Abstract
Rectal etodolac–Poloxamer gel systems composed of Poloxamer and bioadhesive polymers were developed and evaluated. Hydroxypropylmethyl cellulose, poly)vinyl) pyrrolidone, methyl cellulose, hydroxyethylcellulose, and carbopol were examined as mucoadhesive polymers. The characteristics of the rectal gels differed according to the properties of mucoadhesive polymers. The physicochemical properties such as gelation temperature, gel strength, and bioadhesive force of various formulations were investigated. The analysis of release mechanism showed that the release of etodolac was proportional to the square root of time, indicating that etodolac might be released from the suppositories by Fickian diffusion. The anti-inflammatory effect of etodolac–Poloxamer gel system was also studied in rats. Moreover, liquid suppository of etodolac did not cause any morphological damage to the rectal tissues. These results suggested that in situ gelling liquid suppository with etodolac and mucoadhesive polymer was a physically safe, convenient, and effective rectal dosage form for etodolac.
Key words: anti-inflammatory effect, etodolac, mucoadhesive polymers, poloxamer gel, safety study
INTRODUCTION
A conventional suppository is a semi-solid dosage form that melts or softens in the rectum at body temperature. Such suppository gives a feeling of alien, discomfort, and refusal to the patients, possibly lowering patient compliance. Furthermore, if the solid suppositories reach the end of the rectum, the drugs delivered by the suppositories might undergo the first-pass effect (1). Hence, the ideal suppository should be easy to administer without any pain during insertion and remain at the administration site to avoid the first-pass effect in the liver and the gastrointestinal tract (2). In order to solve the problems of conventional suppository, an attempt was made to develop a liquid rectal dosage form which forms a gel at body temperature, has suitable gel strength not to be leaked out from the anus after administration and has a suitable bioadhesive force so as not to reach the end of the colon. Liquid suppositories had been developed either to improve a local effect or to enhance drug absorption (3–5). As base of liquid suppositories, Poloxamers as surfactants, are copolymers of poly(oxyethylene)-poly(oxypropylene)-poly(oxyethylene), have been studied. Poloxamer solutions are known to exhibit the phenomenon of reverse thermal gelation, remaining as solution at low temperature (4°C) and gelling upon increasing the temperature (25–35°C; 6, 7). The thermosensitive liquid suppositories have many advantages; they are easy to administer to the anus, act as mucoadhesive to the rectal tissues without leakage after the dose (8), and did not cause any damage on mucosal membranes (9). The mucoadhesive polymer such as carbopol, polycarbophil, and sodium alginate were used to control the gel strength and bioadhesive force of Poloxamer gel (6). In addition, non-ionic cellulose ethers derivatives include: hydroxypropylmethylcellulose, hydroxyethylcellulose, and methylcellulose are the most commonly used polymers in the formulation of different dosage forms. Cellulose ethers represent a broad class of polymers which satisfy the key criteria for the development of controlled release dosage forms. The hydration rate of these polymers depends on the nature of the substituent present and the degree of substitution (10, 11). The thermosensitive liquid suppositories showed the enhanced bioavailability of drug such as acetaminophen (12), insulin (13), diclofenac sodium (1), propranolol (14), and quinine (15). However, in the development of liquid suppository containing a poorly water-soluble drug such as ibuprofen, the solubilizing agent must be used, since the drug was not well-absorbed at the rectal tissues (16, 17). Etodolac is a pyranocarboxylic acid-derived nonsteroidal anti-inflammatory drug, with analgesic activity. The recommended adult dosage of etodolac for chronic arthritic pain is 400 to a maximum of 1,200 mg/day in divided dose. A dose of 200 to 400 mg every 6 to 8 h are required and recommended for acute pain. According to Biopharmaceutics Drug Classification System, etodolac is a class II drug with low solubility and high permeability. Dissolution in the gastrointestinal tract is the rate-controlling step in the absorption process (18). The bioavailability of etodolac is expected to be limited by dissolution rate. Many efforts are ongoing to improve the oral absorption by incorporation of the active lipophilic component into inert vehicles such as cyclodextrin or PEG the resulting product might characterized by large weight dosage form which is a factor in decrease patient compliance. Furthermore, it was attempted to develop the alternative dosage form, rectal preparation.
The aim of this work was to develop a mucoadhesive and thermogelling system intended for improving the rectal administration of etodolac. Physicochemical properties such as gelation temperature, gel strength, and bioadhesive force of various formulations composed of etodolac, Poloxamers, and mucoadhesive polymers were investigated. Furthermore, the anti-inflammatory effect and the rectal tissue irritation of the Poloxamer gel were evaluated.
EXPERIMENTAL
Materials
The materials used in this study are: etodolac (Pharco Co., Egypt), Poloxamers (P407 and P188 BASF, Germany), hydroxpropylmethylcellulose (HPMC; Methocel K4M, Dow Chemical Company, USA), Carbopol 934P (CP; M/s Noveon Inc., USA), methylcellulose (MC), hydroxyethyl cellulose (HEC), and polyvinyl pyrolidone (PVP; BDH Laboratory Supplies, UK) and semipermeable cellulose dialysis tubing, MC CO 12,000 Da (Spectra/Pore®, USA). Carrageenan (k-variety, was obtained from Sigma St. Louis, USA). All other reagents and solvents were of analytical grade.
