Abstract
This study is an extrapolation of our previous one (part I) concerned with the formulation and physicochemical evaluation of a novel, simple, monolayer, easy-to-use, cost-effective, and aesthetically acceptable bioadhesive transdermal patch for tramadol hydrochloride. The current work is focused on bioadhesion, skin tolerability, and pharmacodynamic evaluation. Using naked rat skin, chitosan–Eudragit® NE30D (1:1) film attained best bioadhesive properties. During in vivo studies, it also showed a significantly extended analgesic effect compared to both oral formula and chitosan single polymeric film using the hot plate test method. All the polymeric films were skin tolerable for the intended period of application according to the Draize test. The success of our approach can proudly, positively contribute into the world of pain management and arguably push transdermal delivery to realize its great promise.
Key words: analgesia, bioadhesion, pain, tramadol hydrochloride, transdermal
INTRODUCTION
A main concern of health professionals nowadays is to improve the management of pain. Many treatment approaches are being used to reduce and alleviate pain, but unfortunately, there are still many unmet goals (1).
Tramadol is a potent atypical centrally acting analgesic offering many advantages over conventional opioids and nonsteroidal anti-inflammatory drugs, in particular in elderly and high-risk patients. Although not a new opioid, tramadol has been recently rediscovered and widely used; this may be due to its unique dual mechanism of action contributing to its favorable chronic safety profile together with its high potency which is comparable to pethidine. It is extensively metabolized in liver with the M1 metabolite having a higher affinity to opioid receptors than the parent drug (2). It is a freely soluble drug having a short half-life and consequently requires a high administration frequency. Hence, a judicious formulation is crucial to optimize tramadol clinical effect.
Transdermal delivery is a new modality of administration of tramadol offering a dual additional opportunity over all its well-known advantages, which is overcoming the drawback of the drug bitter taste (3) and decreasing any possible abuse and addiction potential by partially avoiding the formation of M1 metabolite, by avoiding peak and trough plasma levels, and by reducing the total amount of medication input.
Our target was to provide patients with an increased control over the management of their distress using an esthetically favored, easy-to-handle, painless, cost-effective, convenient, therapeutically effective, beneficial, and well-tolerated controlled analgesic delivery system ameliorating their functional status, ability to work, and quality of life. This study is complementary to our previous research (part I) and is concerned with bioadhesion study, skin irritation test, and pharmacodynamic evaluation.
Bioadhesion is defined as the attachment of synthetic or biological macromolecules to a biological tissue (4). In the Drug Quality Reporting System, the US Food and Drug Administration has received numerous reports of “adhesion lacking” for transdermal drug delivery systems (TDDS). The adhesion of the TDDS is critical to the safety, efficacy, and quality of the product. Firstly, the therapeutic effect of the drug is linked to the adhesive performance of the TDDS. Reduction in the surface area of contact as a result of patch lift, or even the patch falling off, diminishes the delivery of drug from the patch. In other words, poor adhesion results in improper dosing of patients. Secondly, patches that fail to adhere for their prescribed time period must be replaced more often, thereby increasing the patient’s expenses. All the previous reports provide an overview of the significance of adhesiveness of a transdermal drug delivery system and the necessity for adhesion testing (5). Adequate skin adhesion is one of the most important functional properties of TDDS, to securely, completely, and consistently locate the devices on the skin for the intended period of time as well as to be easily removed when required leaving no, or at least low, residue on the skin. The residue left upon removal is related to both internal cohesion of the film and adhesion to the surfaces or membranes. Adhesion or the lack of adhesion of transdermal systems to the skin is a critical factor directly related to drug delivery and therapeutic effect. Since the drug absorption process is related to the drug partitioning between the TDDS and the skin and the drug permeation process, complete skin contact over the entire delivery surface for the entire application period is essential. If the TDDS lifts or partially detaches, the effective area of TDDS/skin contact and thus the drug absorption changes in an unpredictable manner. Therapeutic failure can then occur. Only a constant TDDS/skin contact over the whole application period allows a consistent delivery and absorption of the drug (6). In other words, the quality of contact between patch and skin is directly reflected in the consistency of drug delivery.
Adhesion to skin, because of interatomic and intermolecular attractive forces, is established at the interface, provided that intimate contact is achieved. To obtain this degree of contact, the film must be soft and flexible. After the initial adhesion, the TDDS/skin bond can be built by stronger interactions (e.g., hydrogen bonding), which will depend on skin characteristics and other parameters (5).
