Abstract
Dilutable nanoemulsions are potent drug delivery vehicles for ophthalmic use due to their numerous advantages as sustained effect and high ability of drug penetration into the deeper layers of the ocular structure and the aqueous humor. The aim of this article was to formulate the antiglaucoma drug dorzolamide hydrochloride as ocular nanoemulsion of high therapeutic efficacy and prolonged effect. Thirty-six systems consisting of different oils, surfactants, and cosurfactants were prepared and their pseudoternary-phase diagrams were constructed by water titration method. Seventeen dorzolamide hydrochloride nanoemulsions were prepared and evaluated for their physicochemical and drug release properties. These nanoemulsions showed acceptable physicochemical properties and exhibited slow drug release. Draize rabbit eye irritation test and histological examination were carried out for those preparations exhibiting superior properties and revealed that they were nonirritant. Biological evaluation of dorzolamide hydrochloride nanoemulsions on normotensive albino rabbits indicated that these products had higher therapeutic efficacy, faster onset of action, and prolonged effect relative to either drug solution or the market product. Formulation of dorzolamide hydrochloride in a nanoemulsion form offers, thus, a more intensive treatment of glaucoma, a decrease in the number of applications per day, and a better patient compliance compared to conventional eye drops.
Key words: dorzolamide hydrochloride, glaucoma, nanoemulsion, pharmacodynamic, physicochemical characterization
INTRODUCTION
Ophthalmic drug delivery is one of the most interesting and challenging endeavors facing the pharmaceutical scientist (1). It is a common knowledge that the application of eye drops as conventional ophthalmic delivery systems result in poor bioavailability and therapeutic response because of lacrimal secretion and nasolacrimal drainage in the eye (2,3). Most of the drug is drained away from the precorneal area in few minutes. As a result, frequent instillation of concentrated solutions is needed to achieve the desired therapeutic effects (4). But, by the tear drainage, the main part of the administered drug is transported via the nasolacrimal duct to the gastric intestinal tract where it may be absorbed, sometimes causing side effects (5). In order to increase the effectiveness of the drug, a dosage form should be chosen which increases the contact time of the drug in the eye. This may then increase the bioavailability, reduce systemic absorption, and reduce the need for frequent administration leading to improved patient compliance.
To overcome these problems, various ophthalmic vehicles such as suspensions, ointments, inserts, and aqueous gels have been investigated to extend the ocular residence time of medications for topical application to the eye (6). These ocular drug delivery systems offer some improvement over conventional liquid dosage forms but, because of blurred vision (e.g., ointments) or lack of patient compliance (e.g., inserts), they have not been universally accepted. As a result, good ocular bioavailability following topical delivery of a drug to the eye remains a challenge yet to be resolved satisfactorily (7).
Glaucoma is a serious eye disorder characterized by an increase in the intraocular pressure which leads gradually to loss of vision due to damage of the optic disk, usually without symptoms and is the second leading cause of blindness worldwide (8–10). It is believed that glaucoma is a result of an imbalance between aqueous humor secretion and drainage processes within the ocular chamber (11).
Drugs used to treat glaucoma work broadly in one of two ways: either to reduce the production or to increase the drainage of aqueous humor. Dorzolamide hydrochloride was synthesized in the 1980s (12) and was shown to be about 20 times more potent than the carbonic anhydrase inhibitor acetazolamide with regard to the inhibition of carbonic anhydrase isoenzyme II (13), which is thought that this isoenzyme plays a major role in aqueous humor secretion (14). The pKa values of dorzolamide hydrochloride are 6.35 and 8.5 (15) and its apparent partition coefficient is 1.96 for the n-octanol/pH 7.4 buffer system (16).
Topically effective aqueous dorzolamide eye drop solution (Trusopt®) has become one of the most widely used medications for the treatment of open-angle glaucoma since it became commercially available in 1995 (17). The concentration of dorzolamide HCl in Trusopt® is 2.2%, corresponding to 2.0% of the free base, pH 5.65. Hydroxyethyl cellulose is used to increase the viscosity of Trusopt® eye drops to 100 cps; this increased viscosity leads to increased corneal contact time and, consequently, to increased bioavailability (15). However, the relatively low pH and high viscosity have been shown to generate local irritation after topical administration of the eye drops (18).
The objective of our study was to formulate dorzolamide hydrochloride as eye drops capable of delivering the drug in a sustained manner, thus avoiding frequent instillation of the drops which may induce toxic side effects and cellular damage at the ocular surface (19–21). In the meantime, the preparation of dorzolamide eye drops of high therapeutic efficacy and lacking the undesirable effects of the market product (Trusopt®) as irritation and blurred vision (22) is an additional aim of our study. Nanoemulsions as a drug delivery system were utilized in our study for formulating dorzolamide hydrochloride as ocular eye drops in virtue of their distinct advantages (23–25). These include sustained release of the drug applied to the cornea, high penetration in the deeper layers of the ocular structure, and aqueous humor as well as ease of sterilization. Thus, these systems can achieve therapeutic action with a smaller dose and a fewer systemic and ocular side effects.
MATERIALS AND METHODS
Materials
Dorzolamide hydrochloride was obtained from Hetero Drugs Ltd., Hetero House, Erragadda, India. Tween 80 (TW80), isopropyl myristate (IPM), triacetin (glycerol triacetate), and dialysis tubing cellulose membrane (molecular weight cutoff 12,000 g/mol) were obtained from Sigma-Aldrich Chemical Company, St. Louis, USA. Cremophor EL (CrEL, polyethoxylated castor oil), Miglyol 812 (Miglyol, caprylic/capric triglyceride), Transcutol P (Transcutol, diethylene glycol monoethyl ether), and Miranol C2M conc NP (Miranol, disodium cocoamphodiacetate) were kindly supplied by Seppic (Paris, France), Sasol Germany GmbH (Witten, Germany), Gattefossé (Saint Priest, France), and Rhodia, Inc. (CA, USA), respectively. Propylene glycol (PG) was purchased from BDH Laboratory Supplies, Poole, England. Dorzolamide hydrochloride market product (Trusopt®, 2.2% dorzolamide hydrochloride) was provided by Merck Sharp and Dohme B.V. (Haarlem, Netherlands). Sodium dihydrogen phosphate, disodium hydrogen phosphate, and sodium chloride were purchased from Sisco research laboratories Pvt. Ltd., Mumbai, India.
Methods
Construction of Pseudoternary-Phase Diagrams
The pseudoternary-phase diagrams of oil (isopropyl myristate, Miglyol 812, and triacetin), surfactant (Tween 80 and Cremophor EL), and cosurfactant (propylene glycol, triacetin, Transcutol P, and Miranol C2M conc NP) were developed using water titration method at 25°C (26). For each combination of surfactant (S) and cosurfactant (CoS), four phase diagrams were constructed with S to CoS weight ratios of 1:1, 2:1, 3:1, and 4:1. In case monophasic, clear, and transparent mixtures were visualized after stirring, the samples were marked as points in the phase diagram. The area covered by these points represents the region where nanoemulsion exists which was calculated using AutoCAD® software (Autodesk, Inc., San Rafael, CA, USA).
Preparation of Dorzolamide Hydrochloride Nanoemulsions
In order to mimic physiological dilution process after ocular administration of the prepared nanoemulsions, nanoemulsions were diluted 1:5 (v/v) with isotonic buffer solution (pH 7.4) and assessed visually for transparency for a period of at least 48 h (27). Diluted systems that showed transparency and no phase separation were considered as true oil/water (o/w) nanoemulsions maintaining their physical integrity and were used for preparing drug-loaded nanoemulsions.
Seventeen nanoemulsion vehicles were prepared at S to CoS weight ratio of 3:1. Dorzolamide hydrochloride (2.22% w/w) was dissolved in the prepared nanoemulsion samples with the aid of vortexing until clear transparent systems were obtained. Drug-loaded nanoemulsions were prepared 48 h before investigation so that drug distribution among the oil, water, and surfactant micelles attains thermodynamic equilibrium and were stored at room temperature. Benzalkonium chloride was added as a preservative in all prepared nanoemulsions in a concentration of 0.01% w/w.
Accelerated Physical Stability Studies
The prepared nanoemulsions were subjected to a series of heating–cooling cycle (28), centrifugation (27), and freeze–thaw cycle tests (28) and the nanoemulsions that were stable were considered for further studies.
Physicochemical Characterization of Nanoemulsions
Particle Size Analysis
A Photon Correlation Spectrometer (Zetasizer 1000HS, Malvern Instruments Ltd., UK) was employed to monitor the particle size of nanoemulsions. Light scattering was monitored at 90° angle and 25°C.
