Abstract
Gonadal steroid hormone receptors play a vital role in transforming ligand signals into gene expression. We have shown previously that gonads from wild-caught juvenile alligators express greater levels of estrogen receptor 1 (ESR1) than estrogen receptor 2 (ESR2). Furthermore, sexually dimorphic ESR2 mRNA expression (female > male) observed in animals from the reference site (Lake Woodruff, FL, USA) was lost in alligators from the contaminated Lake Apopka (FL, USA). We postulated that environmental contaminant exposure could influence gonadal steroid hormone receptor expression. Here, we address questions regarding gonadal estrogen and androgen receptor (AR) mRNA expression in 1-yr-old, laboratory-raised alligators. What are relative expression levels within gonads? Do these levels vary between sexes or incubation temperatures? Can contaminant exposure change these levels? We observed a similar pattern of expression (ESR1 > AR > ESR2) in ovary and testis. However, both incubation temperature and environment modulated expression. Males incubated at 33.5°C expressed greater AR levels than females incubated at 30°C; dimorphic expression was not observed in animals incubated at 32°C. Compared to Lake Woodruff alligators, Lake Apopka animals of both sexes showed lesser ESR2 mRNA expression levels. Employing cluster analyses, we integrated these receptor expression patterns with those of steroidogenic factors. Elevated ESR2 and CYP19A1 expressions were diagnostic of alligator ovary, whereas elevated HSD3B1, CYP11A1, and CYP17A1 expressions were indicative of testis. In contrast, AR, ESR1, and NR5A1 showed variable expressions that were not entirely associated with sex. These findings demonstrate that the mRNA expression of receptors required for steroid hormone signaling are modified by exposure to environmental factors, including temperature and contaminants.
Keywords: alligator, environment, ovary, steroid hormone receptors, testis
There is a consistent pattern of estrogen and androgen receptor mRNA expression in alligator gonads within individual gonads of either sex, but incubation temperature differences and environmental contaminant exposure induce different expression levels between the ovary and testis.
INTRODUCTION
Steroid hormone signaling at the cellular and molecular level is regulated by the availability of ligands, the abundance of ligand-modifying enzymes, the abundance of receptors for these ligands, and molecular response elements, such as DNA response elements and subsequently recruited cofactors. Life stage, sex, and environment modulate gonadal steroid synthesis and receptor expression, resulting in differences in endocrine signaling. Across a wide range of animals, degraded environmental quality negatively influences endocrine activities through a variety of molecular mechanisms, including nuclear receptor signaling [1]. In American alligator (Alligator mississippiensis) gonads, we investigated the effects of sex and the environment on sex steroid receptor mRNA expression.
Previous research from our laboratory has compared the expression of steroidogenic factors and enzymes [2], steroid receptors [3], and circulating steroid concentrations [4–6] between juvenile alligators from Lake Woodruff National Wildlife Refuge, Florida, USA, an area of minimal anthropogenic influence, and the highly polluted Lake Apopka, Florida, USA [7–9]. These data suggest that the gonads of juvenile alligators are physiologically active, that these gonads show sexual dimorphisms at both hormonal and molecular levels of analysis, and that environmental factors influence their endocrine signaling. For instance, the testes of 13-mo-old alligators expressed higher levels of nuclear receptor 5A1 (NR5A1, also named SF-1), steroidogenic acute regulatory protein (STAR), cytochrome P450 11A1 (CYP11A1), hydroxy-delta-5-steroid dehydrogenase, 3beta- and steroid delta-isomerase 1 (HSD3B1), and cytochrome P450 17A1 (CYP17A1) than the ovaries of similar-age animals [2]. Conversely, ovaries of 13-mo-old females expressed greater cytochrome P450 19A1 (CYP19A1, or aromatase) mRNA levels than testes. These sexually dimorphic expression patterns were observed in animals hatched from eggs collected at Lake Woodruff National Wildlife Refuge and raised in a controlled laboratory environment. Within the same study, animals hatched from eggs collected from Lake Apopka did not exhibit the same patterns of sexual dimorphism. Lake Apopka males exhibited lower relative mRNA expression of NR5A1 and STAR compared to Lake Woodruff males, and no difference was detected in the relative abundance of these transcripts between Lake Apopka males and females. In addition, expression levels of CYP11A1 and HSD3B1 mRNAs were not different between Lake Apopka males and females. This lack of sexual dimorphisms was associated with greatly increased posthatching mortality in Lake Apopka animals when compared to Lake Woodruff animals. These results support the hypothesis that the effects of poor environmental quality experienced by the mother are transmitted through her eggs to her offspring and that physiological changes in offspring can be observed without direct exposure to a contaminated environment during incubation or posthatching.
Recently, our laboratory reported sexually dimorphic expression of estrogen receptor mRNA in the gonads of wild-caught juvenile alligators in Lake Woodruff [3]. Juvenile alligators from Lake Apopka did not exhibit sexually dimorphic expression of estrogen receptor mRNA. It was unclear, however, if the absence of sexual dimorphism resulted from posthatching environmental exposure to xenobiotics or from developmental effects caused by differences in egg incubation temperature or maternal contribution to the embryonic environment.
In the current study, we address a series of fundamental questions regarding the effects of egg incubation temperature, sex, and environmental quality on the expression of sex steroid receptor mRNA in the gonad. The sex of all crocodilians is determined by incubation temperature during a critical window of embryonic development. Previous studies using eggs from these populations have shown that 30°C incubation produces only females, 33.5°C produces only males, and 32°C produces both males and females [10]. First, we ask what are the proportions of estrogen receptor α (ESR1), estrogen receptor β (ESR2) and androgen receptor (AR) mRNA expression within gonads of each sex? Second, how do these proportions vary between ovaries and testes produced at different temperatures versus ovaries and testes produced at the same temperature? Third, are there differences in expression level between ovaries and testes of alligators hatched from eggs collected at sites of differing environmental quality?