Skin irritation is a common problem encountered with TDDS limiting its wide acceptance among patients in spite of its obvious benefits; this may be caused by excessive occlusion of the skin and decreased breathing providing an environment for microbial proliferation, exfoliation of the stratum corneum or deeper layers during adhesive removal; these may affect skin integrity (7).
MATERIALS AND METHODS
Materials
Tramadol hydrochloride (T) was kindly provided by ADWIA Corp., Egypt. Chitosan, highly viscous, was purchased from Fluka, BioChemika, Japan. Eudragit® NE 30D, Eudragit® RS PO, and Eudragit® RL PO were generously donated by Rohm Pharma, Darmstadt, Germany. Polyethylene glycol 400 (PEG 400) was purchased from S.D. Fine-Chem Ltd., Mumbai, India. All other reagents were of pharmaceutical grade.
Methods
Bioadhesion Studies
Chatillon® apparatus was used to study the bioadhesive properties of the formulated films. Full-thickness naked abdominal rat skin was attached with cyanoacrylate adhesive to the moving lower platform. Film segment (1 cm2) was attached to the upper probe using double-sided adhesive tap. Adhesion was initiated by adding a defined amount of water (50 μl) over the skin sample. The platform was raised until the patch come in contact with the skin and a force of 20 g was applied for 2 min. The lower probe was then moved downward at a speed of 1 mm/s. Adhesion force (F) was recorded as the force required to detach the sample from the surface of the excised skin, and the work of adhesion (W) was representing the area under the force distance curve (8,9).
Pharmacological Evaluation
Animal experiments were conducted in compliance with local, national, ethical, and regulatory principles and local licensing regulations, per the spirit of Association for Assessment and Accreditation of Laboratory Animal Care.
Skin Irritation Test
The irritancy of the two films selected for pharmacological investigation was evaluated in male Wistar rats (150–200 g) based on the method described by Draize et al. (10). The dorsal side of the rats was shaved 24 h before the beginning of the experiment. In order to transform qualitative observations of physiological effects to reasonably quantitative objective measurements and in order to obtain data easily subject to arithmetical interpretation, Draize et al. have applied the principle of assigning numerical values to physiological phenomena. The animals were divided into different groups: Group A served as control (no treatment), group B received 0.5 ml of a 0.8% v/v aqueous formalin solution as a standard irritant (11), groups C and D received medicated and unmedicated chitosan film, respectively, and groups E and F received medicated and blank E4 [chitosan–Eudragit® NE30D (1:1)] film, respectively. A new patch (or formalin solution) was applied daily for 72 h. The application sites were examined for edema and erythema at 24 and 72 h and graded (0–4) according to a visual scoring scale (Table I); the final score represents the average of the 24- and 72-h readings. The primary irritancy index (PII) was determined for each preparation by adding the edema and erythema scores; the formulations were accordingly classified as nonirritant if PII <2, irritant if PII = 2–5, and highly irritant if PII = 5–8. The data were analyzed statistically by the one-way analysis of variance (ANOVA) test followed by the least significant difference procedure. This statistical analysis was computed using the SPSS® software.
Table I.