Methods
Preparation of Liquid Suppository
The liquid suppository was prepared as previously described by Choi et al., (19). Etodolac (50 mg/g) and mucoadhesive polymer (50–150 mg/g) were dispersed or dissolved in distilled water at room temperature and the solution was cooled down to 4°C. Poloxamers P407 and P188 where then slowly added to the solution with continuous agitation (Yellow Line, 20, Ika-Werke, Germany). The Poloxamer gel was left at 4°C until a clear solution was obtained. The concentrations of etodolac, mucoadhesive polymer, and Poloxamers P407 and P188 in the final solution were 5, 5–15, 15, and 20% (w/w), respectively. All formulations were allowed to equilibrate for 24 h at room temperature before carrying out the studies. The compositions of the tested formulations are summarized in Table I.
Table I.
Effect of Mucoadhesive Polymer Contents on the Physicochemical Properties of Poloxamer gels [Etodolac/P407/P188 (5/15/20)]
| Poloxamer formulations | Gelation temperature (°C) | Gel strength (s) | Bioadhesive force (×102 dyne/cm2) |
|---|---|---|---|
| Control | 34.8 ± 0.3 | 20.4 ± 0.2 | 30.5 ± 6.8 |
| MC/CP | |||
| 5/0 | 30.2 ± 0.3 | 28.8 ± 0.2 | 40.2 ± 4.6 |
| 10/0 | 27.5 ± 0.5 | 42.5 ± 0.3 | 67.3 ± 8.3 |
| 15/0 | 26.6 ± 0.5 | 60.6 ± 0.2 | 83.6 ± 4.5 |
| 5/5 | 36.3 ± 0.2 | 45.5 ± 0.1 | 88.4 ± 9.4 |
| HPMC/CP | |||
| 5/0 | 27.4 ± 0.2 | 28.4 ± 0.1 | 41.6 ± 5.9 |
| 10/0 | 25.7 ± 0.1 | 30.1 ± 0.3 | 50.3 ± 4.8 |
| 15/0 | 23.6 ± 0.2 | 50.2 ± 0.6 | 74.6 ± 8.8 |
| 5/5 | 38.3 ± 0.1 | 47.4 ± 0.1 | 68.4 ± 4.7 |
| PVP/CP | |||
| 5/0 | 28.4 ± 0.1 | 30.2 ± 0.4 | 33.6 ± 7.8 |
| 10/0 | 25.6 ± 0.2 | 44.5 ± 0.2 | 48.3 ± 7.9 |
| 15/0 | 22.4 ± 0.6 | 61.3 ± 0.6 | 66.4 ± 10.3 |
| 5/5 | 38.8 ± 0.3 | 42.7 ± 0.5 | 100.3 ± 11.5 |
| HEC/CP | |||
| 5/0 | 23.4 ± 0.2 | 52.3 ± 0.4 | 60.4 ± 5.9 |
| 10/0 | 21.1 ± 0.7 | 67.3 ± 0.2 | 82.4 ± 4.9 |
| 15/0 | 20.4 ± 0.4 | 88.3 ± 0.2 | 110.3 ± 9.9 |
| 5/5 | 34.5 ± 0.3 | 61.4 ± 0.2 | 98.6 ± 6.7 |
Each value represent the mean ± SD (n = 3)
P407 Poloxamer 407, P188 Poloxamer 188, MC methylecellulose, CP carbopol 947, HPMC hydroxypropylmethylcellulose, PVP polyvinyl pyrolidone, HEC hydroxyethylcellulose
Measurement of Gelation Temperature
The gelation temperature was measured according to the method reported by Yun et al. (13). Briefly, 10 g of each Poloxamer gel was placed in a 20-ml transparent glass vial with a magnetic bar (15 × 6 mm). The preparation was placed in a low-temperature thermostat water bath (GFL, Germany). Poloxamer gel was gradually heated, from 20°C, at an increased temperature of 1°C/min with a constant stirring of 50 rpm. The temperature at which the magnetic bar stopped moving was taken as the gelation temperature. All gelation temperature results are the mean of n = 3 experiments.
Measurement of Gel Strength
Liquid suppository base (50 g) was put in a 100-ml graduated cylinder and gelled in a water bath kept at 36 ± 0.5°C for 30 min. A disk for measuring gel strength (1.5 cm diameter, weight 35 g) was placed on the surface of the gelled base in the cylinder. The time (s) required for the disk to move 5 cm down through the cylinder was measured and taken as an arbitrary index of gel strength. In cases that it took more than 5 min to drop the apparatus into the gel, various weights were placed on top of the apparatus and gel strength was described by the minimal weights that pushed the apparatus 5 cm down through the gel (12).
