Abstract
Progestagenic sex steroid hormones play critical roles in reproduction across vertebrates, including teleost fish. To further our understanding of how progesterone modulates testis functions in fish, we set out to clone a progesterone receptor (pgr) cDNA exhibiting nuclear hormone receptor features from zebrafish testis. The open reading frame of pgr consists of 1854 bp, coding for a 617-amino acid-long protein showing the highest similarity with other piscine Pgr proteins. Functional characterization of the receptor expressed in mammalian cells revealed that zebrafish Pgr exhibited progesterone-specific, dose-dependent induction of reporter gene expression, with 17alpha,20beta-dihydroxy-4-pregnen-3-one (DHP), a typical piscine progesterone, showing the highest potency. Expression of pgr mRNA: 1) appeared in embryos at 8 h after fertilization; 2) was significantly higher in developing ovary than in early transforming testis at 4 wk of age but vice versa in young adults at 12 wk of age, and thus resembling the expression pattern of the germ cell marker piwil1; and, 3) was restricted to Leydig and Sertoli cells in adult testis. Zebrafish testicular explants released DHP concentration dependently in response to high concentrations of recombinant zebrafish gonadotropins. In addition, DHP stimulated 11-ketotestosterone release from zebrafish testicular explants, but only in the presence of its immediate precursor, 11beta-hydroxytestosterone. This stimulatory activity was blocked by a Pgr antagonist (RU486), suggesting that 11beta-hydroxysteroid dehydrogenase activity was stimulated by DHP via Pgr. Our data suggest that DHP contributes to the regulation of Leydig cell steroidogenesis, and potentially—via Sertoli cells—also to germ cell differentiation in zebrafish testis.
Keywords: gonad development, nuclear progesterone receptor, steroid hormones, steroid release, testis
The zebrafish progesterone receptor (DHP) is expressed by Leydig and Sertoli cells and regulates steroidogenesis and possibly germ cell development. DHP is best activated by its natural ligand produced under strong gonadotropin stimulation.
INTRODUCTION
Progestagenic sex steroid hormones play critical roles in vertebrate reproduction. In mammals, progesterone signaling regulates multiple reproductive processes in females, including follicle growth, oocyte maturation, ovulation, implantation, and the maintenance of pregnancy [1]. In male mice, however, loss of progesterone receptor (PGR) function does not result in a testis phenotype, and the animals are fertile, although plasma luteinizing hormone (LH) levels are higher than normal [2], reflecting a negative feedback effect of progesterone on LH release that is used in hormonal male contraception [3]. It has also been reported that progesterone stimulates the acrosome reaction [4, 5].
In many teleost fish, the biologically active progesterone molecule is 17α,20β-dihydroxy-4-pregnen-3-one (DHP), which plays crucial roles during the resumption of meiosis in final oocyte maturation [6]. However, also in male fish, DHP plays multiple and significant roles in reproductive physiology. Plasma DHP levels increase during the reproductive cycle [7, 8], when germ cells enter into meiosis and—in a later stage—when attaining full maturity and spawning activity. Studies on testicular steroid metabolism in rainbow trout showed that the DHP precursor 17α-hydroxy-4-pregnen-3-one (17α(OH)P4) was efficiently converted to DHP during three periods; namely, when testis tissue was immature and contained spermatogonia only, when germ cells entered meiosis, and in fully mature fish [9]. Regarding the final stages of sperm maturation, DHP has been reported to stimulate sperm hydration [10] and acquisition of sperm motility [11, 12]. More recently, it was found that DHP induces the entry of male germ cells into meiosis [13]. Finally, DHP is a highly potent pheromone in fish [14, 15]. Therefore, fish are an interesting vertebrate group to study the spectrum of progesterone actions in male reproduction.
The biological activity of progesterone is mediated via specific receptors. A single hormone can interact with different receptor types. For estrogens [16], retinoids [17], or prostaglandins [18], it is known that next to members of the nuclear receptor family, there are also membrane-associated receptors. Also for progesterones, different receptor types have been reported, belonging either to the nuclear hormone receptor superfamily or to the membrane-associated receptor family [19, 20], although the functions mediated by membrane-associated progesterone receptors are still a matter of discussion [21]. The present study deals with the nuclear progesterone receptor of zebrafish.
Zebrafish (Danio rerio, Cyprinidae) is a vertebrate model system offering the attractive combination of being simple to maintain and suitable for studies on development, genetics, diseases, and physiology [22, 23]. Zebrafish are also used for basic [23, 24] and applied [25–27] studies on the biology of reproduction. Recently, we presented a detailed and quantitative description of testis structure and the different stages of germ cell development during spermatogenesis [28]. To develop our understanding of the two main testicular functions, spermatogenesis and steroidogenesis, and to elucidate the possible Pgr role(s) in this context, we set out to clone the zebrafish nuclear progesterone receptor (pgr) cDNA. We report the pharmacological characterization of the zebrafish Pgr, pgr mRNA expression patterns during ontogenesis and in different adult tissues. We also studied the capacity of zebrafish testicular explants to produce DHP under gonadotropin stimulation and the ability of DHP to modulate androgen release in a Pgr-dependent manner.
MATERIALS AND METHODS
Animals and Source of Steroid Hormones
Tübingen AB strain zebrafish, outbred zebrafish from a mixed background, or transgenic zebrafish (AB background) expressing enhanced green fluorescent protein under the control of the germ cell-specific vas promoter (vas::egfp) [29] were used. Animal culture [30] and experimentation were consistent with Dutch regulations and were approved by the Utrecht University Life Sciences Committee for Animal Care and Use. Under the conditions of constant photoperiod and temperature in our aquarium facility, we see no evidence for a seasonality of reproductive parameters [28].
The following steroids were used in the current study: DHP, 17α,20β,21-trihydroxy-4-pregnen-3-one (20β-S), progesterone (P4), 17α(OH)P4, testosterone, 11-ketotestosterone (11-KT), 17β-estradiol (E2), cortisol, 11β-hydroxytestosterone (11β-OHT), synthetic progestin promegestone (R5020), and mifepristone (RU486). All steroids were purchased from Sigma-Aldrich (Zwijndrecht, The Netherlands) except for R5020, which was obtained from Perkin Elmer (Waltham, MA).
Cloning and Sequence Analysis of Zebrafish pgr cDNA
Total RNA was extracted from adult zebrafish testes using the FastRNA Pro Green kit (MP Biomedicals, Solon, OH). Poly(A)-rich zebrafish testis RNA was isolated using Dynabeads-oligo(dT25) (Dynal A.S., Oslo, Norway) and reverse transcribed to 5′ and 3′ rapid amplification of cDNA ends (RACE)-ready cDNA using a SMART RACE cDNA amplification kit (Clontech, Mountain View, CA) following the manufacturers' instructions.
Two partial zebrafish pgr cDNAs (GenBank accession numbers DQ017620 and XM_001343705) were obtained from GenBank by BLAST searches [31], using the human PGR cDNA sequence (GenBank accession number M15716) as query sequence. Based on these two sequences, a full-length zebrafish pgr cDNA sequence was predicted, which was confirmed with BLAST searches in the D. rerio Ensembl database (http://www.ensembl.org). To generate a zebrafish pgr expression vector construct, the predicted pgr open reading frame (ORF) was PCR amplified using primers overlapping the start and stop codons (2783, 5′-TTGCCACCATGGACACGGTGAACACTTCTCCCGCTGATT-3′; 2784, 5′-TCGTCCGGTCGGCCTTCATTTGTGGTGA-3′), cloned into pcDNA3.1/V5-His TOPO (Invitrogen, Carlsbad, CA) in the correct orientation, and sequence verified using Dye Terminator cycle sequencing chemistry (Applied Biosystems, Foster City, CA).
After obtaining the zebrafish pgr cDNA sequence, a homology search was performed using the BLAST program [31]. The alignment of multiple nuclear PGR sequences was performed using the Megalign program of the Lasergene software package (DNASTAR Inc., Madison, WI) with the Clustal W (PAM250) algorithm [32], and percentage identity was calculated. The percentage identity is a measure of similarity between the zebrafish and other PGR sequences, derived by taking the matches over the matches, mismatches, and gaps, according to the formula: similarity = (100 × consensus length)/(consensus length + mismatches + gaps). For comparison with the zebrafish Pgr, we only selected (deduced) PGR amino acid sequences from studies that experimentally demonstrated P4 binding to the receptors; the respective GenBank accession numbers are available as Supplemental Data (all Supplemental Data are available online at www.biolreprod.org). A phylogenetic tree was constructed from the aligned sequences using the neighbor-joining method [33].
