Abstract
The prevalence of human obesity and related chronic disorders such as diabetes, cardiovascular diseases, and cancer is rapidly increasing. Human studies have shown a direct relationship between obesity and infertility. The objective of the current work was to examine the effect of diet-induced obesity on male fertility and the effect of obesity on susceptibility to chemical-induced reproductive toxicity. From 5 to 30 wk of age, genetically intact male C57Bl/6J mice were fed a normal diet or one in which 60% of the kilocalories were from lard. Obese mice exhibited significant differences in the mRNA of several genes within the testes in comparison to lean males. Pparg was increased 2.2-fold, whereas Crem, Sh2b1, Dhh, Igf1, and Lepr were decreased 6.7, 1.4, 3.2, 1.6, and 7.2-fold, respectively. The fertility of male mice was compared through mating with control females. Acrylamide (AA)-induced reproductive toxicity was assessed in obese or lean males treated with water or 25 mg AA kg−1 day−1 via gavage for 5 days and then mated to control females. Percent body fat and weight were significantly increased in mice fed a high-fat vs. a normal diet. Obesity resulted in significant reduction in plugs and pregnancies of control females partnered with obese vs. lean males. Serum leptin and insulin levels were each approximately 5-fold higher in obese vs. age-matched lean mice. Sperm from obese males exhibited decreased motility and reduced hyperactivated progression vs. lean mice. Treatment with AA exacerbated male infertility of obese and lean mice; however, this effect was more pronounced in obese mice. Further, females partnered with AA-treated obese mice exhibited a further decrease in the percentage of live fetuses, whereas the percentage of resorptions increased. This work demonstrated that diet-induced obesity in mice caused a significant reduction in male fertility and exacerbated AA-induced reproductive toxicity and germ cell mutagenicity.
Keywords: acrylamide, diet-induced obesity, dominant lethal mutations, hyperinsulinemia, insulin, leptin, male mice infertility
High-fat diet-induced obesity reduces fertility in male mice and increases susceptibility to acrylamide-induced reproductive toxicity.
INTRODUCTION
It is estimated that greater than 1 billion adults around the world are overweight (body mass index (BMI) greater than 25 kg/m2) and at least one-third of this population has a BMI that exceeds 30 kg/m2, classifying them as obese [1]. While genetic predisposition, age, and environmental factors may contribute to a person's tendency to gain weight, it is generally accepted that the two primary causes of obesity are increased intake of energy-rich foods and reduced physical activity. It is well established that the higher the BMI, the greater the risks for humans to develop chronic diseases, including type 2 diabetes, infertility, hypertension, stroke, cardiovascular diseases, and cancer [2–10]. Obese and overweight men exhibit a high incidence of infertility in association with metabolic disturbances and hormonal dysregulation [5, 11–14]. In order to investigate obesity and obesity-related illnesses, in vivo studies in mouse models have recreated this obesity phenotype primarily through genetic manipulation. Obesity was associated with infertility in the ob/ob mouse in association with leptin deficiency [15–17]. Subsequent studies then demonstrated that leptin replacement therapy rescued the sterility of genetically obese ob/ob male mice [17]. Experiments in obese mice possessing lethal yellow mutations (C57BL/6J-Ay/a) demonstrated an association between reduced fertility, leptin resistance, and hyperleptinemia [18]. In contrast, high-fat diet consumption caused obesity in DBA/2J male mice; however, these mice exhibited no impairment in fertility despite enduring insulin resistance and hyperleptinemia [19]. On the other hand, female DBA/2J mice consuming a diet rich in fat developed obesity as well as reduced fertility in association with hyperleptinemia [19].
Using the genetically intact C57BL/6J mouse model of diet-induced obesity, the current study was undertaken to assess the impact of obesity produced by the consumption of a high-fat diet on the fertility of male C57BL/6J mice. In a second facet of this study, we have investigated our hypothesis that diet-induced obesity may influence host susceptibility to environmental chemicals. A strong correlation exists between the etiology of some human diseases and exposure to environmental pollutants [20], and it is conceivable that obese humans or individuals suffering from chronic pathophysiological conditions may exhibit varied responses to environmental pollutants. With obesity at epidemic proportions, we compared the reproductive toxic effects of orally administered acrylamide (AA) in lean and high-fat diet-induced obese mice. AA was selected because of the great potential for human exposure to this chemical. Recent reports showed that AA is present in baked and fried carbohydrate-rich foods, including French fries, potato chips, bread, and cereals. The concern over the potential for human exposure to this chemical through the consumption of AA-containing foodstuffs was compounded by the fact that AA is a known animal carcinogen, germ and somatic cell mutagen, and potent neurotoxicant [21–23]. Earlier studies have shown a relationship among AA exposure, sperm toxicity, and dominant lethality in both mice and rats [23–25]. Furthermore, recent work in this laboratory using Cyp2e1−/− mice demonstrated that AA metabolism to glycidamide via CYP2E1 is a prerequisite for AA-induced somatic and germ cell mutagenicities [21, 22]. In addition, increased expression of CYP2E1 has been reported in association with obesity, type 2 diabetes, and consumption of a high-fat diet [26–28]. It is our hypothesis that high-fat diet-induced obesity renders mice more susceptible to the bioactivation and toxicity of environmental CYP2E1 substrates such as AA. The novelty of the current work is the evaluation of reproductive toxicity, specifically, the assessment of dominant lethal mutations in diet-induced obese male mice exposed to orally administered AA.
MATERIALS AND METHODS
Chemicals
AA (CAS no. 79-06-1; >99.5% pure) was manufactured by Fluka Chemie GmbH and purchased from Sigma-Aldrich Laborchemikalien GmbH (Milwaukee, WI). All other chemicals used were of the highest commercially available purity.
Animals and Treatments
Male C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Starting at 5 wk of age, groups of male mice were fed a diet in which either 60% kcal (high-fat diet) or 10% kcal (normal diet) was attributed to fat (D12492i and D12450Bi obtained from Research Diets, Inc., New Brunswick, NJ). Ad libitum consumption of diets occurred through age 30 wk. Female mice were fed the normal diet throughout the study. All animal care and experimentation were conducted in accordance with the National Research Council publication Guide for Care and Use of Laboratory Animals [29].
Weight and body fat measurements were determined for male mice at age 30 wk just prior to use in the fertility studies. Body weight of female mice was also determined just prior to mating at 8–9 wk of age. Body fat composition of male mice fed a normal or high-fat diet was determined by dual-energy x-ray absorptiometry (DEXA) using the Piximus densitometer (GE Medical Systems, Waukesha, WI). During the analysis, mice were anesthetized using a mixture of 2.5%–3% isoflurane. This mixture enabled the quick recovery of each animal once removed from the densitometer.