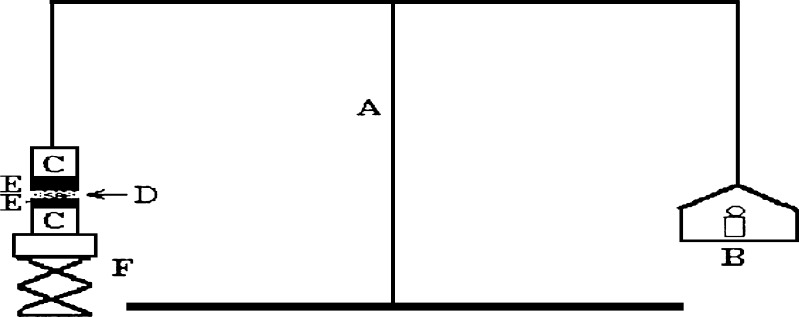
Measurement of Mucoadhesive Force
The mucoadhesive force of Poloxamer gel was measured using a modified balance (Fig. 1) according to previously reported method (17). A section of rectal tissues was cut from the fundus of the rabbit (New Zealand White) and instantly secured with mucosal side out onto glass vials using a rubber band and an aluminum cap. The diameter of each exposed mucosal membrane was 6.3 mm. The vials with the rectal tissues were stored at 36.5°C for 10 min before measuring the mucoadhesive force. One vial was connected to the balance and the other vial was placed on a height-adjustable pan. A 0.15 g sample of liquid suppository was then spread between the mucosal membranes on the vials to attach them. The weights on the other side of the balance required to separate the vials’ membranes was read. The mucoadhesive force of the liquid suppository per unit area (dyne/cm2) of mucosa was calculated using the following equation
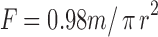 |
1 |
where m and r represent the balance weight (g) and radius of the vial (i.e., 6.3 mm), respectively.
Fig. 1.
Bioadhesive force-measuring device: (A) modified balance, (B) weights, (C) glass vial, (D) poloxamer gel, (E) rectal tissue, (F) height-adjustable pan
In vitro Release from Rectal Formulations
In vitro release of etodolac from rectal formulations was monitored by the USP paddle method at a rotating speed of 100 rpm in 500 ml phosphate buffer as a dissolution medium, pH 7.4 at 37 ± 0.5°C. One gram of each formulation containing 50 mg etodolac was placed into a semipermeable cellulose membrane tube (5 × 1.5 cm, length × diameter). Both sides of the tube were tied up with a thread to prevent leakage. The semipermeable membrane tube was placed in a dissolution tester (DST-600, Erweka, Germany). Five-milliliter samples were withdrawn at predetermined time over a 4-h period and replaced with the same volume of fresh dissolution medium. The samples were analyzed spectrophotometrically at λmax 274 nm using UV/visible variable wavelength detector (Ultrospec II, Biochrom, Cambridge, England)
Analysis of the Release Mechanism
To understand the dissolution mechanism of etodolac from Poloxamer gel, the release profiles between 10% and 70% release were characterized by fitting the following equation (20):
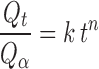 |
2 |
where Qt is the amount of drug released at time t, Q∞ is the total drug dose, k is a characteristic constant of the liquid suppository, and n is an indicative of release mechanism. As the k value becomes higher, the drug is released faster. The n value of 1 corresponds to zero order release kinetics, 0.5 < n < 1 means a non-Fickian release model and n = 0.5 indicates Fickian diffusion, i.e., Higuchi model. The dissolution time for 50% (DT50) of the initial drug content of each Poloxamer systems were extracted from the dissolution profiles.
Anti-inflammatory Effect of Etodolac Liquid Suppository
Experiments were carried out on 24 male Sprague-Dawley rats weighing 200 ± 10 g. The animals were fasted for 18 h prior to treatment but had free access to tap water. They were randomly assigned in four groups with six rats in each group. A volume of 0.1 ml of 1% carrageenan solution was injected into the right-hand paw. One hour after carrageenan injection, the first group was given placebo Poloxamer gel [P407/P188/MC/CP (15/20/5/5% w/w)] (1.0 g/kg). Second group was given oral etodolac suspension at 50 mg/kg. Third group was given the Poloxamer gel [etodolac/P407/P188/MC/CP (5/15/20/5/5% w/w)] (0.5 ml equivalent to etodolac 50 mg/kg). Poloxamer gel is inserted into the rectum 4 cm above the anus through a polyethylene rectal cannula. The fourth group received 100 mg of etodolac solid suppository [etodolac/PEG 4000 (10/90%)] equivalent to 50 mg/kg. Before the rectal administration, the rectum of the rat was emptied using a Foley cannula and after rectal administration, the rectum of the rat was clamped with a clip in order to prevent leakage of the product. All experiments were performed according to the Guideline for Experimental Animal Research Committee of King Saud University. Paw thickness was measured from ventral to dorsal surfaces, using a dial caliper immediately prior to carrageenan injection and then at hourly intervals for 6 h. The mean swelling for six rats was calculated. The inflammation responses are expressed as percentage increase in paw thickness compared with pre-injection values and plotted against time.
Cytotoxicity Study
Male Sprague–Dawley rats weighing 200 ± 10 g were fasted for 24–36 h prior to the experiments but allowed free access to water. The rectal Poloxamer gel [etodolac/P 407/P 188/MC/CP (5/15/20/5/5)] was administered at 1.5 g/kg into the rectum 4 cm above the anus. Control animal was administered normal saline. The entrance of the anus was then blocked with a cyanoacrylate adhesive to prevent leakage out of from the anus. At 2 and 6 h after administration, the rectum was isolated, rinsed with a saline solution, fixed in 10% neutral carbonated buffered formaldehyde, embedded in paraffin using an embedding center, and cut into slices. The slices were stained with hematoxylin-eosin (8) and observed under a light microscope (Motic B Series digital laboratory optical microscope, China, equipped with a Moticam 2220 digital camera, 2.0 M Pixel, USB, at ×40 magnification power).
Statistics
All data were expressed as mean value ± SD. Statistical analysis was performed using ANOVA followed by post hoc Tukey test. Mean differences were considered statistically significant at a level P < 0.05.