Finally, to expand our understanding of steroid signaling in these juvenile animals, we address associations between the mRNA expression of steroid receptors, regulators of steroidogenesis, and steroidogenic enzymes. To this end, we employ cluster analysis to identify novel, multivariate patterns in the data sets [11]. This technique suspends the use of experimental independent variables and finds inherent patterns of mRNA expression in the data set, forming de novo groups based on similarities in gene expression profiles. These de novo groupings of individual animals with similar expression profiles are employed to define and statistically test relationships that are not apparent when animals are clustered by experimentally defined, independent variables alone. These patterns help us to understand a “gene expression landscape” within the experiment and to identify variability in response or gene expression plasticity between the clusters.
MATERIALS AND METHODS
Experimental Design and Animal Care
All fieldwork was conducted under permits from the Florida Fish and Wildlife Conservation Commission and the U.S. Fish and Wildlife Service (permit WX01310). All work involving alligators was performed under the guidelines specified by the Institutional Animal Care and Use Committee at the University of Florida. Egg collection, handling, and incubation methods have been published previously in detail [2, 12]. In brief, soon after oviposition, six complete clutches of eggs were collected from Lake Apopka and Lake Woodruff National Wildlife Refuge during June 2001. At least one egg per clutch was opened to confirm the embryonic stage of development according to criteria set forth by Ferguson [13]. All eggs were incubated at 32°C until assigned to their respective incubation cohort at stage 19, which precedes the thermosensitive period of sex determination.
Viable eggs, as determined by candling, were allocated into one of two study designs. The first study design used only eggs from Lake Woodruff. In this design, 13 eggs were incubated at a female-producing temperature (30°C), and 17 eggs were incubated at a male-producing temperature (33.5°C). These groups were assembled from nine different clutches, with a maximum of three eggs from any clutch at either incubation temperature. The imbalance in sample size is an artifact of using these animals in several developmental studies, one of which required additional males. The second study design consisted of 60 eggs from Lake Apopka and 60 eggs from Lake Woodruff, all incubated at 32°C, a temperature that produces males and females. These groups were assembled using 10 eggs from each of six clutches collected from each study site, as described previously [2].
Incubation of Lake Woodruff eggs at 30 and 33.5°C resulted in 92% and 94% hatch rates, respectively. Hatching success and posthatching mortality from eggs incubated at 32°C have been published previously [2]. In summary, 90% of eggs from Lake Woodruff and 80% of eggs from Lake Apopka hatched. After hatching, alligators were web-tagged and housed in tanks within a greenhouse enclosure under natural lighting for 13 mo at the University of Florida. Animals were fed commercial alligator chow (Burris Mill and Feed) ad libitum. Health was checked daily, and water changes were performed every other day. Sex, as determined by visual inspections of gonad morphology and the presence or absence of oviducts at necropsy, was true to incubation temperature expectations for all animals. At necropsy, 100% (n = 12) of 30°C females and 81% (n = 13) of 33.5°C putative males survived 13 mo. In the 32°C cohort, only 66% of the animals that hatched from Lake Apopka eggs survived to 13 mo of age, compared to 96% of animals that hatched from eggs obtained from Lake Woodruff. This mortality was most extreme in females from Lake Apopka, which exhibited a 40% survival rate. At necropsy, Lake Woodruff was represented by 33 females and 19 males, whereas Lake Apopka was represented by 8 females and 19 males. Before necropsy, body mass (BM), snout-vent length (SVL), and total length (TL) were measured. Condition indices (CI) were calculated for each animal by dividing BM by SVL3 (i.e., the SVL CI) or by TL3 (i.e., the TL CI).
Tissue Collection, RNA Isolation, and Quantitative Real-Time PCR
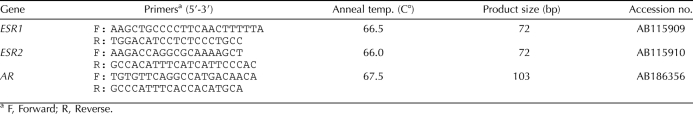
At necropsy, gonads were removed, flash-frozen in liquid nitrogen, and stored at −80°C until RNA extraction was performed. RNA isolation and reverse transcription procedures have been described previously in detail [2]. Quantitative real-time PCR has been used to measure mRNA expression of each gene of interest in the American alligator [3, 14, 15], and primer sequence information, annealing temperatures, and accession numbers are reported in Table 1. Quantitative real-time PCR of steroid receptors (ESR1, ESR2, and AR) was performed in the MyiQ single-color detection system (Bio-Rad) following the manufacturer's protocol using iQ SYBR Green Supermix (Bio-Rad) in triplicate reaction volumes of 15 μl with 2 μl of RT product and specific primer pairs. Expression levels of steroid receptor mRNA were calculated using gene-specific absolute standard curves, which contain the target cDNA at known concentrations. The use of absolute standard curves allows statistical comparisons of mRNA expression levels of different genes within and among samples. Measurements of steroidogenic factors (NR5A1 and STAR) and steroidogenic enzymes (CYP11A1, HSD3B1, CYP17A1, and CYP19A1) were performed using relative standard curves of serially diluted cDNA as previously reported [2]. Values for each measured steroidogenic factor or enzyme mRNA expression were normalized to a mean female expression level of one. All sample means were normalized using ribosomal protein L8 (Rpl8) expression [2, 15].
TABLE 1.
Quantitative real-time PCR primers for alligator steroid receptors.
Statistical Analysis
JMP for Windows (version 7.0.2; SAS Institute) was used for statistical analyses. To achieve homogeneous variances, morphometric data were log transformed and gene expression ratios were arcsin transformed, as needed. Significance for all tests was set at P < 0.05. One-way ANOVA was used to compare relative expression of steroid receptors within a gonad. Unpaired Student t-tests were used to compare means from females produced at 30°C to means from males produced at 33.5°C. The eggs from Lake Woodruff used in the two study designs did not originate from the same clutches; therefore, statistical comparisons between incubation temperatures within a sex (e.g., high-temperature vs. intermediate-temperature males) could not be made. Two-way ANOVA was used to compare the effects of sex and lake of origin among alligators incubated at 32°C, and least square means were analyzed using Tukey-Kramer post hoc comparisons. Significant differences by two-way ANOVA were statistically analyzed further using independent t-tests to identify sexual or lake-of-origin dimorphic expressions.