Transactivation Assays for Zebrafish Pgr
HEK293T cells were used to express the zebrafish Pgr protein. Cells were seeded in 10-cm dishes (∼2 × 106 cells per dish) in Dulbecco modified Eagle medium (DMEM) supplemented with 10% v/v fetal bovine serum (FBS), glutamine, and penicillin/streptomycin (Gibco, Breda, The Netherlands) at 37°C in a 5% CO2 incubator. After 24 h, the cells were cotransfected using a standard calcium phosphate precipitation method [34] with 400 ng of the zebrafish pgr expression plasmid and 7 μg of pGL3-MMTV-Luc plasmid, containing the mouse mammary tumor virus-long terminal repeat (MMTV-LTR) and the Photinus pyralis luciferase gene [35]. After 5–6 h, the transfected cells were transferred to 24-well plates coated with poly-l-lysine hydrobromide (Sigma-Aldrich). The next day, the medium was replaced by transactivation assay medium (DMEM without phenol red, supplemented with 0.2% v/v charcoal-stripped FBS, glutamine, and nonessential amino acids) containing different steroids (in duplicates) with final concentrations ranging between 0.1 nM and 10 μM (n = 2 per condition tested; see Fig. 2) or with different concentrations of DHP (10 pM to 1 μM) in the presence of the mammalian PGR antagonist RU486 (1–100 μM; see Fig. 8) [36]. After incubation at 37°C for 24–36 h, the cells were harvested in lysis mix (100 mM potassium phosphate buffer, pH 7.7; 1% v/v Triton X-100 [Sigma-Aldrich]; 15% v/v glycerol; and 2 mM dithiotreitol [DTT]) and stored at −80°C. Luciferase activity was determined by adding an equal volume of substrate mix (100 mM potassium phosphate buffer, pH 7.7; 250 mM d-luciferin [Invitrogen]; 1 mM DTT; 2 mM ATP [Roche, Woerden, The Netherlands]; and 15 mM magnesium sulfate [Promega, Leiden, The Netherlands]) to thawed samples, and luminescence was measured in a Packard TopCount NXT luminometer (Perkin Elmer Life Sciences, Meriden, CT). Each compound was tested in three independent experiments using cells from different transfections.
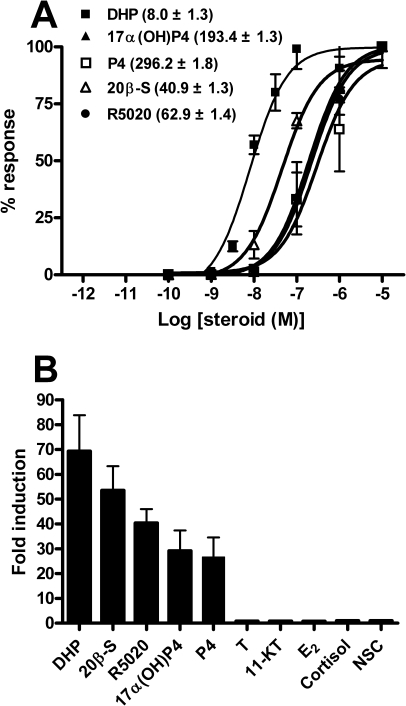
FIG. 2.
Ligand-induced transactivation properties of the zebrafish Pgr. HEK293T cells were transiently cotransfected with the pGL3-MMTV-Luc vector and the zebrafish pgr expression vector construct. A) Transfected cells were incubated with increasing concentrations of various progesterones (from 0.1 nM to 10 μM). Percentage (%) of response: values are given relative to the maximal amount of luciferase activity for each condition. Each point represents the mean ± SEM of three independent experiments, with duplicates for each steroid concentration. The EC50 (nM) value of each progesterone is given between brackets. Curves were generated using nonlinear regression (GraphPad Prism 4.0). B) Transfected cells were incubated with or without 1 μM of the steroids indicated. Data are expressed as the ratio of steroid:no-steroid control (NSC). Each column represents the mean ratio of three independent experiments, with the vertical bar representing the SEM, if not too small for the scale. T, testosterone.
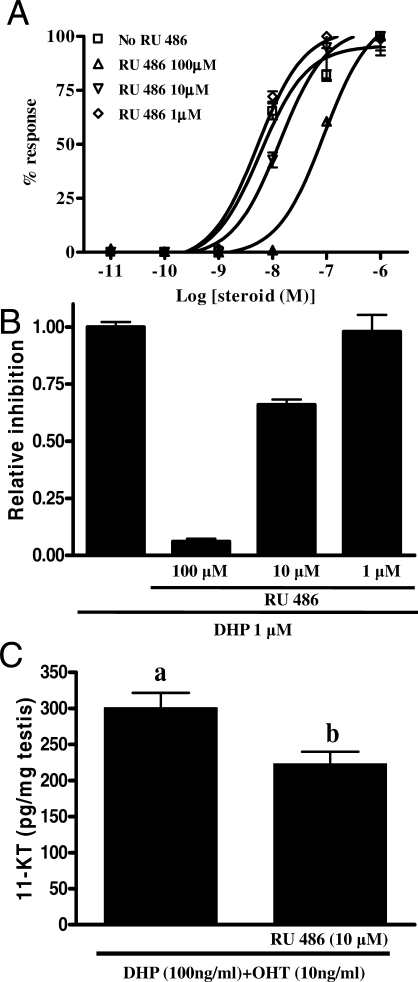
FIG. 8.
Effects of RU486 on DHP-stimulated 11-KT release. A) Inhibition of DHP-induced, zebrafish Pgr-mediated transactivation of the MMTV promoter by RU486. The cells were incubated for 24 h with increasing concentrations of DHP (10 pM to 1 μM) with or without 1, 10, or 100 μM RU486. Percentage (%) of response: values are given relative to the maximal amount of luciferase activity for each condition. B) The cells were incubated for 24 h with fixed concentrations of DHP (1 μM) with or without 1, 10, or 100 μM RU486. Data are expressed as the ratio of RU486:DHP. C) Amounts of 11-KT released by zebrafish testis during overnight exposure to either DHP (100 ng/ml) plus 11β-OHT (10 ng/ml) or DHP (100 ng/ml) plus 11β-OHT (10 ng/ml) with RU486 (10 μM). Data are expressed as mean ± SEM (n = 6). Bars marked with different letters are significantly different from each other (P < 0.05; Student t-test).
Tissue and Ontogenic Analysis of Zebrafish pgr mRNA Expression
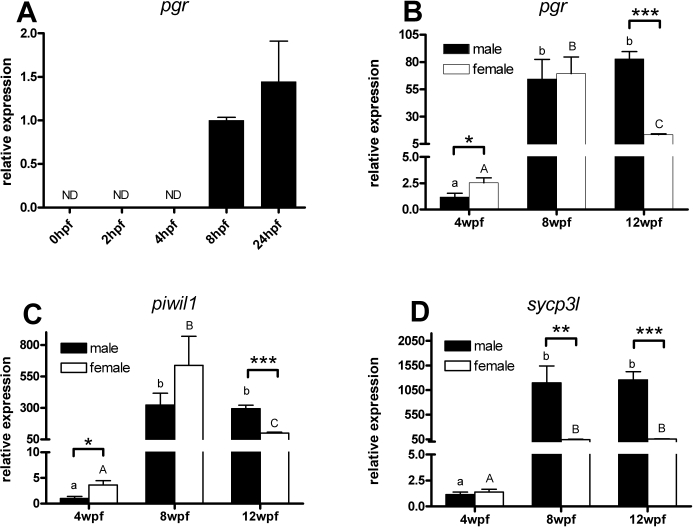
First, relative zebrafish pgr mRNA expression levels were examined in different organs obtained from adult zebrafish (n = 3 individuals per sex). Second, changes in zebrafish pgr mRNA expression were analyzed from 0 to 24 h after fertilization (hpf) in whole-zebrafish embryos (n = 3 pools of 20 embryos for each time point) to investigate whether pgr mRNA is among the maternally contributed mRNAs or when pgr mRNA expression starts during early embryonic development. Finally, relative gonadal pgr mRNA expression levels were studied during zebrafish sex differentiation. Zebrafish is an “undifferentiated” gonochoristics species (i.e., gonads initially develop as ovaries at ∼2–3 wk after fertilization [wpf]), but in future males, ovarian tissue soon degenerates and gonadal tissue transforms into testis starting at ∼3–5 wpf [25, 37]. Using gonad samples from vas::egfp zebrafish to sort for testicular and ovarian tissue [29], we selected three sampling points during the ovary-to-testis transformation process: 1) initial phases of the sex-reversal process at 4 wpf (fish at this age were classified as developing females or transforming males) [26, 37], 2) completion of testicular differentiation and start of meiosis/spermiogenesis at 8 wpf, and 3) young adults at 12 wpf. To investigate the relative pgr mRNA expression levels in relation to early stages of germ cell development, we also examined the relative expression levels of the germ cell marker piwil1 [38] and of the meiosis-specific marker sycp3l [39].