Comparison of the Fertility and Reproduction of Mice Fed High-Fat and Normal Diets
Individually housed lean and obese mice at 30 wk of age were cohabited with three virgin female B6C3F1 mice (8–9 wk old) for a period of 5 days, after which males and females were separated. Every day during the cohabitation, females were examined for plugs as evidence of mating. Approximately 13 days after the last day of cohabitation, each female was humanely euthanized with CO2/O2, and uterine contents were carefully examined for the following endpoints: total number of implantation sites, number of live fetuses, resorptions, early and late dead embryos, and dead fetuses [21]. Live fetuses were humanely euthanized after examination. The aforementioned fertility study was repeated three times with different lean and obese male mice. The number of lean mice per study was 16, 22, and 35 (N = 73), and the number of obese mice per study was 16, 21, and 34 (N = 71).
Comparison of the Reproductive Toxicity of AA in Lean and Obese Male Mice
AA dosing solutions were freshly prepared and administered by gavage to groups of obese and lean males age 30 wk at 25mg/kg for 5 consecutive days. Dosing solutions were prepared in water at a dose volume of 5 ml/kg. Matching vehicle control mice were administered water at 5ml/kg by the same route.
Published studies from this laboratory, as well as others, have demonstrated a positive dose-response correlating an increasing percentage of resorptions per litter (an indication of dominant lethality), with escalating doses of AA (0–60 mg kg−1 day−1) administered intraperitoneally or by gavage [21, 23, 30]. The current dosage was chosen based on results from oral disposition studies conducted in our laboratory demonstrating enzyme saturation following daily gavage with AA at doses higher than 25 mg kg−1 day−1. Moreover, sterility in wild-type male mice was observed following a consecutive 5-day treatment of AA at 50 mg/kg [21]. Subsequent studies in wild-type mice were performed in order to determine lower doses of AA that would induce dominant lethal mutations while preserving fertility at normal levels [21] and limiting possible complications of neurotoxicity [30]. Within the current work, age-matched vehicle controls from each group, obese and lean mice, were administered 5 ml kg−1 day−1 of water via gavage for 5 consecutive days. Because neurotoxic effects have been ascribed to AA exposure [31], each male was cohabited with three virgin B6C3F1 females (8–9 wk old) for a period of 5 days beginning 48 h after the final dose of AA or water had been given. Female mice were maintained on normal diet throughout the study. Half-life determination studies have shown that AA is cleared from plasma within 6 h after dosing in mice, long before mating [32]. At the end of the 5-day mating period, females and males were separated and housed separately. Approximately 13 days from the last day of cohabitation, females were humanely euthanized with CO2/O2, and uterine contents were carefully examined as described above. Live fetuses were humanely euthanized after examination. This study was repeated twice, each time with different males. The number of lean male mice per study in the vehicle-treated group was 16 and 22 (N = 38), while the number per study of vehicle-treated obese and AA-treated lean and obese mice were 16 and 21 (N = 37) for each group. Results from both studies were comparatively similar and, therefore, the data were pooled and presented.
Fasted Serum Glucose, Cholesterol, Triglycerides, Insulin, Leptin, and Testosterone Measurements
Under CO2/O2 anesthesia, whole blood sampled from the retro-orbital venous sinus was collected in BD Microtainer Serum Separator Tubes (Becton, Dickinson, and Company, Suwanee, GA), allowed to clot at room temperature, and centrifuged for serum harvest. Biochemical variables (serum glucose, triglycerides, and cholesterol) were measured in all serum samples on an Olympus AU400e Clinical Chemistry Analyzer (Olympus America, Inc., Irvin, TX) using reagents obtained from the instrument manufacturer. Serum insulin and testosterone were determined using a radioimmunoassay kit obtained from Linco Research (St. Charles, Missouri) on an Apex Gamma Counter (ICN Micromedic Systems, Inc., Huntsville, AL). Serum leptin concentrations were determined using a Sector Imager 2400 Multi-Spot multiplex kit from Meso Scale Discovery (MSD; Gaithersburg, MD). Mouse-specific reagents for detection of leptin were acquired in the MSD Multi-Spot multiplex kits, and procedures were obtained from the instrument manufacturer.
Cauda Epididymal Sperm Count Measurements
Male C57BL/6J mice age 30 wk were weighed and anesthetized. The left testis, epididymis, and vas deferens were immediately removed. The testis was weighed following its dissection from the epididymis and fat. Subsequently, the epididymis and vas deferens were dissected away from the fat, and both were weighed independently. In a 12-well plate, the epididymis and vas deferens from each animal were placed in a well containing 1.0 ml of M2 buffer. Using a watch glass and tweezers, any remaining fat and blood vessels were removed from both the epididymis and vas deferens. The epididymis was then cut at the junction between the corpus and cauda epididymis, and the cauda was placed into a well with 1.0 ml of M2 buffer. Several cuts were made in the cauda epididymis with scissors, and sperm was gently pressed. Sperm was also expressed from the vas deferens in a separate well and then removed from the plate. The pressed sperm from the cauda epididymis was then collected in an Eppendorf tube. Using a hemocytometer (15 μl per side), sperm counts were determined as number of sperm per microliter.
Sperm Motility and Progressive Measurements
Quantitative parameters of sperm motility were determined by computer-assisted sperm analysis (CASA). Sperm from the cauda epididymis were incubated in 1.0 ml of M2 medium at room temperature for 30 min and loaded into CASA assay chambers (Hamilton Thorne Research, Beverly, MA). Sperm tracks (1.5 sec, 30 frames) were captured at 60 Hz and analyzed using HTM-IVOS Sperm Analyzer software (version 12.2L; Hamilton Thorne). The parameters during measurements were: minimum contrast, 30; minimum cell size, 4 pixels; straightness threshold, 50.0%; path velocity cutoff, 10.0 μm/s; progressive minimum path velocity, 50.0 μm/s; static head size, 0.13 to 2.43; static head intensity, 0.10 to 1.52; and static elongation, 5 to 80.
To prepare the hyperactivated sperm, sperm were allowed to disperse into 1.0 ml PBS for 5 min at 37°C. After a brief low-speed centrifugation (100 × g for 15 sec at room temperature) to remove debris, the sperm were centrifuged at 500 × g for 8 min at 4°C and resuspended in 2 ml of M16 medium (Sigma), and the aliquots were either processed as below or incubated at 37°C in 5% CO2/95% air for 1.5 h. Hyperactivated sperm were measured using the following sorting parameters: track speed >170 μm/s, amplitude of lateral head displacement >9.0 μm/s, and linearity <30%.