Hierarchical/agglomerative cluster analysis of mRNA expression data was performed using Ward linkage clustering to explore relationships in mRNA expressions between alligators of each study. Before clustering, mRNA expression data were standardized so that all variables had a mean of zero and an SD of one. Elbow criteria were used to determine the number of clusters from scree plots (not shown). Cluster analyses produced matrices of relative gene expression levels, presented here in gray-scale, colorimetric gradients, with dark squares denoting high expression levels and light squares denoting low expression levels. This expression matrix is in conjunction with adjacent distance dendrograms showing relationships based on mRNA expression similarities for individual animals in the horizontal axis and hierarchical clustering of similar gene expressions between all animals in the vertical axis. Clusters derived from the analysis were used as independent variables to reexamine the mRNA expression data using one-way ANOVA, followed by Tukey-Kramer post hoc comparisons when applicable. Pairwise linear correlation analyses were performed to further investigate specific relationships between gene expressions and are reported along with corresponding Pearson r values.
RESULTS
Body Morphometrics
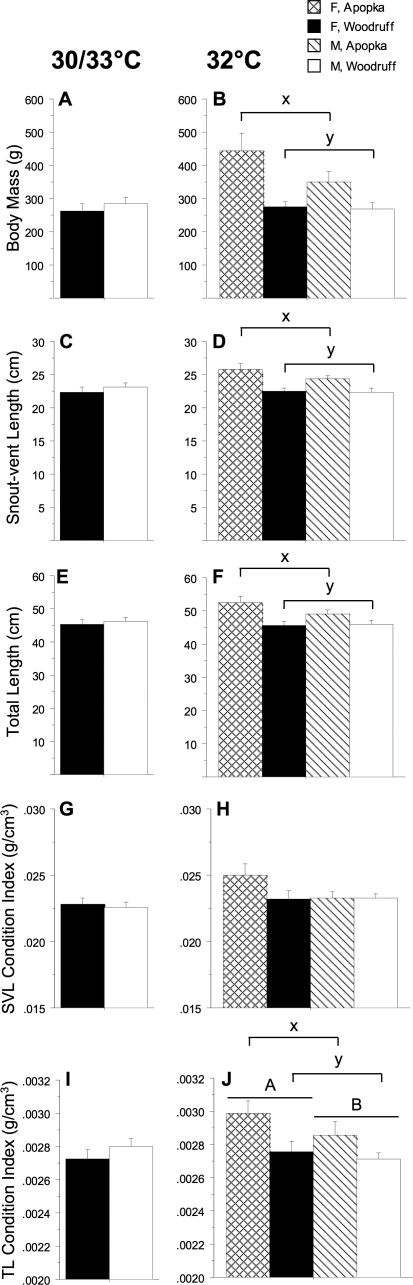
Body mass, snout-vent length, total length, SVL CI, and TL CI were not different between 30°C females and 33.5°C males (Fig. 1, A, C, E, G, and I). Lake Apopka animals had greater body mass, snout-vent length, and total length than Lake Woodruff animals at 32°C, but within each lake no difference between sexes was detected (lake effect, P < 0.001 for each measurement) (Fig. 1, B, D, and F). Whereas the SVL CI did not vary by lake or sex, the TL CI was different between both lake and sex (lake effect: P = 0.01; sex effect, P = 0.02) (Fig. 1, H and J).
FIG. 1.
Body morphometrics measurements from 13-mo-old alligators. Incubation periods were 30 or 33.5°C (A, C, E, G, and I) and 32°C (B, D, F, H, and J). Crosshatched bars are Lake Apopka females (F), and black bars are Lake Woodruff females. Diagonal lined bars are Lake Apopka males (M), and white bars are Lake Woodruff males. Bars represent the mean ± SEM for body mass (A and B), snout-vent length (C and D), and total length (E and F). Condition indices (body mass/length measurement3) were calculated using either snout-vent length (SVL; G and H) or total length (TL; I and J). In J, an uppercase A and B over bars denotes a significant sex effect; in B, D, F, and J, a lowercase x and y over bars denotes a significant lake-of-origin effect.
Steroid Receptor Expression
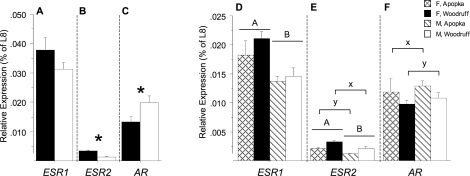
Detectable levels of ESR1, ESR2, and AR mRNA expression were measured in ovaries and testes. The mRNA expression levels of steroid receptors within each gonad exhibited the pattern ESR1 > AR > ESR2 in all incubation groups except Lake Apopka males, in which ESR1 = AR > ESR2. The expression of ESR1 mRNA was not different between 33.5°C males and 30°C females, whereas ESR2 mRNA expression was greater in ovaries than in testes and AR expression was greater in testes than in ovaries (P = 0.2, P < 0.001, and P = 0.03, respectively) (Fig. 2, A–C).
FIG. 2.
Mean (± SEM) mRNA expression of ESR1 (A and D), ESR2 (B and E), and AR (C and F) in gonads of 13-mo-old alligators incubated at 30 or 33.5°C (A–C) or at 32°C (D–F). Crosshatched bars are Lake Apopka females (F), and black bars are Lake Woodruff females. Diagonal lined bars are Lake Apopka males (M), and white bars are Lake Woodruff males. In A–C, asterisks (*) denote a significant difference in expression between sexes. In D–F, an uppercase A or B over bars denotes a sex effect, and a lowercase x or y over brackets denotes a lake-of-origin effect. In A–C and D–F, respectively, expression measurements separated by dotted lines are proportional. L8, ribosomal protein L8 (Rpl8).