Depending on the size of the tissue samples, either the FastRNA Pro Green kit (MP Biomedicals) or the RNAqueous-Micro Kit (Ambion, Austin, TX) was used for total RNA extraction. Synthesis of cDNA from total RNA samples was performed as described previously [40]. Primers to detect zebrafish pgr, piwil1, and sycp3l mRNA were designed and tested before use for specificity and amplification efficiency on serial dilutions of testis cDNA (Supplemental Table S1), as described elsewhere [40]. Primers and a 6-carboxy-fluorescein-labeled probe were acquired to detect the endogenous control, 18S rRNA (TaqMan gene expression assays; Applied Biosystems). All real-time, quantitative PCRs (qPCRs) were performed in 20-μl reactions, and Ct values were determined in a 7900HT Real-Time PCR System (Applied Biosystems) using default settings, as described previously [35]. Relative pgr, piwil1, and sycp3l mRNA levels were calculated as reported previously [40].
Cellular Localization of pgr Expression in Zebrafish Testis
The localization of pgr mRNA expression in zebrafish testis was investigated by in situ hybridization and by qPCR analysis of laser-microdissected testis tissue fractions and of testis tissue samples from germ cell-less, homozygous piwil1 mutants [38].
A zebrafish pgr-specific PCR product was generated with primers 2737 (5′-GGGCGGGTGTTATTAACCCTCACTAAAGGGCTTGAAGAGTCAAACACAGTTTGATG-3′) and 2738 (5′-CCGGGGGGTGTAATACGACTCACTATAGGGACTGATTCTAATTCTTTCTCCACTCTCTGAA-3′), which contained the T3 or T7 RNA polymerase promoter sequence (underlined) attached at their 5′ ends, respectively. The ∼465-bp PCR product obtained was gel purified and served as template for digoxigenin-labeled cRNA probe synthesis, as described previously [41]. In situ hybridization was performed on 10-μm-thick cryosections from adult zebrafish testis, as reported previously [35], except that a 48-h hybridization period was used.
Laser microdissection of zebrafish testis sections was carried out similar to the procedure described recently for African catfish (Clarias gariepinus) testis [42]. In brief, two testis tissue fractions were microdissected from freshly obtained cryosections and collected using a PALM MicroBeam Instrument (PALM Microlaser Technologies, Bernried, Germany): interstitial tissue, identified by means of the 3β-hydroxysteroid dehydrogenase (3β-HSD) staining of Leydig cells, and intratubular tissue, containing spermatogenic cysts (germ/Sertoli cell units) with germ cells at all three major stages of spermatogenesis (mitotic, meiotic, and spermiogenic phase). Total RNA extraction of laser-microdissected samples (RNAqueous-Micro Kit; Ambion), linear amplification (MessageAmpTM II aRNA Amplification Kit; Ambion), and reverse transcription to cDNA were performed as reported previously [42]. The relative pgr mRNA expression levels were quantified in zebrafish interstitial and intratubular tissue fractions. In this RNA amplification technique, poly(A)+ mRNA is reverse transcribed and converted into double-stranded cDNA using an oligo(dT) primer containing a promoter for T7 RNA polymerase. The second-strand cDNA serves as a transcription template for amplified antisense RNA (aRNA) production. Therefore, the target amplicons for pgr and actb1 were designed in their last exons. Primers to detect the endogenous control actb1 mRNA as well as the pgr mRNA were tested before use for specificity and amplification efficiency on serial dilutions of testis cDNA as described above (Supplemental Table S1) [40], whereas the RNA samples were DNAse I treated before cDNA synthesis.
Homozygous piwil1 mutant zebrafish [piwil1(−/−)] have germ cell-depleted testes [38]. The relative zebrafish pgr mRNA expression levels were compared between testis of piwil1(−/−) and wild-type zebrafish by qPCR. Total RNA extraction and reverse transcription to cDNA were performed as described above, and 18S rRNA served as an endogenous control gene in this series.
Short-Term In Vitro Steroid Secretion by Zebrafish Testicular Explants
Testicular tissue explants from sexually mature, outbred zebrafish were used in the experiments described below. Both testes from six fish were used per condition to be tested. For each individual, one testis served as control for the contralateral one, as described previously [43], hence representing biologically independent sample sets. Moreover, two series of similar experiments were carried out. Incubations lasted 18 h in a humidified air atmosphere at 25°C in 96-well flat-bottom plates (Corning), using a final volume of 200 μl. Basal culture medium consisted of 15 g/L Leibovitz L-15 (Invitrogen) supplemented with 10 mM HEPES (Merck), 0.5% w/v bovine serum albumin fraction V (Roche, Mannheim, Germany), 0.4 mg/L amphotericin B (Fungizone; Invitrogen), and 200 000 units/L penicillin/streptomycin (Invitrogen); pH was adjusted to 7.4. The different solvents used (dimethyl sulfoxide [DMSO] <0.5%; PBS <0.4%; ethanol <0.001%) for different test substances always were identical between control and treated testes, and the different solvents had no significant effect on basal steroid release (see below). After incubation, the tissue explants were weighed. The medium was heated at 80°C for 1 h, centrifuged for 30 min (16 000 × g), and the supernatant collected and stored at −20°C until quantification of levels of different steroids by radioimmunoassay [44]. The results are expressed as amount of steroid released into the medium per milligram of testis tissue incubated.
First, testicular explants were challenged with increasing concentrations of single-chain recombinant zebrafish Fsh (rec-zfFsh; from 50 to 1000 ng/ml), single-chain recombinant zebrafish Lh (rec-zfLh; from 100 to 2000 ng/ml), or the adenylate cyclase activator forskolin (from 0.1 to 25 μM; Sigma-Aldrich). Details on the production and purification by affinity chromatography of recombinant zebrafish gonadotropins will be published separately. Gonadotropin stocks were prepared in PBS, whereas the forskolin stock was prepared in DMSO. After incubation, DHP levels in the medium were quantified by radioimmunoassay [44]. Significant differences among the different concentrations of each test substance were identified by one-way ANOVA followed by the Student-Newman-Keuls test. DHP release in basal medium and in media containing low gonadotropin concentrations was below the detection limit of the assay (4 pg per 50 μl) and was excluded from the statistical analyses.
Second, the ability of DHP to stimulate 11-KT production by zebrafish testis tissue was studied by incubating testicular explants with either DHP (100 ng/ml), 11β-OHT (10 ng/ml), or DHP plus 11β-OHT. Steroid stocks were prepared in ethanol. Our previous studies have shown that the main steroidogenic pathway in zebrafish testis leads from the conversion of 11β-hydroxyandrostenedione to 11-ketoandrostenedione, catalyzed by 11β-HSD, followed by conversion of 11-ketoandrostenedione to 11-KT, mediated by 17β-HSD [35]. To circumvent this main steroidogenic pathway, we used 11β-OHT as substrate, which can be converted to 11-KT by 11β-HSD. After incubation, 11-KT levels in the medium were quantified by radioimmunoassay [44]. Because of the experimental design (incubation of one testis under basal conditions, the contralateral one under experimental conditions), we obtained a basal 11-KT release dataset for each condition assayed. Homogeneity of basal androgen release among the different groups of replicates was tested by one-way ANOVA. No significant differences were identified (P > 0.05), and therefore basal release data were compiled into one single basal 11-KT release condition. Thereafter, significant differences among the different treatments were identified by one-way ANOVA followed by the Student-Newman-Keuls test (P < 0.05).
Effects of RU486 on DHP-Stimulated 11-KT Release
To investigate whether the DHP-stimulated 11-KT production was Pgr dependent, we incubated testicular explants with DHP (100 ng/ml) and 11β-OHT (10 ng/ml) or with DHP and 11β-OHT together with RU486 (10 μM). This concentration of RU486 was chosen because it partially inhibited DHP-stimulated and Pgr-mediated reporter gene expression, whereas it did not inhibit androgen production in the presence of a concentration of rec-zfLh (500 ng/ml) that stimulated 11-KT release but not yet DHP release.
RESULTS
Isolation and Sequence Analysis of Zebrafish pgr cDNA
The ORF of the zebrafish pgr consisted of 1854 nucleotides (GenBank accession number FJ409244), encoding a protein of 617 amino acids (Supplemental Fig. S1). The comparison of the deduced amino acid sequence of the zebrafish Pgr with PGRs from other species is shown in Supplemental Table S2. The zebrafish Pgr sequence could be subdivided into four domains. An N-terminal transactivation domain showed low homology (7.1%–23.9%), whereas the putative DNA-binding domain (DBD) and ligand-binding domain (LBD) showed high homology (DBD, 83.3%–97.2%; LBD, 65.3%–83.2%) with PGRs of other vertebrates. The overall homology of zebrafish Pgr with PGRs from other species is 43.6%–66.3%.