Evaluation of Skin Reactions
| Skin reaction | Score |
|---|---|
| Erythema formation | |
| None | 0 |
| Very slight erythema | 1 |
| Well-defined erythema | 2 |
| Moderate to severe erythema | 3 |
| Severe erythema and scar formation | 4 |
| Edema formation | |
| None | 0 |
| Very slight edema | 1 |
| Slight edema (edges of area well defined by definite raising) | 2 |
| Moderate edema (area raised approximately 1 mm) | 3 |
| Severe edema (raised more than 1 mm and extending beyond area of exposure) | 4 |
Evaluation of the Pharmacodynamic Activity
The pharmacodynamic activity of the selected monolithic matrix films in terms of analgesic effect was evaluated using the hot plate method in rats according to the method of Woolfe and MacDonald (12) and the modified method of O’Neill (13). A commercially available apparatus consisting of acrylic resin cage and a thermocontrolled aluminum plate maintained at 55 ± 0.5°C was used (14). All the experiments were performed with adult male healthy Wistar rats (National Research Center, Dokki, Egypt) weighing between 150 and 200 g. Before using them in this study, the animals were housed for 7 days under constant environmental and nutritional conditions. Prior to the experiment, the rats were fasted overnight and were uniformly hydrated each with 3 ml water before the administration of the test formulae to reduce variation. The rats were randomly allocated to four groups (control, oral, and two transdermal groups) of six rats each. The selected adhesive films, chitosan and chitosan–Eudragit NE (1:1) [E4] films, were applied to the rats naked dorsal region (group 3 and 4, respectively) which was shaved 24 h before the experiment and were compared with Amadol® capsules (ADWIA Drug Company, Egypt) which was given to group 2 by oral gavage. Each rat received the specified formula in a dose equivalent to 80 mg/kg (14,15). The rats were placed on the thermocontrolled aluminum plate, and the response latency was evaluated on the basis of either hind paw lick or jump reaction. Basal reaction times (t = 0) were recorded before drug administration and were between 4 and 7 s, and the cutoff time was 30 s (15). The latency time was recorded again after drug administration at 1, 2, 3, 4, 5, 6, 24, and 48 h. The mean and standard deviation were calculated for each group.
The paw of mice and rats are very sensitive to heat at temperatures which are not damaging to skin. The responses are jumping, withdrawal, or licking of the paws. The time taken until these responses occur (latency time) is prolonged after administration of, specifically, centrally acting analgesics (16). The latency time was recorded and the percentage increase of latency time was calculated as follows: 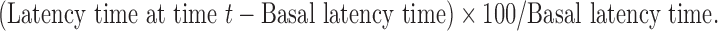 The percentage increase of latency time was plotted against time. The area under the plasma concentration–time curve from 0 to 48 h (AUC(0–48 h)), Emax (maximum analgesic effect), and Tmax (time for peak effect) were calculated from the curves. The data were analyzed statistically by the one-way ANOVA test followed by the least significant difference procedure using the SPSS® software.
The percentage increase of latency time was plotted against time. The area under the plasma concentration–time curve from 0 to 48 h (AUC(0–48 h)), Emax (maximum analgesic effect), and Tmax (time for peak effect) were calculated from the curves. The data were analyzed statistically by the one-way ANOVA test followed by the least significant difference procedure using the SPSS® software.
RESULTS AND DISCUSSION
Bioadhesion Test
Although in vivo human skin testing is the most reliable method for evaluation of TDDS, the time, safety, and money involved in human trials prohibit the extensive use of in vivo testing methods (5). The obtained results were generally more or less correlating together with the preliminary screening in terms of macroscopic and organoleptic properties done on human skin (part I), which is very promising.
Table II is showing the bioadhesion behavior of the films under investigation. The bioadhesive properties of chitosan are well known (17). Chitosan showed the highest adhesive force, also the distance to separate the bioadhesive film from the naked rat skin was large; therefore, the work of adhesion was markedly high. According to one of theories, hydration of the polymer causes mobilization of the polymer chains and hence influences polymeric adhesion (18,19). The adequate water absorption capacity together with the cationic nature which promote binding to the negative surface of the skin (20) can interpret the good adhesion behavior of chitosan. The plasticizer glycerin can also enhance bioadhesion (18).
Table II.
Bioadhesion of Polymeric Monolithic Matrix Films
| Film | Adhesive force (N) ± SD | Work of adhesion (N cm) ± SD |
|---|---|---|
| Chitosan | 1.20 ± 0.04 | 3.84 ± 0.33 |
| Eudragit NE 30D | 0.27 ± 0.03a | 0.41 ± 0.04a |
| Eudragit RL PO | 0.40 ± 0.03a | 0.57 ± 0.03a |
| Eudragit RS PO | 0.30 ± 0.01a | 0.42 ± 0.02a |
| E4 [chitosan–Eudragit NE (1:1)] | 0.70 ± 0.06b,e | 1.72 ± 0.06b,f,g |
| E5 [chitosan–Eudragit® RL PO (1:1)] | 0.70 ± 0.05c | 1.12 ± 0.05c |
| E6 [chitosan–Eudragit® RS PO (1:1)] | 0.60 ± 0.04d | 0.89 ± 0.08d |
Each experiment was done in triplicate
SD standard deviation
a p < 0.001 significant when compared to chitosan film;
b p < 0.001 significant when compared to Eudragit NE 30D film;
c p < 0.001 significant when compared to Eudragit RL PO film;
d p < 0.001 significant when compared to Eudragit RS PO film;
e p < 0.05 significant when compared to E6 film;
f p < 0.05 significant when compared to E5 film;
g p < 0.01 significant when compared to E6 film
Eudragit® NE film showed the least adhesive force; this may be due to the absence of plasticizer in NE film which does not need it according to Rohm Pharma specification. RL type showed more satisfactory adhesion than RS type, this may be due to the more hydrophilic nature of RL (21) which favor water uptake (previously discussed in part I) and consequently adhesion (18,19). The cationic nature of Eudragits® together with the presence of PEG 400 (in RL and RS types) may have a positive effect on their adhesive properties. The addition of RL or RS types to NE type ameliorates the adhesive properties of the latter. As expected, because chitosan film has attained the highest adhesion compared to other single polymer films, its blending with other polymers ameliorated the bioadhesion of the latter.