In animals incubated at 32°C, sex and lake of origin had significant interactions on expression of ESR1, ESR2, and AR mRNA (P < 0.001 for each) (Fig. 2, D–F). ESR1 and ESR2 mRNA expression was greater in ovary than in testis (sex effect, P < 0.001 for both) (Fig. 2, D and E). Gonads of Lake Woodruff animals expressed greater ESR2 mRNA levels compared to Lake Apopka animal gonads (two-way ANOVA: lake effect, P = 0.002) (Fig. 2E). Conversely, Lake Apopka animal gonads expressed greater amounts of AR mRNA than Lake Woodruff animal gonads (two-way ANOVA: lake effect, P = 0.05) (Fig. 2F).
Upon further refinement of statistical analysis by independent t-tests, ESR2 expression levels were different within each sex, with greater expression levels in Lake Woodruff males and females when compared to Lake Apopka animals of the same sex (P = 0.001 and 0.03, respectively). Furthermore, within animals from each lake, female ESR2 expression levels were greater than male levels (P = 0.004 for both Lake Woodruff and Lake Apopka animals). In contrast, lake-of-origin differences were not observed in AR expression levels (independent t-tests: P = 0.12 and 0.21 for males and females, respectively) as observed by two-way ANOVA.
Steroidogenic Gene Expression
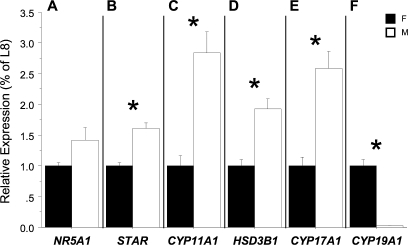
The expression of NR5A1 mRNA was not different between 30°C females and 33.5°C males (P = 0.07) (Fig. 3A), whereas expression of STAR, CYP11A1, HSD3B1, and CYP17A1 was greater in testes than in ovaries (P < 0.001 for each) (Fig. 3, B–E). On the other hand, CYP19A1 mRNA expression was much greater in ovary relative to testis (P < 0.001) (Fig. 3F).
FIG. 3.
Mean (± SEM) mRNA expression of (A) NR5A1, (B) STAR, (C) CYP11A1, (D) HSD3B1, (E) CYP17A1, and (F) CYP19A1 in gonads of 13-mo-old, 30 or 33.5°C incubated alligators. Black bars represent females (F), and white bars represent males (M). Asterisks (*) denote a significant difference in expression between sexes. Expression measurements are not proportionally comparable. L8, ribosomal protein L8 (Rpl8).
Multivariate Cluster Analysis of Individual Animals by mRNA Expression
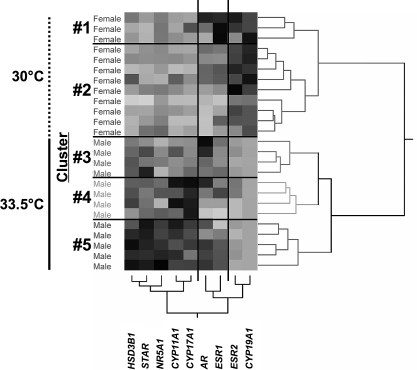
Cluster analysis of females and males from 30 and 33.5°C, respectively, yielded five clusters of animals (Fig. 4, numbered clusters defined by horizontal lines on the matrix). The first node of the dendrogram of individual alligators separated males from females. Females further divided into two clusters, whereas males divided into three clusters (Fig. 4 and Table 2).
FIG. 4.
Hierarchical cluster analysis of mRNA expression data from gonads of 13-mo-old Lake Woodruff alligators incubated at 30°C (vertical dotted line) or 33.5°C (vertical solid line) using Ward linkage clustering. The gray-scale, colorimetric gradient matrix displays lower expression in lighter colors and higher expressions in darker colors. Dendrograms show distance scales for individual animals in the horizontal and mRNA expressions in the vertical. Sex of individual animals is noted to the left of matrix. Horizontal lines separate numbered clusters. Vertical lines separate groups of similar gene expressions.
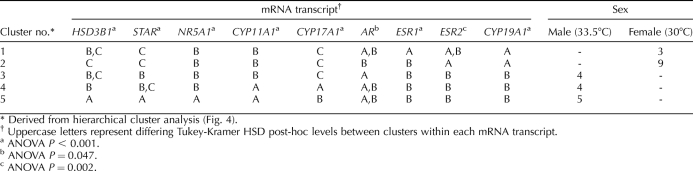
TABLE 2.
Tukey-Kramer HSD post-hoc analysis of mRNA expressions from gonads of alligators from Lake Woodruff, incubated at 30°C (Female) and 33.5°C (Male).
The ANOVA of the transcript level data by clusters and subsequent post hoc comparisons revealed differing expression patterns among the clusters (Table 2). The dendrogram of mRNA expression patterns divided into three groups (Fig. 4, groups defined by vertical lines on the matrix). Post hoc analysis showed that ESR2 and CYP19A1 mRNA expression levels displayed sexually dimorphic patterns, with females exhibiting elevated mRNA expressions. The AR and ESR1 mRNA expression group had roughly sex-equivalent mRNA expressions. Male clusters exhibited considerably more variation in HSD3B1, STAR, NR5A1, CYP11A1, and CYP17A1 mRNA levels compared to female clusters. Transcript levels in cluster 3 were similar to those in female clusters, whereas clusters 4 and 5 showed elevated, but variable, mRNA expressions of these steroidogenic factors.