Rheological Measurements
Rheological measurements were performed at 25 ± 0.1°C using a Bohlin rheometer (Model CS 100, Bohlin Instruments, UK) equipped with a cone/plate apparatus 40 mm per 4°. For each sample, continuous variation of shear rate γ (80–400 s−1) was applied and the resulting shear stress σ was measured. Viscosity η of dispersions with Newtonian flow properties was calculated according to the relation: η = σ/γ.
Refractive Index
Refractive index was determined at 25°C using refractometer M 46.17/63707 supplied by Higler and Walts Ltd., England.
Surface Tension
Surface tension measurements were carried out at 20°C using a thermostatically controlled processor tensiometer K100 (Kruss GmbH, Germany) provided with a Du Nouy ring (ring radius 9.545 mm, wire diameter 0.37 mm).
pH and Osmotic Pressure
pH was measured at 25°C using JENWAY model 350 (JENWAY Ltd., UK) and the osmotic pressure was measured using Micro Osmometer model 3300, Advanced Instruments Inc., USA.
In Vitro Drug Release Studies
These studies were performed using US Pharmacopeia dissolution apparatus type II (SR8 PLUS, Handson dissolution tester, USA). The release medium was 900 ml of phosphate buffer (pH 7.4), the temperature was set at 34 ± 0.5°C (the ocular surface temperature; 29,30) and the paddle revolution speed was 50 rpm. Release experiments were conducted for 6 h as all tested preparations attained 100% release within this time period.
A 0.5 ml of aqueous drug solution (pH 5.5), market product, or the drug-loaded nanoemulsion was instilled in the dialysis bag which was secured with two clamps at each end. At definite time intervals, a 5-ml sample was withdrawn and replaced by fresh buffer; these samples were assayed for dorzolamide hydrochloride by Shimadzu UV spectrophotometer (2401/PC), Japan at 252.6 nm. Triplicate experiments were carried out for each release study and the mean value of release efficiency (RE) was calculated. The release efficiency was calculated from the area under the release curve at time t. It is expressed as a percentage of the area of the rectangle corresponding to 100% release, for the same total time, according to the following equation (31):
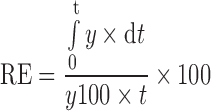 |
Where y is the percentage drug released at time t.
Ocular Irritation Studies
Six groups, each of six New Zealand albino rabbits weighing 1.5–2 kg, were kept in an air-conditioned room at 25 ± 0.5°C and fed a standard pellet diet and water with artificial fluorescent light providing a cycle of night and day, 12 h each. All animals were healthy and free of clinically observable abnormalities. The experimental procedures conform to the ethical principles of the National Research Center, Cairo (Egypt), on the use of animals.
The right eye received 50 μl of the tested formulation, while the left eye was used as a control. Application of the tested formulation onto the rabbit’s cornea was repeated every 2.5 h through a period of 7.5 h per day for three successive days and once on the fourth day. After 1 and 24 h from last instillation, eyes were examined under general anesthesia (35 mg/kg ketamine and 5 mg/kg xylazine) utilizing Draize technique (32). The eyelids, cornea, iris, conjunctiva, and anterior chamber were inspected for inflammation or toxic reaction. Furthermore, both eyes were stained with fluorescein and examined under UV light to verify possible corneal lesion.
After corneal examination, the corneas were separated, washed with saline phosphate buffer (pH 7.4), and immediately fixed in Bouin’s solution [85 ml picric acid, 10 ml formalin (37–40%) and 5 ml acetic acid] for 24 h. The corneas were then dehydrated with an ethyl alcohol gradient (70– 90–100%) and xylene, put in melted paraffin, and solidified in block forms. Cross sections were cut, stained with hematoxylin and eosin, and microscopically examined for pathological modifications (n = 3).
Therapeutic Efficacy Studies
This study was of a single-dose crossover design and was performed on aqueous drug solution (pH 5.5), the market product (Trusopt®, as a reference standard) in addition to the selected formulations. Sterility of formulations was achieved by filtration through sterile 0.22-μm pore size pyrogen-free cellulose filters.
Male albino rabbits weighing 2–2.5 kg were used; the animals were housed as previously described under “Ocular Irritation Studies” and the experimental procedures conform to the ethical principles of the National Research Center, Cairo (Egypt), on the use of animals. Intraocular pressure (IOP) measurements were performed with a Schiötz Tonometer (Rudolf Riester GmbH and Co. KG, Germany). No more than three repeated readings for any eye were performed at each measurement. Only measurements in which two consecutive readings were identical were included. Animals which showed a consistent difference of more than 2 mmHg between IOP of both eyes, showed any signs of irritation, or were agitated during handling were excluded.
Eyedrops were instilled topically into the upper quadrant of the eye and the eye was manually blinked three times; one eye received 50 μl of the preparation and the other served as control. IOP was measured immediately prior to giving the drug and at different time intervals following the treatment. All measurements were done three times at each interval and the mean values were taken.
The percentage decrease in IOP was determined according to the following equation:
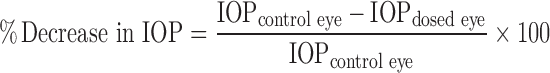 |
The pharmacodynamic parameters taken into consideration were maximum percentage decrease in IOP, time for maximum response (tmax), area under percentage decrease in IOP versus time curve (AUC0–10 h), and mean residence time (MRT). These parameters were calculated using WinNonlin® software (Pharsight Co., CA, USA).
Statistical analysis of the results was performed using one-way analysis of variance followed by the least-significant difference test. Statistical analysis was computed with the SPSS® software (SPSS Inc., Chicago, USA).
RESULTS AND DISCUSSION
Construction of Pseudoternary-Phase Diagrams
For the present study, one type of oil from different categories such as long-chain triglycerides (isopropyl myristate), medium-chain triglycerides (Miglyol 812) as well as short-chain triglycerides (triacetin) were selected. These oils are well tolerated by the eye (25,33–35).
Tween 80 and Cremophor EL were used as examples of nonionic surfactants. Tween 80 is widely used in ophthalmic preparations due to its safety. It is listed in US Pharmacopeia-National Formulary, European Pharmacopeia, and the Japanese Pharmacopeia (36). An ocular irritation evaluation test was made by Alany et al. (37) and classified Tween 80 as practically nonirritant. On the other hand, according to information provided by the manufacturer, the instillation of 0.05 ml of Cremophor EL in the rabbit’s conjunctival sac caused only slight reddening of the conjunctiva, and this disappeared within a few hours (38). The application of a 50% aqueous solution of this product caused slight irritation with lacrimation, which disappeared rapidly; 30% aqueous solutions had no irritant effect (38).
An additional important criterion for selection of the surfactants is their HLB values. The HLB value required to form o/w nanoemulsions should be greater than 10 (39). Tween 80 and Cremophor EL have HLB values of 15 and 12–14, respectively, thus fulfilling this requirement.
Propylene glycol, triacetin, Transcutol P, and Miranol C2M conc NP were used as cosurfactants. Solutions of up to 50% propylene glycol caused no irritations to the rabbit eye, whereas the undiluted application was associated with a weak conjunctival redness (40,41). Triacetin, as reported by Hughes (33), is well tolerated by the rabbit eye. Undiluted triacetin has no or only minor effect on the rabbit eye (34,35). On the other hand, Transcutol P and the amphoteric surfactant, Miranol C2M conc NP, are known as common emulsion excipients suitable for dissolving or dispersing lipophilic drugs in ocular preparations (42).
Thirty-six systems were prepared; the pseudoternary-phase diagrams were mapped with the water titration method at 25°C to identify the area of nanoemulsion regions.
