Abstract
It was hypothesized that histone deacetylase (HDAC) inhibitors may increase survival after total-body irradiation (TBI) based on previous reports demonstrating that HDAC inhibitors stimulate the proliferation of bone marrow stem cells. Using the time for mice to lose 20% or more of their weight as the end point, two HDAC inhibitors, valproic acid and trichostatin-A, were found to reduce lethality in a dose-dependent manner. HDAC inhibitors were effective at reducing lethality when given either 24 h before or 1 h after TBI. The results indicate that HDAC inhibitors have potential for protecting against and mitigating radiation-induced lethality.
INTRODUCTION
The development of an effective mitigator of radiation injury after a total-body irradiation (TBI) has the potential to benefit three groups of individuals: victims of a radiological attack or nuclear disaster, clean-up workers exposed after such events, and patients undergoing radiation therapy. With the first group, the mitigator must be effective when administered after the radiation exposure.
Chung and coworkers (1) showed that skin injury could be reduced within 2 weeks of a radiation exposure when a high topical dose of a histone deacetylase (HDAC) inhibitor was given twice daily starting 1 day after the radiation exposure. Although no previous study supported the systemic use of HDAC inhibitors to reduce injuries after TBI, we investigated their use for this purpose for two reasons. First, HDAC inhibitors have a documented safety record for systemic use in humans with a variety of neurodegenerative diseases and have recently received considerable interest for their direct anti-cancer effects and as a therapy to reduce graft versus host disease after an allogenic bone marrow transplant. Second, HDAC inhibitors have been reported to stimulate the proliferation of stem cells of the bone marrow (2, 3). Consequently, we tested the hypothesis that HDAC inhibitors could decrease lethality after TBI, presumably by increasing bone marrow stem cell populations.
METHODS AND MATERIALS
All studies were performed with prior approval and in accordance with institutional and national standards of animal care. The studies were approved by the Institutional Animal Care and Use Committee (IACUC) and were performed in an accredited AAALAC facility. Male BALB/c mice (Charles River Laboratories) 8 to 10 weeks old were allowed to acclimate for 1 week prior to the start of the study. Mice were maintained in environment-controlled (temperature and lighting) animal facilities, provided food and water ad libitum, and randomly assigned to experimental groups.
Histone Deacetylase (HDAC) Inhibitors
Two HDAC inhibitors were studied, valproic acid (VPA), a class I HDAC inhibitor, and trichostatin-A (TSA), a non-selective HDAC inhibitor. VPA (Depakene, Abbott Laboratories, Abbott Park, IL) was obtained from the hospital pharmacy. TSA was purchased from Sigma-Aldrich (St. Louis, MO).
Total-Body Irradiation (TBI)
TBI was delivered using a 185 TBq (5000 Ci) 137Cs source (Mark I, J. L. Shepherd and Associates, San Fernando, CA). Doses ranged from sublethal exposures of 5.5 Gy to lethal exposures of 6.5 Gy and a supralethal exposure of 7.0 Gy. The dose rate was approximately 1 Gy per minute.
HDAC Activity
Western blot analyses were used to confirm the activity of VPA and TSA. Histone H3 and H4 acetylation activity of fresh mouse spleen and bone marrow after administration of VPA or TSA was measured by Western blot analyses ex vivo. Mice were administered two doses of HDAC inhibitors subcutaneously, one dose at 0 h (TSA, 0.5 mg/kg; VPA, 600 mg/kg) and one dose at 5 h (TSA, 0.5 mg/kg; VPA, 300 mg/kg). Blood marrow or spleens were harvested at 10 h.
Blood Counts
Twelve days after exposure to 5.5 Gy alone or in combination with either valproic acid (400 mg/kg administered i.p. 1 h after radiation exposure) or trichostatin A (0.5 mg/kg i.p. administered 1 h after radiation exposure), mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) for blood collection. Blood (0.5 ml) obtained by cardiac puncture with a 25-gauge needle was placed into heparinized anticoagulant tubes and shipped to ANTECH Diagnostics (Alsip, IL) for analysis of red and white blood cell counts using their CBC differential small mammalian protocol. The same mice were used for the spleen colony-forming assay.