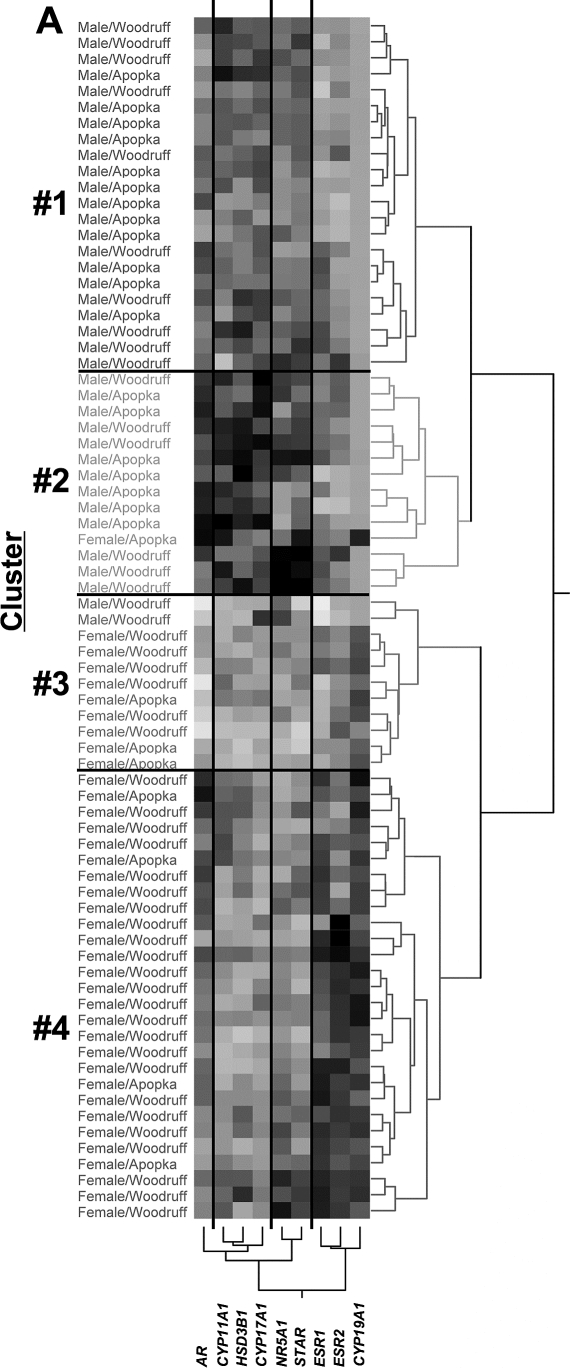
Cluster analysis of males and females from 32°C yielded four clusters of animals (Fig. 5, numbered clusters defined by horizontal lines on the matrix). The first node of the dendrogram of individual alligators segregated all male alligators except two and included one female. Relatively equal proportions of male alligators from Lake Woodruff and Lake Apopka populated clusters 1 and 2. Clusters 3 and 4 were predominantly female, with the majority of Lake Woodruff females allocated to cluster 4, whereas Lake Apopka females distributed relatively equally between clusters 3 and 4 (Fig. 5 and Table 3).
FIG. 5.
Hierarchical cluster analysis of mRNA expression data from gonads of 13-mo-old Lake Woodruff and Lake Apopka alligators incubated at 32°C using Ward linkage clustering. The gray-scale, colorimetric gradient matrix displays lower expressions in lighter colors and higher expressions in darker colors. Dendrograms show distance scales for individual animals in the horizontal and mRNA expressions in the vertical. Sex and lake of origin of individual animals are noted to the left of the matrix. Horizontal lines separate numbered clusters. Vertical lines separate groups of similar gene expressions.
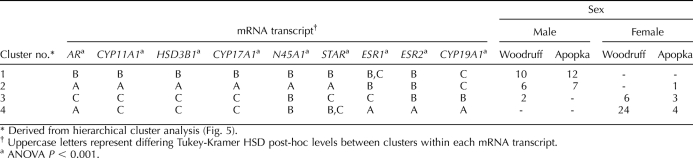
TABLE 3.
Tukey-Kramer HSD post-hoc analysis of mRNA expressions from gonads of alligators from Lakes Woodruff and Apopka, incubated at 32°C; sex, number of animals, and lake of origin are also shown.
Significant differences in mRNA expressions between clusters of animals incubated at 32°C were also observed, with post hoc analysis showing a variety of sex-related expression dimorphisms (Table 3). Cluster analysis defined four groups of mRNA expression (Fig. 5, groups defined by vertical lines on the matrix). Expression of ESR1, ESR2, and CYP19A1 grouped as sexually dimorphic and female-elevated. The female-only cluster 4 had greater ESR1, ESR2, and CYP19A1 mRNA expression when compared to all other clusters, including cluster 3, which was composed of 82% females. Notably, the ESR1 mRNA expression level for the female-rich cluster 3 was lower than that for the mostly male cluster 2 and not different from the that for the all-male cluster 1. Expression levels of AR, STAR, and NR5A1 mRNA varied between clusters, but in a non-sexually dimorphic manner. Compared to female-biased clusters, HSD3B1, CYP11A1, and CYP17A1 mRNA expression levels were greater in the male-biased clusters. These steroidogenic enzymes had similar mRNA expression patterns, in which the mostly male cluster 2 displayed the greatest expressions, followed by the all-male cluster 1, and the lowest expression levels were observed in the all-female clusters.
DISCUSSION
Alligators lack sex chromosomes [16], and sex is determined by the temperature experienced during incubation in ovo [10]. Therefore, each embryo putatively has an equal potential to develop either testes or ovaries that produce sex-appropriate gene expressions. The sexually dimorphic mRNA expressions of steroidogenic factors and enzymes from gonads of Lake Woodruff animals incubated at 30 or 33.5°C reported here (Fig. 3) are similar to those reported previously in laboratory-raised Lake Woodruff animal ovaries and testes incubated at 32°C [2]. The one exception is NR5A1 expression dimorphism observed in the 32°C cohort, but not in the 30 or 33.5°C cohort. These results support a hypothesis that variation in incubation temperature does not markedly effect the establishment of sex-specific mRNA expressions within gonads of a given sex.