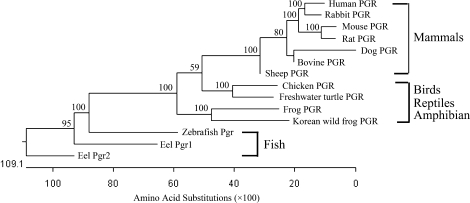
A phylogenetic tree, constructed from the aligned amino acid sequences using the neighbor-joining method [33], revealed that the known PGRs are divided into three major clades (Fig. 1). The first clade consisted of fish Pgrs; the second clade contained avian, reptilian, and amphibian PGRs; and the last clade contained mammalian PGRs.
FIG. 1.
Phylogenetic tree of PGRs. The Jotun Hein method was used to perform multiple-sequence alignment. The phylogenetic tree was constructed by the neighbor-joining method using the MegAlign program (Lasergene software package; DNASTAR Inc.), including only sequences where progesterone binding had been demonstrated experimentally. The horizontal distances to the branching points are proportional to the number of amino acid substitutions. The numbers beside the branches indicate bootstrap values from 1000 replicates.
Steroid-Specific Transactivation of the Zebrafish Pgr
To determine the steroid-dependent transactivation properties of the zebrafish Pgr, HEK 293T cells were transfected with the pGL3-MMTV-Luc reporter construct alone or together with the zebrafish pgr expression vector construct. Next, transfected cells were stimulated with increasing concentrations of different steroid hormones. When transfected with the empty vector, none of the steroid hormones tested (i.e., DHP, 20β-S, P4, 17α(OH)P4, and R5020) increased luciferase activity (data not shown), indicating that HEK 293T cells do not express an endogenous PGR. Dose-dependent, Pgr-mediated activation of the MMTV promoter was shown for all aforementioned progesterone-related steroids (Fig. 2A), the one with the lowest effective concentration at 50% (EC50) value being DHP (8.0 ± 1.3 nM). Also, at a fixed concentration of 1 μM, DHP was the most potent inducer of luciferase activity (69-fold above control; Fig. 2B). The other four progesterone-related hormones tested elicited significant increases of luciferase activity as well (from 26- to 53-fold above control), whereas other steroid hormones assayed (testosterone, 11-KT, E2, or cortisol) were ineffective at the dose tested (1 μM).
Tissue Distribution of Zebrafish pgr mRNA
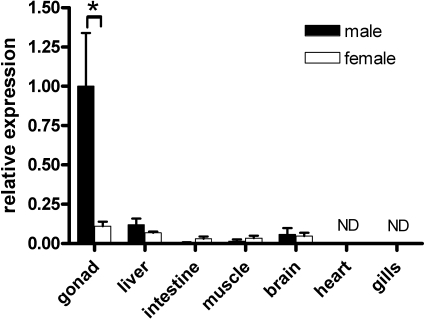
Real-time quantitative PCR analysis of several tissues from adult male and female zebrafish showed that pgr mRNA was predominantly expressed in the testis (Fig. 3). Significantly lower pgr mRNA levels were found in the ovary and in most other tissues tested, without showing significant differences between sexes. Heart and gill tissue did not express detectable pgr mRNA level.
FIG. 3.
Relative expression of zebrafish pgr mRNA in adult organs. Total RNA was extracted from various tissues of male (black columns) and female (white columns) zebrafish. The expression level was normalized to the expression of 18S rRNA. Values represent mean ± SEM (n = 3) relative to testicular pgr mRNA levels. The asterisk indicates a significant difference between testicular and ovarian (P < 0.05) tissue (Student t-test). ND, not detectable.
Ontogenic Analysis of Zebrafish pgr mRNA Relative Expression
Ontogenic changes in zebrafish pgr mRNA expression were analyzed during early embryogenesis in whole embryos, and in sex-differentiating and sexually mature gonads by qPCR. Analysis of zebrafish embryos showed that pgr mRNA became detectable from 8 hpf onward (Fig. 4A) (i.e., there was no maternal contribution of pgr mRNA). Expression analysis in early sex-differentiating gonads at 4 wpf revealed that pgr mRNA expression was significantly higher in ovarian than in testicular tissue. At 8 wpf, when sex differentiation is completed and pubertal gonad development has started, pgr mRNA was increased more than 20-fold and showed similar levels in both sexes (Fig. 4B). High testicular expression levels were maintained in young adults (12 wpf), whereas ovarian pgr mRNA levels decreased significantly compared with ovaries at 8 wpf.
FIG. 4.
The relative expression of zebrafish pgr (A and B), piwil1 (C), and sycp3l (D) mRNAs during ontogeny. A) RNA was extracted from whole embryos at different stages of development. The level of expression was determined by qPCR and normalized to the expression of 18S rRNA. Data are expressed as mean ± SEM (n = 3) relative to pgr mRNA levels in 8-hpf embryo. ND, not detectable. B–D) RNA was extracted from developing gonads of individual vas::egfp transgenic zebrafish and classified into testis or ovary according to their EGFP expression pattern. Relative levels of pgr (B), piwil1 (C), and sycp3l (D) mRNA were determined by qPCR after normalization to the levels of 18S rRNA. Data are expressed as mean ± SEM (n = 6) relative to pgr mRNA levels in 4-wpf male testis. The asterisks indicate a significant difference in relative expression between male and female (*P < 0.05; **P < 0.01; ***P < 0.001). Bars marked with different letters are significantly different between each other (P < 0.01; lowercase for males, uppercase for females).
In addition, the expression levels of the specific germ cell transcripts piwil1 (predominantly expressed during the mitotic and early meiotic germ cell stages [38]) and sycp3l (exclusively expressed in meiotic cells [39]) were quantified during zebrafish sex differentiation. The expression pattern of piwil1 mRNA was similar to that observed for pgr mRNA (Fig. 4C). Gonadal sycp3l mRNA expression showed similarly low levels in both sexes at 4 wpf (Fig. 4D). At 8 and 12 wpf, sycp3l mRNA amounts increased significantly in both testes and ovaries, although the levels measured in testis tissue were ∼20-fold higher than in ovarian tissue (P < 0.01).
Cellular Localization of pgr mRNA Expression in Zebrafish Testis
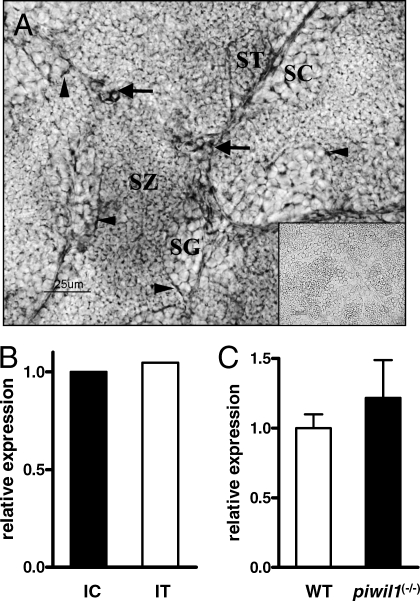
Identification of specific cell types expressing the zebrafish pgr mRNA was accomplished by in situ hybridization using zebrafish testis cryosections. A strong signal was observed in Leydig cells in addition to a weaker signal in Sertoli cells (Fig. 5A). No signal was observed when adjacent sections were hybridized with the sense cRNA pgr probe (Fig. 5A).
FIG. 5.
Cellular localization of pgr mRNA expression in zebrafish testis. A) In situ hybridization for pgr mRNA on testis sections of sexually mature zebrafish. The antisense cRNA probe showed strong staining in Leydig cells (arrows) and weak staining in Sertoli cells (arrowheads). Germ cells (spermatogonia [SG]; spermatocytes [SC]; spermatids [ST]; spermatozoa [SZ]) were devoid of signal. Inset shows the sense cRNA probe; note the absence of specific staining. Bar = 25 μm. B) Relative expression levels of zebrafish pgr from interstitial (IC) and intratubular (IT) tissue fractions. The level of pgr expression was normalized to the expression of actb1. C) Relative expression levels of zebrafish pgr from wild-type (WT) and piwil1 mutant [piwil1(−/−)] testis. The level of pgr expression was normalized to the expression of 18S rRNA.
Confirmation of the pgr mRNA expression by Sertoli cells was obtained by qPCR analysis of laser-microdissected samples (Fig. 5B). The levels of pgr mRNA in the intratubular fraction were similar to those of the interstitial fraction. Sertoli cell expression in the intratubular fraction, and somatic cell expression in general, was further supported by analyzing pgr mRNA expression in testis samples of sterile piwil1(−/−) mutants. The pgr mRNA levels in the germ cell-free piwil1(−/−) testis were similar to those in wild-type testis (Fig. 5C), demonstrating that pgr mRNA in the intratubular fraction is associated with Sertoli cells, the only other intratubular cell type next to germ cells.