RESULTS AND DISCUSSION
Effect of Mucoadhesive Polymers on the Physicochemical Properties of Liquid Suppository
Gelation Temperature
Gelation temperature is the temperature at which the liquid phase makes a transition to gel. For liquid suppository, gelation temperature ranged 30–36°C. If it is lower than 30°C, gelation occurs at room temperature leading to difficulty in manufacturing, handling, and administering, while higher than 36°C, the suppository still stays as a liquid at body temperature, resulting in leakage from the anus. Based on this theory, Poloxamers P407 and P188 in a ratio 15/20 showed a gelation temperature of 34.8°C which is similar to that found in previous study (14). The concentration of etodolac was fixed as 5% throughout the experiment. On the other hand, presence of etodolac slightly increased the gelation temperature of Poloxamer solutions (37.1°C).
The impact of mucoadhesive polymers on the gelation temperatures depends on the nature of bioadhesive polymers in the formulations. The mucoadhesive polymers namely, HPMC, MC, HEC, and PVP were examined in different concentrations ranged from 5–15% w/w. All the mucoadhesive polymers showed significant effect on the gelation temperature at the examined concentrations. Increasing the concentration of mucoadhesive polymers resulted in decreasing the gelation temperature of all Poloxamer systems. The gelation temperatures of all Poloxamer systems were 21–30°C. This indicates that these concentrations of mucoadhesive polymers could not provide the suitable gelation temperature. HEC exhibited the largest decrease in gelation temperature (20.4–23.4°C) while addition of carbopol to the mucoadhesive polymer (1:1 ratio) showed higher gelation temperature (34.5–38.8°C; Table I).
Gel Strength
Gel strength is important parameter in finding the suitable condition which allows the easy insertion of the suppositories and no leakage from the anus. It was previously reported that the optimal liquid suppository must have suitable gel strength (10–50 s) (12). The gel strength of etodolac–Poloxamer gels was affected by the nature and composition of mucoadhesive polymers. All mucoadhesive polymers abruptly increased the gel strength as the concentration increased from 5–15%. The more increased the concentration of the mucoadhesive polymer, the greater the gel strength of Poloxamer gels. This finding was in agreement with Yong et al. (7), where they found that sodium alginate had a great effect on the gel strength of acetaminophen liquid suppository when added in the range 0.2–1.0%. It was speculated that the mucoadhesive polymer could bind strongly with the cross-linked Poloxamer gel by placing the polymer in the gel matrix (21). HEC exhibited the largest increase in the gel strength (52.3–88.3 s), upon increasing the concentration from 5% to 15%; while HPMC showed the least effect on gel strength (28.4–50.2 s) at the same concentrations. The increment of strength might be related to hydrogen bonding between Poloxamers and mucoadhesive polymers in the suppository (21).
The results are in agreement with that of Lehr et al. (22). Addition of carbopol to the tested mucoadhesive polymer in ratio 1:1 showed the suitable gel strength of (42.7–61.4 s; Table I).
Mucoadhesive Force
Mucoadhesive force is known to be dependent on the nature and the concentration of mucoadhesive polymers. The stronger the mucoadhesive force is, the more it can prevent the gelled suppositories from reaching the end of the colon, the pathway for the first-pass effect. But if the mucoadhesive force is too excessive, the gel can damage the rectal mucous membrane (19). Therefore, liquid suppository must have the suitable mucoadhesive force. The Poloxamers gel itself has a moderate bioadhesive force due to binding of the hydrophilic oxide group to oligosaccharide chains (23). It is indicated that the mucoadhesive polymers which enhanced gel strength efficiently increased the mucoadhesive force (Table I). In all Poloxamer gels, there was a substantial increase of mucoadhesive force from 33.6–110.3 dyne/cm2 as the mucoadhesive polymers’ concentration increased from 5% to 15%. Such effects on the mucoadhesive force might be due to the binding of these polymers with the oligosaccharide chains of rectal mucous lining. HEC 15%-containing Poloxamer gel showed the greatest mucoadhesive force (110.3 × 102 dyne/cm2) followed by MC 15% (83.6 × 102 dyne/cm2). The charges of mucoadhesive polymers seem not to play a major role in affecting the physicochemical properties of Poloxamer-based liquid suppository. Inclusion of carbopol in the Poloxamer systems exerted higher effects on the mucoadhesive force of the gel (68.4–91.6 × 102 dyne/cm2). The stronger effects of carbopol on the physicochemical properties seem to be due to its carboxyl groups which could bind strongly with the cross-linked reticular Poloxamer gel by putting its molecules in between the gel. Furthermore, the mucoadhesive force-strengthening effects appear to be contributed by its strong hydrogen bonding with the oligosaccharide chain of the rectal mucous lining (24).
