Abstract
Maternal obesity coupled with Western-style high-energy diets represents a special problem that can result in poor fetal development, leading to harmful, persistent effects on offspring, including predisposition to obesity and type 2 diabetes. Mechanisms linking maternal obesity to the increased incidence of obesity and other metabolic diseases in offspring remain poorly defined. Because skeletal muscle is the principal site for glucose and fatty acid utilization and composes 40%–50% of total body mass, changes in the properties of offspring skeletal muscle and its mass resulting from maternal obesity may be responsible for the increase in type 2 diabetes and obesity. Fetal stage is crucial for skeletal muscle development because there is no net increase in the muscle fiber number after birth. Fetal skeletal muscle development involves myogenesis, adipogenesis, and fibrogenesis, which are all derived from mesenchymal stem cells (MSCs). Shifting commitment of MSCs from myogenesis to adipogenesis and fibrogenesis will result in increased intramuscular fat and connective tissue, as well as reduced numbers of muscle fiber and/or diameter, all of which have lasting negative effects on offspring muscle function and properties. Maternal obesity leads to low-grade inflammation, which changes the commitment of MSCs in fetal muscle through several possible mechanisms: 1) inflammation downregulates wingless and int (WNT) signaling, which attenuates myogenesis; 2) inflammation inhibits AMP-activated protein kinase, which promotes adipogenesis; and 3) inflammation may induce epigenetic modification through polycomb group proteins. More studies are needed to further explore the underlying mechanisms associated with maternal obesity, inflammation, and the commitment of MSCs.
Keywords: adipogenesis, fetus, inflammation, maternal obesity, mesenchymal stem cells, myogenesis, skeletal muscle
Maternal obesity leads to low grade inflammation and changes fetal muscle development through down-regulation of WNT signaling, inhibition of AMP-activated protein kinase, and epigenetic modifications.
INTRODUCTION
Maternal Obesity and Offspring Health
Obesity is a growing, serious problem in developed and certain developing countries. According to the latest National Health and Nutrition Examination survey (1999–2002), 26% of nonpregnant women ages 20–39 yr are overweight, and 29% are obese [1]. More importantly, there is a shift toward higher gestational weight gain [2], which indicates excessive nutrient intake during gestation in affluent countries. In addition to maternal obesity (MO), an alarming trend in childhood obesity is also recorded. Epidemiological studies clearly establish a strong association between MO and obesity in offspring. Maternal obesity might adversely affect fetal development, producing lasting effects on offspring, including predisposition to obesity and diabetes [3–6]. Obesity and insulin resistance are closely linked. A growing body of evidence demonstrates that obesity and insulin resistance have a fetal origin in many patients. Insulin resistance indicated by slower glucose removal rates and higher insulin levels is observed in offspring of parents with type 2 diabetes [7–9].
Skeletal muscle and liver are the two key insulin-responsive organs [10]. Skeletal muscle composes 40%–50% of body mass, making it the most important tissue for glucose and fatty acid utilization. The fetal stage is crucial for skeletal muscle development because there is no increase in muscle fiber numbers after birth. Poor fetal skeletal muscle development impairs glucose and fatty acid metabolism by skeletal muscle in response to insulin stimulation, and thus predisposes offspring to diabetes and obesity later in life [11, 12]. Human infants who are small at birth are at greater risk for type 2 diabetes and obesity [13–15]; decreased muscle mass is a major factor in low birth weight [13, 16]. On the other hand, mice with enhanced fetal skeletal muscle growth due to a muscle-specific myostatin knockout have resistance to diabetes and obesity induced by high-glucose and high-fat diets [17, 18]. Skeletal muscle mass and oxidative capacity are positively related to the resting energy expenditure, and low resting energy expenditure is associated with increased incidence of obesity and diabetes [19, 20]. Therefore, changes in fetal skeletal muscle development are likely to provide a link between MO and progeny obesity.
In fetal muscle, a large number of mesenchymal stem cells (MSCs) exist. Although the vast majority of MSCs commit to myogenesis, MSCs are also capable of differentiating into other cell types, such as adipocytes or fibroblasts [21, 22]. A shift from myogenesis to adipogenesis or fibrogenesis will replace muscle fibers with adipocytes or fibrous tissues, impairing the physiological functions of skeletal muscle, such as reduction in muscle force [23] and oxidative capacity [24]. In addition, enhanced adipogenesis within muscle leads to skeletal muscle insulin resistance, which plays a key role in the pathogenesis of type 2 diabetes [21].
Low-grade inflammation accompanies obesity [25–27]. Maternal obesity induces fetal inflammation, which changes fetal skeletal muscle development by promoting adipogenesis [28, 29]. Because the effects of maternal obesity and overnutrition on inflammation and overall fetal developmental programming have been reviewed previously [30–32], this discussion will be limited to the impact on fetal skeletal muscle development of MO-induced fetal inflammatory response.
Fetal Skeletal Muscle Development
Skeletal muscle cells are derived from MSCs, a process controlled by a well-coordinated set of transcription factors, which include wingless and int (WNT), paired box gene 3 (PAX3) and PAX7, and myogenic regulatory factors (MRFs) [33, 34]. WNT signaling is crucial for mesoderm formation. Within the surrounding tissues, a portion of MSCs to become myogenic progenitor cells express PAX3 and PAX7, which then induce the expression of MRFs [35, 36]. Myogenic precursor cells further differentiate into myoblasts and then myotubes under the control of MRFs, which include MYOD, MYF5, MYOG (myogenin), and MYF6 (also known as MRF4) [37]. Skeletal muscle development can be roughly separated into three stages: embryonic, fetal, and postnatal. These stages correspond to primary, secondary, and postnatal myogenesis, respectively [38]. The secondary myogenesis during the fetal stage forms most muscle fibers [38, 39]. Because of the large number of muscle fibers needed to be formed, secondary myogenesis is susceptible to stresses, such as maternal undernutrition, which reduces fetal muscle fiber numbers [24, 40]. Skeletal muscle development has lower priority in nutrient partitioning than does the development of the neural system, internal organs, and bone, making it susceptible to nutrient fluctuation [24].
Formation of secondary myofibers and adipogenesis begins in mid gestation in humans and sheep and in late gestation in rodents [41–43]. There are a large number of MSCs in fetal muscle that can differentiate into adipogenic cells starting at mid gestation. Adipose tissue growth later in life is due to both hypertrophy and hyperplasia [41]. However, new adipocytes generated later in life are mostly located in visceral, retroperitoneal, and subcutaneous fat depots, with few located in intramuscular fat [44]. Thus, adipogenesis occurring inside muscle during the fetal stage has a dominant effect on the number of adipocytes existing inside the muscle, an event associated with skeletal muscle insulin resistance [21]. Enhanced adipogenesis in fetal muscle produces a large number of adipocytes in skeletal muscle, which predisposes the offspring muscle to accumulate intramuscular fat because of the hypertrophy of existing adipocytes [6]. Mechanisms controlling adipogenesis in fetal muscle in vivo are poorly defined, although there are numerous in vitro cell culture studies [45]. These studies identify several transcription factors regulating adipogenesis, which include CCAAT/enhancer-binding protein (CEBPA and CEBPB), peroxisome proliferator-activated receptor (PPARG), and sterol regulatory element-binding transcription factor 1 (SREBF1, also known as SREBP-1c) [46]. CEBPB is the first factor induced by adipogenic stimuli and is followed by an increase in PPARG and CEBPA expression. PPARG and CEBPA are essential transcription factors in adipogenesis that activate many downstream target genes specific to adipocytes [47–49].