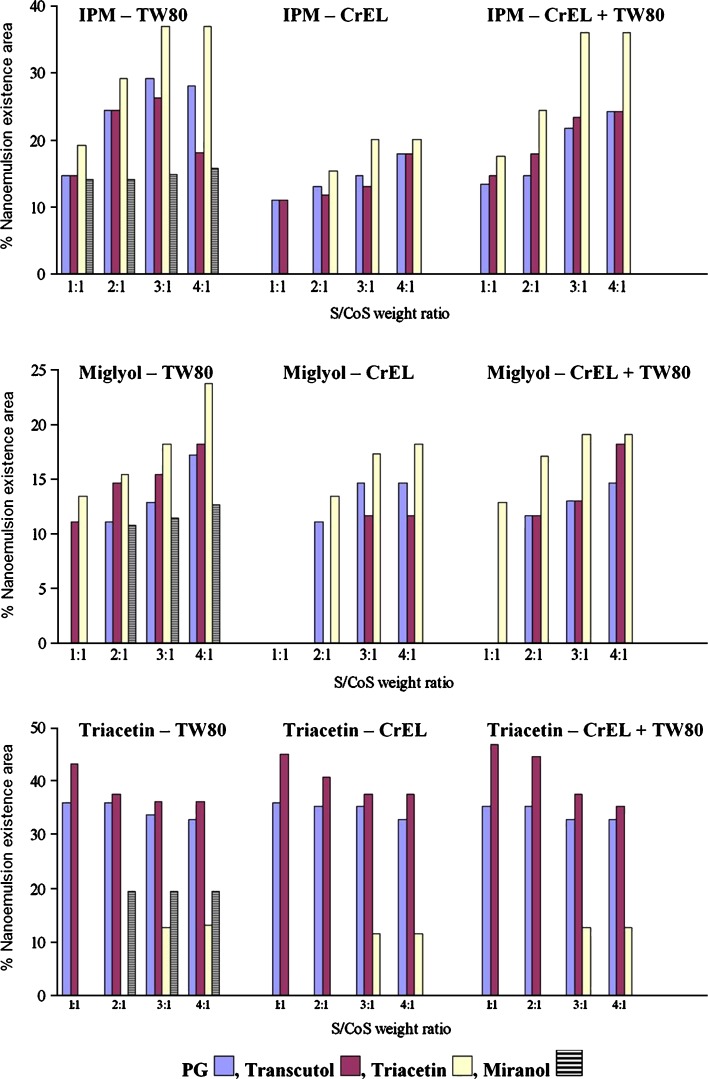
As expected, the phase behavior was strongly influenced by the molecular volume of the oil incorporated within the nanoemulsion (43). Depending on the chain length and on the volume of the molecule, penetration of the surfactant into the hydrocarbon tails will change the hydrocarbon chain volume of the surfactant molecule and, thus, the effective critical packing parameter (44). The molecular volumes of IPM, Miglyol, and triacetin are 529, 572, 188 Å3, respectively; accordingly, the nanoemulsion area was highest in the case of triacetin followed by IPM and then Miglyol, on using PG, Transcutol, or Miranol as cosurfactants (Fig. 1).
Fig. 1.
Dependency of nanoemulsion existence area on types of oil, surfactant, cosurfactant, and surfactant to cosurfactant weight ratio
On using triacetin as a cosurfactant, the highest nanoemulsion area was found for IPM followed by Miglyol and then triacetin (Fig. 1). This might be due to a decrease in the solubilizing capacity of the surfactants as triacetin acts here as an oil beside being a cosurfactant. Oils, as triacetin, possessing a very small chain length compared to the surfactant hydrophobe may be incorporated into the nanoemulsion droplet in a different manner to that originally thought; in other words, an oil may be too small to act as a cosurfactant, possibly preferring to locate more towards the center of the aggregate (45).
The use of Cremophor EL surfactant resulted, in most cases, in smaller nanoemulsion existence areas compared to Tween 80 (Fig. 1). In order to increase the oil-solubilizing capacity of Cremophor EL, a mixture of Cremophor EL and Tween 80 (1:1 weight ratio) was tried. This resulted in an increase in nanoemulsion existence areas (Fig. 1). This might be due to the fact that the flexibility of surfactant layer and its ability to partition at higher levels into the oil–water interface might be enhanced by the combined surfactants; both of which stabilized o/w nanoemulsion formed (27,46–49). Moreno et al. (27) reported that the combined use of Tween 80 and soybean lecithin was found to greatly increase the oil content in microemulsions by threefold. Huibers and Shah (46) also observed synergistic effects of surfactant combinations for w/o microemulsions.
With respect to the cosurfactants used, Fig. 1 denotes that the use of the branched cosurfactant triacetin resulted, generally, in the highest nanoemulsion existence area followed by PG and Transcutol. Taha et al. (50) found that optimal w/o microemulsion stability requires the cosurfactant molecules to be branched and short, such that they can occupy sphere-like gaps between interfacial surfactant molecules. On the other hand, Miranol induced the least nanoemulsion area. This might be due to the steric bulking of Miranol which is expected to disturb the interfacial packing of the nanoemulsion.
It is evident from Fig. 1 that for systems containing IPM or Miglyol, increasing surfactant concentration in relation to cosurfactant concentration led, generally, to an increase in the nanoemulsion existence area together with an increase in the maximum amount of oil that can be incorporated in the system. Kawakami et al. (51) reported that increasing S to CoS ratio enhances micelle formation, which consequently increases the solubilization capacity of the microemulsion (51). However, this trend was, generally, reversed when triacetin was used as the oil phase and PG or Transcutol as cosurfactants since increasing S to CoS ratio from 1:1 to 4:1 resulted in a decrease in the solubilizing capacity of the nanoemulsion (Fig. 1). This might be due to the fact that, at the S to CoS ratio of 1:1, the cosurfactant was inserted into the cavities between the surfactant molecules exactly, and the formed nanoemulsions had the maximum solubilizing capacity (51).
It is clear from the aforementioned results that the nanoemulsion existence area can be modified according to the type and amount of oil, surfactant, and cosurfactant used.
It is noteworthy that the use of o/w nanoemulsions in drug delivery is more straightforward than is the case with w/o nanoemulsions. This is because the droplet structure of o/w nanoemulsions is often retained on dilution by a biological aqueous phase, thereby permitting ocular administration (52). Our goal is to formulate a dilutable ophthalmic nanoemulsion (o/w) having the lowest possible surfactant content and optimal solubilization of the hydrophilic and lipophilic components. Therefore, 17 dilutable nanoemulsions were formulated in which a S to CoS ratio of 3:1 and water content of 77.78% w/w were used. The prepared nanoemulsions were loaded with 2.22% w/w of dorzolamide hydrochloride (Table I).
Table I.
Composition of Dorzolamide Hydrochloride-Loaded Nanoemulsions
| Component (% w/w) | Nanoemulsion (NE) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
| IPM | 2.00 | 2.00 | 2.00 | 2.00 | |||||||||||||
| Triacetin | 4.50 | 4.50 | 2.00 | 4.00 | 2.00 | 4.00 | 2.00 | 2.00 | 4.00 | 2.00 | 4.00 | 2.00 | 4.00 | 2.00 | 4.00 | ||
| TW80 | 13.50 | 13.50 | 13.50 | 6.75 | 13.50 | 12.00 | 13.50 | 12.00 | 13.50 | 6.75 | 6.00 | 6.75 | 6.00 | ||||
| CrEL | 6.75 | 13.50 | 12.00 | 13.50 | 12.00 | 6.75 | 6.00 | 6.75 | 6.00 | ||||||||
| PG | 4.50 | 4.50 | 4.00 | 4.50 | 4.00 | 4.50 | 4.00 | ||||||||||
| Transcutol | 4.50 | 4.50 | 4.00 | 4.50 | 4.00 | 4.50 | 4.00 | ||||||||||
| Miranol | 4.50 | ||||||||||||||||
Bidistilled deionized water was used as the aqueous phase (77.78% w/w). Dorzolamide hydrochloride was used in a concentration of 2.22% w/w
IPM (isopropyl myristate); Tween 80 (TW80); Cremophor EL (CrEL); PG (Propylene glycol)
Accelerated Physical Stability Studies
Stability of nanoemulsions was studied using heating–cooling cycles, centrifugation, and freeze–thaw cycle stress tests. All nanoemulsions were stable after heating–cooling cycles, except NE 4 which displayed alteration in transparency and was thus excluded. The centrifugation test showed that the tested nanoemulsions had good physical stability. Through freeze–thaw cycle stress test, turbidity was observed when the nanoemulsions were stored at −21°C. Coagulation of the internal phase at low temperature might have led to this instability; however, these nanoemulsions were easily recovered by storing at ambient temperature. Chen et al. (53) reported that nanoemulsions should be kept above 15°C at least.
Physicochemical Characterization of Nanoemulsions
Particle Size Analysis
The selected nanoemulsions showed a mean droplet diameter of 8.4–12.7 nm (Table II). This small average diameter was expected since, in nanoemulsions, the cosurfactant molecules penetrate the surfactant film, lowering the fluidity and surface viscosity of the interfacial film, decreasing the radius of curvature of the nanodroplets, and forming transparent systems (54).
Table II.
