Abstract
Leukemia stem cells (LSCs) are responsible for sustaining and propagating malignant disease, and, as such, are promising targets for therapy. Studies of human LSCs have served an important role in defining the major tenets of the cancer stem cell model, which center on the frequencies of cancer stem cells, their potential hierarchical organization, and their degree of maturation. LSCs in acute myeloid leukemia (AML) have recently been studied using mouse syngeneic models of leukemia induced by MLL oncogenes. These studies have revealed that LSCs are more analogous to progenitor cells and employ embryonic stem cell-like genetic programs for their maintenance, prompting a refinement of the original cancer stem cell model with important implications for design of therapies to selectively target LSCs.
Keywords: acute myeloid leukemia, cancer stem cells, embryonic stem cells, expression, leukemic stem cells, xenotransplantation
INTRODUCTION
Given their prospects as potential targets for efficacious therapy, there is considerable interest in defining and characterizing leukemia stem cells (LSCs), which are responsible for sustaining and propagating malignant disease. Much of our knowledge regarding LSCs in human acute myeloid leukemias (AML) is based on studies employing xenotransplantation techniques for their functional characterization. These studies suggest that leukemic hematopoiesis is a caricature of normal hematopoiesis, wherein the leukemic clone appears to be hierarchically organized with a rare population of LSCs at the apex.1 Estimates of LSC frequencies have ranged from 1:10,000 leukemia cells to less than 1:10,000,000. LSCs may also share other properties with normal hematopoietic stem cells (HSCs) in addition to their rarity, including the expression of similar cell surface antigens (CD34+, CD38−) as well as being predominantly quiescent cells. Thus, studies of human LSCs have served an important role in defining the major, albeit controversial, tenets of the cancer stem cell model, which center on the frequencies of cancer stem cells, their potential hierarchical organization, and their degree of maturation.
AML LEUKEMIA STEM CELLS: LESSONS FROM STUDIES IN A MOUSE MODEL
Given the technical complexities of xenotransplantation techniques for studying human LSCs, we addressed these issues in a mouse model that has been historically useful in elucidating the molecular pathogenesis of AML induced by MLL oncogenes.2 Mutations of MLL are associated with de novo and secondary leukemias of adults and children, representing approximately 6%–10% of acute leukemias (myeloid and lymphoblastic).3 Remarkably, MLL is fused with over 50 different partner proteins as a consequence of chromosomal translocations in acute leukemias, however the MLL-AF9 fusion protein is most commonly encountered in AML and was utilized for the studies described here. The molecular pathogenesis of MLL leukemias, regardless of the fusion protein involved, reflects excessive HOXA gene expression and inappropriate self-renewal of stem/progenitor cells.4
LSCs can be more frequent and mature than predicted from xenotransplantation studies
In earlier studies using a retroviral transduction/transplantation model of MLL leukemia, we demonstrated that AML can arise from transduction of normal HSCs as well as committed progenitors that otherwise lack unlimited self-renewal potential.5 This was the first demonstration that cells without an inherent ability for unlimited self-renewal are capable of giving rise to AML. However, these studies, which identified the potential leukemia initiating cells, did not necessarily define the cell responsible for sustaining and propagating disease, which by definition is the LSC.
The identification and characterization of LSCs, was facilitated by our discovery that they are synonymous with colony forming cells (CFCs) in this model of AML. 6 This provided an alternative method for measuring LSC activity independent of transplantation, thus circumventing many of the limitations associated with the latter technique. Using CFC enumeration as a readout, LSCs were observed to constitute as much as 25% of total leukemia cells in AML induced by the MLL-AF9 oncogene. By comparison, limit dilution analysis, the conventional method for quantifying LSCs, underestimated LSC frequencies by at least 30-fold. These studies indicated that AML stem cells do not necessarily engraft with high efficiency, even in a syngeneic setting. Our studies, which suggest that LSCs engraft with ~3% efficiency (compared with ~30% for HSCs), have profound cautionary implications for previous estimates of LSC frequencies in human AMLs based on xenotransplantation, which would be expected to be even less efficient than syngeneic transplantation.