Fetal stage is also associated with fibrogenesis. Fibroblasts developed during this stage synthesize connective tissue that forms perimysium and epimysium in fetal skeletal muscle during late gestation. Limited studies have also indicated that maternal nutrition affects the connective tissue content in skeletal muscle. In pigs, runts are smaller than their littermates and experienced maternal nutrient restriction during the fetal stage. When compared to their counterparts, grown runts have a higher concentration of collagen in their skeletal muscle [50]. Additional studies on the association between maternal nutrition, fibrogenesis, and collagen accumulation in offspring muscle are apparently needed.
OBESITY, INFLAMMATION, AND FETAL MUSCLE DEVELOPMENT
Inflammatory Signaling in Fetal Muscle of MO Mothers
Inflammation has received extensive attention recently because of its association with several diseases, including cancer, diabetes, and obesity. Obesity induces chronic low-grade inflammation that may be the primary cause of diseases associated with obesity [51]. Generally, inflammation is classified as acute or chronic [52]. Cellular and molecular mechanisms in acute inflammatory response are well studied. Events involved in chronic inflammation and their physiological consequences are beginning to be appreciated [53]. Interleukin 6 (IL6) and tumor necrosis factor α (TNF) are among the most studied inflammatory mediators associated with increased body fat [54–56].
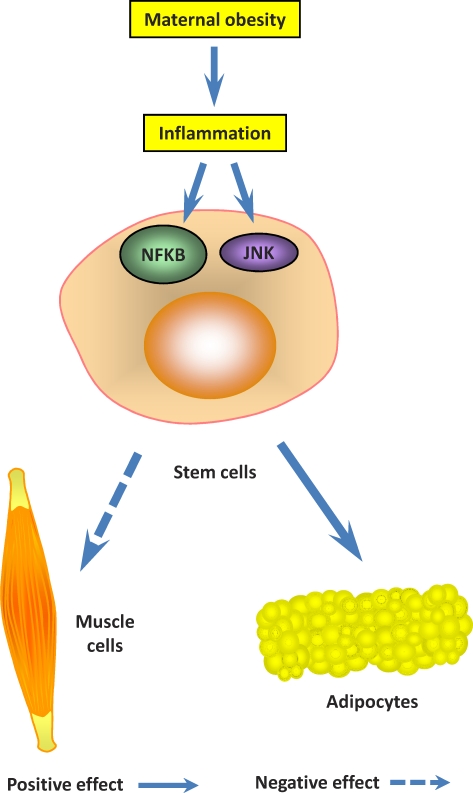
Inflammatory signaling is primarily mediated by the nuclear factor-κB (NFKB) pathway (Fig. 1) [57]. Conserved helix-loop-helix ubiquitous kinase (CHUK, also known as IKKα) and IκB kinase β (IKBKB, also known as IKKβ) phosphorylate IκB, which results in its ubiquitination, and then degradation. This process releases NFKB from IκB and allows translocation of NFKB to the nucleus, where it activates the transcription of specific genes [58]. There are several NFKB target genes, such as IL6, TNF, and chemokine (C-C motif) ligand 2 (CCL2, also known as monocyte chemotactic protein-1 [MCP-1]), and their expression enhances inflammation [51].
FIG. 1.
Inflammation and fetal skeletal muscle development. Inflammation inhibits stem cell differentiation into myocytes but promotes differentiation into adipocytes.
The c-Jun N-terminal kinase (JNK) is another mediator of inflammation (Fig. 1) [59, 60]. Obesity activates the JNK signaling pathway [60]. JNK signaling activates JUN, which induces expression of inflammatory-related genes [61, 62].
Toll-like receptors (TLRs) function as pattern-recognition receptors in mammals and play an important role in the recognition of microbial components [63]. More than 10 members have been discovered in the TLR family [64]. Among these TLRs, TLR4 functions as a receptor of lipopolysaccharide (LPS) in Gram-negative bacterial cell walls [65]. When LPS binds to TLR4, the adaptor protein myeloid differentiation factor-88 (MYD88) is attracted to the TLR4 receptor. This leads to the autophosphorylation of IL1R-associated kinases (IRAKs). The phosphorylated IRAKs then bind to TNF-associated factor 6 (TRAF6), causing the activation of the NFKB [65] and JNK signaling pathways [66, 67]. Recent evidence indicates that fatty acids activate TLR4 signaling [68–70] and associate dietary fatty acids with inflammation.
Inflammatory Signaling, Myogenesis, and Adipogenesis
Inflammation changes fetal skeletal muscle development by downregulating myogenesis. Inactivation of NFKB restores myogenesis, which suggests a negative role for NFKB in myogenesis [71]. Ablation of NFKB is associated with induction of myogenic genes [72]. Mutant mice lacking RELA, a member of the NFKB family, exhibit enhanced myogenesis [73]. Furthermore, TNF, which activates NFKB signaling, inhibits myogenesis [74].
Inflammation promotes adipogenesis [28, 29]. NFKB is upregulated during fat cell differentiation [75]. Loss-of-function mutation in TLR4, a receptor known to induce the NFKB signaling pathway, prevents diet-induced obesity [60]. However, contradictory reports also exist in which NFKB signaling inhibited the expression of adipocyte-specific genes [76] through reducing PPARG expression in 3T3-L1 cells [77] and MSCs [78, 79]. The possible reason for such controversy might be due to the inflammatory drug doses used in cell culture studies. Acute inflammatory response is known to inhibit cell growth and induce apoptosis, not only to adipocytes but cells in general, which is quite different from obesity-induced inflammation, which is low grade and chronic. Indeed, in vivo studies support the role of NFKB in promoting adipogenesis [75, 79–81].
The possible role of inflammation in MSC differentiation was further evidenced by JNK signaling. JUN is activated by JNK [82]. c-Jun dimerizes with protein JDP2, which inhibits the transcriptional activity of JUN (also known as activator protein 1 [AP-1]), and thus myogenesis [83]. Less studied is the function of JNK in adipogenesis. The absence of JNK is reported to decrease adipogenesis [84]. JNK scaffold protein JNK-interacting protein 1, which binds to JNK signaling molecules, plays a critical role in JNK activation in adipocytes of obese mice [85]. In summary, accumulating data indicate that chronic inflammation downregulates myogenesis and enhances adipogenesis in fetal skeletal muscle [29].
INFLAMMATION, WNT SIGNALING, AND FETAL SKELETAL MUSCLE DEVELOPMENT
Introduction of WNT Signaling
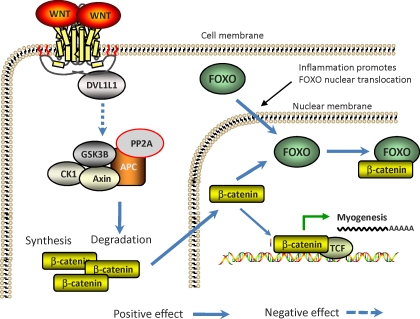
The canonical WNT/β-catenin signaling pathway is well studied [86]. Binding of WNT to the Frizzled proteins activates Disheveled family proteins, which inhibit a destruction complex consisting of axin, glycogen synthase kinase GSK3B, and anaphase-promoting complex (APC), which degrades β-catenin [87]. As a result of inhibition, a pool of cytoplasmic β-catenin stabilizes, enters the nucleus, and interacts with members of the T-cell factor/Lymphoid enhancer factor (TCF/LEF) family of transcription factors to activate the transcription of specific target genes (Fig. 2) [88, 89].
FIG. 2.