Chitosan–Eudragit® composite films showed satisfactory bioadhesion with E4 film attaining the best. The combination of chitosan and Eudragit® had a positive impact on bioadhesion of the resultant films when compared to Eudragit® films. The beneficial intrinsic properties and bioadhesive nature of chitosan (17,20), the positive charge on Eudragit®, the effect of the plasticizer (22) together with the internal cohesive strength of Eudragit® matrix (detected in part I) may account for the acceptable bioadhesion of E4, E5 [chitosan–Eudragit® RL PO (1:1)] and E6 [chitosan–Eudragit® RS PO (1:1)] films, with the E4 film showing the highest work of adhesion. The peak adhesive force was found to be equal to 0.70 ± 0.06, 0.70 ± 0.05, and 0.60 ± 0.04 N, respectively, although it was expected for E5 to attain the highest value due to its more hydrophilic nature by presence of RL type (21) promoting hydration and perhaps adhesion (18,19). This may be due to the inhabitual presence of glycerin with NE molecules which have certainly allowed for certain polymer(s)–plasticizer and drug–plasticizer interaction to take place, favoring its binding to skin. The additional presence (compared to NE film) of a plasticizer which is water-soluble and so can easily penetrate E4 film structure renders the film more hydrophilic, favoring more water absorption into the polymer, which may have a positive impact on bioadhesion. Another interpretation is that with the addition of the plasticizer, the network may become less dense because of an increase in the mobility of the polymeric chains and in the free volume between the chains, causing the polymer network to relax (23) and therefore may better conform to skin.
Blending of chitosan with a synthetic polymer, Eudragit® NE 30D, forming E4 composite film, allowed us to attain the targeted physicochemical properties (part I) together with adequate bioadhesion, and therefore, it was subjected to further pharmacological investigation to guarantee the aimed tramadol delivery system performance.
Statistical analysis shown in Table II is done using the one-way ANOVA followed by the least significant difference procedure.
Pharmacological Activity
E4 film was selected for further pharmacological investigation because it attained the most optimized bioadhesiveness as well as further physicochemical properties studied in part I.
Skin Irritation Test
Testing the blank and active formulations allows the characterization of the contribution of each component of the transdermal therapeutic system (TTS) to any observed dermal effect (24). Table III shows the erythema and edema rating scores observed after application of the proposed formulae or the formalin solution (standard irritant). According to Draize et al. (10), all the tested transdermal films were considered to be nonirritant [PII <2]. Statistical analysis using the one-way ANOVA followed by the least significant difference procedure (Table III) showed that, compared to the control, the formalin solution was found to be significantly irritant [PII = 6.25 ± 1.24] (p < 0.001), while all transdermal preparations were nonirritant (p > 0.05). Statistical analysis has also shown that the formalin solution was significantly irritant (p < 0.001) compared to all the transdermal films, while there was no significant difference (p > 0.05) between all the transdermal films. These results proved the nonirritancy of the drug or any of the film components and showed that the innovated films are safe to be applied to the skin for the intended period of time.
Table III.