Testicular Gene Expression: RNA Preparation and Quantitative RT-PCR
Total RNA was isolated from total testicular extracts of 30-wk-old untreated obese or lean mice using TRIzol Reagent (Invitrogen, Carlsbad, CA) and purified with the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The total RNA yield and quality was evaluated by measuring its absorbance at A260/A280. The first strand cDNA was synthesized by using RT2 First Strand Kit (SuperArray Bioscience Corporation, Frederick, MD) with 1 μg of total RNA in each reaction. The cDNA was amplified by PCR using RT2 Real-Time SYBR Green PCR master mix (SuperArray) according to the manufacturer's instructions. The expression profile for each gene was determined using the comparative CT method. Normalization was established by subtracting the endogenous control or reference (an average of four genes) from the CT values of each gene in the array to get ΔCT. Calculation of ΔΔCT was the difference in the ΔCT of genes expressed in obese vs. lean mice. The final gene expression levels were reported as fold change = 2−ΔΔCT. Gene expressions were considered significant at values with fold changes ± 1.5 and P ≤ 0.05. All studies were done in triplicate.
Statistical Analysis
Selecting the appropriate unit of analysis was the initial step in the assessment. Because males were mated to more than one female, it was possible for litter data to be “overdispersed,” that is, the variability of litters across males could be substantially greater than the variability of litters within males; in this case the unit of analysis would be the male. On the other hand, if the variability of litters across males is no greater than the variability of litters within males, the litter could be considered the unit of analysis. A test for overdispersion across males was conducted on pregnancy rates and on each litter characteristic, and no significant over-dispersion was detected. Therefore, the litter was considered to be the unit of analysis.
Body weights of adult males and females were approximately normally distributed and were compared between groups using two-sample t-tests. Pregnancy rates were compared between groups using chi-square tests, or Fisher's exact test when frequencies were small. Number of implants per litter and numbers and percentages of live births, resorptions, and dead fetuses per litter were compared between groups using Mann-Whitney tests.
RESULTS
Body Weight and Body Fat
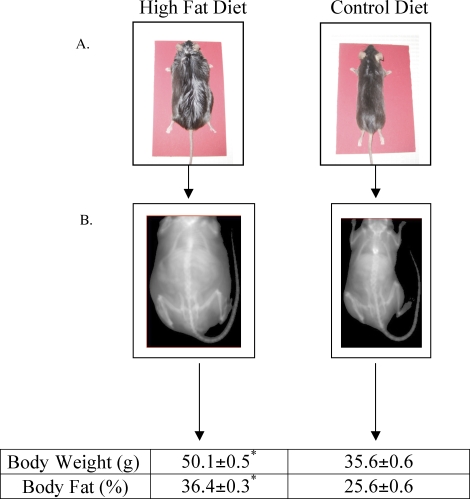
A significant difference in body weight was observed in male C57Bl/6J mice consuming the high-fat diet in comparison to age-matched littermates eating a normal diet. Mice consuming the high-fat diet swelled to weights averaging 50 grams at 30 wk of age, whereas littermates on the normal diet at the same age averaged 36 grams (Fig. 1). Body fat percentages were also significantly higher in high-fat-fed vs. normal-diet-fed 30-wk mice, averaging 36.4% and 25.6%, respectively (Fig. 1). Therefore, mice fed a normal diet were classified as lean and mice fed a high-fat diet were classified as obese throughout the study.
FIG. 1.
Photographs (A) and PIXIMUS (middle) densitometry images (B) of mice at age 30 wk that consumed either the high-fat or control diet since age 5 wk. Average body weight and percent body fat measurements of mice at age 30 wk. *Denotes a statistical difference in mice consuming the high fat vs. control diet at age 30 wk. Data represents the mean ± SEM of 24 male mice per group. Values were considered significantly different at P ≤ 0.05.
Effect of Diet on Copulation and Fertility
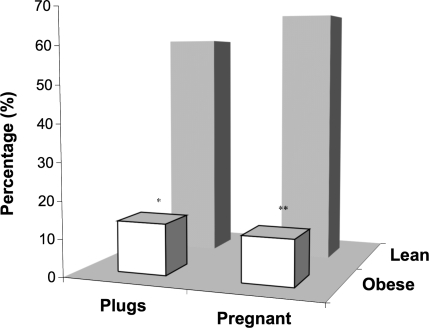
Female B6C3F1 virgin mice that were partnered with obese male mice exhibited significantly reduced numbers of plugs and pregnancies, in contrast to female mice mated with lean males (Fig. 2). The distribution of plugs between females mated with lean and obese mice was 61.9% and 13.7%, respectively. Subsequent pregnancy rates between the two groups mirrored plug data, that is, 68.8% vs. 12.2%, respectively (Fig. 2).
FIG. 2.
Percentage of plugs and pregnancies in female B6C3F1 mice as a result of mating with either obese or lean males. *Denotes statistical difference in the percentage of plugs resulting from mating with lean vs. obese males (P < 0.003). **Denotes statistical difference in the percentage of pregnancies resulting from mating with lean vs. obese males (P < 0.0001). N = 102 females used to determine percentage of plugs resulting from copulation with obese males. N = 105 used to determine percentage plugs resulting from copulation with lean males. N = 215 females used to determine percentage of pregnancies as a result of mating with lean males. N = 213 females used to determine percentage of pregnancies as a result of mating with obese males.
Effect of Diet on Sperm Count, Motility, and Progressive Measurements
Obese and lean male mice exhibited neither significant differences in the average weight of either testes or epididymis nor disparities in morphology or total sperm count collected from the cauda epididiymis (data not shown). However, obese males exhibited a 20% decrease in sperm motility (Fig. 3A) and no significant hyperactivated progression, an indicator of sperm viability and potential for fertilization (Fig. 3B). In contrast, lean mice exhibited a 3-fold increase in sperm hyperactivity at 90 min after incubation (Fig. 3B). Measurement of testosterone produced inconclusive results in both obese and lean mice (data not shown) but seemed to be comparatively similar.
FIG. 3.
A) Mean percentage of sperm motility. *Denotes a statistical difference in sperm motility in obese vs. lean mice. B) Mean percentage of hyperactivated sperm in lean and obese mice at age 30 wk determined at 0 and 60 min. *Denotes a statistical difference in the percentage of hyperactivated sperm from lean mice at 0 vs. 60 min. Data represents the mean ± SEM of four male mice per group. Values were considered significantly different at P ≤ 0.05.