Unexpectedly, greater than 99.8% of LSCs expressed the myeloid lineage antigen MAC1 (in variable combinations with other myeloid antigens), which provided compelling evidence that they were committed myeloid cells, as opposed to HSCs, based on phenotype. Nevertheless, despite their high frequencies and relative maturation, MLL LSCs are organized into a hierarchy based on morphologic and phenotypic criteria.6
Revision of the cancer stem cell model
Taken together, our observations provide strong support that the cancer stem cell model as originally proposed needs to be refined but not entirely discarded (Figure 1). The observation that LSC frequencies are much higher in a syngeneic model of AML than predicted from xenotransplantation studies is consistent with recent observations in other mouse models of AML and lymphoma,7 some of which have shown transplantation of disease following single cell transfers. In contrast to historic assumptions that cancer stem cells (CSCs) represent corrupted tissue stem cells, LSCs in this model display features of aberrantly self-renewing myeloid precursors, not tissue stem cells. Despite these significant variations from the originally proposed CSC model, AMLs can display a hierarchical organization. However, in the case of MLL leukemias, the cellular hierarchy appears to be separate from the normal progenitor compartment.
Figure 1.
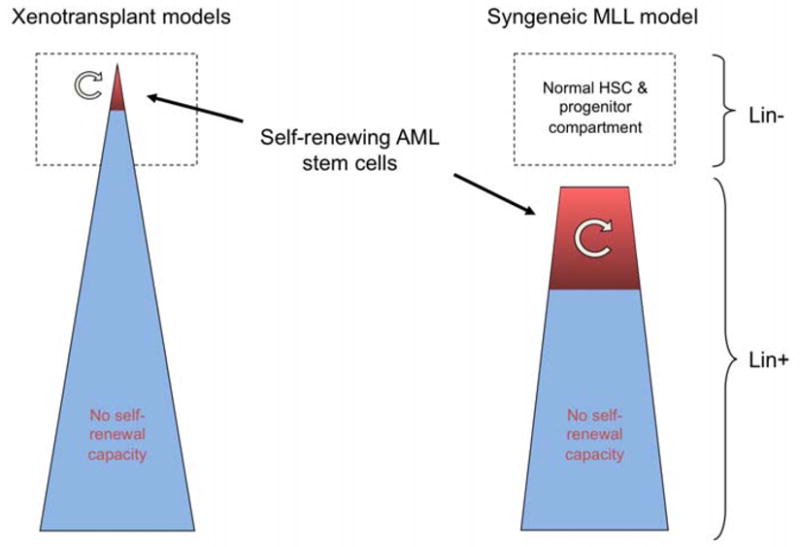
Schematic illustration of the nature and frequency of leukemia stem cells as deduced from xenotransplant versus syngeneic models of AML.
LEUKEMIA STEM CELLS SHARE FEATURES WITH EMBRYONIC STEM CELLS
The foregoing studies demonstrate that LSCs in a mouse model of AML are more frequent and more mature than would be predicted from the original CSC model. Nevertheless, LSCs appear to result at least in part from the corrupted expression of an HSC-specific genetic subprogram. Most notably, the MLL oncoprotein results in the inappropriate expression of HOXA genes, which normally are highly expressed in HSCs and progressively downregulated with myeloid maturation.8 When compared to their likely normal cellular counterparts, ie, myeloblasts and/or granulocyte macrophage progenitors, the levels of HOXA gene expression in MLL LSCs are markedly upregulated to levels similar to those in HSCs. Despite the expression of this HSC-specific subprogram, it is unclear on a broader scale whether an adult stem cell program maintains the downstream precursor-like LSCs in the self-renewing compartment of the leukemia clone.