WNT/β-catenin signaling, inflammation, and myogenesis. Wnt signaling enhances β-catenin nuclear translocation, but inflammation promotes the formation of β-catenin/FOXO complexes, which divert β-catenin from forming a complex with TCF to induce myogenesis. DVL1L1, disheveled, dsh homolog 1; GSK-3B, glycogen synthase kinase 3; PP2A, protein phosphatase 2A.
WNT Signaling and Myogenesis
WNT signaling is required for early embryonic myogenesis [90]. Activation of the WNT signaling pathway leads to the transformation of nonmyogenic cells into the myogenic lineage [91, 92]. Myogenesis in the mesoderm and somites is inhibited by the WNT antagonist [93]. β-Catenin is a primary mediator of the canonical WNT/β-catenin signaling pathway [94, 95]. Activation of WNT/β-catenin signaling pathway leads to the stabilization of β-catenin, which enters the nucleus to activate target genes, including MYOD and MYF5 [29, 89]. Blocking the β-catenin pathway reduces the total number of myocytes [96, 97]. Overexpression of β-catenin leads to increased myoblast proliferation and enhanced muscle repair following ischemia-induced muscle damage [92, 98]. β-Catenin is necessary for the growth response to mechanical overload in skeletal muscle [99]. β-Catenin regulates the expression of transcription factors PAX3 and GLI1 [100, 101]. PAX3 is essential for skeletal myogenesis and acts upstream of MYOD during skeletal muscle development, whereas GLI1 mediates MYF5 expression [102, 103]. In summary, β-catenin is sufficient to induce skeletal muscle development, which suggests that WNT signaling acts through the canonical pathway to promote myogenesis (Fig. 2) [104].
WNT Signaling and Adipogenesis
Adipocytes arise from MSCs during mid to late gestation [41, 45]. Many proadipogenic and antiadipogenic transcription factors function in a coordinated and sequential manner to control various steps in adipogenesis [105, 106]. Activation of WNT/β-catenin signaling suppresses MSC commitment to the adipogenic lineage and terminal differentiation [105]. The canonical WNT/β-catenin pathway suppresses both white and brown adipogenesis by blocking induction of PPARG and CEBPA. This pathway also blocks the thermogenic program through suppression of PPARG coactivator 1-α (PPARGC1A, also known as PGC1α). Several studies indicate that WNT10B activates antiadipogenic WNT signaling. The WNT10B gene is highly expressed in preadipocytes and declines rapidly during differentiation [107, 108]. Ectopic expression of WNT10B in 3T3-L1 preadipocytes stabilizes free cytosolic β-catenin and blocks adipogenesis. WNT10B antiserum added to 3T3-L1 media promotes adipocyte differentiation [105, 109]. Transgenic mice overexpressing Wnt10b showed a 50% decline in total body fat and were resistant to the high-fat diet-induced accumulation of white fat [110]. On the contrary, Wnt10b deficiency displayed increased adipogenic gene expression and contributed to increased lipogenic potential of myoblasts and excessive lipid accumulation in myofibers [111]. Activation of the WNT signaling pathway enhanced myogenesis and inhibited adipogenesis in cultured MSCs [112].
Inflammation, β-Catenin, and MSC Differentiation
Oxidative stress and inflammatory responses are inseparable [113], and both are associated with obesity [114]. Inflammatory responses attract monocytes that secrete reactive oxygen species and induce oxidative stress [115]. On the other hand, oxidative stress leads to inflammatory response [116–118]. In response to inflammation, β-catenin serves as a cofactor of forkhead transcription factors (FOXOs) [119]. β-Catenin binds directly to FOXO and enhances FOXO transcriptional activity in mammalian cells [120]. In OB6 cells, inflammation and oxidative stress cause a diversion of the limited pool of β-catenin from TCF-mediated transcription to FOXO-mediated transcription (Fig. 2) [121]. FOXO competes with TCF for interaction with β-catenin, thereby inhibiting TCF transcriptional activity and the expression of its targeted genes, like MYOD. Reduced binding between TCF and β-catenin is observed after FOXO overexpression and cellular oxidative stress [122]. Oxidative stress and inflammation decrease the amount of nuclear β-catenin and TCF/LEF-dependent transcription [123]. In an obese sheep model, an inflammatory response was observed in fetal skeletal muscle, which enhanced the formation of FOXO/β-catenin complex, downregulating myogenesis and upregulating adipogenesis [29].
INFLAMMATION, AMP-ACTIVATED PROTEIN KINASE, AND MSC DIFFERENTIATION
PRKA Introduction
PRKA (also known as AMPK) is a serine-threonine kinase consisting of a catalytic subunit (α) and two regulatory subunits (β and γ). PRKA serves as the energy status guardian within cells. PRKA is activated after ATP depletion or, more accurately, a rise in the AMP:ATP ratio within the cell, and responds by adjusting the rates of ATP-consuming (anabolic) and ATP-generating (catabolic) pathways in an attempt to restore and maintain cellular energy levels [124]. Activated PRKA enhances fatty acid oxidation and inhibits de novo synthesis of fatty acids [125]. PRKA activation is associated with phosphorylation of the PRKAA subunit at Thr172 by LKB1 and calcium/calmodulin-dependent protein kinase kinases (CAMKKs) [126–130]. Protein phosphatase 2C (PP2C) dephosphorylates the Thr172 phosphorylation of PRKAA subunit, inactivating PRKA [131].
PRKA Activation Enhances Myogenesis but Inhibits Adipogenesis
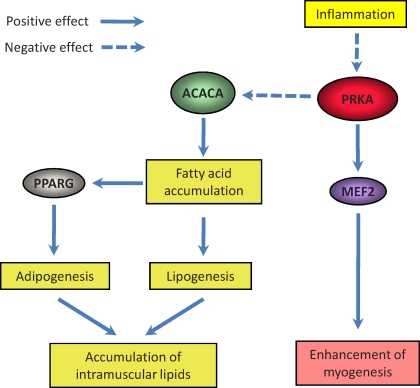
Existing data suggest that PRKA mediates myogenesis. Activation of PRKA by AICAR increases the expression of myogenic enhancer factor 2 (MEF2), which enhances myogenesis [132]. In our previous studies in cattle, PRKA activity was positively associated with muscularity and negatively associated with the content of intramuscular adipocytes [133, 134], indicating that PRKA switches MSCs in skeletal muscle from adipogenesis to myogenesis (Fig. 3).
FIG. 3.
PRKA myogenesis and adipogenesis. PRKA inhibition by inflammation downregulates myogenesis but enhances adipogenesis.
Studies also point to the important role of PRKA in regulating adipogenesis. Activation of PRKA inhibits the expression of PPARG and CEBPs in 3T3-L1 cells and also in obese mice [48, 135]. Genistein inhibits adipocyte differentiation through activation of PRKA [136]. Overnutrition in pregnant ewes inhibited PRKA activity in fetal skeletal muscle and enhanced expression of PPARG, a marker of adipogenesis. In addition, activation of PRKA by 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside (ATIC, also known as AICAR), a specific activator of PRKA, inhibited adipogenesis in cultured 3T3-L1 cells [6, 137]. A plausible explanation for the inhibition of adipogenesis by PRKA is through regulation of PPARG activity. PRKA phosphorylates acetyl-coenzyme A-carboxylase (ACACA, also known as ACC) at Ser79, which inhibits the activity of ACACA and reduces malonyl-coenzyme A (malonyl-CoA) formation [138]. Accumulation of malonyl-CoA decreases fatty acid oxidation and increases lipogenesis [139], resulting in intracellular fatty acid accumulation. To be known ligands of PPARG, accumulated fatty acids promote adipogenesis (Fig. 3).