Short-Term In Vitro Steroid Secretion by Zebrafish Testicular Explants
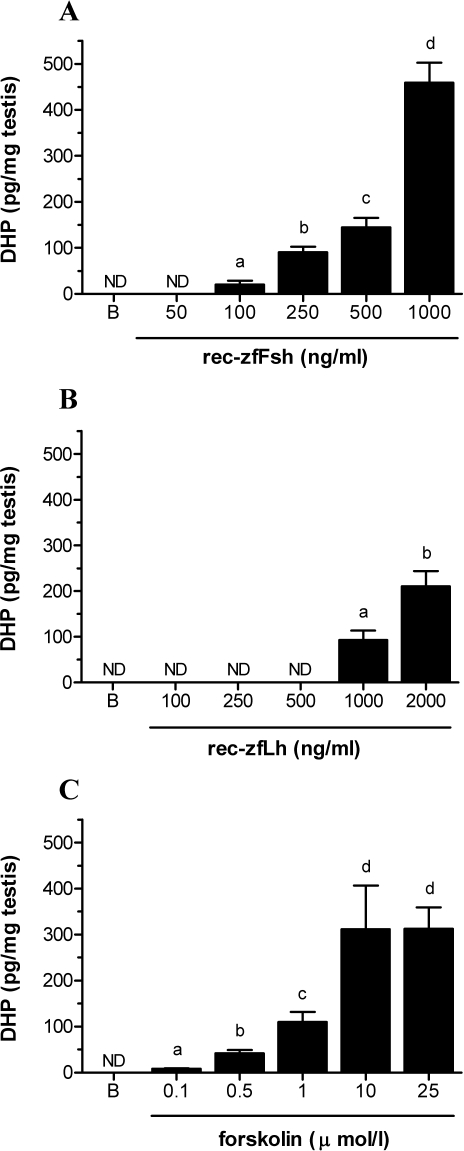
The capacity of zebrafish testis tissue to release DHP when stimulated by zebrafish gonadotropins or the adenylate cyclase activator forskolin was evaluated in overnight primary testis tissue cultures. DHP release under basal conditions as well as in the presence of low to intermediate concentrations of recombinant zebrafish gonadotropins was below the detection limit of the assay (4 pg per 50 μl; Fig. 6). The lowest rec-zfFsh concentration eliciting a detectable DHP release was 100 ng/ml, whereas for rec-zfLh, this concentration was 1000 ng/ml (Fig. 6, A and B). Also, at higher concentrations, rec-zfFsh was significantly more potent in stimulating DHP release than rec-zfLh (P < 0.05). The profile of DHP release in the presence of increasing amounts of forskolin (Fig. 6C) showed a clear dose dependency, and 0.1 μM forskolin induced the first significant DHP release response. The DHP release induced by 10 and 25 μM forskolin was not significantly different from that induced by 1000 ng/ml rec-zfFsh (P > 0.05), and thus can be considered as the maximum response.
FIG. 6.
Stimulation of DHP release by zebrafish testicular explants. Amounts of DHP (mean ± SEM) released by zebrafish testis during overnight exposure to increasing concentrations of recombinant zebrafish Fsh (rec-zfFsh; A), recombinant zebrafish Lh (rec-zfLh; B) or the adenylate cyclase activator forskolin (C). B, basal release; ND, not detectable. The values shown are data compiled from two independent experiments with six replicates per ligand concentration each. Bars marked with different letters are significantly different from each other (P < 0.05).
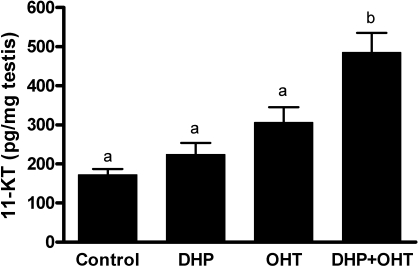
The ability of DHP to stimulate 11-KT release by zebrafish testicular explants was evaluated to test whether an observation made in juvenile eel testis [45] also applied to adult zebrafish testis. Neither the presence of 100 ng/ml DHP nor the presence of 10 ng/ml 11β-OHT (a steroid precursor of 11-KT) alone increased the amount of 11-KT released compared with control (Fig. 7). However, when the testicular explants were incubated with both DHP and 11β-OHT, 11-KT production increased by 2.5-fold compared with control (P < 0.001), suggesting that DHP is able to increase 11β-HSD activity.
FIG. 7.
Release of 11-KT from zebrafish testicular explants. Amounts of 11-KT (mean ± SEM) released by zebrafish testis during overnight exposure to different conditions. Control, testis incubated without steroid; DHP, testis incubated with DHP (100 ng/ml); OHT, testis incubated with 11β-OHT (10 ng/ml); DHP + OHT, testis incubated with DHP (100 ng/ml) and 11β-OHT (10 ng/ml). Values represent compiled data from two independent experiments with six replicates per condition. Bars marked with different letters are significantly different from each other (P < 0.05).
Effects of RU486 on DHP-Stimulated 11-KT Release
Transactivation of the MMTV promoter via the DHP-stimulated zebrafish Pgr was inhibited by RU486. The antagonistic effect of RU486 on Pgr-mediated transactivation by increasing doses of DHP (10 pM to 1 μM) was reflected in 2- or 10-fold higher concentrations of DHP needed to reach half-maximal reporter gene activation with DHP in the presence of 10 μM (EC50 = 14 nM) or 100 μM (EC50 = 60 nM) RU486, respectively, compared with the condition where no RU486 was included (Fig. 8A). Also, the luciferase activity induced by DHP (at 1 μM) was inhibited by RU486 (Fig. 8B).
Although RU486 showed best inhibitory effect at 100 μM, we found that at this concentration it interfered with androgen production, whereas 10 μM RU486 did not (data not shown). Therefore, we used RU486 at 10 μM to test whether the ability of DHP to stimulate 11-KT production was Pgr dependent. In the presence of RU486 (10 μM), the testicular 11-KT production induced by DHP plus 11β-OHT was significantly decreased (P < 0.01; Fig. 8C), suggesting that DHP increased 11β-HSD activity via a Pgr-dependent mechanism.
DISCUSSION
In the present study, we cloned the ORF of a zebrafish pgr cDNA, which encodes a protein of 617 amino acids. The N-terminal domain of the deduced zebrafish Pgr protein displayed low homology (7%–24%; Supplemental Table S2) with PGRs from other vertebrate species. In contrast, the DBD (89%–97%) and LBD (65%–83%) are highly conserved between the zebrafish Pgr and other PGRs. The highly conserved DBD contains cysteine residues, constituting the two zinc finger motifs, as well as the P box (GSCKV) and D box (AGRND) sequences, which are important regions for the recognition of target gene sequences that are all conserved in the zebrafish Pgr. A proline-rich motif in the N-terminal domain of the human PGR, responsible for the interaction with the c-Src family of tyrosine kinases [46], was not found in the zebrafish Pgr, so a Pgr-mediated Mos/MAPK activation may not occur in zebrafish.
The result of our comparative analysis of Pgr amino acid sequences was congruent with the phylogenetic relationships among the major vertebrate clades [47]. The zebrafish Pgr formed a clade with other piscine Pgr proteins, whereas amphibian, reptilian, and avian Pgr proteins, on the one hand, and mammalian PGRs, on the other, formed two separate clades. Our phylogenetic analysis is in accordance with the phylogenetic trees produced by other authors [48] prior to the characterization of the zebrafish Pgr.
Two isoforms (forms A and B) encoded by the same gene but originating from different translational initiation at two in-phase ATG codons have been reported for chicken and human progesterone receptor homologues [49, 50]. In Japanese eel, Anguilla japonica, two distinct pgr genes have been reported [51, 52]. However, experimental trials to isolate additional pgr cDNAs or in silico approaches (e.g., searches of the D. rerio ENSEMBL database [version 44.6e]; data not shown) to identify related sequences did not provide evidence for the existence of additional pgr-like genes or mRNA isoforms from one gene in zebrafish.
We demonstrated that zebrafish Pgr is able to transactivate target genes in a progesterone-dependent manner. In the presence of DHP, zebrafish Pgr activated the transcription of a luciferase gene under control of the progesterone-regulated MMTV-LTR promoter [53]. Moreover, transactivation was progesterone specific, and DHP was the most effective steroid (EC50 = 8 nM). In mammals and chicken, P4 is considered to be a ligand for their PGRs. However, in teleost fish, DHP and/or 20β-S (the latter mainly for marine species) are the major progestins [54–56], and P4 is an intermediate in the synthesis of these steroids [57]. Our experiments showed that zebrafish testis tissue produced DHP in response to gonadotropic stimulation (see below). Although no information is available on DHP plasma levels in zebrafish, 3–8 nM DHP was measured in blood plasma samples of spawning males in closely related fish species [58, 59]. Taken together, these results support the view that DHP is the native ligand for the zebrafish Pgr.