Data of the Skin Irritation Test
| Rat | Gp A | Gp B | Gp C | Gp D | Gp E | Gp F | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Er. | Ed. | Er. | Ed. | Er. | Ed. | Er. | Ed. | Er. | Ed. | Er. | Ed. | |
| 1 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 2 | 0 | 0 | 3 | 2.5 | 0 | 0 | 1 | 0 | 1 | 0 | 1.5 | 0 |
| 3 | 0 | 0 | 4 | 4 | 0.5 | 0 | 0.5 | 0 | 0.5 | 0 | 1 | 0 |
| 4 | 0 | 0 | 4 | 3 | 1 | 0 | 1 | 0 | 1 | 0 | 0.5 | 0 |
| 5 | 0 | 0 | 4 | 2.5 | 0 | 1 | 0 | 0.5 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 3.5 | 2 | 1 | 1 | 0.5 | 0 | 1 | 0 | 1 | 0 |
| Av. | 0.00 | 0.00 | 3.58 | 2.67 | 0.42 | 0.33 | 0.50 | 0.08 | 0.58 | 0.00 | 0.83 | 0.00 |
| SD | 0.00 | 0.00 | 0.49 | 0.75 | 0.49 | 0.52 | 0.45 | 0.20 | 0.49 | 0.00 | 0.52 | 0.00 |
| PII | 0.00 ± 0.00 | 6.25 ± 1.24a | 0.58 ± 0.49b,c | 0.83 ± 0.52b,c | 0.38 ± 1.01b,c | 0.29 ± 0.65b,c | ||||||
GP group, Er. erythema, Ed. edema, Av average, SD standard deviation, PII primary irritancy index
a p < 0.001 significant when compared to group A;
b p < 0.001 significant when compared to group B;
c p > 0.05 nonsignificant when compared to group A
Pharmacodynamic Efficacy
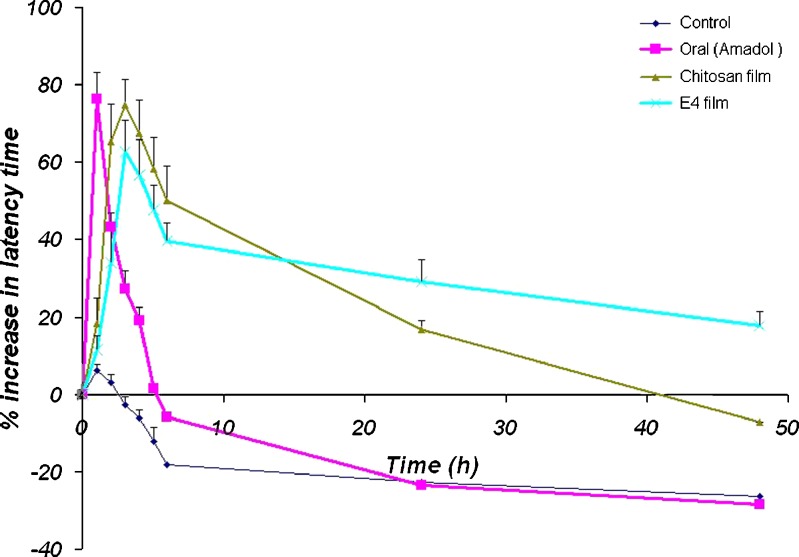
From the examination of the percentage increase of latency time data (Fig. 1), we can notice that the control group showed a very small increase in latency time, as the rats can withstand more heat after two trials; this returned to normal after 3 h, while the oral group attained a sudden and sharp increase in latency time (peak time = 1 h) which then detrimentally decreased quickly to reach basal level after about 4.5 h and while the transdermal groups showed a reasonably gradual increase in latency time till reaching peak effect, after about 3 h, which then decreased slowly and gradually. The latency time in chitosan group decreased to basal values after about 40 h. The latency time in chitosan–Eudragit® (1:1) [E4] groups did not attain basal values till the end of the experiment at 48 h. These results attributed a good correlation with the in vitro results considering the release and permeation profiles (part I) of the selected patches and evidenced the feasibility of avoiding sharp peak and trough drug effects and its associated limitations and arguably provided an alternative simplified dosing regimen for administration of tramadol.
Fig. 1.
Antinociceptive effect of tramadol HCl in male Wistar rats using the hot plate method
Statistical analysis of the data of Emax presented in Table IV using one-way ANOVA test followed by the least significant difference procedure showed a significant difference (p < 0.001) between the control group and all other groups. Also, there was a significant difference (p < 0.01) between group 2 and group 4, while there is a nonsignificant difference (p > 0.05) between group 2 and group 3. In between the transdermal groups, statistical analysis showed a significant difference (p < 0.05) between group 4 and group 3.