Tumor necrosis factor α reduces PRKA activity in skeletal muscle [140]. Increase in TNF production is a hallmark of the inflammatory response. Tumor necrosis factor α regulates PRKA activity by upregulation of PP2C, leading to PRKA dephosphorylation [140]. In MO sheep fetuses, circulating TNF was dramatically increased, which provides a primary reason for PRKA inhibition in the muscle of these fetuses [6]. Chronic oxidative stress in combination with low-grade inflammation associated with obesity leads to PRKA inhibition [6]. Ketone bodies that are enhanced under obesity and diabetic conditions inhibit the PRKA signaling pathway [141]. PRKA activity was also inhibited in obese rats [142]. In summary, an increasing body of evidence supports the notion that obesity inhibits PRKA, which provides another mechanism for the downregulation of myogenesis and enhancement of adipogenesis in fetal skeletal muscle due to MO (Fig. 3).
INFLAMMATION AND EPIGENETIC MODIFICATIONS
Because myogenesis, adipogenesis, and fibrogenesis from MSCs are controlled by the expression of one or more crucial genes, maternal nutrition might change fetal muscle development through epigenetic modifications. Depending on the nature of modifications, epigenetic modifications have different degrees of plasticity. Histone modification only passes through several cell generations [143], but histone modifications can guide DNA methylation, leading to stable alterations in gene expression [144, 145].
Polycomb group proteins (PcGs) and trithorax (trxG) group proteins regulate histone methylation, which leads to additional epigenetic modifications during cell differentiation [145]. Polycomb group proteins and trxGs regulate the methylation of histone H3 by binding to PcG and trxG response elements in the genome. Polycomb group proteins possess H3K27-specific trimethylase activity, which mediates gene expression repression, whereas trxG complexes have H3K4 trimethylase activity, which mediates activation of genes [146]. The crucial development is the demonstration that PcG-mediated gene repression leads to DNA methylation of the targeted genes [144]. The PcG protein enhancer of zeste homolog 2 (EZH2) interacts with DNA methyltransferases and serves as a recruitment platform for DNA methyltransferases, which convert plastic histone modifications to stable DNA methylation [144]. DNA methylation leads to the silence of genes by the following mechanisms: 1) recruitment of histone deacetylases, which remove histone acetylation. Deacetylation increases the affinity between histones and DNA and inhibits gene expression because acetylation of the lysine residues at the histone neutralizes its positive charges. 2) DNA methylation can interfere directly with the binding of transcription factors. 3) DNA methylation leads to the formation of inactive chromatin structure.
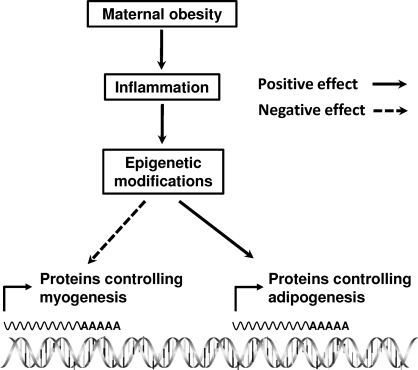
Currently, no studies are available linking maternal nutrition to epigenetic modifications in fetal muscle. However, indirect evidence does support epigenetic modification in key genes controlling fetal development. It is likely that maternal undernutrition permanently changes the insulin/insulinlike growth factor-1 signaling in fetal muscle [147], very likely through epigenetic modifications. Maternal diet alters the expression of PPARs in fetal muscle through DNA methylation [148]. Maternal cocaine administration caused epigenetic modification to a key protein kinase gene in rat heart [149]. Maternal obesity was recently shown to induce epigenetic changes in genes crucial for energy metabolism in primate liver [150]. NFKB p65 might inhibit myogenesis by stimulating expression of the PcG protein YY1 [72, 73], resulting in H3K27 trimethylation and inhibition of myogenic gene expression. This recent evidence points to the association between inflammation and epigenetic modification of key genes regulating myogenesis and adipogenesis, providing an additional mechanism for inflammation and altered fetal skeletal muscle development (Fig. 4).
FIG. 4.
Inflammation, epigenetic modification, myogenesis, and adipogenesis. Inflammation may induce epigenetic modifications that alter the expression of genes involved in myogenesis and adipogenesis in mesenchymal stem cells.
OTHER POSSIBLE SIGNALING PATHWAYS LINKING INFLAMMATION AND FETAL SKELETAL MUSCLE DEVELOPMENT
There are other pathways likely involved in the differentiation of MSCs in fetal muscle resulting from MO. One important pathway is the transforming growth signaling pathway. Transforming growth factor β has immunosuppressive effects [151] and is involved in skeletal muscle development [152]. More importantly, TGFB1 contributes to the conversion of MSCs to fibroblasts [153]. In injured skeletal muscle, differentiation of MSCs into fibroblasts is enhanced through autocrine production of TGFB1 [154], enhancing fibrogenesis. However, until now, the role of TGFB1 signaling in fetal skeletal muscle development has not been studied, although a related growth factor, myostatin, has been extensively studied for its role as a negative regulator of fetal skeletal muscle development [155]. In addition, mitogen-activated protein kinase (MAPK) phosphatases (MKPs) are negative regulators of MAPK, which is involved in immune suppression and negatively controls cell proliferation and growth [156]. Although there is no direct evidence linking MKPs to MSC differentiation in fetal skeletal muscle, further studies may establish such a relationship.
CONCLUSIONS AND FUTURE STUDIES
Proper fetal skeletal muscle development is crucial for offspring health. Maternal obesity changes fetal muscle development by shifting MSC differentiation from myogenesis toward adipogenesis. This shift is expected to have permanent effects on offspring skeletal muscle properties. Existing evidence points to the important role of inflammation in changes to fetal skeletal muscle development. Chronic inflammation associated with MO may alter fetal skeletal muscle development through three major mechanisms, which include: 1) downregulation of WNT signaling, 2) inhibition of PRKA activity, and 3) induction of epigenetic modifications. Additional pathways need to be further explored. Future studies should focus on mechanisms leading to fetal skeletal muscle inflammation due to MO and develop strategies to prevent such inflammation. Possible epigenetic modification of key genes regulating myogenesis and adipogenesis due to inflammation induced by MO is an exciting field to explore. It is possible that both WNT/β-catenin signaling and PRKA regulate MSC differentiation partially by inducing epigenetic modifications of these key genes, which awaits further exploration.
Footnotes
Supported by National Institutes of Health grant R03HD060076-01 and U.S. Department of Agriculture Research Initiative Grants 2008-35206-18826 and 2007-35203-18065.