Spleen Colony-Forming Assay
The numbers of spleen colony-forming units were measured to assess the in vivo effect of HDAC inhibitors on bone marrow cell survival. Groups of BALB/c mice were exposed to 5.5 Gy alone or in combination with HDAC inhibitors as described. Spleens were excised and examined for colony formation 12 days after the sublethal TBI using a dissecting microscope.
Lethality
Survival was measured in mice after TBI with and without HDAC inhibitors. Groups of mice were exposed to 6.5 or 7.0 Gy alone or in combination with HDAC inhibitors, as described above. Survival was defined as the time from irradiation to the time of euthanasia, which occurred when animals had lost 20% or more of their body weight.
RESULTS
HDAC Inhibitors Activity in Bone Marrow of Mice
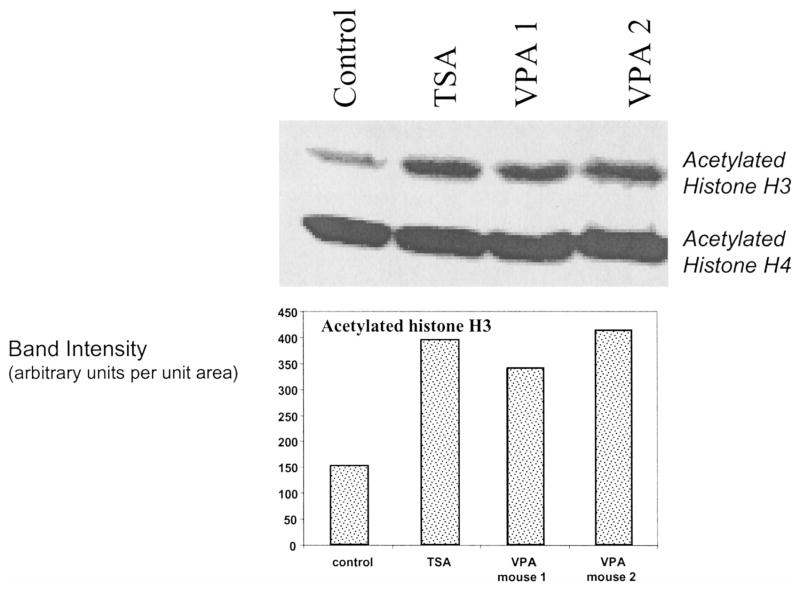
VPA is a class I HDAC inhibitor and is expected to increase acetylation of histone H3. TSA is not selective and is expected to increase acetylation of histones non-specifically. Western blot analyses for acetylated histones in cells derived from the bone marrow done 10 h after the first dose of the HDAC inhibitor (TSA, 0.5 mg/kg; VPA, 600 mg/kg) and 5 h after the second dose (TSA, 0.5 mg/kg; VPA, 300 mg/kg) showed that the acetylated form of histone H3 was increased three- to fourfold relative to the levels of the beta-actin control (Fig. 1). These studies established that both TSA and VPA acetylated histones in our animal model at the doses used.
FIG. 1.
Two- to threefold increases in acetylation of histone H3 relative to the beta-actin control were observed in the bone marrow of mice 10 h after the first dose (TSA: 0.5 mg/kg; VPA: 600 mg/kg) and 5 h after the second dose (TSA: 0.5 mg/kg; VPA: 300 mg/kg) of HDAC inhibitors. Each blot is from an individual mouse.