The LSC maintenance program is more akin to embryonic rather than adult stem cells
To address this issue, gene expression profiling was employed to identify the genes differentially expressed in populations enriched or depleted of self-renewing LSCs.9 This approach was uniquely facilitated by our ability to highly enrich for LSCs based on their expression of the c-KIT antigen. In excess of 5000 differentially expressed genes were identified. The data set of differentially expressed genes was then compared using gene set enrichment analysis 10 with gene sets in cyberspace previously reported to be associated with various biological phenomena, including different stem cell subsets as well as clinical prognostic features. Unexpectedly, gene sets previously shown to be characteristic of embryonic stem cells (ESC), as opposed to adult tissue stem cells, were highly expressed in the MLL LSC-enriched cell fraction.
These ESC-like gene sets were previously identified by module map analyses of gene expression signatures that distinguish embryonic stem cells and adult tissue stem cells,11 as well as studies identifying ESC-associated transcriptional regulators that are highly expressed in poorly differentiated human tumors. 12 Contrary to original suggestions, there does not appear to be a universal “stemness” signature for all classes of stem cells. Rather, these recent studies define adult tissue stem versus embryonic stem cell programs. Of note, the ESC-like signature was reported to be a feature of poorly differentiated human tumors. Our studies extend these observations, by demonstrating that the gene expression program associated with maintenance of LSCs in the self-renewing compartment is also highly similar to an ESC-like program.
The ESC-like LSC maintenance program correlates with poor prognosis in human cancers
Gene set enrichment analysis also demonstrated that genes that predict for poor prognosis in various cancers are enriched in the high LSC data set. Notably, this included genes that predict for poor prognosis in pediatric AML,13 in addition to gene sets associated with poor outcome in breast cancer and hepatocellular carcinoma. These bioinformatic results, which reach the level of strong statistical significance, suggest that there are shared programs between different lineage cancers and different species. Previous studies have indicated a link between the ESC-like signature and prognosis in various human cancers.11,12 Our data critically extend these observations by establishing additional functional and biological interrelationships with the frequency of self-renewing progenitor-like cancer stem cells (Figure 2).
Figure 2.
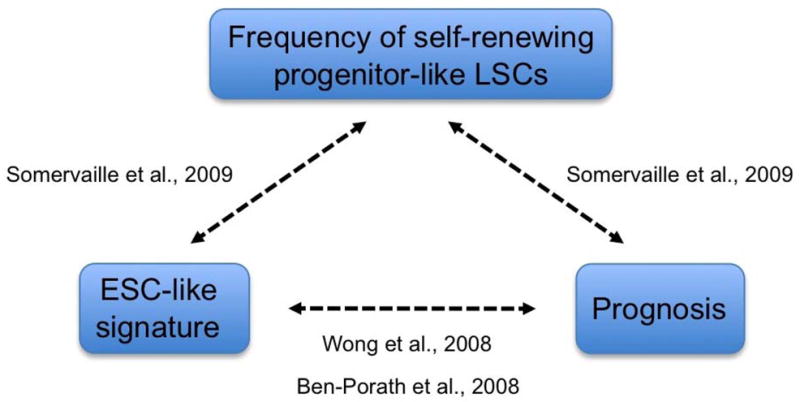
The link between ESC-like transcriptional signature, prognosis, and cancer stem cell frequency.9,11,12
Leukemia stem cells in human versus mouse AML
How can these results in a mouse model of AML be reconciled with the original CSC model as proposed based on xenograft studies of human AML? It is likely that the technical complexity of xenotransplantation may account for some of the differences. Xenograft potential may be selective for a specific subgroup of AMLs. Many (~30%–50%) human AMLs do not engraft NOD/SCID mice, and those that do engraft tend to be associated with adverse prognosis,14 suggesting that xenotransplantation may selectively readout a particularly poor prognosis subset of AMLs. Nevertheless, there are rare reports of t(9;11) AML, the human equivalent of MLL- AF9, in which the CD34− fraction of leukemia, which would correspond to downstream progenitors, engraft NOD/SCID mice.15 Finally, recent studies suggest that human progenitors may be more prevalent LSCs than previously suspected due to technical limitations of the xenotransplantation technique as originally employed to define the CSC model.16 Going forward, the challenge will be to firmly establish which human AML cells serve to sustain and propagate the disease in comparable detail to recent studies of mouse AML.