REFERENCES
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM.Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 2004; 291: 2847–2850. [DOI] [PubMed] [Google Scholar]
- Siega-Riz AM, Siega-Riz AM, Laraia B.The implications of maternal overweight and obesity on the course of pregnancy and birth outcomes. Matern Child Health J 2006; 10: 153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ.Fetal programming of coronary heart disease. Trends Endocrinol Metab 2002; 13: 364–368. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Ward JW, Wooding FP, Forhead AJ, Constancia M.Programming placental nutrient transport capacity. J Physiol 2006; 572: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanielsz PW.Animal models that elucidate basic principles of the developmental origins of adult diseases. ILAR J 2006; 47: 73–82. [DOI] [PubMed] [Google Scholar]
- Zhu MJ, Han B, Tong J, Ma C, Kimzey JM, Underwood KR, Xiao Y, Hess BW, Ford SP, Nathanielsz PW, Du M.AMP-activated protein kinase signalling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J Physiol 2008; 586: 2651–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR.Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med 1990; 113: 909–915. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI.Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 2004; 350: 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Shulman GI.Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med 2005; 2: e233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Shulman GI.Mitochondrial dysfunction and type 2 diabetes. Science 2005; 307: 384–387. [DOI] [PubMed] [Google Scholar]
- Stannard SR, Johnson NA.Insulin resistance and elevated triglyceride in muscle: more important for survival than “thrifty” genes? J Physiol 2004; 554: 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriguez-Gonzalez GL, Guzman C, Larrea F, Nathanielsz PW.Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol 2005; 566: 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JG, Yliharsila H, Forsen T, Osmond C, Barker DJ.Exercise protects against glucose intolerance in individuals with a small body size at birth. Prev Med 2004; 39: 164–167. [DOI] [PubMed] [Google Scholar]
- Forsen T, Eriksson J, Tuomilehto J, Reunanen A, Osmond C, Barker D.The fetal and childhood growth of persons who develop type 2 diabetes. Ann Intern Med 2000; 133: 176–182. [DOI] [PubMed] [Google Scholar]
- Harding JE.Nutrition and growth before birth. Asia Pac J Clin Nutr 2003; 12(suppl):S28 15023631 [Google Scholar]
- Hediger ML, Overpeck MD, Kuczmarski RJ, McGlynn A, Maurer KR, Davis WW.Muscularity and fatness of infants and young children born small- or large-for-gestational-age. Pediatrics 1998; 102: E60 [DOI] [PubMed] [Google Scholar]
- Yang J, Zhao B.Postnatal expression of myostatin propeptide cDNA maintained high muscle growth and normal adipose tissue mass in transgenic mice fed a high-fat diet. Mol Reprod Dev 2006; 73: 462–469. [DOI] [PubMed] [Google Scholar]
- Zhao B, Wall RJ, Yang J.Transgenic expression of myostatin propeptide prevents diet-induced obesity and insulin resistance. Biochem Biophys Res Commun 2005; 337: 248–255. [DOI] [PubMed] [Google Scholar]
- Zurlo F, Larson K, Bogardus C, Ravussin E.Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest 1990; 86: 1423–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illner K, Brinkmann G, Heller M, Bosy-Westphal A, Muller MJ.Metabolically active components of fat free mass and resting energy expenditure in nonobese adults. Am J Physiol Endocrinol Metab 2000; 278: E308–E315. [DOI] [PubMed] [Google Scholar]
- Aguiari P, Leo S, Zavan B, Vindigni V, Rimessi A, Bianchi K, Franzin C, Cortivo R, Rossato M, Vettor R, Abatangelo G, Pozzan T, et al. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci U S A 2008; 105: 1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR.Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol 2004; 166: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayol SA, Macharia R, Farrington SJ, Simbi BH, Stickland NC.Evidence that a maternal “junk food” diet during pregnancy and lactation can reduce muscle force in offspring. Eur J Nutr 2009; 48: 62–65. [DOI] [PubMed] [Google Scholar]
- Zhu MJ, Ford SP, Means WJ, Hess BW, Nathanielsz PW, Du M.Maternal nutrient restriction affects properties of skeletal muscle in offspring. J Physiol 2006; 575: 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg GR.Inflammation in obesity is the common link between defects in fatty acid metabolism and insulin resistance. Cell Cycle 2007; 6: 888–894. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Obin MS.Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 2006; 83: 461S–465S. [DOI] [PubMed] [Google Scholar]
- Wei Y, Chen K, Whaley-Connell AT, Stump CS, Ibdah JA, Sowers JR.Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am J Physiol Regul Integr Comp Physiol 2008; 294: R673–R680. [DOI] [PubMed] [Google Scholar]
- Bayol SA, Simbi BH, Bertrand JA, Stickland NC.Offspring from mothers fed a ‘junk food' diet in pregnancy and lactation exhibit exacerbated adiposity that is more pronounced in females. J Physiol 2008; 586: 3219–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong JF, Yang X, Zhu MJ, Ford SP, Nathanielsz PW, Du M.Maternal obesity downregulates myogenesis and β-catenin signaling in fetal skeletal muscle. Am J Physiol Endocrinol Metab 2009; 296: E917–E924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage JA, Taylor PD, Poston L.Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol 2005; 565: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Ozanne SE.Mechanisms by which poor early growth programs type-2 diabetes, obesity and the metabolic syndrome. Physiol Behav 2006; 88: 234–243. [DOI] [PubMed] [Google Scholar]
- Reusens B, Ozanne SE, Remacle C.Fetal determinants of type 2 diabetes. Curr Drug Targets 2007; 8: 935–941. [DOI] [PubMed] [Google Scholar]
- Fehrer C, Lepperdinger G.Mesenchymal stem cell aging. Exp Gerontol 2005; 40: 926–930. [DOI] [PubMed] [Google Scholar]
- Westerweel PE, Verhaar MC.Directing myogenic mesenchymal stem cell differentiation. Circ Res 2008; 103: 560–561. [DOI] [PubMed] [Google Scholar]
- Maroto M, Reshef R, Munsterberg AE, Koester S, Goulding M, Lassar AB.Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue. Cell 1997; 89: 139–148. [DOI] [PubMed] [Google Scholar]
- Hyatt JP, McCall GE, Kander EM, Zhong H, Roy RR, Huey KA.PAX3/7 expression coincides with myod during chronic skeletal muscle overload. Muscle Nerve 2008; 38: 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchley WR, Fitch WM.A natural classification of the basic helix-loop-helix class of transcription factors. Proc Natl Acad Sci U S A 1997; 94: 5172–5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland NC.A quantitative study of muscle development in the bovine foetus (Bos indicus). Anat Histol Embryol 1978; 7: 193–205. [DOI] [PubMed] [Google Scholar]
- Du M, Zhu MJ. Fetal Programming of Skeletal Muscle Development. Boca Raton, FL:: CRC Press;; 2009. [Google Scholar]
- Zhu MJ, Ford SP, Nathanielsz PW, Du M.Effect of maternal nutrient restriction in sheep on the development of fetal skeletal muscle. Biol Reprod 2004; 71: 1968–1973. [DOI] [PubMed] [Google Scholar]
- Feve B.Adipogenesis: cellular and molecular aspects. Best Pract Res Clin Endocrinol Metab 2005; 19: 483–499. [DOI] [PubMed] [Google Scholar]
- Gnanalingham MG, Mostyn A, Symonds ME, Stephenson T.Ontogeny and nutritional programming of adiposity in sheep: potential role of glucocorticoid action and uncoupling protein-2. Am J Physiol Regul Integr Comp Physiol 2005; 289: R1407–R1415. [DOI] [PubMed] [Google Scholar]