Physicochemical Properties of Dorzolamide Hydrochloride Nanoemulsions (mean ± SD)
| NE | Physicochemical properties | |||||
|---|---|---|---|---|---|---|
| Particle diameter (nm) | Viscosity (mPa s) | Refractive index | Surface tension (mN/m) | pH | Osmolality (mOsm/Kg) | |
| 1 | 11.8 ± 0.8 | 4.63 ± 0.32 | 1.356 ± 0.001 | 47.6 ± 0.07 | 6.36 ± 0.53 | 1,050 ± 31 |
| 2 | 12.7 ± 0.9 | 4.50 ± 0.28 | 1.357 ± 0.001 | 45.5 ± 0.31 | 6.20 ± 0.59 | 758 ± 35 |
| 3 | 12.5 ± 0.7 | 4.46 ± 0.24 | 1.357 ± 0.003 | 44.1 ± 0.28 | 5.22 ± 0.48 | 500 ± 36 |
| 5 | 9.2 ± 0.8 | 4.54 ± 0.29 | 1.357 ± 0.001 | 51.1 ± 0.07 | 5.20 ± 0.64 | 1,195 ± 27 |
| 6 | 9.7 ± 0.6 | 4.19 ± 0.29 | 1.357 ± 0.002 | 49.3 ± 0.39 | 5.42 ± 0.51 | 1,205 ± 30 |
| 7 | 8.8 ± 0.7 | 5.19 ± 0.56 | 1.357 ± 0.003 | 49.9 ± 0.14 | 5.67 ± 0.58 | 886 ± 25 |
| 8 | 9.2 ± 0.7 | 4.22 ± 0.26 | 1.356 ± 0.001 | 49.0 ± 0.08 | 5.43 ± 0.61 | 920 ± 25 |
| 9 | 8.4 ± 0.4 | 4.68 ± 0.26 | 1.356 ± 0.002 | 49.1 ± 0.09 | 6.66 ± 0.53 | 709 ± 10 |
| 10 | 10.5 ± 0.7 | 7.06 ± 0.85 | 1.357 ± 0.002 | 51.3 ± 0.13 | 4.34 ± 0.47 | 1,269 ± 25 |
| 11 | 10.5 ± 0.8 | 5.56 ± 0.22 | 1.356 ± 0.003 | 51.4 ± 0.21 | 4.22 ± 0.51 | 1,320 ± 36 |
| 12 | 11.2 ± 0.8 | 9.24 ± 0.11 | 1.358 ± 0.003 | 51.7 ± 0.16 | 4.34 ± 0.31 | 1,032 ± 31 |
| 13 | 11.1 ± 0.8 | 6.65 ± 0.13 | 1.358 ± 0.001 | 51.9 ± 0.40 | 4.18 ± 0.42 | 969 ± 25 |
| 14 | 9.5 ± 0.7 | 5.45 ± 0.86 | 1.357 ± 0.001 | 49.6 ± 0.14 | 4.55 ± 0.47 | 1,238 ± 26 |
| 15 | 10.1 ± 0.6 | 4.79 ± 0.23 | 1.356 ± 0.002 | 49.6 ± 0.14 | 4.47 ± 0.45 | 1,247 ± 28 |
| 16 | 9.6 ± 0.7 | 5.51 ± 0.21 | 1.357 ± 0.003 | 50.3 ± 0.13 | 4.52 ± 0.48 | 929 ± 25 |
| 17 | 9.8 ± 0.5 | 4.96 ± 0.26 | 1.356 ± 0.001 | 50.6 ± 0.21 | 4.45 ± 0.52 | 687 ± 29 |
Rheological Measurements
It has been assumed that ophthalmic instillation of a formulation should influence the normal behavior of tears as little as possible. Systems with low viscosity allow good tolerance with little blinking pain. In contrast, systems with enhanced viscosity, although less tolerant, induce an increase in ocular contact time by reducing the drainage rate and, as a consequence, improve bioavailability (55). Viscosity of eyedrops is required to be not higher than 20.0 mPa s (56).
Dorzolamide hydrochloride nanoemulsions exhibited a Newtonian behavior. The viscosity values of all dorzolamide hydrochloride nanoemulsions were less than 10.0 mPa s (Table II).
It is obvious that increasing oil concentration of dorzolamide hydrochloride nanoemulsions, on the expense of the S–CoS mixture, decreased the average viscosity of the nanoemulsion (NEs 6, 8, 11, 13, 15, and 17 versus NEs 5, 7, 10, 12, 14, and 16, respectively, Table II). It is also observed that the viscosity of the nanoemulsions differed, generally, with the surfactant used; nanoemulsions containing Cremophor EL (NEs 10–13) had higher viscosity values relative to those containing Tween 80 (NEs 5–9). This might be because Cremophor EL is semisolid while Tween 80 is liquid at room temperature.
Refractive Index
Refractive index measurements detect possible impairment of vision or discomfort to the patient after administration of eyedrops (57). Refractive index of tear fluid is 1.340 to 1.360 (58). It is recommended that eye drops should have refractive index values not higher than 1.476 (59).
Table II depicts that dorzolamide hydrochloride nanoemulsions had refractive index values ranging from 1.356 to 1.358 which are within the recommended values.
Surface Tension
The tear film is destabilized when the surface tension of eyedrops is much lower than the surface tension of the lachrymal fluid (60,61) which ranges from 40 to 50 mN/m (62).
The surface tension of the prepared dorzolamide hydrochloride nanoemulsions ranged from 44.1 to 51.9 mN/m (Table II), which is more or less similar to that of the lachrymal fluid. Low surface tension of nanoemulsions guarantees good spreading effect on the cornea and mixing with the precorneal film constituents, thus possibly improving the contact between the drug and the corneal epithelium (63).
pH
The ideal pH for maximum comfort when an ophthalmic preparation is instilled in the eye should be in the order of 7.2 ± 0.2 (64). In some cases, the instillation of a solution with a pH different from tears is irritant and causes a painful sensation. This depends on the volume instilled, the buffering capacity, composition of the solution, and the contact time with the eye surface (65). However, different pH values can be tolerated if the preparation is not or is only very slightly buffered because in this case the limited buffering capacity of the tears is able to adjust the pH to physiologic levels on administration (57). The pH of therapeutic substances applied as eyedrops can vary from 3.5 to 8.5 (66).
The pH values of the prepared dorzolamide hydrochloride nanoemulsions were within the acceptable range (4.2 to 6.7, Table II).
Osmolality
The osmolality of lachrymal fluid is between 280 and 293 mOsm/kg on waking. As a result of evaporation when the eyes are open, osmolality may vary between 231 and 446 mOsm/kg (67). Depending on the drop size, solutions with an osmolality lower than 100 mOsm/kg or higher than 640 mOsm/kg are irritant; however, the original osmolality is restored 1 or 2 min after instillation of the nonisotonic solution depending on the drop size (65).
The osmolality of the prepared dorzolamide hydrochloride nanoemulsions ranged from 500 to 1,320 mOsm/kg (Table II). Nanoemulsions containing propylene glycol (NEs 1, 5, 6, 10, 11, 14, and 15) had higher osmolality compared to those containing other cosurfactants as Transcutol, triacetin, or Miranol (1,050–1,320 versus 500–1,032 mOsm/kg, Table II). Hasse and Keipert (63) formulated ocular nanoemulsions with osmolality which ranged from 1,200 to 2,400 mOsm/kg and found that they were nonirritant using hen’s egg test on the chorioallantoic membrane and Draize test on rabbits’ eyes.
In Vitro Drug Release Studies
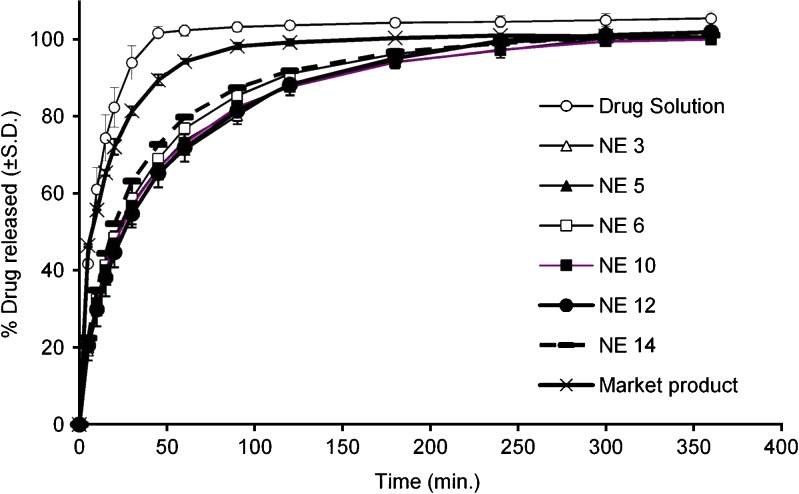
Drug release from nanoemulsions containing IPM and Tween 80 together with Transcutol or triacetin (NEs 2 and 3, respectively) was lower (p < 0.05) than that from nanoemulsion containing propylene glycol (NE 1, Fig. 2a). Due to the lower osmolality of NE 3 compared to NE 2 (Table II), the former one was taken into consideration for subsequent investigations.
