando H, Sakurada M, Ohta K, et al. Exploratory eye movements and neuropsychological tests in schizophrenic patients. Schizophrenia Bulletin. 1992;18(1):85–94. doi: 10.1093/schbul/18.1.85. [DOI] [PubMed] [Google Scholar]
- Lang KY, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: A meta-analysis. Journal of Abnormal Psychology. 2005;114(4):599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Lencz T, Bilder RM, Turkel E, Goldman RS, Robinson D, Kane JM, et al. Impairments in perceptual competency and maintenance on a visual delayed match-to-sample test in first-episode schizophrenia. Archives of General Psychiatry. 2003;60(3):238–243. doi: 10.1001/archpsyc.60.3.238. [DOI] [PubMed] [Google Scholar]
- Li CS, Lin WH, Yang YY, Huang CC, Chen TW, Chen YC. Impairment of temporal attention in patients with schizophrenia. Neuroreport. 2002;13(11):1427–1430. doi: 10.1097/00001756-200208070-00016. [DOI] [PubMed] [Google Scholar]
- Matsushima E, Kojima T, Ohbayashi S, Ando H, Ando K, Shimazono Y. Exploratory eye movements in schizophrenic patients and patients with frontal lobe lesions. European Archives of Psychiatry and Clinical Neuroscience. 1992;241(4):210–214. doi: 10.1007/BF02190255. [DOI] [PubMed] [Google Scholar]
- Matsushima E, Kojima T, Ohta K, Obayashi S, Nakajima K, Kakuma T, et al. Exploratory eye movement dysfunctions in patients with schizophrenia: Possibility as a discriminator for schizophrenia. Journal of Psychiatric Research. 1998;32(5):289–295. doi: 10.1016/S0022-3956(98)00019-3. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Carter C, Goldman R, Green MF, Marder SR, Xie H, et al. The effects of clozapine and risperidone on spatial working memory in schizophrenia. American Journal of Psychiatry. 2005;162(5):1013–1016. doi: 10.1176/appi.ajp.162.5.1013. [DOI] [PubMed] [Google Scholar]
- Myles-Worsley M, Park S. Spatial working memory deficits in schizophrenia patients and their first degree relatives from Palau, Micronesia. American Journal of Medicine Genet. 2002;114(6):609–615. doi: 10.1002/ajmg.10644. [DOI] [PubMed] [Google Scholar]
- Neufeld RW, Vollick D, Carter JR, Boksman K, Jette J. Application of stochastic modeling to the assessment of group and individual differences in cognitive functioning. Psychol Assess. 2002;14(3):279–298. [PubMed] [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Archives of General Psychiatry. 1992;49(12):975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS, Goldman-Rakic PS. Spatial working memory deficits in the relatives of schizophrenic patients. Archives of General Psychiatry. 1995;52(10):821–828. doi: 10.1001/archpsyc.1995.03950220031007. [DOI] [PubMed] [Google Scholar]
- Park S, Hooker C. Increased repetition blindness in schizophrenia patients and first-degree relatives of schizophrenia patients. Schizophrenia Research. 1998;32(1):59–62. doi: 10.1016/s0920-9964(98)00035-8. [DOI] [PubMed] [Google Scholar]
- Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status. New York: The Psychological Corporation, Harcourt Brace & Company; 1998. [Google Scholar]
- Rassovsky Y, Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Modulation of attention during visual masking in schizophrenia. American Journal of Psychiatry. 2005;162(8):1533–1535. doi: 10.1176/appi.ajp.162.8.1533. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Harris MS, Keshavan MS, Sweeney JA. Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Archives of General Psychiatry. 2006;63(11):1189–1197. doi: 10.1001/archpsyc.63.11.1189. [DOI] [PubMed] [Google Scholar]
- Rund BR, Landro NI, Orbeck AL. Stability in backward masking performance in schizophrenics, affectively disturbed patients, and normal subjects. Journal of Nervous and Mental Disease. 1993;181(4):233–237. doi: 10.1097/00005053-199304000-00004. [DOI] [PubMed] [Google Scholar]
- Ryu H, Morita K, Shoji Y, Waseda Y, Maeda H. Abnormal exploratory eye movements in schizophrenic patients vs healthy subjects. Acta Neurologica Scandinavica. 2001;104(6):369–376. doi: 10.1034/j.1600-0404.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- Schechter I, Butler PD, Silipo G, Zemon V, Javitt DC. Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophrenia Research. 2003;64(2–3):91–101. doi: 10.1016/s0920-9964(03)00008-2. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav Res Methods Instrum Comput. 1999;31(1):137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Tek C, Gold J, Blaxton T, Wilk C, McMahon RP, Buchanan RW. Visual perceptual and working memory impairments in schizophrenia. Archives of General Psychiatry. 2002;59(2):146–153. doi: 10.1001/archpsyc.59.2.146. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Woodman GF, Luck SJ. The time course of consolidation in visual working memory. Journal of Experimental Psychology: Human Perception and Performance. 2006;32(6):1436–1451. doi: 10.1037/0096-1523.32.6.1436. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test 3. Wilmington, DE: Wide Range, Inc; 1993. [Google Scholar]
- Wynn JK, Breitmeyer B, Nuechterlein KH, Green MF. Exploring the short term visual store in schizophrenia using the attentional blink. Journal of Psychiatric Research. 2006;40(7):599–605. doi: 10.1016/j.jpsychires.2006.06.002. [DOI] [PubMed] [Google Scholar]