- Muhlhausler BS, Duffield JA, McMillen IC.Increased maternal nutrition stimulates peroxisome proliferator activated receptor-γ, adiponectin, and leptin messenger ribonucleic acid expression in adipose tissue before birth. Endocrinology 2007; 148: 878–885. [DOI] [PubMed] [Google Scholar]
- Tong J, Zhu MJ, Underwood KR, Hess BW, Ford SP, Du M.AMP-activated protein kinase and adipogenesis in sheep fetal skeletal muscle and 3T3-L1 cells. J Anim Sci 2008; 86: 1296–1305. [DOI] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA.Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 2006; 7: 885–896. [DOI] [PubMed] [Google Scholar]
- Kim JB, Spiegelman BM.ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev 1996; 10: 1096–1107. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM.AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A 2007; 104: 12017–12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri S, Rattan R, Haq E, Khan M, Yasmin R, Won JS, Key L, Singh AK, Singh I.AICAR inhibits adipocyte differentiation in 3T3L1 and restores metabolic alterations in diet-induced obesity mice model. Nutr Metab (Lond) 2006; 3: 31–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YC, Jefcoate CR.PPARgamma1 synthesis and adipogenesis in C3H10T1/2 cells depends on S-phase progression, but does not require mitotic clonal expansion. J Cell Biochem 2004; 91: 336–353. [DOI] [PubMed] [Google Scholar]
- Karunaratne JF, Ashton CJ, Stickland NC.Fetal programming of fat and collagen in porcine skeletal muscles. J Anat 2005; 207: 763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Herrero L, Naaz A.Obesity, inflammation, and insulin resistance. Gastroenterology 2007; 132: 2169–2180. [DOI] [PubMed] [Google Scholar]
- Moraes LA, Piqueras L, Bishop-Bailey D.Peroxisome proliferator-activated receptors and inflammation. Pharmacol Ther 2006; 110: 371–385. [DOI] [PubMed] [Google Scholar]
- Medzhitov R.Origin and physiological roles of inflammation. Nature 2008; 454: 428–435. [DOI] [PubMed] [Google Scholar]
- Kubaszek A, Pihlajamaki J, Komarovski V, Lindi V, Lindstrom J, Eriksson J, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Tuomilehto J, Uusitupa M, et al. Promoter polymorphisms of the TNF-alpha (G-308A) and IL-6 (C-174G) genes predict the conversion from impaired glucose tolerance to type 2 diabetes: the Finnish Diabetes Prevention Study. Diabetes 2003; 52: 1872–1876. [DOI] [PubMed] [Google Scholar]
- Yudkin JS.Adipose tissue, insulin action and vascular disease: inflammatory signals. Int J Obes Relat Metab Disord 2003; 27(suppl 3):S25–S28. [DOI] [PubMed] [Google Scholar]
- Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, Kalhan SC, Catalano PM.TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes 2002; 51: 2207–2213. [DOI] [PubMed] [Google Scholar]
- Dhindsa G, Bhatia R, Dhindsa M, Bhatia V.Insulin resistance, insulin sensitization and inflammation in polycystic ovarian syndrome. J Postgrad Med 2004; 50: 140–144. [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB.Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest 2001; 107: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappas M, Permezel M, Rice GE.Mitogen-activated protein kinase proteins regulate LPS-stimulated release of pro-inflammatory cytokines and prostaglandins from human gestational tissues. Placenta 2007; 28: 936–945. [DOI] [PubMed] [Google Scholar]
- Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, Velloso LA, Saad MJ.Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007; 56: 1986–1998. [DOI] [PubMed] [Google Scholar]
- Trouche D, Grigoriev M, Lenormand JL, Robin P, Leibovitch SA, Sassone-Corsi P, Harel-Bellan A.Repression of c-fos promoter by MyoD on muscle cell differentiation. Nature 1993; 363: 79–82. [DOI] [PubMed] [Google Scholar]
- Strle K, Broussard SR, McCusker RH, Shen WH, LeCleir JM, Johnson RW, Freund GG, Dantzer R, Kelley KW.C-jun N-terminal kinase mediates tumor necrosis factor-alpha suppression of differentiation in myoblasts. Endocrinology 2006; 147: 4363–4373. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T.Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2001; 2: 675–680. [DOI] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE.Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 2001; 293: 1673–1677. [DOI] [PubMed] [Google Scholar]
- Reyna SM, Ghosh S, Tantiwong P, Meka CS, Eagan P, Jenkinson CP, Cersosimo E, Defronzo RA, Coletta DK, Sriwijitkamol A, Musi N.Elevated Toll-like receptor 4 expression and signaling in muscle from insulin resistant subjects. Diabetes 2008; 57: 2595–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM.A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 2007; 282: 35279–35292. [DOI] [PubMed] [Google Scholar]
- Frost RA, Nystrom GJ, Lang CH.Lipopolysaccharide stimulates nitric oxide synthase-2 expression in murine skeletal muscle and C(2)C(12) myoblasts via Toll-like receptor-4 and c-Jun NH(2)-terminal kinase pathways. Am J Physiol Cell Physiol 2004; 287: C1605–C1615. [DOI] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS.TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006; 116: 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW.Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 2007; 100: 1589–1596. [DOI] [PubMed] [Google Scholar]
- Schaeffler A, Gross P, Buettner R, Bollheimer C, Buechler C, Neumeier M, Kopp A, Schoelmerich J, Falk W.Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology 2009; 126: 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardite E, Barbera JA, Roca J, Fernandez-Checa JC.Glutathione depletion impairs myogenic differentiation of murine skeletal muscle C2C12 cells through sustained NF-kappaB activation. Am J Pathol 2004; 165: 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Hertlein E, Bakkar N, Sun H, Acharyya S, Wang J, Carathers M, Davuluri R, Guttridge DC.NF-kappaB regulation of YY1 inhibits skeletal myogenesis through transcriptional silencing of myofibrillar genes. Mol Cell Biol 2007; 27: 4374–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkar N, Wang J, Ladner KJ, Wang H, Dahlman JM, Carathers M, Acharyya S, Rudnicki MA, Hollenbach AD, Guttridge DC.IKK/NF-kappaB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J Cell Biol 2008; 180: 787–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen RC, Van Der Velden JL, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM.Tumor necrosis factor-alpha inhibits myogenic differentiation through MyoD protein destabilization. FASEB J 2004; 18: 227–237. [DOI] [PubMed] [Google Scholar]
- Berg AH, Lin Y, Lisanti MP, Scherer PE.Adipocyte differentiation induces dynamic changes in NF-kappaB expression and activity. Am J Physiol Endocrinol Metab 2004; 287: E1178–E1188. [DOI] [PubMed] [Google Scholar]
- Ruan H, Hacohen N, Golub TR, Van Parijs L, Lodish HF.Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes 2002; 51: 1319–1336. [DOI] [PubMed] [Google Scholar]
- Chae GN, Kwak SJ.NF-kappaB is involved in the TNF-alpha induced inhibition of the differentiation of 3T3-L1 cells by reducing PPARgamma expression. Exp Mol Med 2003; 35: 431–437. [DOI] [PubMed] [Google Scholar]
- Li X, Makarov SS.An essential role of NF-kappaB in the “tumor-like” phenotype of arthritic synoviocytes. Proc Natl Acad Sci U S A 2006; 103: 17432–17437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmrich K, Thomas GP, Abberton KM, Thompson EW, Rophael JA, Penington AJ, Morrison WA.Monocyte chemoattractant protein-1 and nitric oxide promote adipogenesis in a model that mimics obesity. Obesity (Silver Spring) 2007; 15: 2951–2957. [DOI] [PubMed] [Google Scholar]
- Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME.Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity (Silver Spring) 2008; 16: 1248–1255. [DOI] [PubMed] [Google Scholar]