In adult zebrafish, pgr mRNA is expressed predominantly in testis but is detectable at low levels in other tissues, although it has a less broad expression pattern than the zebrafish androgen [35] or estrogen receptor [60] mRNAs. In mammals, PGRs were detected in uterus, ovary, vagina, testis, breast, brain, vascular endothelium, thymus, pancreatic islet, osteoblastlike cells, and lung [61]. In nonmammalian species, PGRs were also detected in testis and oviduct of chicken [49, 62] or oviduct and liver of turtle [63, 64]. In Japanese eel, pgr2 mRNA was detected in gill, spleen, testis, brain, and ovary, whereas pgr1 mRNA was observed in kidney, spleen, liver, and testis [52]. In a frog species, pgr mRNA has a broad expression pattern [65].
In zebrafish embryos, the pgr mRNA cannot be detected at 0, 2, and 4 hpf; pgr mRNA is first detected at 8 hpf, and then pgr mRNA levels increase at 24 hpf. This shows that in zebrafish, pgr mRNA is not maternally deposited in oocytes, but shows zygotic expression and may have a role during late embryonic development. In the mouse, there is little expression of Pgr mRNA until the blastocyst stage [66], and Pgr expression is not essential for embryonic viability [67].
During zebrafish gonad development, all individuals first develop an ovary containing oogonia and oocytes [25]. At approximately 3 wpf, this initial ovary either develops further into a mature ovary or starts transforming into a testis. At 4 wpf, the ovary contains numerous oocytes, whereas testes develop into three different types [37]. In this experiment, type I testes were used (i.e., threadlike gonads with low intensity of EGFP fluorescence) to represent males at 4 wpf [37]. Our results revealed that pgr and piwil1 mRNA levels were higher in the developing ovary than in type I testis, whereas the meiosis marker sycp3l was found at similar levels in both sexes. Because germ cell proliferation starts earlier in females [25], and because Piwil1 protein is expressed in oogonia and early oocytes [38], the higher germ cell number in females may explain the higher level of both piwil1 and sycp3l in ovaries, whereas the detection of sycp3l mRNA in testes may reflect the presence of residual, perhaps degenerating, oocytes in the transforming testis (spermatocytes are still absent). At 8 wpf, the pgr mRNA levels had increased significantly in both sexes, whereas the difference between sexes disappeared. At this age, meiosis had started in males [25]. Miura et al. [13] showed that a function for DHP in male eel is to stimulate entry of germ cells into meiosis. In Japanese eel [52] and chicken [62], pgr mRNA levels were also higher in testes of mature than of immature animals. We therefore speculate that first reaching (8 wpf) and then surpassing (12 wpf) female pgr mRNA expression levels may reflect the entry of numerous germ cells into meiosis in the maturing testis; after all, there are many more spermatocytes than oocytes in (young) adult gonads. In ovarian tissue, however, pgr mRNA levels decreased significantly when the females developed toward young adults. This may be based on a dilution effect, because ovarian tissue mass increased considerably in context with increases in oocyte growth due to vitellogenesis from 8 to 12 wpf, which is associated with stockpiling large amounts of maternal mRNAs in the oocytes [68], not including pgr mRNA, as we have shown in the present study.
We have no information on circulating DHP levels during gonad development in zebrafish, whereas respective data are available from larger fish species. In male Japanese huchen, plasma DHP levels increased above the detection limit with the appearance of meiotic cells in the testis [8]. In rainbow trout, a similar observation was made by Scott and Sumpter [7]. Dépéche and Sire [9] reported that rainbow trout testis tissue showed three periods of DHP production from 17α(OH)P4: in immature fish before the start of rapid spermatogonial proliferation, during the entry into meiosis, and in fully mature, spawning fish. Taken together, our data suggest that gonadal pgr mRNA expression patterns in zebrafish may be functionally related to the entry into meiosis, as has been demonstrated previously for Japanese eel [13]. The early presence of pgr mRNA levels in zebrafish testis and DHP production in immature rainbow trout males might indicate that additional functions are fulfilled during the initial stages of spermatogenesis, whereas there is already information available on the role for DHP during final maturation stages (e.g., composition of seminal fluid [10–12] and reproductive behavior [14, 15]).
In boar testes, the PGR protein locates to type A and B spermatogonia [69], to primary spermatocytes and spermatids in rat [70], and to fully mature spermatozoa in dog [71]. In eel, pgr1 mRNA was expressed in germ cells, Sertoli cells, and interstitial cells of testis, whereas pgr2 mRNA was detected only in germ cells [13]. Human testicular PGR expression was found in some but not all germ cell types, in Sertoli cells, and in Leydig cells in one study [72], whereas a much more restricted distribution to peritubular cells and to Leydig cells was reported in a study using four different antibodies and examining human and nonhuman primate testes [73]. In the present study, we found a strong in situ hybridization signal in the cytoplasm of Leydig cells and a weak staining in Sertoli cells. However, we found no evidence for pgr mRNA expression in germ cells, so DHP effects on germ cells development are likely to be mediated by testicular somatic cells.
We have demonstrated that zebrafish testis tissue produced DHP in vitro when exposed to relatively high concentrations of rec-zfFsh, rec-zfLh, or forskolin. In the steroidogenic pathways leading to androgens or DHP, 17α(OH)P4 holds a central position as substrate for both 20β-hydroxysteroid dehydrogenase (catalyzing DHP production) and Cyp17a1 (catalyzing androgen production), whereas the production of 17α(OH)P4 depends on the StAR-mediated, gonadotropin-dependent conversion of cholesterol to pregnenolone in the mitochondria. In salmonids and eel, it has been suggested that gonadotropin stimulates the testicular somatic cells to produce DHP precursor, probably 17α(OH)P4, which is then converted to DHP via the 20β-HSD activity of spermatozoa [12, 57]. However, 20β-HSD activity is also present in immature rainbow trout testis when spermatozoa are still absent [74]. Ongoing work in our laboratory indicates that a significant stimulation of androgen release occurs already at 4- to 8-fold lower gonadotropin concentrations than an increase of DHP release (García-López and Schulz, unpublished data). These results are compatible with the model that strong gonadotropic stimulation, and hence high levels of the precursor 17α(OH)P4, is required to allow DHP production, whereas moderate gonadotropic stimulation would mainly result in androgen production.
In juvenile eel testis, DHP increases 11β-HSD activity, the enzyme catalyzing the final step in the production of the main androgen in fish, 11-KT [45]. Our results suggest that this stimulation occurs via a Prg-dependent manner also in adult zebrafish testis. On the other hand, androgens were shown to stimulate DHP production in Japanese eel [13] and to downregulate Cyp17a activity in Japanese eel [13] and African catfish [75]. In the latter species, this downregulation depended on the type of androgen and the stage of maturity; although testosterone shows downregulatory effects in both immature and mature fish, 11-KT was only active in immature fish [76]. This leads to a model in which androgen and progesterone production exert mutual control of their biosynthesis, provided that gonadotropic stimulation is sufficiently strong, possibly leading to a phased oscillation of DHP and androgen production.
In conclusion, we identified a progesterone receptor cDNA exhibiting nuclear hormone receptor features from zebrafish testis. The zebrafish progesterone receptor is expressed by Leydig and Sertoli cells, is best activated by its natural ligand (DHP), which is produced under strong gonadotropin stimulation, and may regulate germ cell differentiation (e.g., meiosis) and steroidogenesis.
Supplementary Material
Acknowledgments
The authors thank Wytske van Dijk and Joke Granneman (both from the Division of Endocrinology and Metabolism, Utrecht University, Utrecht, The Netherlands) for technical support. Roland Romijn and Wieger Hemrika (both of U-Protein Express BV, Utrecht, The Netherlands) are acknowledged because of their assistance during the preparation of recombinant zebrafish gonadotropins. The authors also thank DSM Food Specialties (Delft, The Netherlands) and SenterNovem (Utrecht, The Netherlands) for the use of their PALM MicroBeam Instrument at the Department of Cell Architecture and Dynamics (Utrecht University, Utrecht, The Netherlands). The continuous support of H.C. Schriek and J. van Rootselaar from the Biology Department's aquarium facility is highly appreciated.
Footnotes
1Supported by Norwegian Research Council grant 159662/S40 to R.W.S., National Institutes of Health grant DK69711 to J.B., China Scholarship Council grant 2007101952 to S.X.C., and the Ramón Areces Foundation (Spain) to A.G.L.