Table IV.
Maximum Antinociceptive Effect of Tramadol HCl
| Rat | E max | |||
|---|---|---|---|---|
| Control | Oral | Transdermal | ||
| Chitosan | E4 | |||
| Group 1 | Group 2 | Group 3 | Group 4 | |
| 1 | 3.33 | 65.67 | 70.00 | 69.23 |
| 2 | 4.84 | 73.91 | 63.16 | 63.79 |
| 3 | 3.57 | 72.58 | 64.18 | 58.33 |
| 4 | 6.56 | 81.03 | 72.41 | 74.55 |
| 5 | 4.24 | 61.76 | 69.64 | 58.33 |
| 6 | 1.89 | 66.67 | 68.42 | 51.61 |
| Mean | 4.07 | 70.27 | 67.97 | 62.64 |
| SD (±) | 1.57 | 6.34 | 7.20 | 7.59 |
SD standard deviation
Some recent studies had mentioned that a once-daily modified release formula of tramadol HCl would be advantageous compared to the immediate release preparations as it prevents drug plasma peaks responsible for more severe adverse effects while providing a 24-h pain relief; this may benefit patients experiencing pain throughout the dosing interval and improve pain-related sleep parameters and physical functions (2,25). As a result of the additional simplified dosing regimen, patient compliance should be improved.
Statistical analysis of the data of Tmax listed in Table V showed a nonsignificant difference (p > 0.05) between group 1 and group 2, but a significant difference (p < 0.05) between group 1 and group 3 or 4. Statistical analysis between oral and transdermal groups showed significant difference (p < 0.05) between oral group and groups 3 and 4 confirming a somewhat satisfactory gradual increase in the analgesic effect of the transdermal formulae till reaching its peak followed then by a gradual decline in effect, compared to a sudden and sharp increase in effect associated with oral administration followed by a rapid decrease. In between the transdermal groups, there was no significant difference (p > 0.05).
Table V.
Time for Maximum Antinociceptive Effect of Tramadol HCl
| Rat | T max (h) | |||
|---|---|---|---|---|
| Control | Oral | Transdermal | ||
| Group 1 | Group 2 | Group 3 | Group 4 | |
| 1 | 1.00 | 1.00 | 3.00 | 3.00 |
| 2 | 1.00 | 1.00 | 4.00 | 3.00 |
| 3 | 1.00 | 1.00 | 2.00 | 3.00 |
| 4 | 2.00 | 1.00 | 3.00 | 3.00 |
| 5 | 2.00 | 1.00 | 3.00 | 3.00 |
| 6 | 1.00 | 1.00 | 3.00 | 3.00 |
| Mean | 1.33 | 1.00 | 3.00 | 3.00 |
| SD (±) | 0.47 | 0.00 | 0.58 | 0.00 |
SD standard deviation
The antinociceptive effect expressed in terms of AUC0–48 h manifested very promising results (Table VI). The AUC of all transdermal groups was far higher than the commercial oral formula, with that of E4 > chitosan films, running in parallel with the release and permeation patterns of each, monitored during physicochemical evaluation. Statistical analysis of the data using one-way ANOVA test followed by the least significant difference procedure showed a nonsignificant difference (p > 0.05) between control and oral group and a significant difference (p < 0.001) between the control and all transdermal groups. Also, there is a significant difference (p < 0.001) between the oral group and all the transdermal groups. In between the transdermal groups, we can notice a significant difference (p < 0.001) between group 3 and group 4.
Table VI.
Antinociceptive Effect of Tramadol HCl
| Rat | AUC0–48 h | |||
|---|---|---|---|---|
| Control | Oral | Transdermal | ||
| Group 1 | Group 2 | Group 3 | Group 4 | |
| 1 | 6.60 | 104.98 | 720.00 | 1,547.23 |
| 2 | 9.68 | 133.15 | 685.27 | 1,404.76 |
| 3 | 7.14 | 148.90 | 785.41 | 1,235.84 |
| 4 | 14.76 | 146.37 | 881.46 | 1,600.74 |
| 5 | 7.63 | 113.24 | 816.57 | 1,205.18 |
| 6 | 3.78 | 126.11 | 608.00 | 1,410.47 |
| Mean | 8.27 | 128.71 | 749.45 | 1,400.70 |
| SD (±) | 3.38 | 16.22 | 89.66 | 145.54 |
AUC (0–48 h) area under the plasma concentration–time curve from 0 to 48 h, SD standard deviation
Besides being more efficient with regard to the amount of medication input relative to the total amount of medication present in the transdermal system and therefore decreasing abuse liability secondary to less medication actually administered (26), these investigations supply us with hopeful results about the efficiency of transdermal delivery of opioids, particularly tramadol in an effort to optimize effect while limiting diversion and abuse. A recent study has focused on the proven efficacy and safety profile and the low potential for abuse which make extended-release tramadol a viable therapeutic option for the management of chronic/persistent nonmalignant pain in some patients (25).