In contrast to sexually dimorphic expressions of steroidogenic factors and enzymes observed in these gonads, the expression of estrogen and androgen receptor mRNA in ovary and testis did not present as pronounced sex-specific differences (Fig. 2). Measurement of gonadal steroid receptor mRNA expression levels in 13-mo-old alligators revealed relatively consistent expression patterns, regardless of sex or incubation temperature. In testis or ovary, the level of ESR1 mRNA expression was greatest, whereas AR mRNA expression was intermediate and ESR2 mRNA expression was lowest. The exception to this pattern was Lake Apopka males incubated at 32°C: These males exhibited equivalent ESR1 and AR mRNA expression. Our laboratory has demonstrated previously a similar estrogen receptor mRNA expression pattern of ESR1 > ESR2 mRNA in gonads of wild-caught Lake Woodruff juvenile alligators approximately 5–7 yr of age [3]. This present study is the first to report gonadal AR mRNA expression levels that are intermediate in relation to those of ESR1 and ESR2. In support of our findings, newborn mouse ovary ESR1 expression is approximately 17-fold greater than that of ESR2 [17].
Taking into account this expression-level hierarchy of estrogen and androgen receptors, we did observe sexually dimorphic steroid receptor expressions. In Lake Woodruff animals incubated at 30 or 33.5°C, ESR2 mRNA expression was greater than in ovary than in testis; however, ESR1 expression was not sexually dimorphic. A similar pattern of sexually dimorphic ESR2 expression has been observed in gonads of wild-caught Lake Woodruff animals [3]. Additionally, in the present study, AR mRNA expression was greater in testes than in ovaries. In the 32°C incubation cohort, irrespective of lake of origin, these ovaries expressed greater levels of both ESR1 and ESR2 when compared to testes, whereas AR did not differ by sex. Gonads from Lake Woodruff animals, irrespective of sex, expressed higher levels of ESR2 mRNA. Two-way ANOVA showed a lake-of-origin difference in AR expression levels, with greater expression levels in Lake Apopka animals, but subsequent independent t-tests by sex did not show similar differences. Therefore, the biological significance of this difference requires further examination.
Our findings support a hypothesis that sex, differing incubation temperatures, and environmental quality may influence steroid receptor expression, though to a lesser degree than that of the relative overall ESR1, ESR2, and AR level hierarchy observed in both ovary and testis. On average, estrogen receptor mRNA expressions were greater in ovaries than in testes. Androgen receptor expression was sexually dimorphic between 30 and 33.5°C gonads, but not between 32°C gonads. The environmental exposures of these 13-mo-old animals were only through maternal contribution to the egg, the oviductal environment, and a brief exposure to the nesting materials. Therefore, differences in ESR2 mRNA expression observed at dissection are contingent on factors present well before these tissues were collected and may be organizational alterations with long-term impacts on gonadal functioning.
In the present study, we measured receptor expressions from gonad homogenates. Currently, the distribution of these steroid receptors within alligator gonads is unknown; therefore, relatively small dimorphic expressions from whole-gonad homogenates may translate into larger differences in expression in specific gonad compartments or cell types. In light of this provision, we propose that both estrogen and androgen signaling are active and equally necessary for appropriate function of juvenile alligator ovary and testis.
Literature evaluating steroid receptor expressions between sexes from nonmammalian vertebrate studies is sparse and usually focuses on embryonic development [18, 19]. AR immunoreactivity has been observed in granulosa, theca, and fibroblast cells of chicken ovarian follicles [20] and in Sertoli, Leydig, and myeloid cells of chicken and duck testes [21]. During development, AR mRNA is expressed in the left chicken ovary at higher levels than in testis, and AR levels increase in both ovary and testis before hatching [22]. Antagonism of androgen signaling in embryonic chicken ovary by flutamide treatment results in a disorganized cortex; proper ovarian development is rescued by cotreatment with either testosterone or estradiol [22]. Flutamide treatment was shown to decrease aromatase expression, leading to a hypothesis that androgen-AR signaling may regulate aromatase expression in the embryonic ovary. In posthatchling quail, turkey, duck, and goose gonads, ESR1 mRNA expression is not sexually dimorphic, in spite of concomitant ovarian aromatase expression that is orders of magnitude greater than testis expression [23, 24]. In light of this, our finding of a correlation between AR (but not ESR1 or ESR2) and sexually dimorphic CYP19A1 mRNA expression in the ovaries incubated at 32°C is notable.
In mammals, the roles of both estrogens and androgens in maintaining gonadal health, regardless of sex, is becoming more apparent. In neonatal mouse testes, inactivation of ESR2 increases the number of gonocytes and testosterone production, whereas an inactivation of ESR1 hypertrophies Leydig cells and increases the expression of steroidogenic enzymes [25]. In contrast, neonatal estradiol benzoate treatment of rats alters testicular steroid receptor expression during subsequent postnatal/peripubital development, in which mRNA expressions of Esr1 and Ar decrease and that of Esr2 increases [26]. Recent reviews highlight a growing understanding of the role androgen receptor activity plays in enhancing mammalian ovarian follicle development [27, 28]. Female mice that are deficient in androgen receptor are subfertile, have impaired folliculogenesis, show accelerated follicle depletion similar to premature ovarian failure, and exhibit reduced levels of specific growth factors [27–29]. Conversely, appropriate estrogenic signaling is vital to the establishment and maintenance of testis health [25, 30–32]. Adult Esr1 knockout mice have impaired spermatogenesis, which is marked by decreased numbers of developing germ cells, decreased testis weights, larger Leydig cells, and increased levels of both circulating and testicular testosterone [33]. Additionally, testes of ESR2 knockout adult mice have increased numbers of smaller Leydig cells and increased numbers of spermatagonia per testis. In summary, this research supports the idea that in bird and mammals, both estrogen and androgen signaling play necessary roles in ovary and testis alike, and it demonstrates that extreme sexual dimorphic steroid receptor expressions in juvenile alligator gonads should not be expected.