Effect of Diet on Serum Glucose, Cholesterol, Triglycerides, Leptin, Insulin, and Pregnancy
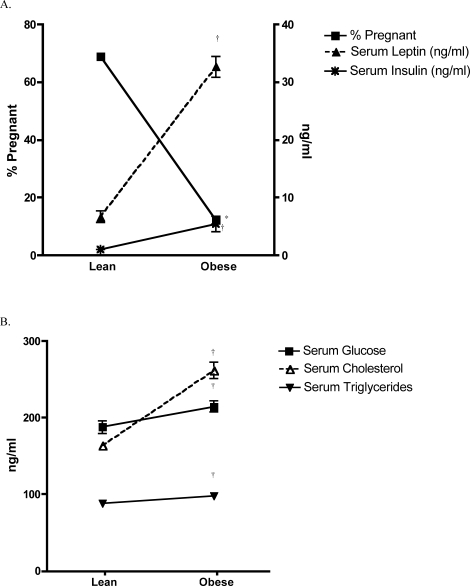
Obese male mice exhibited notably elevated fasting levels of leptin (32.6 ± 1.8 vs. 6.6 ± 1.0 ng/ml) and insulin (5.4 ± 1.3 vs. 1.0 ± 0.3 ng/ml) at age 30 wk when compared to age-matched lean littermates (Fig. 4A). As hyperleptinemia and hyperinsulinemia were evidenced in obese males, pregnancy rates diminished in females that were mated with them, when compared to the same rates when mated with lean males (12.2% vs. 68.8%, respectively; Fig. 4A). The associations are striking and account for an approximate 5-fold increase in both insulin and leptin in association with a 5-fold decrease in the pregnancy rate. Significantly elevated serum glucose, cholesterol, and triglycerides were also determined in obese vs. lean mice (Fig. 4B).
FIG. 4.
A) Comparison of serum leptin and insulin levels in fasted obese and lean male mice at age 30 wk and the percentage of pregnancies resulting from mating with B6C3F1 females. †Denotes a significant increase in serum leptin and insulin in obese vs. lean mice. *Denotes a significant decrease in the percentage of pregnant females mated to obese vs. control males. B) Serum cholesterol, glucose, and triglycerides from fasted obese and lean male mice at age 30 wk. †Denotes a statistical increase of these parameters in fasted obese vs. lean mice. Data represents the mean ± SEM of four to eight male mice per group. Values were considered significantly different at P ≤ 0.05.
Effect of Diet on Testicular Gene Expression Determined by RT-PCR Arrays
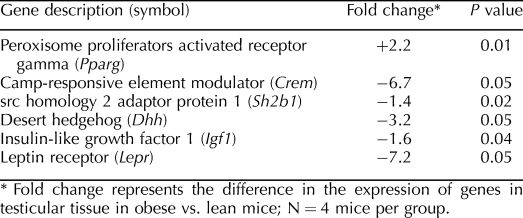
Further investigation of mechanisms that may hinder male fertility as a consequence of diet-induced obesity was achieved through the analysis of mRNA from total testicular extracts. In addition to altered serum biochemistry, the expression of several genes important to competent reproduction was also seemingly impacted by diet. Peroxisome proliferator activated receptor gamma (Pparg) was upregulated, 2.2-fold, while the mRNA of camp-responsive element modulator (Crem), src homology 2 adaptor protein 1 (Sh2b1), desert hedgehog (Dhh), insulin-like growth factor 1 (Igf1), and leptin receptor (Lepr) were downregulated 6.7, 1.4, 3.2, 1.6, and 7.2-fold, respectively (Table 1).
TABLE 1.
Messenger RNA expression of testicular genes in obese vs. lean mice at age 30 wk.
Effect of AA on the Fertility of Obese and Lean Male Mice
Diet-induced male obesity resulted in a significant reduction in fertility in comparison to age-matched lean littermates (Fig. 5). Further, obesity resulted in significant potentiation of AA-induced inhibition of male fertility in both obese and lean mice. However, the effect of AA was more severe in obese vs. lean littermates (Fig. 5). There were no significant differences in the average number of implants per litter determined in female mice impregnated by vehicle-treated lean or obese males (8.5 vs. 8.9, respectively; Fig. 6A). In contrast, treatment of lean and obese males with AA significantly decreased the number of implants in mated females (approximately 30% and 60%, respectively; Fig. 6A). Similarly, in the absence of AA, the number of live fetuses per litter was not affected as a consequence of diet. Females mated to either vehicle-treated lean or obese males produced litters in which >95% of the fetuses were live, but AA treatment of both lean and obese males severely inhibited the development of live fetuses by an estimated 60% and 89%, respectively (Fig. 6B). The percentage of resorption in litters that were the outcome of vehicle-treated obese vs. lean males was statistically similar (1.5 vs. 2.8%, respectively; Fig. 6C). However, litters resulting from matings between AA-treated lean males and untreated females produced an average of 63.5% resorption per litter (Fig. 6C), and mating of AA-treated obese males with untreated females produced litters with >90% resorption (Fig. 6C).
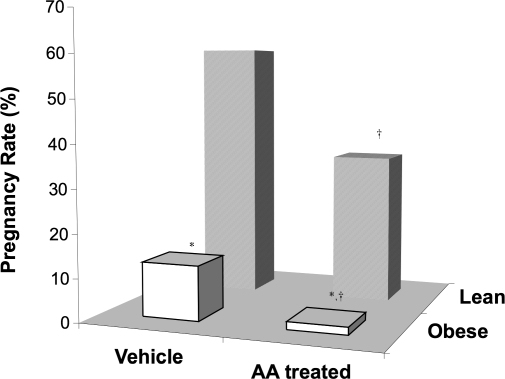
FIG. 5.
Pregnancy rate of female B6C3F1 mice mated to obese or lean males treated with either AA or vehicle. *Denotes statistical decrease in the pregnancy rate of females mated to vehicle- and AA-treated obese males vs. vehicle- and AA-treated lean males, respectively (P < 0.0001). †Denotes significant decreases in the pregnancy rates of females mated to AA-treated vs. vehicle-treated males (P < 0.0001). Data represents the mean ± SEM of 111 female mice per group.