CONCLUSION
This study has supplied us with brightening results not only concerning the questionable equipotent therapeutic efficacy of transdermal versus oral tramadol but also concerning the intertransdermal formulae variability. The results showed that we are in front of a patient-friendly flexible delivery system which allows obtaining versatile properties and performances by just simple, yet of high significance, modifications. Therefore, although already proved, this study can assure that the use of polymeric systems appears to be an attractive way enabling the tailoring of the intended formula. Also, switching to a transdermal formula seems to improve patient acceptance of the drug from which many complain during oral administration due to its bitter taste and some reported gastrointestinal side effects (3,27). A patch seems to be a new look among tramadol pharmaceutical dosage forms providing additional privileges in parallel with the obvious well-known advantages of transdermal delivery.
Contributor Information
Hussein O. Ammar, Phone: +20-12-4472851, FAX: +20-2-3370931, Email: husseinammar@hotmail.com
Rabab Kamel, Email: drrababk@hotmail.com.
References
- 1.Guindon J, Walczak J, Beaulieu P. Recent advances in the pharmacological management of pain. Drugs. 2007;67:2121–2133. doi: 10.2165/00003495-200767150-00002. [DOI] [PubMed] [Google Scholar]
- 2.Malonne H, Coffiner M, Fontaine D, Sonet B, Sereno A, Peretz A. Long-term tolerability of tramadol LP, a once-daily formulation, in patients with osteoarthritis or low back pain. J Clin Pharm Therap. 2005;30:113–120. doi: 10.1111/j.1365-2710.2004.00624.x. [DOI] [PubMed] [Google Scholar]
- 3.Radbruch L, Grond S, Lehmann KA. A risk–benefit assessment of tramadol in the management of pain. Drug Safety. 1996;15:8–29. doi: 10.2165/00002018-199615010-00002. [DOI] [PubMed] [Google Scholar]
- 4.Peppas NA, Buri PA. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J Cont Rel. 1985;2:257–275. doi: 10.1016/0168-3659(85)90050-1. [DOI] [Google Scholar]
- 5.Wokovich AM, Prodduturi S, Doub WH, Hussain AS, Buhse LF. TDDS adhesion as a critical safety, efficacy and quality attribute. Eur J Pharm Biopharm. 2006;64:1–8. doi: 10.1016/j.ejpb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Minghetti P, Cilurzo F, Montanari L. Evaluation of adhesive properties of patches based on acrylic matrices. Drug Dev Ind Pharm. 1999;25:1–6. doi: 10.1081/DDC-100102135. [DOI] [PubMed] [Google Scholar]
- 7.Zhai H, Maiback H. Effects on skin occlusion on percutaneous absorption: an overview. Skin Pharmacol Appl Skin Physiol. 2001;14:1–10. doi: 10.1159/000056328. [DOI] [PubMed] [Google Scholar]
- 8.McCarron PA, Donnelly RF, Zawislak A, Woolfson AD. Design and evaluation of a water-soluble bioadhesive patch formulation for cutaneous delivery of 5-aminolevulinic acid to superficial neoplastic lesions. Eur J Pharm Sci. 2006;27:268–279. doi: 10.1016/j.ejps.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Repka MA, Gutta K, Prodduturi S, Munjal M, Stodghill SP. Characterization of cellulosic hot-melt extruded films containing lidocaine. Eur J Pharm Biopharm. 2005;59:189–196. doi: 10.1016/j.ejpb.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Draize J, Woodward G, Calvery H. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82:377–379. [Google Scholar]
- 11.Ubaidulla U, Reddy MVS, Ruckmani K, Ahmad FJ, Khar RK. Transdermal therapeutic system of carvedilol: effect of hydrophilic and hydrophobic matrix on in-vitro and in-vivo characteristics. AAPS Pharm Sci Tech. 2007;8:Article 2. [DOI] [PMC free article] [PubMed]