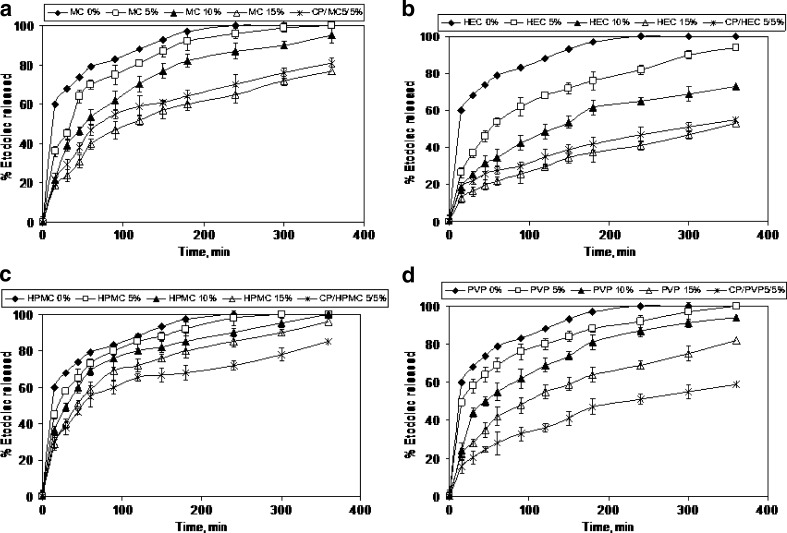
In vitro Release of Etodolac from Liquid Suppository
Release profiles from the various Poloxamer formulations are shown in Fig. 2 (A–D) and the main release kinetic characteristics are reported in Table II. The release of etodolac was variously affected by mucoadhesive polymer concentrations. Drug release was generally retarded by the addition of mucoadhesive polymers compared with liquid suppository without the adhesive polymers (control formulation of liquid suppository containing etodolac/P407/P188 in 5/15/20% w/w ratio). This finding was confirmed by the DT 50% values (10 min for control liquid suppository and 15–330 min for mucoadhesive liquid suppository formulations). HEC and MC exhibited the highest retardation, followed by PVP and HPMC. Burst effect was observed with the lowest concentration of the polymers (5%); more than 40% released in 1 h. Ryu et al. by associating mucoadhesive substance (sodium alginate, polycarophil, carbopol, poly(vinyl pyrolidone), and hydroxypropylcellulose) to a mixture of Poloxamer 407 and 188, showed that these substances slowed down the in vitro release of propanolol (14). The cumulative amount of etodolac released over the 6 h was significantly higher from control liquid suppository (94.3–100%) than from mucoadhesive liquid suppository formulations (53.7–100%). As the k value becomes higher, the drug is released faster. The k values indicate that etodolac was released more slowly from liquid suppositories with higher concentrations of mucoadhesive polymer. Such a slow release of etodolac appears to be partly caused by the higher gel strength of the liquid suppository. Table II shows that most of n values are close to 0.5, suggesting that etodolac is released from the liquid suppositories by Fickian diffusion through extramicellar aqueous channels of the gel matrix, which means the outer layer of Poloxamer cross-linking system (Poloxamer micelle). The increase in gel strength (Table I) and/or molecular interaction between etodolac and Poloxamers appeared to be involved in the retarded release of the drug by the addition of mucoadhesive polymers. As shown in Fig. 2 and Table I, the liquid suppository with 5% methyl cellulose and 5% carbopol showed the optimal gelation temperature (36.3 ± 0.2°C), gel strength (45.5 ± 0.1 s), bioadhesive force (88.4 ± 9.4 × 102 dyne/cm2), and a retardant effect on etodolac release from liquid suppository formulation.
Fig. 2.
Release of etodolac from liquid suppositories composed of etodolac: P407: P188: mucoadhesive polymer (5:15:20:5–15% w/w)
Table II.
Release Kinetic Parameters of Etodolac from Different Rectal Formulations Composed of [Etodolac/P407/P188 (5/15/20)]
| Poloxamer formulations | Kinetic constant (k, %/minn) | Release exponent (n) | Correlation coefficient (r) | DT50% (min) | Etodolac released 6 h (%) |
|---|---|---|---|---|---|
| Control | 39.843 | 0.435 | 0.9818 | 10 | 100 |
| MC/CP | |||||
| 5/0 | 16.864 | 0.312 | 0.9968 | 25 ± 3.8 | 100 ± 3.2 |
| 10/0 | 13.833 | 0.447 | 0.9949 | 50 ± 3.5 | 95.1 ± 2.1 |
| 15/0 | 7.993 | 0.399 | 0.9850 | 110 ± 4.6 | 77.3 ± 4.3 |
| 5/5 | 10.004 | 0.478 | 0.9939 | 80 ± 2.4 | 81.2 ± 3.6 |
| HPMC/CP | |||||
| 5/0 | 17.349 | 0.338 | 0.9795 | 25 ± 2.3 | 100 ± 2.4 |
| 10/0 | 13.862 | 0.467 | 0.9886 | 30 ± 2.9 | 100 ± 5.1 |
| 15/0 | 11.196 | 0.437 | 0.9971 | 45 ± 1.8 | 96.3 ± 4.8 |
| 5/5 | 9.829 | 0.323 | 0.9882 | 55 ± 3.2 | 85.4 ± 5.2 |
| PVP/CP | |||||
| 5/0 | 27.516 | 0.220 | 0.9965 | 15 ± 4.2 | 100 ± 5.4 |
| 10/0 | 10.054 | 0.456 | 0.9979 | 45 ± 3.5 | 94.7 ± 4.8 |
| 15/0 | 7.345 | 0.469 | 0.9807 | 100 ± 3.9 | 82.5 ± 3.8 |
| 5/5 | 5.437 | 0.483 | 0.9980 | 240 ± 5.3 | 59.2 ± 2.5 |
| HEC/CP | |||||
| 5/0 | 9/735 | 0.397 | 0.9906 | 55 ± 5.2 | 94.3 ± 4.1 |
| 10/0 | 5.431 | 0.453 | 0.9967 | 130 ± 6.1 | 73.3 ± 3.9 |
| 15/0 | 3.352 | 0.458 | 0.9968 | 330 ± 4.8 | 53.7 ± 3.6 |
| 5/5 | 9.648 | 0.498 | 0.9690 | 280 ± 6.1 | 55.3 ± 2.7 |
Each value represent the mean ± SD (n = 3)
P407 Poloxamer 407, P188 Poloxamer 188, MC methylecellulose, CP carbopol 947, HPMC hydroxypropylmethylcellulose, PVP polyvinyl pyrolidone, HEC hydroxyethylcellulose
Compared to solid suppository, etodolac release showed a similar release pattern and k value was 11.8% min−n (data not shown). Thus, the liquid suppository, [etodolac/P407/P188/MC/CP (5/15/20/5/5% w/w)] was selected as a test sample for anti-inflammatory study in rats.