Fig. 2.
a–d Release efficiency for dorzolamide hydrochloride solution, market product and nanoemulsion formulations
Results of dorzolamide hydrochloride release from nanoemulsions containing triacetin and Tween 80 together with different cosurfactants (NEs 5–9) are presented in Fig. 2b. The comparison of the release properties of NEs 5, 7, and 9 which contain the same concentrations of oil, surfactant, and cosurfactant and differ only in the type of cosurfactant (PG, Transcutol, and Miranol, respectively) reveals that the release efficiency of these nanoemulsions follows the order: NE 9 > NE 7 > NE 5 (p < 0.05). On the other hand, comparing dorzolamide hydrochloride release from NE 5 with NE 6 and NE 7 with NE 8 which contain 2% and 4% w/w of triacetin, respectively, denotes lack of significant difference between their release efficiency (p > 0.05). Therefore, NE 5 (2% Triacetin–TW80–PG) and NE 6 (4% Triacetin–TW80–PG) were selected for subsequent studies of the effect of oil concentration of the nanoemulsion on the bioavailability of the drug.
Figure 2c comprises the results of the release study for nanoemulsions containing triacetin, Cremophor EL, and different cosurfactants (NEs 10–13). Comparing the release efficiency of NE 10 with that of NE 12 containing the same oil and surfactant but different cosurfactants (PG and Transcutol, respectively) and of NE 11 with that of NE 12 containing the same oil and surfactant but different cosurfactants (PG and Transcutol, respectively) reveals lack of significant difference (p > 0.05). On the other hand, comparing the release properties of NE 10 with NE 11 and NE 12 with NE 13 which contain 2% and 4% w/w of triacetin, respectively, denotes that nanoemulsions containing higher contents of triacetin had a higher release efficiency (p < 0.01). This might be due to:
The increase of oil content of NEs 11 and 13 relative to NEs 10 and 12 was not accompanied by a similar increase of nanoemulsion particle size (Table II). This resulted in an increase of total number of oil globules and subsequent increase in their surface area which led to an increase in drug release.
The increase in oil content was combined with a decrease in surfactant concentration; this might cause an increase in the thermodynamic activity of the drug (54,68) which acts as a driving force for its release (69).
On the basis of the previous results, NEs 10 and 12 were chosen for subsequent biological investigations.
Figure 2d represents results of the release studies for nanoemulsions containing triacetin, Cremophor EL–Tween 80 (1:1), and PG or Transcutol (NEs 14–17). It is evident that the release efficiency of NE 14 was lower (p < 0.01) than those of NEs 15, 16, and 17 which were more or less similar (p > 0.05); therefore, NE 14 was selected for the bioavailability studies.
The obtained release curves (Fig. 3) reveal that dorzolamide hydrochloride nanoemulsions (NEs 3, 5, 6, 10, 12, and 14) exhibit satisfactory sustained drug release behavior compared with that of drug solution or the market product. The difference in the release pattern between the above-mentioned nanoemulsions and that of the market product indicates that these nanoemulsions might act as a reservoir for the drug since their viscosity values were much lower than that of the market product (4.19–9.24 versus 100 mPa s).
Fig. 3.
Release of dorzolamide hydrochloride from different formulations
On the other hand, comparing the release efficiency of the nanoemulsion formulations with that of the drug solution for the market product indicates a lower efficiency for the nanoemulsions (p < 0.0001). This may suggest that the oil phase in nanoemulsions served as a depot for the drug, while the drug transport occurred primarily from the water phase (70). This phenomenon could have significant implications for the development of ocular systems for sustained delivery.
Based on the results of dorzolamide hydrochloride release studies, NEs 3, 5, 6, 10, 12, and 14 were chosen for the bioavailability studies since these nanoemulsions exhibited relatively low release efficiency and possible sustained release of the drug.
Ocular Irritation Studies
Clinical investigations revealed that the selected nanoemulsion formulations (NEs 3, 5, 6, 10, 12, and 14) were nonirritant and could be tolerated by the rabbit eye (average total score 0–0.33).
Cross sections from the corneas of rabbits’ eye after application of the tested formulations together with a control section showed that both corneal structure and integrity were unaffected. Taking into consideration that the rabbit eye is more susceptible to irritant substances than the human eye (71), this result would be considered very promising.
Therapeutic Efficacy Studies
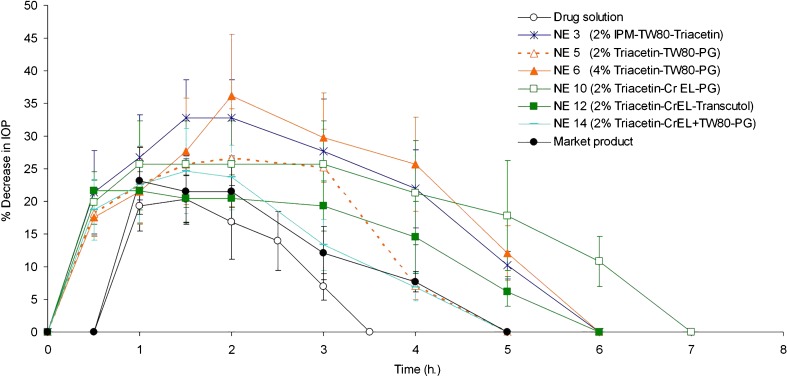
Figure 4 demonstrates the percentage decrease in IOP of normotensive rabbits after administration of a single dose of dorzolamide hydrochloride nanoemulsions (NEs 3, 5, 6, 10, 12, and 14), drug solution, and the market product. It is observed that nanoemulsions, in contrast to the drug solution or the market product, induced a pronounced decrease in IOP already half an hour postinstillation of the eyedrops. This indicates that formulation of dorzolamide hydrochloride as a nanoemulsion led to a faster onset of drug action compared to that of either drug solution or the market product.
Fig. 4.
Percentage decrease in IOP after administration of dorzolamide hydrochloride nanoemulsions, drug solution, and the market product
It is also observed that the mean maximum percentage decrease in IOP occurred 0.5 to 1.6 h after instillation of nanoemulsions, drug solution, or the market product (Fig. 4). In this respect, nanoemulsions 3 (2% IPM–TW80–Triacetin) and 6 (4% Triacetin–TW80–PG) had higher values (p < 0.01) for this parameter compared to that of the drug solution or the market product (Table III). On the other hand, there was no significant difference between the value of this parameter for the other nanoemulsions and that for the drug solution or the market product (p > 0.05).
Table III.
Pharmacodynamic Parameters after Administration of Dorzolamide Hydrochloride Solution, Nanoemulsion Formulations, and the Market Product (mean ± SD)
| Formula | Pharmacodynamic parameter | |||
|---|---|---|---|---|
| Max % decrease in IOP | t max (h) | AUC0–10 h | MRT | |
| Drug solution | 20.93 ± 4.17 | 1.2 ± 0.4 | 38.73 ± 8.38 | 1.73 ± 0.06 |
| NE 3 | 32.83 ± 5.82 | 1.2 ± 0.5 | 124.98 ± 23.35 | 2.41 ± 0.08 |
| NE 5 | 26.68 ± 7.54 | 1.1 ± 0.6 | 85.49 ± 17.15 | 2.01 ± 0.05 |
| NE 6 | 37.23 ± 7.89 | 1.6 ± 0.5 | 128.14 ± 16.32 | 2.55 ± 0.15 |
| NE 10 | 25.71 ± 6.59 | 0.9 ± 0.2 | 130.53 ± 27.43 | 2.86 ± 0.25 |
| NE 12 | 21.63 ± 2.86 | 0.5 ± 0.0 | 87.24 ± 14.99 | 2.29 ± 0.14 |
| Market product | 23.11 ± 5.11 | 1.1 ± 0.2 | 58.20 ± 10.90 | 2.06 ± 0.06 |
AUC0–10h (area under the percentage decrease in IOP − time curve); MRT (mean residence time); NE (nanoemulsion)
With respect to the duration of drug action, it is evident that the effect of nanoemulsions was continued for up to 4–6 h, while that of the drug solution and the market product lasted for only 3 and 4 h, respectively (Fig. 4). This would indicate that dorzolamide hydrochloride nanoemulsions exhibited a more prolonged effect compared to either drug solution or the market product.