CONCLUSIONS
Our studies demonstrate that LSCs in mouse models of AML can be more frequent and mature than predicted by original studies of human AML using xenotransplantation techniques. LSCs have features of aberrantly self-renewing committed progenitors or precursors, as opposed to adult tissue stem cells. Their maintenance in the self-renewing compartment of AML employs a global transcriptional program more akin to embryonic rather than adult stem cells. Expression of LSC maintenance program genes is enriched in poor prognosis human malignancies, suggesting that the frequency of aberrantly self-renewing progenitor-like cancer stem cells may be linked to prognosis in human cancer (Figure 2). The fact that LSCs are more analogous toprogenitors and employ ESC-like genetic programs for their maintenance, may allow for selective therapeutic targeting of LSCs that spares the normal stem cell population required for hematopoiesis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 2.Lavau C, Luo RT, Du C, et al. Retrovirus-mediated gene transfer of MLL-ELL transforms primary myeloid progenitors and causes acute myeloid leukemias in mice. Proc Natl Acad Sci U S A. 2000;97:10984–10989. doi: 10.1073/pnas.190167297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimartino JF, Cleary ML. Mll rearrangements in haematological malignancies: lessons from clinical and biological studies. Br J Haematol. 1999;106:614–626. doi: 10.1046/j.1365-2141.1999.01439.x. [DOI] [PubMed] [Google Scholar]
- 4.Hess JL. MLL: a histone methyltransferase disrupted in leukemia. Trends Mol Med. 2004;10:500–507. doi: 10.1016/j.molmed.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Cozzio A, Passegue E, Ayton PM, et al. Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev. 2003;17:3029–3035. doi: 10.1101/gad.1143403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somervaille TC, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006;10:257–268. doi: 10.1016/j.ccr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Kelly PN, Dakic A, Adams JM, et al. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 8.Krivtsov AV, Twomey D, Feng Z, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 9.Somervaille TC, Matheny CJ, Spencer GJ, et al. Hierarchical maintenance of MLL myeloid leukemia stem cells employs a transcriptional program shared with embryonic rather than adult stem cells. Cell Stem Cell. 2009;4:129–140. doi: 10.1016/j.stem.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong DJ, Liu H, Ridky TW, et al. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2:333–344. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Porath I, Thomson MW, Carey VJ, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yagi T, Morimoto A, Eguchi M, et al. Identification of a gene expression signature associated with pediatric AML prognosis. Blood. 2003;102:1849–1856. doi: 10.1182/blood-2003-02-0578. [DOI] [PubMed] [Google Scholar]
- 14.Pearce DJ, Taussig D, Zibara K, et al. AML engraftment in the NOD/SCID assay reflects the outcome of AML: implications for our understanding of the heterogeneity of AML. Blood. 2006;107:1166–1173. doi: 10.1182/blood-2005-06-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blair A, Sutherland HJ. Primitive acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo lack surface expression of c-kit (CD117) Exp Hematol. 2000;28:660–671. doi: 10.1016/s0301-472x(00)00155-7. [DOI] [PubMed] [Google Scholar]
- 16.Taussig DC, Miraki-Moud F, njos-Afonso F, et al. Anti-CD38 antibody-mediated clearance of human repopulating cells masks the heterogeneity of leukemia-initiating cells. Blood. 2008;112:568–575. doi: 10.1182/blood-2007-10-118331. [DOI] [PubMed] [Google Scholar]