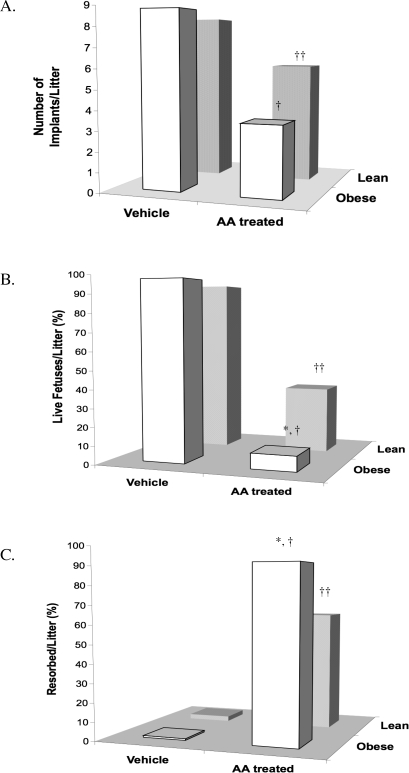
FIG. 6.
Pregnancy outcome of female B6C3F1 mice that were impregnated by either obese or lean male mice treated with either acrylamide (AA) or vehicle. A) Number of implants per litter determined in B6C3F1 mice. †Denotes statistical difference in the number of implants resulting from mating with obese males treated with vehicle vs. AA (P < 0.007). ††Denotes statistical decrease in the number of implants resulting from mating with AA- vs. vehicle-treated lean males (P < 0.0001). B) Number of live fetuses per litter determined in B6C3F1 mice. *Denotes statistical decrease in the number of live fetuses resulting from mating with AA-treated obese vs. lean males (P < 0.045). †Denotes statistical decrease in the number of live fetuses resulting from mating with obese males treated with vehicle vs. AA (P < 0.007). ††Denotes statistical difference in the number of live fetuses resulting from mating with lean males treated with vehicle vs. AA (P < 0.0001). C) Number of resorptions per litter determined in B6C3F1 mice. *Denotes statistical increase in the number of resorptions resulting from mating with AA-treated obese vs. lean males (P < 0.045). †Denotes statistical increase in the number of resorptions resulting from mating with obese males treated with AA vs. vehicle (P < 0.007). ††Denotes statistical difference in the number of resorptions resulting from mating with lean males treated with vehicle vs. AA (P < 0.0001). Data represents the mean ± SEM of 111 female mice per group.
DISCUSSION
Obesity trends in the United States and globally have steadily increased over the past 20 yr [1]. The global epidemic of obesity in both adults and children has also been associated with the global burden of an increased incidence of chronic illness, including physical disability, cardiovascular disease, hypercholesterolemia, hypertension, stroke, type 2 diabetes and other metabolic disorders, and cancer [1]. Further, an association between infertility and obesity has been demonstrated; however, most of these studies have primarily focused on the relationship between elevated BMI, or percent body fat, in females and reduced fertility [19, 33–37]. In contrast, the impact of obesity on male fertility has only recently begun to be explored, and in vivo studies using obese mice have produced varied results [11–13, 38–40]. Studies have reported that men with higher BMIs (>25) have quantitatively and qualitatively inferior sperm, decreased serum testosterone, and increased levels of estrogen and follicle-stimulating hormone than men with BMIs ranging between 20 and 25 [5, 12, 13]. Therefore, it was our hypothesis that diet-induced obesity would be associated with reduced male fertility as well as increased male susceptibility to environmental reproductive toxicants.
In the current study, we have demonstrated a significant reduction in male fertility in association with diet-induced obesity in mice. Although parameters such as sperm morphology and total sperm count were relatively similar in both lean and obese mice, pronounced differences were established between the two groups with regard to sperm motility and hyperactivated progression, indicating that obesity may play an important role in the reproductive capabilities of obese mice. These results support earlier work by Kort et al. [40], who demonstrated a negative correlation between BMI and sperm motility in men: 18.6 million motile sperm in men of normal weight vs. 3.6 million in overweight men vs. 0.7 million in obese men.
Endocrine dysregulation has been shown to be a primary characteristic of male obesity. Of particular focus in the development and progression of disease within this diet-induced obesity model has been the uncoupling of the relationship between leptin and insulin. Earlier work conducted in this laboratory demonstrated that leptin and insulin resistance, exemplified by hyperleptinemia and hyperinsulinemia, respectively, fostered the development of obesity-related lesions, glomerulopathy, early steatohepatitis, and altered hepatic gene expression [41]. Secreted by adipocytes, leptin has been shown to have important regulatory functions in numerous physiological and biochemical processes, including appetite control, suppression of insulin secretion, inflammation, reproduction, and protection of nonadipose tissue from fat accumulation [42–44]. Studies have shown a direct relationship between elevated serum leptin concentrations and increased body fat percentages in both humans and rodents [41, 45]. In the current work, obese mice exhibited dyslipidemia, as evidenced by significantly elevated fasting serum cholesterol and triglycerides. Further, fasting serum leptin and insulin levels were 5-fold higher in obese vs. lean males in concert with a 5-fold reduction in fertility. Interestingly, leptin receptors (Lepr) in the testes of obese males were downregulated approximately 7.2-fold in comparison to control mice, an indication of leptin resistance. These data support earlier trends established in other tissues indicating an inverse relationship between serum leptin and expression of Lepr, specifically in the hypothalamus and liver [41, 46]. Independent mechanisms of oxidative stress through the generation of reactive oxygen species have also been attributed to hyperleptinemia [47, 48]. Thus, the downregulation of leptin signaling in the testes, continuous exposure to elevated serum lipids traversing the blood-testis barrier, and the accumulation of free radicals and fatty acids in the testes of obese mice presented in the current work may have collectively contributed to impaired testicular function and reduced pregnancy outcome. In concert with the aforementioned events, Pparg was upregulated 2-fold in the testes of obese vs. control mice. Elevated expression of Pparg may indicate the activation of a possible compensatory mechanism in order to manage and ultimately decrease excess fatty acids (endogenous ligands of Pparg) amassed in the testes of obese mice.
The increased secretion of insulin, as indicated by hyperinsulinemia, presents another manifestation of the reduced efficacy of leptin in diet-induced obese mice. Studies have also alluded to the suggestion that insulin imparts neuroendocrine regulation to mouse fertility [49, 50]. Mice with a neuron-specific disruption of the insulin receptor gene (NIRKO) displayed an obesity phenotype characterized by elevated triglycerides, insulin resistance, and hyperleptinemia, mirroring the pathologies of the diet-induced obesity model presented in the current work. Matings between male NIRKO mice and control females produced a 30% reduction in the number of offspring in comparison to control matings. The decline in male NIRKO fertility was attributed to decreased sperm count and impaired spermatogenesis as a result of insulin receptors mediating the synthesis and/or secretion of gonadotropin-releasing hormone [49].