If levels of steroid receptors are not expressed in highly sexually dimorphic manners but other steroidogenic enzymes, such as CYP19A1 and CYP11A1, show more pronounced sexually dimorphic expressions, what associations can be found both within and between sex steroid receptor and steroidogenic factor and enzyme expressions? Cluster analysis revealed that these expression patterns are more complex than can be explained simply by sex or environment. In cluster analyses of mRNA expression data from both experimental groups. almost all males and females segregated at the earliest stages of hierarchical clustering. Subsequently, males and females segregated into primarily same-sex clusters of similar gene expression patterns. Analysis of mRNA expression levels across these clusters showed that only CYP19A1 expression is uniformly sexually dimorphic. In comparison, the steroidogenic factors, enzymes, and sex steroid receptors investigated here showed expression patterns that, when compared between clusters, range from partial sexual dimorphisms to no correlation between sex and gene expression. Expressions of steroidogenic enzymes HSD3B1, CYP11A1, and CYP17A1 were greater in all the male clusters of the 32°C incubation cohort (Fig. 5); however, expression levels of these enzymes in some the 33.5°C male clusters were equivalent to 30°C female expression levels (Fig. 4). The prosteroidogenic factors NR5A1 and STAR showed the highest expression in some male clusters, but the expression levels in other male clusters of both incubation cohorts were equivalent to female expression levels. Therefore, elevated CYP19A1 expression is a clear steroidogenic marker of ovary, whereas elevated HSD3B1, CYP11A1, and CYP17A1 mRNA expression is indicative, but not diagnostic, of testis. Expression of the prosteroidogenic factors NR5A1 and STAR is male-biased but displays substantial expression variability.
When examined across clusters, ESR2 showed greater levels of sexual dimorphism and mRNA expression patterns more similar to those of CYP19A1 expression than ESR1 expression in both incubation temperature cohorts. A female cluster always showed the highest expression levels of ESR1 or ESR2, however, ESR1 and ESR2 expression levels were equivalent between some predominantly male and female clusters. AR mRNA expression patterns grouped with CYP19A1 in the 30 or 33.5°C cohort, but more closely with HSD3B1, CYP11A1, and CYP17A1 in the 32°C cohort. Furthermore, AR mRNA expression did not display sexually dimorphic expression between male and female clusters in either study design. Therefore, whereas estrogen receptor expressions trended toward higher expression in ovaries, AR mRNA expression showed greater intrasex than intersex variability.
In the present study, we demonstrated that both ovary and testis show a similar relative mRNA expression pattern of steroid receptors, in which ESR1 > AR > ESR2. However, both sex and environment can modulate expression patterns. Elevated CYP19A1 and ESR2 mRNA expressions are more diagnostic of alligator ovary, whereas elevated HSD3B1, CYP11A1, and CYP17A1 mRNA expressions are most indicative of alligator testis. Other gonadal mRNA expressions, such as those of AR, ESR1, and NR5A1, show variable expression patterns that are not entirely associated with sex.
Acknowledgments
The present study was possible through the continuing logistical support of our alligator research by the Florida Fish and Wildlife Conservation Commission; specifically, we thank Allan Woodward for his continued assistance with fieldwork and permitting. We also thank Elizabeth Swiman and Teresa Bryan for providing animal care.
Footnotes
Supported by NIH R21 HD047885-01 (L.J.G.); NIH R21 ES014053-01 (L.J.G.); University of Florida Opportunity Fund (L.J.G.); Howard Hughes Medical Institute Professors Program (L.J.G.); National Science Foundation and Japanese Society for the Promotion of Science OISE-0413602 (M.R.M.); Japanese Ministry of Environment (Y.K. and T.I.); and Japanese Ministry of Education, Sports, Culture, and Technology (Y.K. and T.I.).
REFERENCES
- Iguchi T, Katsu Y.Commonality in signaling of endocrine disruption from snail to human. Bioscience 2008; 58: 1061–1067. [Google Scholar]
- Milnes MR, Bryan TA, Katsu Y, Kohno S, Moore BC, Iguchi T, Guillette LJ.Increased posthatching mortality and loss of sexually dimorphic gene expression in alligators (Alligator mississippiensis) from a contaminated environment. Biol Reprod 2008; 78: 932–938. [DOI] [PubMed] [Google Scholar]
- Kohno S, Bermudez DS, Katsu Y, Iguchi T, Guillette LJ.Gene expression patterns in juvenile American alligators (Alligator mississippiensis) exposed to environmental contaminants. Aquat Toxicol 2008; 88: 95–101. [DOI] [PubMed] [Google Scholar]
- Guillette LJ, Crain DA, Gunderson MP, Kools SAE, Milnes MR, Orlando EF, Rooney AA, Woodward AR.Alligators and endocrine disrupting contaminants: a current perspective. Am Zool 2000; 40: 438–452. [Google Scholar]
- Guillette LJ, Gunderson MP.Alterations in development of reproductive and endocrine systems of wildlife populations exposed to endocrine-disrupting contaminants. Reproduction 2001; 122: 857–864. [DOI] [PubMed] [Google Scholar]
- Milnes MR, Bermudez DS, Bryan TA, Gunderson MP, Guillette LJ.Altered neonatal development and endocrine function in Alligator mississippiensis associated with a contaminated environment. Biol Reprod 2005; 73: 1004–1010. [DOI] [PubMed] [Google Scholar]
- Fujisaki I, Rice KG, Woodward AR, Percival HF.Possible generational effects of habitat degradation on alligator reproduction. J Wildl Manage 2007; 71: 2284–2289. [Google Scholar]