REFERENCES
- Clarke CL, Sutherland RL.Progestin regulation of cellular proliferation. Endocr Rev 1990; 11: 266–301. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Burgess C, Sleiter NC, DonCarlos LL, Lydon JP, O'Malley B, Levine JE.Enhanced sexual behaviors and androgen receptor immunoreactivity in the male progesterone receptor knockout mouse. Endocrinology 2005; 146: 4340–4348. [DOI] [PubMed] [Google Scholar]
- Kamischke A, Nieschlag E.Progress towards hormonal male contraception. Trends Pharmacol Sci 2004; 25: 49–57. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Burrello N, Barone N, Palermo I, Grasso U, D'Agata R.Effects of progesterone on sperm function: mechanisms of action. Hum Reprod 2000; 15: 28–45. [DOI] [PubMed] [Google Scholar]
- Lösel RM, Falkenstein E, Feuring M, Schultz A, Tillmann HC, Rossol-Haseroth K, Wehling M.Nongenomic steroid action: controversies, questions, and answers. Physiol Rev 2003; 83: 965–1016. [DOI] [PubMed] [Google Scholar]
- Nagahama Y.17α,20β-dihydroxy-4-pregnen-3-one, a maturation-inducing hormone in fish oocytes: mechanisms of synthesis and action. Steroids 1997; 62: 190–196. [DOI] [PubMed] [Google Scholar]
- Scott AP, Sumpter JP.Seasonal variations in testicular germ cell stages and in plasma concentrations of sex steroids in male rainbow trout (Salmo gairdneri) maturing at 2 years old. Gen Comp Endocrinol 1989; 73: 46–58. [DOI] [PubMed] [Google Scholar]
- Amer MA, Miura T, Miura C, Yamauchi K.Involvement of sex steroid hormones in the early stages of spermatogenesis in Japanese huchen (Hucho perryi). Biol Reprod 2001; 65: 1057–1066. [DOI] [PubMed] [Google Scholar]
- Dépéche J, Sire O.In vitro metabolism of progesterone and 17α-hydroxyprogesterone in the testis of the rainbow trout, Salmo gairdneri Rich., at different stages of spermatogenesis. Reprod Nutr Dev 1982; 22: 427–438. [DOI] [PubMed] [Google Scholar]
- Ueda H, Kanbegawa A, Nagahama Y.Involvement of gonadotrophin and steroid hormones in spermiation in the amago salmon, Oncorhynchus rhodurus, and goldfish, Carassius auratus. Gen Comp Endocrinol 1985; 59: 24–30. [DOI] [PubMed] [Google Scholar]
- Miura T, Yamauchi K, Takahashi H, Nagahama Y.Involvement of steroid hormones in gonadotropin-induced testicular maturation in male Japanese eel (Anguilla japonica). Biomed Res 1991; 12: 241–248. [Google Scholar]
- Miura T, Yamauchi K, Takahashi H, Nagahama Y.The role of hormones in the acquisition of sperm motility in salmonid fish. J Exp Zool 1992; 261: 359–363. [DOI] [PubMed] [Google Scholar]
- Miura T, Higuchi M, Ozaki Y, Ohta T, Miura C.Progestin is an essential factor for the initiation of the meiosis in spermatogenetic cells of the eel. Proc Natl Acad Sci U S A 2006; 103: 7333–7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulka JG, Stacey NE, Sorensen PW, van der Kraak GJ.A steroid sex pheromone synchronizes male-female spawning readiness in goldfish. Nature 1987; 325: 251–253. [Google Scholar]
- Hong WS, Chen SX, Zhang QY, Zheng WY.Sex organ extracts and artificial hormonal compounds as sex pheromones to attract broodfish and to induce spawning of Chinese black sleeper (Bostrichthys sinensis Lacépède). Aquac Res 2006; 37: 529–534. [Google Scholar]
- Pang Y, Dong J, Thomas P.Estrogen signaling characteristics of Atlantic croaker G protein-coupled receptor 30 (GPR30) and evidence it is involved in maintenance of oocyte meiotic arrest. Endocrinology 2008; 149: 3410–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu RA, Hu J, Peng J, Bok D, Mata NL, Travis GH.Retinal pigment epithelium-retinal G protein receptor-opsin mediates light-dependent translocation of all-trans-retinyl esters for synthesis of visual chromophore in retinal pigment epithelial cells. J Biol Chem 2008; 283: 19730–19738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, Narumiya S.Prostaglandin E receptors. J Biol Chem 2007; 282: 11613–11617. [DOI] [PubMed] [Google Scholar]
- Thomas P.Characteristics of membrane progestin receptor alpha (mPRα) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol 2008; 29: 292–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmakov NN, Kube M, Reinhardt R, Canario AV.Analysis of the goldfish Carassius auratus olfactory epithelium transcriptome reveals the presence of numerous non-olfactory GPCR and putative receptors for progestin pheromones. BMC Genomics 2008; 9: 429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krietsch T, Fernandes MS, Kero J, Losel R, Heyens M, Lam EW-F, Huhtaniemi I, Brosens JJ, Gellersen B.Human homologs of the putative G protein-coupled membrane progestin receptors (mPRα, β, and γ) localize to the endoplasmic reticulum and are not activated by progesterone. Mol Endocrinol 2006; 20: 3146–3164. [DOI] [PubMed] [Google Scholar]
- Briggs JP.The zebrafish: a new model organism for integrative physiology. Am J Physiol Regul Integr Comp Physiol 2002; 282: R3–R9. [DOI] [PubMed] [Google Scholar]
- McGonnell IM, Fowkes RC.Fishing for gene function-endocrine modelling in the zebrafish. J Endocrinol 2006; 189: 425–439. [DOI] [PubMed] [Google Scholar]
- Santos EM, Workman VL, Paull GC, Filby AL, Van Look KJ, Kille P, Tyler CR.Molecular basis of sex and reproductive status in breeding zebrafish. Physiol Genomics 2007; 30: 111–122. [DOI] [PubMed] [Google Scholar]
- Maack G, Segner H.Morphological development of the gonads in zebrafish. J Fish Biol 2003; 62: 895–906. [Google Scholar]
- Schulz RW, Bogerd J, Male R, Ball J, Fenske M, Olsen LC, Tyler CR.Estrogen-induced alterations in amh and dmrt1 expression signal for disruption in male sexual development in the zebrafish. Environ Sci Technol 2007; 41: 6305–6310. [DOI] [PubMed] [Google Scholar]
- Van den Belt K, Wester PW, van der Ven LT, Verheyen R, Witters H.Effects of ethynylestradiol on the reproductive physiology in zebrafish (Danio rerio): time dependency and reversibility. Environ Toxicol Chem 2002; 21: 767–775. [PubMed] [Google Scholar]
- Leal MC, Cardoso ER, Nóbrega RH, Batlouni SR, Bogerd J, França LR, Schulz RW.Histological and stereological evaluation of zebrafish (Danio rerio) spermatogenesis with an emphasis on spermatogonial generations. Biol Reprod 2009; 81: 177–187. [DOI] [PubMed] [Google Scholar]
- Krovel AV, Olsen LC.Expression of a vas::EGFP transgene in primordial germ cells of the zebrafish. Mech Dev 2002; 116: 141–150. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th ed. Eugene, OR:: University of Oregon Press;; 2000. [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ.Basic local alignment search tool. J Mol Biol 1990; 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Higgins DG, Sharp PM.Fast and sensistive multiple sequence alignments on a microcomputer. Comput Appl Biosci 1989; 5: 151–153. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M.The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 1987; 4: 406–425. [DOI] [PubMed] [Google Scholar]
- Graham FL, van der Eb AJ.A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 1973; 52: 456–467. [DOI] [PubMed] [Google Scholar]
- de Waal PP, Wang DS, Nijenhuis WA, Schulz RW, Bogerd J.Functional characterization and expression analysis of the androgen receptor in zebrafish (Danio rerio) testis. Reproduction 2008; 136: 225–234. [DOI] [PubMed] [Google Scholar]
- Baulieu EE.On the mechanism of action of RU486. Ann NY Acad Sci 1991; 626: 545–560. [DOI] [PubMed] [Google Scholar]
- Wang XG, Bartfai R, Sleptsova-Freidrich I, Orban L.The timing and extent of ‘juvenile ovary' phase are highly variable during zebrafish testis differentiation. J Fish Biol 2007; 70: 1–12. [Google Scholar]
- Houwing S, Kamminga LM, Berezikov E, Cronembold D, Girard A, van den Elst H, Filippov DV, Blaser H, Raz E, Moens CB, Plasterk RHA, Hannon GJ, et al. A role for piwi and piRNAs in germ cell maintenance and transposon silencing in zebrafish. Cell 2007; 129: 69–82. [DOI] [PubMed] [Google Scholar]
- Yano A, Suzuki K, Yoshizaki G.Flow-cytometric isolation of testicular germ cells from rainbow trout (Oncorhynchus mykiss) carrying the green fluorescent protein gene driven by trout vasa regulatory regions. Biol Reprod 2008; 78: 151–158. [DOI] [PubMed] [Google Scholar]
- Bogerd J, Blomenrohr M, Andersson E, van der Putten HH, Tensen CP, Vischer HF, Granneman JC, Janssen-Dommerholt C, Goos HJ, Schulz RW.Discrepancy between molecular structure and ligand selectivity of a testicular follicle-stimulating hormone receptor of the African catfish (Clarias gariepinus). Biol Reprod 2001; 64: 1633–1643. [DOI] [PubMed] [Google Scholar]
- Vischer HF, Teves AC, Ackermans JC, van Dijk W, Schulz RW, Bogerd J.Cloning and spatiotemporal expression of the follicle-stimulating hormone β subunit complementary DNA in the African catfish (Clarias gariepinus). Biol Reprod 2003; 8: 1324–1332. [DOI] [PubMed] [Google Scholar]
- García-López A, Bogerd J, Granneman JC, van Dijk W, Trant JM, Taranger GL, Schulz RW.Leydig cells express follicle-stimulating hormone receptors in African catfish. Endocrinology 2009; 150: 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal MC, de Wall PP, García-López A, Chen SX, Bogerd J, Schulz RW.Zebrafish (Danio rerio) primary testis tissue culture: an approach to study testis function ex vivo. Gen Comp Endocrinol 2009; 162: 134–138. [DOI] [PubMed] [Google Scholar]
- Schulz RW, van der Corput L, Janssen-Dommerholt C, Goos HJ.Sexual steroids during puberty in male African catfish (Clarias gariepinus): serum levels and gonadotropin-stimulated testicular secretion in vitro. J Comp Physiol B 1994; 164: 195–205. [Google Scholar]
- Ozaki Y, Higuchi M, Miura C, Yamaguchi S, Tozawa Y, Miura T.Roles of 11β-hydroxysteroid dehydrogenase in fish spermatogenesis. Endocrinology 2006; 147: 5139–5146. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP.Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family of tyrosine kinases. Mol Cell 2001; 8: 269–280. [DOI] [PubMed] [Google Scholar]
- Carroll RL. Vertebrate Paleontology and Evolution. New York: W. H. Freeman and Company; 1988.