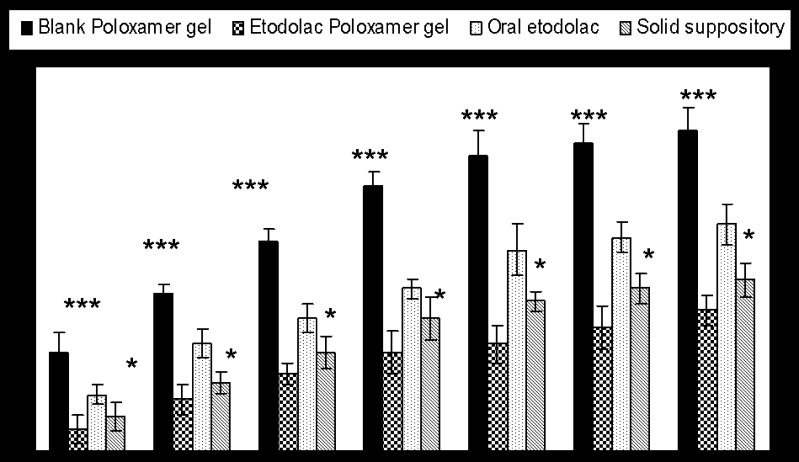
Anti-inflammatory Effect of Etodolac Formulations
Induction of acute inflammation in control rats resulted in a prominent increase in paw thickness, beginning 1 h after intraplanter injection of carrageenan and reaching a peak of inflammation after 4 h. The rank degree of increased in paw thickness is control poloxamer ≻ oral etodolac suspension ≻ conventional etodolac suppository ≻ etodolac–Poloxamer gel. At all the time intervals, P < 0.0001 which mean strong significant effect of etodolac–Poloxamer, oral suspension and conventional suppository compared with the control Poloxamer gel on the increase in paw edema. Administration of etodolac–Poloxamer gel significantly suppressed the maximal edema responses attained during 6 h, from 75 to −35% (Fig. 3). An oral dose of 50 mg/kg of etodolac suspension in water produced little anti-inflammatory effect (56% increases in paw thickness after 6 h). According to the biopharmaceutical classification of drugs defined by Amidon et al. (25) etodolac is a lipophilic drug exhibit low oral absorption as a result of its poor aqueous solubility. Dissolution in the environmental lumen is the rate-controlling step in the absorption process. Our results indicated that the etodolac from Poloxamer gel could absorbed faster than from conventional suppository in rats. The reason for this fast absorption might be dependent upon the dispensability (fluidity) and bioadhesive force (7).
Fig. 3.
Percent increase in paw thickness versus time after administration of different etodolac formulations to rats. *P < 0.05 compared with Etodolac–Poloxamer gel and ***P < 0.001 compared with all poloxamer formulations. Each point represents the average of four experiments (n = 6)
Conventional suppository was not bioadhesive and gradually dissolved and dispersed in the rectal fluid resulting in 40% increase in paw thickness after 6 h. At all time intervals point P < 0.05 compared with etodolac–mucoadhesive Poloxamer gel. In contrast, Poloxamer gel was spread easily in the rectum, gel for improving etodolac deliver in disease people and attached on the rectal mucous membranes, since bioadhesive Poloxamer gel was a fluid initially (17).
Cytotoxicity Study
The safety test of Poloxamer gel was performed by observing any irritation of Poloxamer gel on the rectal tissues. The morphology of rectal tissues shown in Fig. 4 indicated that Poloxamer gel with the mucoadhesive polymers did not irritate or damage the rectal tissues up to 6 h post-administration. Previously, Poloxamers, the non-ionic surfactants, were reported to be inert, giving no damage to mucous membranes (26).
Fig. 4.