VPA Increases Endogenous Spleen Colony Formation
The effect of HDAC inhibition on the survival of bone marrow stem cells was assayed using a variant of the spleen colony assay of Till and McCulloch in which endogenous nodules on the spleens of mice are counted after a sublethal dose of TBI (4–6) (Fig. 2). Twelve days after sublethal irradiation (5.5 Gy) plus HDAC inhibitor (400 mg/kg valproic acid given i.p. 1 h after X rays), the number of spleen colonies was sevenfold higher (P < 0.001) than in mice exposed to 5.5 Gy alone. As expected, leukopenia and erythropenia were also significantly improved in mice receiving VPA in combination with radiation (Fig. 2). Also as expected from the spleen colony-forming assay, spleen weights in mice, which were reduced after radiation alone, were significantly increased in mice receiving the HDAC inhibitor plus radiation (data not shown). These results are consistent in showing that HDAC inhibitors mitigate radiation injury to the bone marrow.
FIG. 2.
HDAC inhibition mitigates radiation injury to bone marrow stem cells as well as leukopenia and erythropenia. VPA (400 mg/kg) was administered 1 h after TBI (5.5 Gy).
Effect of HDAC Inhibitor Dose on Lethality of TBI
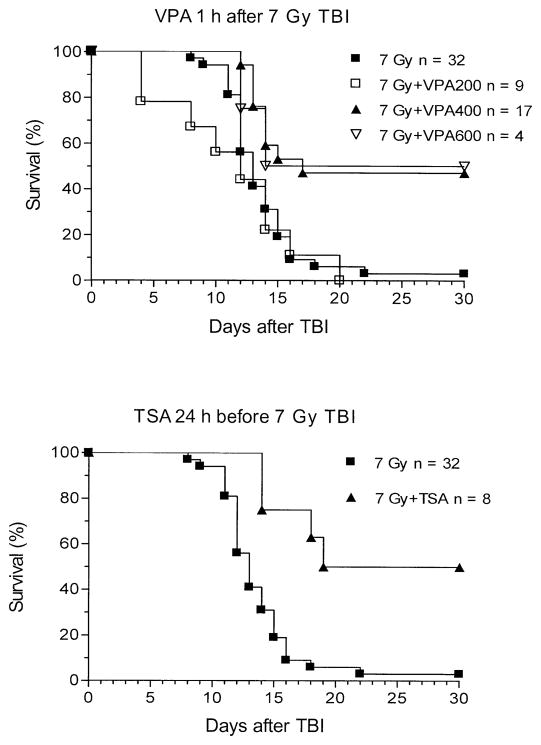
The effect of different doses of HDAC inhibitors on the survival of irradiated mice was studied. VPA given 24 h before radiation increased the chance that mice would survive a lethal dose TBI (Fig. 3). Without VPA, no mice survived a radiation dose of 7 Gy, and half of the mice had died by 12 days after irradiation. A single dose of 200 mg/kg VPA yielded no benefit. A dose of 400 or 600 mg/kg extended the median survival to 14 to 17 days respectively, resulted in about half of the mice surviving for at least 2 months.
FIG. 3.
Groups of BALB/c mice were exposed to TBI alone (7 Gy) alone or in combination with VPA at 200, 400 or 600 mg/kg. When VPA followed TBI by 1 h, a dose of at least 400 mg/kg resulted in increased survival. VPA at 200 mg/kg was not effective (P < 0.05 compared with VPA at 400 mg/kg, log rank test). The figure illustrates the increased survival after 7 Gy TBI when TSA (0.5 mg/kg) was administered 24 h before TBI.
Time and Sequence of Treatment with HDAC Inhibitors on Lethality of TBI
The effect of HDAC inhibitors on the survival of mice after a radiation exposure was studied for various treatment sequences and times. For both the lethal (6.5 Gy), and the supralethal (7 Gy) radiation dose, VPA administration enhanced the survival of irradiated mice. Less than half of a group of 18 mice survived 6.5 Gy TBI alone, whereas VPA (600 mg/kg) administered 24 h before 6.5 Gy resulted in 100% survival (n = 4). Similarly, 50% of a group of 32 mice exposed to 7 Gy were dead 13 days after exposure (i.e. ED50= 13 days), whereas VPA (400 mg/kg) administered 24 h before or 1 h after the radiation exposure resulted in an improved ED50 of 17 days (n = 17) and 26.5 days (n = 12), respectively. All mice that survived until day 17 or day 18 after radiation exposure appeared to be healthy and lived until the end of the study (at least 30 days).