- Guillette LJ, Brock JW, Rooney AA, Woodward AR.Serum concentrations of various environmental contaminants and their relationship to sex steroid concentrations and phallus size in juvenile American alligators. Arch Environ Contam Toxicol 1999; 36: 447–455. [DOI] [PubMed] [Google Scholar]
- Heinz GH, Percival HF, Jennings ML.Contaminants in American alligator eggs from Lake Apopka, Lake Griffin, and Lake Okeechobee, Florida. Environ Monit Assess 1991; 16: 277–285. [DOI] [PubMed] [Google Scholar]
- Ferguson MWJ, Joanen T.Temperature-dependent sex determination in Alligator mississippiensis. J Zool 1983; 200: 143–177. [Google Scholar]
- Hand DJ, Heard NA.Finding groups in gene expression data. J Biomed Biotechnol 2005; 2: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milnes MR, Allen D, Bryan TA, Sedacca CD, Guillette LJ.Developmental effects of embryonic exposure to toxaphene in the American alligator (Alligator mississippiensis). Comp Biochem Physiol C Toxicol Pharmacol 2004; 138: 81–87. [DOI] [PubMed] [Google Scholar]
- Ferguson MW.Reproductive biology and embryology of the crocodilians. Gleason PJ.Biology of the Reptilia, vol. 14 New York:John Wiley and Sons;1985: 329–491. [Google Scholar]
- Gunderson MP, Kohno S, Blumberg B, Iguchi T, Guillette LJ.Up-regulation of the alligator CYP3A77 gene by toxaphene and dexamethasone and its short term effect on plasma testosterone concentrations. Aquat Toxicol 2006; 78: 272–283. [DOI] [PubMed] [Google Scholar]
- Katsu Y, Bermudez DS, Braun EL, Helbing C, Miyagawa S, Gunderson MP, Kohno S, Bryan TA, Guillette LJ, Iguchi T.Molecular cloning of the estrogen and progesterone receptors of the American alligator. Gen Comp Endocrinol 2004; 136: 122–133. [DOI] [PubMed] [Google Scholar]
- Valleley EMA, Harrison CJ, Cook Y, Ferguson MWJ, Sharpe PT.The karyotype of Alligator mississippiensis, and chromosomal mapping of the zfy/x homolog, zfc. Chromosoma 1994; 103: 502–507. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Katsu Y, Watanabe H, Iguchi T.Estrogen receptor subtypes selectively mediate female mouse reproductive abnormalities induced by neonatal exposure to estrogenic chemicals. Toxicology 2008; 253: 117–124. [DOI] [PubMed] [Google Scholar]
- Ramsey M, Crews D.Steroid signaling system responds differently to temperature and hormone manipulation in the red-eared slider turtle (Trachemys scripta elegans), a reptile with temperature-dependent sex determination. Sex Dev 2007; 1: 181–196. [DOI] [PubMed] [Google Scholar]
- Rhen T, Metzger K, Schroeder A, Woodward R.Expression of putative sex-determining genes during the thermosensitive period of gonad development in the snapping turtle, Chelydra serpentina. Sex Dev 2007; 1: 255–270. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Chang C, Okamoto T, Tamura T.Immunolocalization of androgen receptor in the small, preovulatory, and postovulatory follicles of laying hens. Gen Comp Endocrinol 1993; 91: 81–89. [DOI] [PubMed] [Google Scholar]
- Dornas RAP, Oliveira AG, Dias MO, Mahecha GAB, Oliveira CA.Comparative expression of androgen receptor in the testis and epididymal region of roosters (Gallus domesticus) and drakes (Anas platyrhynchos). Gen Comp Endocrinol 2008; 155: 773–779. [DOI] [PubMed] [Google Scholar]
- Katoh H, Ogino Y, Yamada G.Cloning and expression analysis of androgen receptor gene in chicken embryogenesis. FEBS Lett 2006; 580: 1607–1615. [DOI] [PubMed] [Google Scholar]
- Koba N, Mori M, Ha Y, Mizushima S, Tsukada A, Saito N, Ono T, Shimada K.Effects of aromatase inhibitor (fadrozole)-induced sex reversal on gonadal differentiation and mRNA expression of p450arom, AMH and ER alpha in embryos and growth in posthatching quail. J Poultry Sci 2008; 45: 116–124. [Google Scholar]
- Koba N, Ohfuji T, Ha Y, Mizushima S, Tsukada A, Saito N, Shimada K.Expression of p450arom, AMH and ER alpha mRNA in gonads of turkey, duck and goose within one week of age. J Poultry Sci 2008; 45: 220–226. [Google Scholar]
- Delbes G, Levacher C, Habert R.Estrogen effects on fetal and neonatal testicular development. Reproduction 2006; 132: 527–538. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M, Navarro J, Pinilla L, Gonzalez LC, Huhtaniemi I, Aguilar E.Neonatal exposure to estrogen differentially alters estrogen receptor alpha and beta mRNA expression in rat testis during postnatal development. J Endocrinol 2000; 165: 345–357. [DOI] [PubMed] [Google Scholar]
- Walters KA, Allan CM, Handelsman DJ.Androgen actions and the ovary. Biol Reprod 2008; 78: 380–389. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Shiina H, Kawano H, Sato T, Kato S.Androgen receptor functions in male and female physiology. J Steroid Biochem Mol Biol 2008; 109: 236–241. [DOI] [PubMed] [Google Scholar]
- Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, Chambon P, Kanno J, et al. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci U S A 2006; 103: 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell L, Robertson KM, Jones ME, Simpson ER.Estrogen and spermatogenesis. Endocr Rev 2001; 22: 289–318. [DOI] [PubMed] [Google Scholar]
- Sierens JE, Sneddon SF, Collins F, Millar MR, Saunders PTK.Estrogens in testis biology. Ann NY Acad Sci 2005; 1061: 65–76. [DOI] [PubMed] [Google Scholar]
- Akingbemi BT.Estrogen regulation of testicular function. Reprod Biol Endocrinol 2005; 3: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould ML, Hurst PR, Nicholson HD.The effects of estrogen receptors alpha and beta on testicular cell number and steroidogenesis in mice. Reproduction 2007; 134: 271–279. [DOI] [PubMed] [Google Scholar]