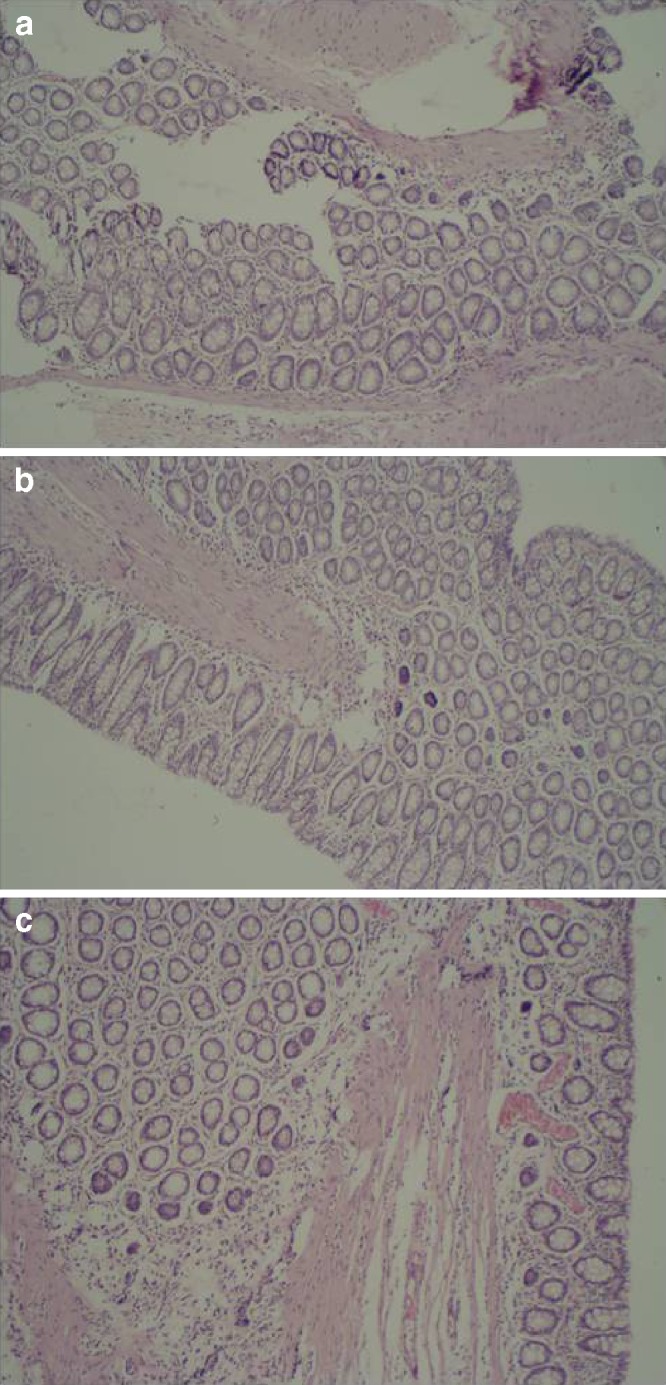
Photomicrograph of rectal mucosa of rats after rectal administration of etodolac–loaded Poloxamer gel (×40). a Control, b 2 h after the dose, and c 6 h after the dose
CONCLUSION
Modulation of the physicochemical and adhesive properties of Poloxamer 407P and 188P mixtures by mucoadhesive polymers showed a prolonged in vitro release of etodolac. It is concluded that liquid suppository [etodolac/P407/P188/MC/CP (5/15/20/5/5)], which remained at the administered sites due to strong gel strength and mucoadhesive force, could improve absorption of etodolac and the anti-inflammatory effect of this drug in rats, without damaging the rectum. The gelation temperatures of etodolac rectal formulations could be easily modulated to adjust the gelation just below the body temperature. This allows obtaining liquid systems at room temperature which can be conveniently handled and administered and that develop an intimate contact with the rectal mucosa. This system might be applicable for the development of in situ gelling and mucoadhesive liquid suppository for humans as a more conventional and effective rectal dosage form.
Acknowledgements
This research is partly supported by the grant from the Research Institute of Pharmaceutical Sciences in College of Pharmacy, King Saud University, Riyadh, SA (AT. 23 461). The author wants to thank Dr. Hanan El-Gawaly Department of Pharmacology and Miss Hanan Mossa, Department of Pathology, Faculty of Medicine, Alexandria University, Egypt, for their assistance in performing the animal studies.
References
- 1.Park YJ, Yong CS, Kim HM, Rhee JD, Oh YK, Kim CK, Choi HG. Effect of sodium chloride on the release, absorption, and safety of diclofenac sodium delivered by poloxamer gel. Int J Pharm. 2003;263:105–111. doi: 10.1016/S0378-5173(03)00362-4. [DOI] [PubMed] [Google Scholar]
- 2.Kim CK, Lee SW, Choi HG, Lee MK, Gao ZG, Kim IS, Park KM. Trials of in-situ gelling and mucoadhesive acetaminophen liquid suppository in human subjects. Int J Pharm. 1998;174:201–207. doi: 10.1016/S0378-5173(98)00258-0. [DOI] [Google Scholar]
- 3.Lee JW, Park JH, Robinson JR. Bioadhesive-based dosage forms; the next generation. J Pharm Sci. 2000;89:850–866. doi: 10.1002/1520-6017(200007)89:7<850::AID-JPS2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Chang JY, Oh TK, Choi HG, Kim YB, Kim CK. Rheological evaluation of thermosensitive and mucoadhesive vaginal gels in physiological conditions. Int J Pharm. 2002;241:155–163. doi: 10.1016/S0378-5173(02)00232-6. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Parsons DL, Navarre C, Kompella UB. Development and in vitro evaluation of sustained release Poloxamer 407 (P407) gel formulations of ceftiotur. J Control Rel. 2002;85:73–81. doi: 10.1016/S0168-3659(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 6.Choi HG, Kim MH, Lee MK, Kim CK. Effect of additives on the physicochemical properties of liquid suppository. Int J Pharm. 1999;190:13–19. doi: 10.1016/S0378-5173(99)00225-2. [DOI] [PubMed] [Google Scholar]
- 7.Yong CS, Choi JS, Quan QZ, Rhee JD, Kim CK, Lim SJ, Kim KM, Oh P, Choi SHG. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of Poloxamer gels containing diclofenac sodium. Int J Pharm. 2001;226:195–205. doi: 10.1016/S0378-5173(01)00809-2. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki S, Suisha F, Kawasaki N, Shirakawa M, Yamatoya K, Attwood D. Thermally reversible xyloglucan gels as vehicles for rectal drug delivery. J Control Rel. 1998;56:75–83. doi: 10.1016/S0168-3659(98)00079-0. [DOI] [PubMed] [Google Scholar]
- 9.Dumortier G, Zuber M, Courarraze G, Chaumeil JC, Grossiord JL. Rheological study of a thermoreversible morphine gel. Drug Dev Ind Pharm. 1991;17:1255–1265. doi: 10.3109/03639049109043858. [DOI] [Google Scholar]
- 10.Wade A, Weller PJ. Handbook of Excipients, 2nd edn. London, England: The American Pharmaceutical Association (USA) and the Pharmaceutical Press, 306:223–229; 1994.