Table III demonstrates that the time for maximum percentage decrease in IOP (tmax) for the tested formulations, drug solution, and the market product varied from 0.5 to 1.6 h. Nanoemulsion 12 had the least value for tmax. Dorzolamide hydrochloride nanoemulsions, with the exception of NEs 6 and 12, drug solution, and the market product showed similar values for tmax (p > 0.05).
Concerning the parameter of area under the percentage decrease in IOP − time curve (AUC0–10 h, Table III), it is evident that the value of this parameter, if compared to that of the drug solution, follows the order: NEs 3 (2% IPM–TW80–Triacetin), 6 (4% Triacetin–TW80–PG) and 10 (2% Triacetin–CrEL–PG) > NEs 5 (2% Triacetin–TW80–PG), 12 (2% Triacetin–CrEL–Transcutol), and 14 (2% Triacetin–CrEL + TW80–PG) > drug solution (p < 0.05). On the other hand, all dorzolamide hydrochloride nanoemulsions, except NE 14, showed higher values for AUC0–10h compared to that of the market product (p < 0.05), indicating a higher drug bioavailability.
Table III denotes that the mean residence time for percentage decrease in IOP (MRT) follows the order: NE 10 (2% Triacetin–CrEL–PG) > NE 6 (4% Triacetin–TW80–PG) > NEs 3 (2% IPM–TW80–Triacetin) and 12 (2% Triacetin–CrEL–Transcutol) > NE 5 (2% Triacetin–TW80–PG) and the market product > NE 14 (2% Triacetin–CrEL + TW80–PG) and drug solution (p < 0.05). This would indicate that, with the exception of NE 14, all dorzolamide hydrochloride nanoemulsions exhibited a more prolonged effect compared to drug solution.
As regards the effect of nanoemulsion components on drug efficacy, it is observed that NE 3 (2% IPM–TW80–Triacetin) was characterized by an enhanced drug bioavailability. This might be due to the inclusion of triacetin as a cosurfactant in this nanoemulsion. Triacetin, being an oil, increased the oil content of the nanoemulsion which acts as a drug reservoir and so, more oil was available to adhere to the lipophilic surface of the corneal epithelium and so, this could promote dorzolamide hydrochloride penetration (72).
The effect of the oil content of nanoemulsions on ocular bioavailability of the drug is also evident on comparing NE 5 (2% Triacetin–TW80–PG) with NE 6 (4% Triacetin–TW80–PG). The latter nanoemulsion contained twice the amount of oil present in the former one and had superior therapeutic efficacy. This might be due to a delayed residence time of nanoemulsions containing high oil contents in the conjunctival sac, following a more significant contact time with the cornea (73).
The effect of surfactant type on therapeutic efficacy of dorzolamide hydrochloride was studied on the examples of NEs 5 (2% Triacetin–TW80–PG) and 10 (2% Triacetin–CrEL–PG). It was found that the presence of Cremophor EL led to more drug bioavailability compared to Tween 80. This may agree with the previously reported data concerning enhancement of permeation of cyclosporin A through human corneas by Cremophor EL (74).
The comparison of NE 12 (2% Triacetin–CrEL–Transcutol) with NE 10 (2% Triacetin–CrEL–PG) indicates that the presence of Transcutol decreased (p < 0.0001) the drug bioavailability (Table III). This is in agreement with the observation of Liu et al. (75) that Transcutol decreased the apparent rabbit corneal permeability coefficient value of oxaprozin.
It is worthy to note that increasing the oil content of the prepared nanoemulsions improved their therapeutic efficacy. On the other hand, presence of Cremophor EL in the preparations led to an enhancement of therapeutic efficacy, while the use of Transcutol as a cosurfactant decreased the therapeutic efficacy.
On the basis of the results of bioavailability studies, it can be concluded that formulation of dorzolamide hydrochloride as nanoemulsion led to improvement of the therapeutic efficacy of the drug. This might be due to greater penetration of the drug from nanoemulsions due to the presence of surfactants and cosurfactants which increase the membrane permeability, thereby increasing drug uptake. In other words, nanoemulsions act as penetration enhancers by removing the mucus layer and disrupting tight junctional complexes to facilitate corneal drug delivery (76,77). Furthermore, the submicron particles penetrate into the corneal epithelium cells by endocytosis (78).
CONCLUSION
The formulated nanoemulsion eye drops of dorzolamide hydrochloride were characterized by thermodynamic stability, acceptable physicochemical properties, and ability to retain the drug. Moreover, the developed nanoemulsions showed fast onset of drug action and prolonged effect as well as enhanced drug bioavailability compared to the market product. Such formulations offer a more intensive treatment of glaucoma, a decrease in the number of applications per day and a better patient compliance.
Acknowledgements
We gratefully appreciate the generous supply of dorzolamide hydrochloride by Jamjoom Pharma, Jeddah, KSA and the kind help for assessment of the ocular irritation test by Dr. Maali A. Halim, Faculty of Medicine, Cairo University. We also acknowledge the financial support provided by the National Research Center, Egypt.
Contributor Information
Hussein O. Ammar, Phone: +20-2-0124472851, FAX: +20-2-0233370931, Email: husseinammar@hotmail.com
A. A. Mahmoud, Phone: +20-2-0103061120, Email: azzaamahmoud@yahoo.com
References
- 1.Hughes PM, Mitra AK. Overview of ocular drug delivery and iatrogenic ocular cytopathologies. In: Mitra AK, editor. Ophthalmic drug delivery systems. New York: Marcel Dekker; 1993. pp. 1–27. [Google Scholar]
- 2.Patton TF, Robinson JR. Quantitative precorneal disposition of topically applied pilocarpine nitrate in rabbit eyes. J Pharm Sci. 1976;65:1295–301. doi: 10.1002/jps.2600650909. [DOI] [PubMed] [Google Scholar]
- 3.Sieg JW, Robinson JR. Vehicle effects on ocular drug bioavailability II: evaluation of pilocarpine. J Pharm Sci. 1977;66:1222–8. doi: 10.1002/jps.2600660905. [DOI] [PubMed] [Google Scholar]
- 4.Chein YW, Cabana BE, Mares SE. Ocular controlled release drug administration. In: Chein YW, editor. Novel drug delivery systems; fundamentals, development concepts, biomedical assessments (drugs and the pharmaceutical sciences) New York: Marcel Dekker; 1982. pp. 13–55. [Google Scholar]
- 5.Middleton DL, Leung SS, Robinson JR. Ocular bioadhesive delivery systems. In: Lenaerts V, Gurny R, editors. Bioadhesive drug delivery systems. Boca Raton: CRC; 1990. pp. 179–202. [Google Scholar]
- 6.Desai SD, Blanchard J. Ocular drug formulation and delivery. In: Swarbick J, Boylan JC, editors. Encyclopedia of pharmaceutical technology. New York: Marcel Dekker; 1994. pp. 43–75. [Google Scholar]
- 7.Felt O, Furrer P, Mayer JM, Plazonnet B, Buri P, Gurny R. Topical use of chitosan in ophthalmology: tolerance, assessment and evaluation of precorneal retention. Int J Pharm. 1999;180:185–93. doi: 10.1016/S0378-5173(99)00003-4. [DOI] [PubMed] [Google Scholar]
- 8.Munier A, Gunning T, Kenny D, O’Keefe M. Causes of blindness in the adult population of the Republic of Ireland. Br J Ophthalmol. 1998;82:630–3. doi: 10.1136/bjo.82.6.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blomdahl S, Calissendorff BM, Tengrowth B, Wallin O. Blindness in glaucoma patients. Acta Ophthalmol (Copenh) 1997;75:589–91. doi: 10.1111/j.1600-0420.1997.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaur IP, Smitha R, Aggarwal D, Kapil M. Acetazolamide: future perspective in topical glaucoma therapeutics. Int J Pharm. 2002;248:1–14. doi: 10.1016/S0378-5173(02)00438-6. [DOI] [PubMed] [Google Scholar]