In the current model, adjuvant to elevated serum biochemistry in obese males, further analysis of testicular gene expression identified several more altered genes in obese vs. control mice. In particular, an approximate 2-, 3-, and 1.4-fold downregulation of Crem, Dhh, and Sh2b1, respectively, was determined in obese males (Table 1). All three genes have a critical influence on male fertility. Studies have identified Crem in male germ cells of both mouse [51, 52] and men [53]. It has been reported that mice and men without a functional Crem gene demonstrated impaired spermatid differentiation and were ultimately infertile [38, 52, 54, 55]. The importance of a fully functional Dhh gene in the reproductive health of male mice has also been shown in several studies [56, 57]. Using Dhh−/− mice, Clark et al. [57] demonstrated that in the absence of Dhh, male mice were rendered incapable of spermatogenesis as a consequence of dysfunctional myoid and peritubular cells. Sh2b1 has been identified as a contributor to sperm maturation, motility, and fertilization [38]. Earlier reports have also implied leptin signaling may play a regulatory role in sperm motility [58]. Considering these findings, in association with results from the present work, it appears the resistance of these mice to the actions of leptin in concert with the downregulation of Sh2b1 may have additively decreased motility and hyperactivated progression of sperm in obese mice. Additionally, studies using Sh2b1−/− mice have reported reduced fertility and developmental defects in gonadal organs [59]. In addition to its direct effects on fertility, Sh2b1 has functioned as a positive regulator of signaling pathways induced by insulin, Igf1, and leptin [60, 61]. Conversely, deletion of Sh2b1 has been associated with insulin and leptin resistance and promotion of obesity and diabetes [61]. Studies have shown that CYP2E1 plays an important role in the bioactivation of numerous environmental chemicals including toxins and procarcinogens [62]. In particular, CYP2E1-mediated metabolism of AA to its epoxide intermediate, glycidamide (GA), was shown to be a prerequisite step in AA-induced germ cell mutagenicity. Using Cyp2e1-null (Cyp2e1−/−) and wild-type (Cyp2e1+/+) male mice, earlier studies in this laboratory were performed in order to investigate the hypothesis that the formation of GA was responsible for the germ cell mutagenicity attributed to AA exposure [21]. Untreated female mice mated with AA-treated Cyp2e1+/+ demonstrated an increase in the number of chromosomally aberrant embryos (resorptions) and a subsequent decrease in the proportion of live fetuses, number of implants, and rate of pregnancy [21]. In contrast, female mice mated to AA-treated Cyp2e1−/− males exhibited no significant adverse effects, indicating that AA-induced germ cell mutagenicity was dependent upon CYP2E1-mediated metabolism of AA [21]. In addition, studies have shown that CYP2E1 expression is induced in some disease states, including obesity [26, 28, 63, 64]. Therefore, we propose that CYP2E1 induction associated with obesity increases susceptibility to environmental chemicals and may specifically enhance the germ cell toxicity of AA.
Concurrent studies exhibited an approximate 40% increase in CYP2E1 expression in obese vs. lean males (Hoffler, Churchwell, Twaddle, Woodling, Bai, Doerge, Ghanayem, unpublished data). The present work demonstrated that the impact of orally administered AA, reproductive toxicity, and germ cell mutagenicity was exacerbated in obese male mice. Untreated females partnered with AA-treated obese males experienced a further decline in their rate of pregnancy and percentage of live embryos per litter. In contrast, the percentage of resorptions per litter escalated dramatically, approaching 92% in comparison to litters produced from matings between untreated females and vehicle-treated obese males or AA-treated lean males (1.5% and 63.5% resorptions per litter, respectively). These current findings support earlier results that showed the percentage of resorptions per litter approached 50% in matings between untreated females and average weight wild-type male mice treated with AA at 25 mg kg−1 day−1 [21]. The combination of diet-induced obesity in male mice and AA exposure at the same dosage produced mating outcomes in which the number of implants and live fetuses per litter were reduced approximately 50% and 32%, respectively, whereas resorptions per litter increased an estimated 40% in comparison to earlier mouse studies in which obesity was not a variable [21]. Although there were no significant differences in the sperm count of AA-treated lean or obese mice in comparison to their age-matched vehicle-treated littermates, it is apparent that the reduced fertility associated with AA exposure was worsened by the obesity. Therefore, it is likely that the potentiation of AA-induced reproductive toxicity evidenced by the exaggerated percentage of resorptions per litter was mediated by increased bioactivation of AA to its proximate metabolite, GA, in obese mice resulting from CYP2E1 upregulation.
In conclusion, our work has demonstrated that diet-induced obesity leads to significant reduction in male fertility. Furthermore, current studies have shown, for the first time, an exacerbation of reproductive toxicity and germ cell mutagenicity resulting from oral exposures to AA in diet-induced obese male mice. Although testicular gene expression within the current work was determined using mRNA isolated from total testes, whereas the use of purified populations of testicular cells would have been ideal, it is probable that collectively, hyperinsulinemia, hyperleptinemia, and dysregulation of testicular gene expression associated with the consumption of a high-fat diet contributed to the impaired fertility of obese male mice. The present findings also suggest that obese men may be more susceptible to the effects of reproductive toxicants and possibly other environmental pollutants and carcinogens, especially those chemicals metabolized by CYP2E1. As the incidence of obesity continues to escalate as a consequence of lifestyle choices, decreased exercise, and consumption of diets rich in fat, male infertility is also increasing. It is possible that increased sensitivity of obese males to environmental chemicals such as AA, which are present in widely consumed foods, in particular French fries, may be one important factor contributing to the worldwide increase in the incidence of male infertility. Thus, continued exposure to environmental chemicals may impact human reproductive health, especially since the adverse effects of these chemicals appear to be exacerbated in states of metabolic disorder. With these considerations in mind, our diet-induced obesity animal model should be an effective tool for the further investigation of the role of diet in the development and progression of chronic disorders in humans.
Acknowledgments
We extend our sincerest thanks to Drs. Retha Newbold, Gordan Flake, and Mitch Eddy for their careful review of this manuscript and to Dr. Noriko Nakamura for her expert guidance in sperm analysis. The authors declare that they have no competing financial interests.
Footnotes
Supported by the Division of Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences.