- Reyna SM, Ghosh S, Tantiwong P, Meka CS, Eagan P, Jenkinson CP, Cersosimo E, Defronzo RA, Coletta DK, Sriwijitkamol A, Musi N.Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 2008; 57: 2595–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengal E, Ransone L, Scharfmann R, Dwarki VJ, Tapscott SJ, Weintraub H, Verma IM.Functional antagonism between c-Jun and MyoD proteins: a direct physical association. Cell 1992; 68: 507–519. [DOI] [PubMed] [Google Scholar]
- Ostrovsky O, Bengal E, Aronheim A.Induction of terminal differentiation by the c-Jun dimerization protein JDP2 in C2 myoblasts and rhabdomyosarcoma cells. J Biol Chem 2002; 277: 40043–40054. [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS.A central role for JNK in obesity and insulin resistance. Nature 2002; 420: 333–336. [DOI] [PubMed] [Google Scholar]
- Jaeschke A, Czech MP, Davis RJ.An essential role of the JIP1 scaffold protein for JNK activation in adipose tissue. Genes Dev 2004; 18: 1976–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Birchmeier W.New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev 2001; 11: 547–553. [DOI] [PubMed] [Google Scholar]
- Liu X, Rubin JS, Kimmel AR.Rapid, Wnt-induced changes in GSK3beta associations that regulate beta-catenin stabilization are mediated by Galpha proteins. Curr Biol 2005; 15: 1989–1997. [DOI] [PubMed] [Google Scholar]
- Dierick H, Bejsovec A.Cellular mechanisms of wingless/Wnt signal transduction. Curr Top Dev Biol 1999; 43: 153–190. [DOI] [PubMed] [Google Scholar]
- Hecht A, Kemler R.Curbing the nuclear activities of beta-catenin. Control over Wnt target gene expression. EMBO Rep 2000; 1: 24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M.Skeletal muscle formation in vertebrates. Curr Opin Genet Dev 2001; 11: 440–448. [DOI] [PubMed] [Google Scholar]
- Polesskaya A, Seale P, Rudnicki MA.Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell 2003; 113: 841–852. [DOI] [PubMed] [Google Scholar]
- Otto A, Schmidt C, Luke G, Allen S, Valasek P, Muntoni F, Lawrence-Watt D, Patel K.Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J Cell Sci 2008; 121: 2939–2950. [DOI] [PubMed] [Google Scholar]
- Borello U, Coletta M, Tajbakhsh S, Leyns L, De Robertis EM, Buckingham M, Cossu G.Transplacental delivery of the Wnt antagonist Frzb1 inhibits development of caudal paraxial mesoderm and skeletal myogenesis in mouse embryos. Development 1999; 126: 4247–4255. [DOI] [PubMed] [Google Scholar]
- Mermelstein CS, Portilho DM, Mendes FA, Costa ML, Abreu JG.Wnt/beta-catenin pathway activation and myogenic differentiation are induced by cholesterol depletion. Differentiation 2007; 75: 184–192. [DOI] [PubMed] [Google Scholar]
- Armstrong DD, Esser KA.Wnt/beta-catenin signaling activates growth-control genes during overload-induced skeletal muscle hypertrophy. Am J Physiol Cell Physiol 2005; 289: C853–C859. [DOI] [PubMed] [Google Scholar]
- Yamanouchi K, Hosoyama T, Murakami Y, Nishihara M.Myogenic and adipogenic properties of goat skeletal muscle stem cells. J Reprod Dev 2007; 53: 51–58. [DOI] [PubMed] [Google Scholar]
- Pan W, Jia Y, Wang J, Tao D, Gan X, Tsiokas L, Jing N, Wu D, Li L.Beta-catenin regulates myogenesis by relieving I-mfa-mediated suppression of myogenic regulatory factors in P19 cells. Proc Natl Acad Sci U S A 2005; 102: 17378–17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KI, Cho HJ, Hahn JY, Kim TY, Park KW, Koo BK, Shin CS, Kim CH, Oh BH, Lee MM, Park YB, Kim HS.Beta-catenin overexpression augments angiogenesis and skeletal muscle regeneration through dual mechanism of vascular endothelial growth factor-mediated endothelial cell proliferation and progenitor cell mobilization. Arterioscler Thromb Vasc Biol 2006; 26: 91–98. [DOI] [PubMed] [Google Scholar]
- Armstrong DD, Wong VL, Esser KA.Expression of beta-catenin is necessary for physiological growth of adult skeletal muscle. Am J Physiol Cell Physiol 2006; 291: C185–C188. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Tabin C, Johnson RL.Control of dorsoventral somite patterning by Wnt-1 and beta-catenin. Dev Biol 1998; 193: 182–194. [DOI] [PubMed] [Google Scholar]
- Borycki A, Brown AM, Emerson CP., JrShh and Wnt signaling pathways converge to control Gli gene activation in avian somites. Development 2000; 127: 2075–2087. [DOI] [PubMed] [Google Scholar]
- Ridgeway AG, Skerjanc IS.Pax3 is essential for skeletal myogenesis and the expression of Six1 and Eya2. J Biol Chem 2001; 276: 19033–19039. [DOI] [PubMed] [Google Scholar]
- Gustafsson MK, Pan H, Pinney DF, Liu Y, Lewandowski A, Epstein DJ, Emerson CP., JrMyf5 is a direct target of long-range Shh signaling and Gli regulation for muscle specification. Genes Dev 2002; 16: 114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos H, Skerjanc IS.Beta-catenin is essential and sufficient for skeletal myogenesis in P19 cells. J Biol Chem 2002; 277: 15393–15399. [DOI] [PubMed] [Google Scholar]
- Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A.Adipogenesis and WNT signalling. Trends Endocrinol Metab 2009; 20: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM.Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol 2000; 16: 145–171. [DOI] [PubMed] [Google Scholar]
- Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA.Inhibition of adipogenesis by Wnt signaling. Science 2000; 289: 950–953. [DOI] [PubMed] [Google Scholar]
- Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, Harrison SD, MacDougald OA.Regulation of Wnt signaling during adipogenesis. J Biol Chem 2002; 277: 30998–31004. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Bryant HU, Macdougald OA.Regulation of bone mass by Wnt signaling. J Clin Invest 2006; 116: 1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo KA, Wright WS, Kang S, Gerin I, Chiang SH, Lucas PC, Opp MR, MacDougald OA.Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem 2004; 279: 35503–35509. [DOI] [PubMed] [Google Scholar]
- Vertino AM, Taylor-Jones JM, Longo KA, Bearden ED, Lane TF, McGehee RE, Jr, MacDougald OA, Peterson CA.Wnt10b deficiency promotes coexpression of myogenic and adipogenic programs in myoblasts. Mol Biol Cell 2005; 16: 2039–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang YC, Zhang C, Wang SH, Xiong F, Zhao CP, Peng FN, Feng SW, Yu MJ, Li MS, Zhang YN.Activated beta-catenin induces myogenesis and inhibits adipogenesis in BM-derived mesenchymal stromal cells. Cytotherapy 2007; 9: 667–681. [DOI] [PubMed] [Google Scholar]
- Gumieniczek A, Hopkala H, Rolinski J, Bojarska-Junak A.Antioxidative and anti-inflammatory effects of repaglinide in plasma of diabetic animals. Pharmacol Res 2005; 52: 162–166. [DOI] [PubMed] [Google Scholar]
- Grimble RF.Inflammatory response in the elderly. Curr Opin Clin Nutr Metab Care 2003; 6: 21–29. [DOI] [PubMed] [Google Scholar]
- Sell H, Eckel J.Monocyte chemotactic protein-1 and its role in insulin resistance. Curr Opin Lipidol 2007; 18: 258–262. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Kim EK, Kim H, Claycombe KJ.Obesity-associated mouse adipose stem cell secretion of monocyte chemotactic protein-1 (MCP-1). Am J Physiol Endocrinol Metab 2007; 293: E1153–E1158. [DOI] [PubMed] [Google Scholar]
- Chapple IL, Milward MR, Dietrich T.The prevalence of inflammatory periodontitis is negatively associated with serum antioxidant concentrations. J Nutr 2007; 137: 657–664. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Murray AR, Gorelik O, Arepalli S, Castranova V, Young SH, Gao F, Tyurina YY, Oury TD, Kagan VE.Vitamin E deficiency enhances pulmonary inflammatory response and oxidative stress induced by single-walled carbon nanotubes in C57BL/6 mice. Toxicol Appl Pharmacol 2007; 221: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC, Almeida M.Gone with the Wnts: beta-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol Endocrinol 2007; 21: 2605–2614. [DOI] [PubMed] [Google Scholar]
- Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC.Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science 2005; 308: 1181–1184. [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, O'Brien CA, Manolagas SC.Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem 2007; 282: 27298–27305. [DOI] [PubMed] [Google Scholar]
- Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM.Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity. J Biol Chem 2008; 283: 9224–9230. [DOI] [PubMed] [Google Scholar]
- Shin SY, Kim CG, Jho EH, Rho MS, Kim YS, Kim YH, Lee YH.Hydrogen peroxide negatively modulates Wnt signaling through downregulation of beta-catenin. Cancer Lett 2004; 212: 225–231. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL.TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003; 115: 577–590. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Hawley SA, Scott JW.AMP-activated protein kinase–development of the energy sensor concept. J Physiol 2006; 574: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momcilovic M, Hong SP, Carlson M.Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem 2006; 281: 25336–25343. [DOI] [PubMed] [Google Scholar]
- Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, Mann DL, Taffet GE, Baldini A, Khoury DS, Schneider MD.A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci U S A 2006; 103: 17378–17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witcsak CA, Fujii N, Brandauer J, Seifer MM, Nozaki N, Goodyear LJ.CaMKKalpha activates AMPK signaling in mouse skeletal muscle in vivo. FASEB J 2006; 20: A820 [Google Scholar]
- Witczak CA, Fujii N, Hirshman MF, Goodyear LJ.Ca2+/calmodulin-dependent protein kinase kinase-α regulates skeletal muscle glucose uptake independent of AMP-activated protein kinase and Akt activation. Diabetes 2007; 56: 1403–1409. [DOI] [PubMed] [Google Scholar]
- Jensen TE, Rose AJ, Jorgensen SB, Brandt N, Schjerling P, Wojtaszewski JF, Richter EA.Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am J Physiol Endocrinol Metab 2007; 292: E1308–E1317. [DOI] [PubMed] [Google Scholar]
- Marley AE, Sullivan JE, Carling D, Abbott WM, Smith GJ, Taylor IW, Carey F, Beri RK.Biochemical characterization and deletion analysis of recombinant human protein phosphatase 2C alpha. Biochem J 1996; 320(pt 3):801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khalili L, Chibalin AV, Yu M, Sjodin B, Nylen C, Zierath JR, Krook A.MEF2 activation in differentiated primary human skeletal muscle cultures requires coordinated involvement of parallel pathways. Am J Physiol Cell Physiol 2004; 286: C1410–C1416. [DOI] [PubMed] [Google Scholar]
- Underwood KR, Means WJ, Zhu MJ, Ford SP, Hess BW, Du M.AMP-activated protein kinase is negatively associated with intramuscular fat content in longissimus dorsi muscle of beef cattle. Meat Sci 2008; 79: 394–402. [DOI] [PubMed] [Google Scholar]
- Underwood KR, Tong J, Zhu MJ, Shen QW, Means WJ, Ford SP, Paisley SI, Hess BW, Du M.Relationship between kinase phosphorylation, muscle fiber typing, and glycogen accumulation in longissimus muscle of beef cattle with high and low intramuscular fat. J Agric Food Chem 2007; 55: 9698–9703. [DOI] [PubMed] [Google Scholar]
- Habinowski SA, Witters LA.The effects of AICAR on adipocyte differentiation of 3T3-L1 cells. Biochem Biophys Res Commun 2001; 286: 852–856. [DOI] [PubMed] [Google Scholar]
- Hwang JT, Park IJ, Shin JI, Lee YK, Lee SK, Baik HW, Ha J, Park OJ.Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem Biophys Res Commun 2005; 338: 694–699. [DOI] [PubMed] [Google Scholar]
- Dagon Y, Avraham Y, Berry EM.AMPK activation regulates apoptosis, adipogenesis, and lipolysis by eIF2alpha in adipocytes. Biochem Biophys Res Commun 2006; 340: 43–47. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Pan DA.Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans 2002; 30: 1064–1070. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM.Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes 2006; 55: 2277–2285. [DOI] [PubMed] [Google Scholar]
- Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Gorgun CZ, Carling D, Hotamisligil GS, Febbraio MA, et al. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab 2006; 4: 465–474. [DOI] [PubMed] [Google Scholar]
- Pelletier A, Coderre L.Ketone bodies alter dinitrophenol-induced glucose uptake through AMPK inhibition and oxidative stress generation in adult cardiomyocytes. Am J Physiol Endocrinol Metab 2007; 292: E1325–E1332. [DOI] [PubMed] [Google Scholar]
- Sriwijitkamol A, Ivy JL, Christ-Roberts C, DeFronzo RA, Mandarino LJ, Musi N.LKB1-AMPK signaling in muscle from obese insulin-resistant Zucker rats and effects of training. Am J Physiol Endocrinol Metab 2006; 290: E925–E932. [DOI] [PubMed] [Google Scholar]
- Rando OJ, Verstrepen KJ.Timescales of genetic and epigenetic inheritance. Cell 2007; 128: 655–668. [DOI] [PubMed] [Google Scholar]
- Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden JM, Bollen M, Esteller M, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature 2006; 439: 871–874. [DOI] [PubMed] [Google Scholar]
- Reik W.Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 2007; 447: 425–432. [DOI] [PubMed] [Google Scholar]
- Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G.Genome regulation by polycomb and trithorax proteins. Cell 2007; 128: 735–745. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Wang CL, Coleman N, Smith GD.Altered muscle insulin sensitivity in the male offspring of protein-malnourished rats. Am J Physiol 1996; 271: E1128–E1134. [DOI] [PubMed] [Google Scholar]
- Rees WD, McNeil CJ, Maloney CA.The roles of PPARs in the fetal origins of metabolic health and disease. PPAR Res 2008; 2008: 459030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Darwanto A, Linkhart TA, Sowers LC, Zhang L.Maternal cocaine administration causes an epigenetic modification of protein kinase Cepsilon gene expression in fetal rat heart. Mol Pharmacol 2007; 71: 1319–1328. [DOI] [PubMed] [Google Scholar]
- Aagaard-Tillery KM, Grove K, Bishop J, Ke X, Fu Q, McKnight R, Lane RH.Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol 2008; 41: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida K.Activins, myostatin and related TGF-beta family members as novel therapeutic targets for endocrine, metabolic and immune disorders. Curr Drug Targets Immune Endocr Metabol Disord 2004; 4: 157–166. [DOI] [PubMed] [Google Scholar]
- McLennan IS, Koishi K.The transforming growth factor-betas: multifaceted regulators of the development and maintenance of skeletal muscles, motoneurons and Schwann cells. Int J Dev Biol 2002; 46: 559–567. [PubMed] [Google Scholar]
- Gosselin LE, Williams JE, Deering M, Brazeau D, Koury S, Martinez DA.Localization and early time course of TGF-beta 1 mRNA expression in dystrophic muscle. Muscle Nerve 2004; 30: 645–653. [DOI] [PubMed] [Google Scholar]
- Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J.Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol 2004; 164: 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ.Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997; 387: 83–90. [DOI] [PubMed] [Google Scholar]
- Zakkar M, Chaudhury H, Sandvik G, Enesa K, Luong le A, Cuhlmann S, Mason JC, Krams R, Clark AR, Haskard DO, Evans PC.Increased endothelial mitogen-activated protein kinase phosphatase-1 expression suppresses proinflammatory activation at sites that are resistant to atherosclerosis. Circ Res 2008; 103: 726–732. [DOI] [PubMed] [Google Scholar]