- 11.Hoyng PF, van Beek LM. Pharmacological therapy for glaucoma: a review. Drugs. 2000;59:411–34. doi: 10.2165/00003495-200059030-00003. [DOI] [PubMed] [Google Scholar]
- 12.Maren TH. The development of topical carbonic anhydrase inhibitors. Glaucoma. 1995;4:49–62. [PubMed] [Google Scholar]
- 13.Sugrue MF. The preclinical pharmacology of dorzolamide hydrochloride, a topical carbonic anhydrase inhibitor. J Ocul Pharmacol Ther. 1996;12:363–76. doi: 10.1089/jop.1996.12.363. [DOI] [PubMed] [Google Scholar]
- 14.Sugrue MF. Pharmacological and ocular hypotensive properties of topical carbonic anhydrase inhibitors. Prog Retin Eye Res. 2000;19:87–112. doi: 10.1016/S1350-9462(99)00006-3. [DOI] [PubMed] [Google Scholar]
- 15.Sigurdsson HH, Stefansson E, Gudmundsdottir E, Eysteinsson T, Thorsteinsdottir M, Loftsson T. Cyclodextrin formulation of dorzolamide and its distribution in the eye after topical administration. J Control Release. 2005;102:255–62. doi: 10.1016/j.jconrel.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Quint MP, Grove J, Thomas SM. Dorzolamide hydrochloride. In: Brittain HG, editor. Analytical profiles of drug substances and excipients. New York: Academic; 1999. pp. 283–316. [Google Scholar]
- 17.Rusk C, Sharpe E, Laurence J, Polis A, Adamsons I. Comparison of the efficacy and safety of 2% dorzolamide and 0.5% betaxolol in the treatment of elevated intraocular pressure. Dorzolamide comparison study group. Clin Ther. 1998;20:454–66. doi: 10.1016/S0149-2918(98)80055-6. [DOI] [PubMed] [Google Scholar]
- 18.Silver LH. Ocular comfort of brinzolamide 1.0% ophthalmic suspension compared with dorzolamide 2.0% ophthalmic solution: results from two multicenter comfort studies. Brinzolamide Comfort Study Group. Surv Ophthalmol. 2000;44(Suppl 2):S141–45. doi: 10.1016/S0039-6257(99)00111-3. [DOI] [PubMed] [Google Scholar]
- 19.Salminen L. Review: systemic absorption of topically applied ocular drugs in humans. J Ocul Pharmacol Ther. 1990;6:243–9. doi: 10.1089/jop.1990.6.243. [DOI] [PubMed] [Google Scholar]
- 20.Baudouin C. Side effects of antiglaucomatous drugs on the ocular surface. Curr Opin Ophthalmol. 1996;7:80–6. doi: 10.1097/00055735-199604000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Arici MK, Arici DS, Topalkara A, Guler C. Adverse effects of topical antiglaucoma drugs on the ocular surface. Clin Experiment Ophthalmol. 2000;28:113–7. doi: 10.1046/j.1442-9071.2000.00237.x. [DOI] [PubMed] [Google Scholar]
- 22.Konowal A, Morrison JC, Brown SV, Cooke DL, Maguire LJ, Verdier DV, et al. Irreversible corneal decompensation in patients treated with topical dorzolamide. Am J Ophthalmol. 1999;127:403–6. doi: 10.1016/S0002-9394(98)00438-3. [DOI] [PubMed] [Google Scholar]
- 23.Silva-Cunha A, Fialho SL, Carneiro LB, Oréfice F. Microemulsões como veículos de drogas para administração ocular tópica. Arq Bras Oftalmol. 2003;66:385–91. [Google Scholar]
- 24.Alany RG, Rades T, Nicoll J, Tucker IG, Davies NM. W/O microemulsions for ocular delivery: evaluation of ocular irritation and precorneal retention. J Control Release. 2006;111:145–52. doi: 10.1016/j.jconrel.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Vandamme TF. Microemulsions as ocular drug delivery systems: recent developments and future challenges. Prog Retin Eye Res. 2002;21:15–34. doi: 10.1016/S1350-9462(01)00017-9. [DOI] [PubMed] [Google Scholar]
- 26.Garti N, Aserin A, Tiunova I, Fanun MA. DSC study of water behavior in water-in-oil microemulsions stabilized by sucrose esters and butanol. Colloids Surf A Physicochem Eng Asp. 2000;170:1–18. doi: 10.1016/S0927-7757(00)00486-6. [DOI] [Google Scholar]
- 27.Moreno MA, Ballesteros MP, Frutos P. Lecithin-based oil-in-water microemulsions for parenteral use: pseudoternary phase diagrams, characterization and toxicity studies. J Pharm Sci. 2003;92:1428–37. doi: 10.1002/jps.10412. [DOI] [PubMed] [Google Scholar]
- 28.Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 2007;66:227–43. doi: 10.1016/j.ejpb.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki S, Suzuki S, Kawasaki N, Endo K, Takahashi A, Attwood D. In situ gelling xyloglucan formulations for sustained release ocular delivery of pilocarpine hydrochloride. Int J Pharm. 2001;229:29–36. doi: 10.1016/S0378-5173(01)00825-0. [DOI] [PubMed] [Google Scholar]
- 30.Wu C, Qi H, Chen W, Huang C, Su C, Li W, Hou S. Preparation and evaluation of a carbopol/HPMC-based in situ gelling ophthalmic system for puerarin. Yakugaku Zasshi. 2007;127:183–91. doi: 10.1248/yakushi.127.183. [DOI] [PubMed] [Google Scholar]
- 31.Khan KA, Rhodes CT. Effect of compaction pressure on the dissolution efficiency of some direct compression systems. Pharm Acta Helv. 1972;47:594–607. [PubMed] [Google Scholar]
- 32.Draize JH, Woodard G, Calvey HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82:377–90. [Google Scholar]
- 33.Hughes WFJ. The tolerance of rabbit cornea for various chemical substances. Bull Johns Hopkins Hosp. 1948;82:338–49. [PubMed] [Google Scholar]
- 34.Laillier J, Plazonnet B, Le Douarec JC. Evaluation of an objective method of studying eye irritation. Proc Eur Soc Toxicol. 1976;17:336–50. [Google Scholar]
- 35.Conquet P, Durand G, Laillier J, Plazonnet B. Evaluation of ocular eye irritation in the rabbit: objective versus subjective assessment. Tox App Pharmacol. 1977;39:129–39. doi: 10.1016/0041-008X(77)90185-5. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi M, Yasueda S, Isowaki A, Yamamoto M, Kimura M, Inada K, et al. Formulation of an ophthalmic lipid emulsion containing an anti-inflammatory steroidal drug, difluprednate. Int J Pharm. 2005;301:121–8. doi: 10.1016/j.ijpharm.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 37.Alany RG, Tucker IG, Davies NM, Rades T. Characterizing colloidal structures of pseudoternary phase diagrams formed by oil/water/amphiphile systems. Drug Dev Ind Pharm. 2001;27:31–8. doi: 10.1081/DDC-100000125. [DOI] [PubMed] [Google Scholar]
- 38.Chemicals BF . Cremophor® EL. Technical leaflet, ME 074 e. Ludwigshafen: BASF Fine Chemicals; 1997. [Google Scholar]
- 39.Kommuru TR, Gurley B, Khan MA, Reddy IK. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: formulation development and bioavailability assessment. Int J Pharm. 2001;212:233–46. doi: 10.1016/S0378-5173(00)00614-1. [DOI] [PubMed] [Google Scholar]
- 40.Grant WM, Schuman JS. Toxicology of the eye. 4. Springfield: Charles C. Thomas; 1993. [Google Scholar]
- 41.Bietti GB, Virno M, Pecori-Giraldi J. Propylene glycol: a new osmotic agent for ophthalmic uses. Doc Ophthalmol. 1973;34:77–92. doi: 10.1007/BF00151798. [DOI] [PubMed] [Google Scholar]
- 42.Tamilvanan S, Benita S. The potential of lipid emulsion for ocular delivery of lipophilic drugs. Eur J Pharm Biopharm. 2004;58:357–68. doi: 10.1016/j.ejpb.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 43.Aboofazeli R, Lawrence MJ. Investigations into the formation and characterization of phospholipid microemulsions. II. Pseudo-ternary phase diagrams of systems containing water–lecithin–isopropyl myristate and alcohol: influence of purity of lecithin. Int J Pharm. 1994;106:51–61. doi: 10.1016/0378-5173(94)90275-5. [DOI] [Google Scholar]