REFERENCES
- World Health Organization. Joint WHO/FAO Expert Consultation on Diet, Nutrition, and the Prevention of Chronic Diseases Geneva, Switzerland:World Health Organization;2003: 160 [Google Scholar]
- Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Nelson H, Whittom R, Hantel A, Thomas J, Fuchs CS.Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol 2008; 26: 4109–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio DM, Newgard CB.Obesity-related derangements in metabolic regulation. Annu Rev Biochem 2006; 75: 367–401. [DOI] [PubMed] [Google Scholar]
- Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH.Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol 2006; 26: 968–976. [DOI] [PubMed] [Google Scholar]
- Sallmen M, Sandler DP, Hoppin JA, Blair A, Baird DD.Reduced fertility among overweight and obese men. Epidemiology 2006; 17: 520–523. [DOI] [PubMed] [Google Scholar]
- McTiernan A.Obesity and cancer: the risks, science, and potential management strategies. Oncology (Williston Park) 2005; 19: 871–881;.discussion 881–885. [PubMed] [Google Scholar]
- Wolk R, Somers VK.Obesity-related cardiovascular disease: implications of obstructive sleep apnea. Diabetes Obes Metab 2006; 8: 250–260. [DOI] [PubMed] [Google Scholar]
- Calle EE, Thun MJ.Obesity and cancer. Oncogene 2004; 23: 6365–6378. [DOI] [PubMed] [Google Scholar]
- Bianchini F, Kaaks R, Vainio H.Overweight, obesity, and cancer risk. Lancet Oncol 2002; 3: 565–574. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP.The spread of the obesity epidemic in the United States, 1991–1998. JAMA 1999; 282: 1519–1522. [DOI] [PubMed] [Google Scholar]
- Nguyen RH, Wilcox AJ, Skjaerven R, Baird DD.Men's body mass index and infertility. Hum Reprod 2007; 22: 2488–2493. [DOI] [PubMed] [Google Scholar]
- Hammoud AO, Gibson M, Peterson CM, Meikle AW, Carrell DT.Impact of male obesity on infertility: a critical review of the current literature. Fertil Steril 2008; 90: 897–904. [DOI] [PubMed] [Google Scholar]
- Hammoud AO, Wilde N, Gibson M, Parks A, Carrell DT, Meikle AW.Male obesity and alteration in sperm parameters. Fertil Steril 2008; 90: 2222–2225. [DOI] [PubMed] [Google Scholar]
- Janssen I, Katzmarzyk PT, Ross R.Duration of overweight and metabolic health risk in American men and women. Ann Epidemiol 2004; 14: 585–591. [DOI] [PubMed] [Google Scholar]
- Chehab FF.A broader role for leptin. Nat Med 1996; 2: 723–724. [DOI] [PubMed] [Google Scholar]
- Chehab FF, Lim ME, Lu R.Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet 1996; 12: 318–320. [DOI] [PubMed] [Google Scholar]
- Mounzih K, Lu R, Chehab FF.Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology 1997; 138: 1190–1193. [DOI] [PubMed] [Google Scholar]
- Brannian JD, Furman GM, Diggins M.Declining fertility in the lethal yellow mouse is related to progressive hyperleptinemia and leptin resistance. Reprod Nutr Dev 2005; 45: 143–150. [DOI] [PubMed] [Google Scholar]
- Tortoriello DV, McMinn JE, Chua SC.Increased expression of hypothalamic leptin receptor and adiponectin accompany resistance to dietary-induced obesity and infertility in female C57BL/6J mice. Int J Obes 2007; 31: 395–402. [DOI] [PubMed] [Google Scholar]
- Franco R, Panayiotidis MI.Environmental toxicity, oxidative stress, human disease and the “black box” of their synergism: how much have we revealed? Mutat Res 2009; 674: 1–2. [DOI] [PubMed] [Google Scholar]
- Ghanayem BI, Witt KL, El-Hadri L, Hoffler U, Kissling GE, Shelby MD, Bishop JB.Comparison of germ cell mutagenicity in male CYP2E1-null and wild-type mice treated with acrylamide: evidence supporting a glycidamide-mediated effect. Biol Reprod 2005; 72: 157–163. [DOI] [PubMed] [Google Scholar]
- Ghanayem BI, Witt KL, Kissling GE, Tice RR, Recio L.Absence of acrylamide-induced genotoxicity in CYP2E1-null mice: evidence consistent with a glycidamide-mediated effect. Mutat Res 2005; 578: 284–297. [DOI] [PubMed] [Google Scholar]
- Tyl RW, Marr MC, Myers CB, Ross WP, Friedman MA.Relationship between acrylamide reproductive and neurotoxicity in male rats. Reprod Toxicol 2000; 14: 147–157. [DOI] [PubMed] [Google Scholar]
- Working PK, Bentley KS, Hurtt ME, Mohr KL.Comparison of the dominant lethal effects of acrylonitrile and acrylamide in male Fischer 344 rats. Mutagenesis 1987; 2: 215–220. [DOI] [PubMed] [Google Scholar]
- Tyl RW, Friedman MA.Effects of acrylamide on rodent reproductive performance. Reprod Toxicol 2003; 17: 1–13. [DOI] [PubMed] [Google Scholar]
- Dey A, Cederbaum AI.Induction of cytochrome P450 2E1 [corrected] promotes liver injury in ob/ob mice. Hepatology 2007; 45: 1355–1365. [DOI] [PubMed] [Google Scholar]
- O'Shea D, Davis SN, Kim RB, Wilkinson GR.Effect of fasting and obesity in humans on the 6-hydroxylation of chlorzoxazone: a putative probe of CYP2E1 activity. Clin Pharmacol Ther 1994; 56: 359–367. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hall SD, Maya JF, Li L, Asghar A, Gorski JC.Diabetes mellitus increases the in vivo activity of cytochrome P450 2E1 in humans. Br J Clin Pharmacol 2003; 55: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council NR. Guide for the Care and Use of Laboratory Animals. Washington, DC:: National Academy Press;; 1996. [Google Scholar]
- Sublet VH, Zenick H, Smith MK.Factors associated with reduced fertility and implantation rates in females mated to acrylamide-treated rats. Toxicology 1989; 55: 53–67. [DOI] [PubMed] [Google Scholar]
- Shipp A, Lawrence G, Gentry R, McDonald T, Bartow H, Bounds J, Macdonald N, Clewell H, Allen B, Van Landingham C.Acrylamide: review of toxicity data and dose-response analyses for cancer and noncancer effects. Crit Rev Toxicol 2006; 36: 481–608. [DOI] [PubMed] [Google Scholar]
- Ghanayem BI, McDaniel LP, Churchwell MI, Twaddle NC, Snyder R, Fennell TR, Doerge DR.Role of CYP2E1 in the epoxidation of acrylamide to glycidamide and formation of DNA and hemoglobin adducts. Toxicol Sci 2005; 88: 311–318. [DOI] [PubMed] [Google Scholar]