Significant radiation protection was observed when VPA was given 24 h before or 1 h after whole-body irradiation (Fig. 3). VPA (600 mg/kg) given 24 h before 6.5 Gy TBI increased survival significantly (one-sided logrank test, P < 0.05). For 7.0 Gy TBI, 600 mg/kg VPA given 24 h before or 1 h after irradiation increased survival significantly (P < 0.0001). However, it is of note that although HDAC inhibitors reduced the lethality of TBI when administered 24 h prior to irradiation and between 1 and 6 h after exposure, when VPA was given 1 h before radiation exposure, survival was not increased (data not shown).
Figure 4 summarizes the effect of the timing and sequence of administration of VPA with respect to radiation exposure. An increase in survival was observed when VPA was administered either 24 h before or shortly (1 h or 4 h) after the exposure.
FIG. 4.
Kinetics of protection against and mitigation of death induced by TBI by an HDAC inhibitor. The mean percentage increase in survival time in days is shown for groups of BALB/c mice receiving TBI and VPI at 400 mg/kg relative to survival time after TBI (7 Gy) alone. Error bars represent the range of survival times. n = 17, 32, 12 and 16 mice per group for 24 h before, 7 Gy alone, 1 h after, and 4 h after, respectively.
DISCUSSION
This report presents the first observation that HDAC inhibitors improve survival when given either before or after TBI. Groups of mice were exposed to whole-body radiation doses between 5.5 and 7.0 Gy alone or in combination with VPA. An increase in survival was observed whether VPA (400 or 600 mg/kg) was administered before or after TBI. HDAC inhibitors such as those we used are FDA approved and have been demonstrated to be safe for humans with minimal toxicities. Their use should be considered for victims exposed to radiation as a result of an accident or terrorism or for patients as part of cancer therapy.
It would appear from the complexities of the timing and sequence of treatment that the biological responses to HDAC inhibitors depend on more than one mechanism. One explanation is that the reduction in lethality with administration of HDAC inhibitors before irradiation is a result of increased repair of DNA damage. It has been shown in vitro that HDAC inhibitors increase the rate of non-homologous end joining of DNA double-strand breaks (7). It is plausible that in vivo a time window of opportunity exists for administration of HDAC inhibitors to protect against a subsequent radiation exposure through a similar increase in DNA repair. The time window would depend on the bio-availability of the drug relative to the radiation exposure. After radiation exposure, our data are consistent with the mitigating effect of HDAC inhibitors on radiation injury being mediated by the proliferative effect of HDAC inhibitors on the proliferation of bone marrow cells.
It has been generally accepted since the early 1900s that stem cells are relatively sensitive to radiation-induced cytotoxicity. Indeed, even after low doses of radiation such as 0.5 Gy, a relatively large proportion of stem cells are killed. However, a normal response of stem cells is self-replication, and in response to a radiation, particularly a large dose, stem cells up-regulate proliferation and self-renewal.
The experimental observations that HDAC inhibitors mitigate the lethality of TBI are consistent with the observations of others that HDAC inhibitors enhance the self-renewal of bone marrow hematopoietic stem cells (2, 3). Furthermore, the effect of HDAC inhibitors on radiation-induced leukopenia, erythropenia, spleen weights and spleen colony-forming units are all consistent with the known effect of HDAC inhibitors on radiation injury. The mechanism of action of HDAC is complex since HDAC inhibition affects numerous mechanisms including apoptosis, differentiation and transcription, etc. The role of HDAC inhibitors in modulating immunity and inflammation has recently been elucidated (8). Wnt-1, a glycoprotein, has a role in many of these processes and is likely a key player in the differential effect of radiation on normal and many tumor cells. Wnt signaling has a major role in self-renewal of hematopoietic stem cells (9), and both VPA and TSA alter Wnt signaling in human and animal cells (7).