- 12.Woolfe G, MacDonald AD. The evaluation of the analgesic action of pethidine hydrochloride. J Pharmacol Exp Ther. 1944;80:300–307. [Google Scholar]
- 13.O’Neill KA. An automated, high-capacity method for measuring jump latencies on a hot plate. J Pharmacol Meth. 1983;10:13–18. doi: 10.1016/0160-5402(83)90010-4. [DOI] [PubMed] [Google Scholar]
- 14.Ide S, Minami M, Ishihara K, Uhl GR, Sora I, Ikeda K. Mu opioid receptor-dependent and independent components in effects of tramadol. Neuropharmacol. 2006;51:651–658. doi: 10.1016/j.neuropharm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Sacerdote P, Bianchi M, Manfredi B, Panerai AE. Effects of tramadol on immune responses and nociceptive thresholds in mice. Pain. 1997;72:325–330. doi: 10.1016/S0304-3959(97)00055-9. [DOI] [PubMed] [Google Scholar]
- 16.Vogel HG, Vogel WH. Drug discovery and evaluation, pharmacological assays. Germany: Springer; 1997. p. 370. [Google Scholar]
- 17.Needleman IG, Smales FC, Martin GP. Characterization of bioadhesives for periodontal and oral mucosal delivery. J Clin Periodontol. 1997;24:394–400. doi: 10.1111/j.1600-051X.1997.tb00203.x. [DOI] [PubMed] [Google Scholar]
- 18.Cafaggi S, Leardi R, Parodi B, Caviglioli G, Russo E, Bignard G. Preparation and evaluation of chitosan–poloxamer 407 based matrix for buccal drug delivery. J Cont Rel. 2005;102:159–169. doi: 10.1016/j.jconrel.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Peh KK, Wong CF. Polymeric films as vehicle for buccal delivery: swelling, mechanical, and bioadhesive properties. J Pharmacol Pharm Sci. 1999;2:53–61. [PubMed] [Google Scholar]
- 20.Sathirakul K, How NC, Stevens WF, Chan S. Application of chitin and chitosan bandages for wound healing. Adv Chitin Sci. 1996;1:490–492. [Google Scholar]
- 21.Akhgari A, Farahmand F, Garekani A, Sadeghi A, Vandamme TF. Permeability and swelling studies on free films containing inulin in combination with different polymethacrylates aimed for colonic drug delivery. Eur J Pharm Sci. 2006;28:307–314. doi: 10.1016/j.ejps.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Chickering DE, Matiowitz E. Definitions, mechanisms and theories of bioadhesion. In: Chickering DE, Matiowitz E, editors. Bioadhesive drug delivery systems. New York: Marcel Dekker; 1999. [Google Scholar]
- 23.Zhang X, Wang Y, Wang J, Wang Y, Li S. Effect of pore former on the properties of casted film prepared from blends of Eudragit® NE 30 D and Eudragit® L 30 D-55. Chem Pharm Bull. 2007;55:1261–1263. doi: 10.1248/cpb.55.1261. [DOI] [PubMed] [Google Scholar]
- 24.Staskin DR. Transdermal systems for overactive bladder. Rev Urol. 2003;5:S26–S30. [PMC free article] [PubMed] [Google Scholar]
- 25.Barkin RL. Extended-release Tramadol (ULTRAM ER): a pharmacotherapeutic, pharmacokinetic, and pharmacodynamic focus on effectiveness and safety in patients with chronic/persistent pain. Am J Ther. 2008;15(2):157–166. doi: 10.1097/MJT.0b013e31815b035b. [DOI] [PubMed] [Google Scholar]
- 26.Fudala PJ, Johnson RE. Development of opioid formulations with limited diversion and abuse potential. Drug Alcohol Depend. 2006;83S:S40–S47. doi: 10.1016/j.drugalcdep.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Tiwari SB, Murthy TK, Pai MR, Mehta PR, Chowdary PB. Controlled release formulation of tramadol hydrochloride using hydrophilic and hydrophobic matrix system. AAPS Pharm Sci Tech. 2003;4:1–6. doi: 10.1208/pt040331. [DOI] [PMC free article] [PubMed] [Google Scholar]