- Pasquali R.Obesity, fat distribution and infertility. Maturitas 2006; 54: 363–371. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Gambineri A.Metabolic effects of obesity on reproduction. Reprod Biomed Online 2006; 12: 542–551. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Patton L, Gambineri A.Obesity and infertility. Curr Opin Endocrinol Diabetes Obes 2007; 14: 482–487. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Spiegelman D, Garland M, Hertzmark E, Hunter DJ, Colditz GA, Willett WC, Wand H, Manson JE.Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology 2002; 13: 184–190. [DOI] [PubMed] [Google Scholar]
- Bolumar F, Olsen J, Rebagliato M, Saez-Lloret I, Bisanti L.Body mass index and delayed conception: a European multicenter study on infertility and subfecundity. Am J Epidemiol 2000; 151: 1072–1079. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ.The biology of infertility: research advances and clinical challenges. Nat Med 2008; 14: 1197–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud AO, Gibson M, Peterson CM, Hamilton BD, Carrell DT.Obesity and male reproductive potential. J Androl 2006; 27: 619–626. [DOI] [PubMed] [Google Scholar]
- Kort HI, Massey JB, Elsner CW, Mitchell-Leef D, Shapiro DB, Witt MA, Roudebush WE.Impact of body mass index values on sperm quantity and quality. J Androl 2006; 27: 450–452. [DOI] [PubMed] [Google Scholar]
- Hoffler U, Hobbie K, Wilson R, Bai R, Rahman A, Malarkey D, Travlos G, Ghanayem BI.Diet-induced obesity in mice is associated with hyperinsulinemia, hepatic steatosis, and glomerulopathy in C57Bl/6J mice. Endocrine 2009: 36: 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluher S, Mantzoros CS.Leptin in reproduction. Curr Opin Endocrinol Diabetes Obes 2007; 14: 458–464. [DOI] [PubMed] [Google Scholar]
- Ronti T, Lupattelli G, Mannarino E.The endocrine function of adipose tissue: an update. Clin Endocrinol 2006; 64: 355–365. [DOI] [PubMed] [Google Scholar]
- Lado-Abeal J, Norman RL.Leptin and reproductive function in males. Semin Reprod Med 2002; 20: 145–151. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF.Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996; 334: 292–295. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Bian J, Liu J, Endoh A.Obesity reduced the gene expressions of leptin receptors in hypothalamus and liver. Horm Metab Res 2007; 39: 489–494. [DOI] [PubMed] [Google Scholar]
- Bouloumie A, Marumo T, Lafontan M, Busse R.Leptin induces oxidative stress in human endothelial cells. FASEB J 1999; 13: 1231–1238. [PubMed] [Google Scholar]
- Stefanovic A, Kotur-Stevuljevic J, Spasic S, Bogavac-Stanojevic N, Bujisic N.The influence of obesity on the oxidative stress status and the concentration of leptin in type 2 diabetes mellitus patients. Diabetes Res Clin Pract 2008; 79: 156–163. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR.Role of brain insulin receptor in control of body weight and reproduction. Science 2000; 289: 2122–2125. [DOI] [PubMed] [Google Scholar]
- Burks DJ, Font de Mora J, Schubert M, Withers DJ, Myers MG, Towery HH, Altamuro SL, Flint CL, White MF.IRS-2 pathways integrate female reproduction and energy homeostasis. Nature 2000; 407: 377–382. [DOI] [PubMed] [Google Scholar]
- Foulkes NS, Mellstrom B, Benusiglio E, Sassone-Corsi P.Developmental switch of CREM function during spermatogenesis: from antagonist to activator. Nature 1992; 355: 80–84. [DOI] [PubMed] [Google Scholar]
- Blocher S, Fink L, Bohle RM, Bergmann M, Steger K.CREM activator and repressor isoform expression in human male germ cells. Int J Androl 2005; 28: 215–223. [DOI] [PubMed] [Google Scholar]
- Behr R, Weinbauer GF.CREM activator and repressor isoforms in human testis: sequence variations and inaccurate splicing during impaired spermatogenesis. Mol Hum Reprod 2000; 6: 967–972. [DOI] [PubMed] [Google Scholar]
- Blendy JA, Kaestner KH, Weinbauer GF, Nieschlag E, Schutz G.Severe impairment of spermatogenesis in mice lacking the CREM gene. Nature 1996; 380: 162–165. [DOI] [PubMed] [Google Scholar]
- Nantel F, Monaco L, Foulkes NS, Masquilier D, LeMeur M, Henriksen K, Dierich A, Parvinen M, Sassone-Corsi P.Spermiogenesis deficiency and germ-cell apoptosis in CREM-mutant mice. Nature 1996; 380: 159–162. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP.Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol 1996; 6: 298–304. [DOI] [PubMed] [Google Scholar]
- Clark AM, Garland KK, Russell LD.Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod 2000; 63: 1825–1838. [DOI] [PubMed] [Google Scholar]
- Glander HJ, Lammert A, Paasch U, Glasow A, Kratzsch J.Leptin exists in tubuli seminiferi and in seminal plasma. Andrologia 2002; 34: 227–233. [DOI] [PubMed] [Google Scholar]
- Ohtsuka S, Takaki S, Iseki M, Miyoshi K, Nakagata N, Kataoka Y, Yoshida N, Takatsu K, Yoshimura A.SH2-B is required for both male and female reproduction. Mol Cell Biol 2002; 22: 3066–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider L, Tao J, Snyder S, Brinley B, Lu J, Diakonova M.Adapter protein sh2b1{beta} cross-links actin filaments and regulates actin cytoskeleton. Mol Endocrinol 2009; 23: 1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maures TJ, Kurzer JH, Carter-Su C.SH2B1 (SH2-B) and JAK2: a multifunctional adaptor protein and kinase made for each other. Trends Endocrinol Metab 2007; 18: 38–45. [DOI] [PubMed] [Google Scholar]
- Ghanayem BI, Hoffler U.Investigation of xenobiotics metabolism, genotoxicity, and carcinogenicity using Cyp2e1(-/-) mice. Curr Drug Metab 2007; 8: 728–749. [DOI] [PubMed] [Google Scholar]
- Varela NM, Quinones LA, Orellana M, Poniachik J, Csendes A, Smok G, Rodrigo R, Caceres DD, Videla LA.Study of cytochrome P450 2E1 and its allele variants in liver injury of nondiabetic, nonalcoholic steatohepatitis obese women. Biol Res 2008; 41: 81–92. [PubMed] [Google Scholar]
- Mantena SK, Vaughn DP, Andringa KK, Eccleston HB, King AL, Abrams GA, Doeller JE, Kraus DW, Darley-Usmar VM, Bailey SM.High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J 2009; 417: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]