One may postulate that a strategy designed to stimulate stem cells exposed to radiation may result in the propagation of a lineage of damaged cells that are potentially susceptible to secondary cancers. Putting aside the argument that immediate survival outweighs the perceived risk of possible future cancers, the issue of increased radiation carcinogenesis was addressed experimentally and was shown not to be a major concern, at least for skin exposures. HDAC inhibitors reduced the number of skin tumors induced in response to a radiation exposure in addition to the late skin fibrosis (1). The reduction of radiation carcinogenesis was a result of down-regulation of oncogenes such as c-Jun, Myc and Bcl-2 by HDAC inhibitors and subsequent reduction in inflammatory cytokines such as interleukins, TNF-α and TGF-β. At least in skin, the potential utility of topically applied HDAC inhibitors to reduce radiation-related toxicities after skin irradiation has been demonstrated (1). We note that the topical doses used by Chung et al. (1) were high compared to the clinically relevant systemic doses used in the present studies, so confirmatory studies regarding the effect of HDAC inhibitors on radiation carcinogenesis are warranted. Despite this uncertainty, the results presented indicate that HDAC inhibitors represent a new class of radiation protectors and mitigators against total-body irradiation and can produce a significant reduction in injury even when administered after the radiation exposure.
The use of HDAC inhibitors as radioprotectors/mitigators in other physiological situations requires further study since HDAC inhibitors appear to have the novel characteristic of simultaneously providing radiation protection/mitigation to normal tissue stem cells while causing tumors to be more sensitive to radiation.
Acknowledgments
These studies were supported by a Center for Medical Countermeasures against Radiation injury (CMCR) grant from NIH/NIAID U19AI067734-020005 (PI of center: John E. Moulder; Director of project: Jae Ho Kim). The authors appreciate the supportive and insightful discussions with Dr. Richard J. Hatchett, NIH/NIAID.
References
- 1.Chung YL, Wang AJ, Yao LF. Antitumor histone deacetylation inhibitors suppress cutaneous radiation syndromes. Implications for increasing therapeutic gain in cancer radiotherapy. Mol Cancer Ther. 2004;3:317–325. [PubMed] [Google Scholar]
- 2.Young JC, Wu S, Hansteen G, Du C, Sambucetti L, Remiszewski S, O’Farrell AM, Hill B, Lavau C, Murray LJ. Inhibitors of histone deacetylase promote hematopoietic stem cells renewal. Cytotherapy. 2004;6:328–336. doi: 10.1080/14653240410004899. [DOI] [PubMed] [Google Scholar]
- 3.De Felice L, Tatarelli C, Mascolo MG, Gregorj C, Agostini F, Fiorini R, Gelmetti V, Pascale S, Padula F, Nervi C. Histone deacetylase inhibitor valproic acid enhances the cytokine-induced expansion of human hematopoietic stem cells. Cancer Res. 2005;65:1505–1513. doi: 10.1158/0008-5472.CAN-04-3063. [DOI] [PubMed] [Google Scholar]
- 4.Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 5.McCulloch EA, Till JE. The radiation sensitivity of normal mouse bone marrow cells, determined by quantitative marrow transplantation into irradiated mice. Radiat Res. 1960;13:115–125. [PubMed] [Google Scholar]
- 6.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 7.Wojewodzka M, Kruszewski M, Buraczewska I, Xu W, Massuda E, Zhang J, Szumiel I. Sirtuin inhibition increases the rate of non-homologous end-joining of DNA double strand breaks. Acta Biochim Pol. 2007;54:63–69. [PubMed] [Google Scholar]
- 8.Brogdon JL, Xu Y, Szabo SJ, An S, Buxton F, Cohen D, Huang Q. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood. 2007;109:1123–1130. doi: 10.1182/blood-2006-04-019711. [DOI] [PubMed] [Google Scholar]
- 9.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]