- 44.Trotta M, Gallarate M, Pattarino F, Carlotti ME. Investigation of the phase behaviour of systems containing lecithin and 2-acyl lysolecithin derivatives. Int J Pharm. 1999;190:83–9. doi: 10.1016/S0378-5173(99)00281-1. [DOI] [PubMed] [Google Scholar]
- 45.Warisnoicharoen W, Lansley AB, Lawrence MJ. Nonionic oil-in-water microemulsions: the effect of oil type on phase behaviour. Int J Pharm. 2000;198:7–27. doi: 10.1016/S0378-5173(99)00406-8. [DOI] [PubMed] [Google Scholar]
- 46.Huibers PD, Shah D. Evidence for synergism in non-ionic surfactant mixtures: enhancement of solubilization in water-in-oil microemulsions. Langmuir. 1997;13:5762–5. doi: 10.1021/la962108r. [DOI] [Google Scholar]
- 47.Engels T, Forster T, Rybinsko WV. The influence of coemulsifier type on the stability of oil-in-water emulsions. Colloids Surf A Physicochem Eng Aspects. 1995;99:141–9. doi: 10.1016/0927-7757(95)03132-W. [DOI] [Google Scholar]
- 48.Weingarten C, Magalhaes NSS, Baszkin A, Benita S, Seiller M. Interaction of a nonionic ABA copolymer surfactant with phospholipid monolayers: possible relevance to emulsion stabilization. Int J Pharm. 1991;75:171–9. doi: 10.1016/0378-5173(91)90191-P. [DOI] [Google Scholar]
- 49.Ninham BW, Chen SJ, Evans DF. Role of oils and other factors in microemulsion design. J Phys Chem. 1984;88:5855–7. doi: 10.1021/j150668a023. [DOI] [Google Scholar]
- 50.Taha MO, Abdel-Halim H, Al-Ghazawi M, Khalil E. QSPR modeling of pseudoternary microemulsions formulated employing lecithin surfactants: application of data mining, molecular and statistical modeling. Int J Pharm. 2005;295:135–55. doi: 10.1016/j.ijpharm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 51.Kawakami K, Yoshikawa T, Hayashi T, Nishihara Y, Masuda K. Microemulsion formulation for enhanced absorption of poorly soluble drugs. II. In vivo study. J Control Release. 2002;81:75–82. doi: 10.1016/S0168-3659(02)00050-0. [DOI] [PubMed] [Google Scholar]
- 52.HaBe A, Keipert S. Development and characterization of microemulsions for ocular application. Eur J Pharm Biopharm. 1997;43:179–83. doi: 10.1016/S0939-6411(96)00036-7. [DOI] [Google Scholar]
- 53.Chen H, Chang X, Weng T, Zhao X, Gao Z, Yang Y, et al. A study of microemulsion systems for transdermal delivery of triptolide. J Control Release. 2004;98:427–36. doi: 10.1016/j.jconrel.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Tenjarla S. Microemulsions: an overview and pharmaceutical applications. Crit Rev Ther Drug Carrier Syst. 1999;16:461–521. [PubMed] [Google Scholar]
- 55.Zignani M, Tabatabay C, Gurny R. Topical semi-solid drug delivery: kinetics and tolerance of ophthalmic hydrogels. Adv Drug Deliv Rev. 1995;16:51–61. doi: 10.1016/0169-409X(95)00015-Y. [DOI] [Google Scholar]
- 56.Radomska-Soukharev A, Wojciechowska J. Microemulsions as potential ocular drug delivery systems: phase diagrams and physical properties depending on ingredients. Acta Pol Pharm. 2005;62:465–71. [PubMed] [Google Scholar]
- 57.Fialho SL, da Silva-Cunha A. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin Experiment Ophthalmol. 2004;32:626–32. doi: 10.1111/j.1442-9071.2004.00914.x. [DOI] [PubMed] [Google Scholar]
- 58.Ciba-Geigy . Wissenschaftliche Tabellen Geigy. 8. Basel: Ciba-Geigy; 1997. [Google Scholar]
- 59.Keipert S, Siebenbrodt I, Lüders F, Bornschein M. Mikroemulsionen und ihre. potenzielle pharmazeutische Nutzung. Pharmazie. 1989;44:433–44. [PubMed] [Google Scholar]
- 60.Miller D. Measurement of the surface tension of tears. Arch Ophthalmol. 1969;82:368–71. doi: 10.1001/archopht.1969.00990020370014. [DOI] [PubMed] [Google Scholar]
- 61.Lin SP, Brenner H. Marangoni convection in a tear film. J Colloid Interface Sci. 1982;85:59–65. doi: 10.1016/0021-9797(82)90235-1. [DOI] [Google Scholar]
- 62.Ciba-Geigy . Wissenschaftliche Tabellen Geigy. 8. Basel: Ciba-Geigy; 1977. [Google Scholar]
- 63.Hasse A, Keipert S. Development and characterization of microemulsions for ocular application. Eur J Pharm Biopharm. 1997;43:179–83. doi: 10.1016/S0939-6411(96)00036-7. [DOI] [Google Scholar]
- 64.Mathis GA. Clinical ophthalmic pharmacology and therapeutics: ocular drug delivery. In: Gelatt KN, editor. Veterinary ophthalmology. Orlando: Lippincott Williams & Wilkins; 1999. pp. 291–7. [Google Scholar]
- 65.Van Ooteghem MM. Formulations of ophthalmic solutions and suspensions. Problems and advantages. In: Edman P, editor. Biopharmaceutics of ocular drug delivery. Boca Raton: CRC; 1993. pp. 31–2. [Google Scholar]
- 66.Polish Pharmaceutical Society . Polish Pharmacopoeia V. Warsaw: Polish Pharmaceutical Society; 1993. p. 39. [Google Scholar]
- 67.Terry JE, Hill RM. Human tear osmotic pressure, diurnal variation and closed eye. Arch Ophthalmol. 1978;96:120–2. doi: 10.1001/archopht.1978.03910050076019. [DOI] [PubMed] [Google Scholar]
- 68.Rhee YS, Choi JG, Park ES, Chi SC. Transdermal delivery of ketoprofen using microemulsions. Int J Pharm. 2001;228:161–70. doi: 10.1016/S0378-5173(01)00827-4. [DOI] [PubMed] [Google Scholar]
- 69.Walters KA, Brain KR, Green DM, James VG, Watkinson AC, Sands RH. Comparison of the transdermal delivery of estradiol from two gel formulations. Maturitas. 1998;29:189–95. doi: 10.1016/S0378-5122(98)00009-7. [DOI] [PubMed] [Google Scholar]
- 70.Lee PJ, Langer R, Shastri VP. Novel microemulsion enhancer formulation for simultaneous transdermal delivery of hydrophilic and hydrophobic drugs. Pharm Res. 2003;20:264–9. doi: 10.1023/A:1022283423116. [DOI] [PubMed] [Google Scholar]
- 71.Roggeband R, York M, Pericoi M, Braun W. Eye irritation responses in rabbit and man after single applications of equal volumes of undiluted model liquid detergent products. Food Chem Toxicol. 2000;38:727–34. doi: 10.1016/S0278-6915(00)00057-0. [DOI] [PubMed] [Google Scholar]
- 72.Muchtar S, Abdulrazik M, Frucht-Pery J, Benita S. Ex vivo permeation study of indomethacin from a submicron emulsion through albino rabbit cornea. J Control Release. 1997;44:55–64. doi: 10.1016/S0168-3659(96)01503-9. [DOI] [Google Scholar]
- 73.Mainardes RM, Urban MC, Cinto PO, Khalil NM, Chaud MV, Evangelista RC, Gremiao MP. Colloidal carriers for ophthalmic drug delivery. Curr Drug Targets. 2005;6:363–71. doi: 10.2174/1389450053765914. [DOI] [PubMed] [Google Scholar]
- 74.van der Bijl P, van Eyk AD, Meyer D. Effects of three penetration enhancers on transcorneal permeation of cyclosporine. Cornea. 2001;20:505–8. doi: 10.1097/00003226-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 75.Liu Z, Li J, Nie S, Guo H, Pan W. Effects of Transcutol P on the corneal permeability of drugs and evaluation of its ocular irritation of rabbit eyes. J Pharm Pharmacol. 2006;58:45–50. doi: 10.1211/jpp.58.1.0006. [DOI] [PubMed] [Google Scholar]
- 76.Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45:89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 77.Kaur IP, Smitha R. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev Ind Pharm. 2002;28:353–69. doi: 10.1081/DDC-120002997. [DOI] [PubMed] [Google Scholar]
- 78.Yu W, Tabosa do Egito ES, Barratt G, Fessi H, Devissaguet JP, Puisieux F. A novel approach to the preparation of injectable emulsions by a spontaneous emulsification process. Int J Pharm. 1993;89:139–46. doi: 10.1016/0378-5173(93)90115-V. [DOI] [Google Scholar]