- Katsu Y, Bermudez DS, Braun E, Helbing C, Miyagawa S, Gunderson MP, Kohno S, Bryan TA, Guillette LJ, Iguchi T.Molecular cloning of the estrogen and progesterone receptors of the American alligator. Gen Comp Endocrinol 2004; 136: 122–133. [DOI] [PubMed] [Google Scholar]
- Schrader WT, O'Malley BW.Progesterone-binding components of chick oviduct IV. characterization of purified subunits. J Biol Chem 1972; 247: 51–59. [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B Stropp U, Tora L, Gronemeyer H, Chambon P.Two distince estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 1990; 9: 1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todo T, Ikeuchi T, Kobayashi T, Kajiura-Kobayashi H, Suzuki K, Yoshikuni M, Yamauchi K, Nagahama Y.Characterization of a testicular 17α,20β-dihydroxy-4-pregnen-3-one (a spermiation-inducing steroid in fish) receptor from a teleost, Japanese eel (Anguilla japonica). FEBS Lett 2000; 465: 12–17. [DOI] [PubMed] [Google Scholar]
- Ikeuchi T, Todo T, Kobayashi T, Nagahama Y.A novel progesterone receptor subtype in the Japanese eel, Anguilla japonica. FEBS Lett 2002; 510: 77–82. [DOI] [PubMed] [Google Scholar]
- Truss M, Beato M.Steroid hormone receptors: interaction with deoxyribonucleic acid and transcription factors. Endocr Rev 1993; 14: 459–479. [DOI] [PubMed] [Google Scholar]
- Scott AP, MacKenzie DS, Stacey NE.Endocrine changes during natural spawning in the white sucker, Catostomus commersoni. II. Steroid hormones. Gen Comp Endocrinol 1984; 56: 349–359. [DOI] [PubMed] [Google Scholar]
- Thomas P.Trant M. Evidence that 17α,20β,21-trihydroxy-4-pregnen-3-one is a maturation-inducing steroid in spotted seatrout. Fish Physiol Biochem 1989; 7: 185–191. [DOI] [PubMed] [Google Scholar]
- King W, V, Ghosh S, Thomas P, Sullivan CV.A receptor for the oocyte maturation-inducing hormone 17α,20β,21-trihydroxy-4-pregnen-3-one on ovarian membranes of striped bass. Biol Reprod 1997; 56: 266–271. [DOI] [PubMed] [Google Scholar]
- Nagahama Y.Endocrine regulation of gametogenesis in fish. Int J Dev Biol 1994; 38: 217–229. [PubMed] [Google Scholar]
- Kobayashi M, Aida K, Hanyu I.Gonadotropin surge during spawning in male goldfish. Gen Comp Endocrinol 1986; 62: 70–79. [DOI] [PubMed] [Google Scholar]
- Barry TP, Santos AJG, Furukawa K, Aida K, Hanyu I.Steroid profiles during spawning in male common carp. Gen Comp Endocrinol 1990; 80: 223–231. [DOI] [PubMed] [Google Scholar]
- Menuet A, Pellegrini E, Anglade I, Blaise O, Laudet V, Kah O, Pakdel F.Molecular characterization of three estrogen receptor forms in zebrafish: binding characteristics, transactivation properties, and tissue distributions. Biol Reprod 2002; 66: 1881–1892. [DOI] [PubMed] [Google Scholar]
- Graham JD, Clarke CL.Physiological action of progesterone in target tissues. Endocr Rev 1997; 18: 502–519. [DOI] [PubMed] [Google Scholar]
- González-Morán MG, Guerra-Araiza C, Campos MG, Camacho-Arroyo I.Histological and sex steroid hormone receptor changes in testes of immature, mature, and aged chickens. Domest Anim Endocrinol 2008; 35: 371–379. [DOI] [PubMed] [Google Scholar]
- Reese JC, Callard IP.Two progesterone receptors in the oviduct of the freshwater turtle Chrysemys picta: possible homology to mammalian and avian progesterone receptor systems. J Steroid Biochem 1989; 33: 297–310. [DOI] [PubMed] [Google Scholar]
- Riley D, Reese JC, Callard IP.Hepatic progesterone receptors: characterization in the turtle Chrysemys picta. Endocrinology 1988; 123: 1195–1201. [DOI] [PubMed] [Google Scholar]
- Wang L, Sanyal S, Oh DY, Kim J, Ju JW, Song K, Kim JW, Kwon HB, Choi H.Molecular cloning and characterization of an amphibian progesterone receptor from Rana dybowskii. Gen Comp Endocrinol 2004; 135: 142–149. [DOI] [PubMed] [Google Scholar]
- Hou Q, Gorski J.Estrogen receptor and progesterone receptor genes are expressed differentially in mouse embryos during preimplantation development. Proc Natl Acad Sci U S A 1993; 90: 9460–9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ.Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol 2001; 179: 97–103. [DOI] [PubMed] [Google Scholar]
- Pelegri F.Maternal factors in zebrafish. Dev Dyn 2003; 228: 535–554. [DOI] [PubMed] [Google Scholar]
- Kohler C, Riesenbeck A, Hoffmann B.Age-dependent expression and localization of the progesterone receptor in the boar testis. Reprod Domest Anim 2007; 42: 1–5. [DOI] [PubMed] [Google Scholar]
- Galena HJ, Pillai AK, Terner C.Progesterone and androgen receptors in non-flagellate germ cells of the rat testis. J Endocrinol 1974; 63: 223–237. [DOI] [PubMed] [Google Scholar]
- Sirivaidyapong S, Bevers MM, Gadella BM, Colenbrander B.Induction of the acrosome reaction in dog sperm cells is dependent on epididymal maturation: the generation of a functional progesterone receptor is involved. Mol Reprod Dev 2001; 58: 451–459. [DOI] [PubMed] [Google Scholar]
- Shah C, Modi D, Sachdeva G, Gadkar S, Puri C.Coexistence of intracellular and membrane-bound progesterone receptors in human testis. J Clin Endocrinol Metab 2005; 90: 474–483. [DOI] [PubMed] [Google Scholar]
- Luetjens CM, Didolkar A, Kliesch S, Paulus W, Jeibmann A, Bocker W, Nieschlag E, Simoni M.Tissue expression of the nuclear progesterone receptor in male non-human primates and men. J Endocrinol 2006; 189: 529–539. [DOI] [PubMed] [Google Scholar]
- Vizziano D, LeGac F.Effect of gonadotropin type II and 17-hydroxy-4-pregnene-3,20-dione on 17,20β-dihydroxy-4-pregnen-3-one production by rainbow trout testes at different developmental stages. Fish Physiol Biochem 1998; 19: 269–277. [Google Scholar]
- Cavaco JEB, Blijswijk Van B, Leatherland JF, Goos HJT, Schulz RW.Androgen-induced changes in Leydig cell ultrastructure and steroidogenesis in juvenile African catfish, Clarias gariepinus. Cell Tissue Res 1999; 297: 291–299. [DOI] [PubMed] [Google Scholar]
- Schulz RW, Liemburg M, Garcia-Lopez A, Dijk W, Bogerd J.Androgens modulate testicular androgen production in African catfish (Clarias gariepinus) depending on the stage of maturity and type of androgen. Gen Comp Endocrinol 2008; 156: 154–163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.