- 11.Vueba ML, Batista de Carvalho LAE, Veiga F, Sousa JJ, Pina ME. Influence of cellulose ether mixtures on ibuprofen release: MC25, HPC and HPMC K100M. Pharm Dev Techn. 2006;11:213–228. doi: 10.1080/10837450600561349. [DOI] [PubMed] [Google Scholar]
- 12.Choi HG, Oh YK, Kim CK. In-situ gelling and mucoadhesive liquid suppository containing acetaminophen: enhanced bioavailability. Int J Pharm. 1998;165:23–32. doi: 10.1016/S0378-5173(97)00385-2. [DOI] [Google Scholar]
- 13.Yun MO, Choi HG, Jung JH, Kim CK. Development of a thermo-reversible insulin liquid suppository with bioavailability enhancement. Int J Pharm. 1999;189:137–145. doi: 10.1016/S0378-5173(99)00227-6. [DOI] [PubMed] [Google Scholar]
- 14.Ryu JM, Chung SJ, Lee MH, Kim CK, Shim CK. Increased bioavailability of propranolol in rats by retaining thermally gelling liquid suppositories in the rectum. J Control Rel. 1999;59:163–172. doi: 10.1016/S0168-3659(98)00189-8. [DOI] [PubMed] [Google Scholar]
- 15.Koffi AA, Agnely F, Besnard M, Brou K, Grossiord JL, Ponchel G. In vitro and in vivo characteristics of a thermogelling and bioadhesive delivery system intended for rectal administration of quinine in children. Eur J Pharm Biopharm. 2008;69:167–175. doi: 10.1016/j.ejpb.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Kokot Z, Zmidzinska H. Solubility and dissolution rate of ibuprofen in ionic and nonionic micellar systems. Acta Pol Pharm. 2001;58:117–120. [PubMed] [Google Scholar]
- 17.Yong CS, Oh YK, Jung SH, Rhee JD, Kim HD, Kim CK, Choi HG. Preparation of ibuprofen-loaded liquid suppository using eutectic mixture system with menthol. Eur J Pharm Sci. 2004;23:347–353. doi: 10.1016/j.ejps.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Balfour JA, Buckley MM-T. Etodolac. A reappraisal of its harmacology and therapeutic use in rheumatic diseases and pain states. Drugs. 1991;42:274–299. doi: 10.2165/00003495-199142020-00008. [DOI] [PubMed] [Google Scholar]
- 19.Choi HG, Jung JH, Ryu JM, Yoon SJ, Oh YK, Kim CK. Development of in-situ gelling and mucoadhesive acetaminophen liquid suppository. Int J Pharm. 1998;165:33–44. doi: 10.1016/S0378-5173(97)00386-4. [DOI] [Google Scholar]
- 20.Korsmeyer RW, Gurny R, Doelker EM, Buri P, Peppas NA. Mechanism of release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. doi: 10.1016/0378-5173(83)90064-9. [DOI] [PubMed] [Google Scholar]
- 21.Kramaric A, Resman A, Kofler B, Zmitek J. Thermoreversible gel as a liquid pharmaceutical carrier for a galenic formulation. European Patent 05551 626 (A1) 1992.
- 22.Lehr CM, Bouwatra JA, Bodde HE, Junginger HE. A surface energy analysis of mucoadhesion: contact angle measurement on polycarbophil and pig intestinal mucosa in physiologically relevant fluids. Pharm Res. 1992;9:70–75. doi: 10.1023/A:1018931811189. [DOI] [PubMed] [Google Scholar]
- 23.Choi HG, Jung JH, Yong CS, Rhee JD, Lee MK, Han JH, Park KM, Kim CK. Formulation and in vivo evaluation of omeprazole buccal adhesive tablet. J Control Rel. 2000;68:405–412. doi: 10.1016/S0168-3659(00)00275-3. [DOI] [PubMed] [Google Scholar]
- 24.Lehr CM, Bouwstra JA, Tukker JJ, Junginger HE. Intestinal transit of bioadhesive microspheres in an in situ loop in the rat: a comparative study with copolymers and blends based on poly (acrylic acid) J Control Rel. 1990;13:51–62. doi: 10.1016/0168-3659(90)90074-4. [DOI] [Google Scholar]
- 25.Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–420. doi: 10.1023/A:1016212804288. [DOI] [PubMed] [Google Scholar]
- 26.Escobar-Chavez JJ, Lopez-Cervantes M, Naik A, Kalia YN, Quintanar-Guerrero D, Ganem-Quintanar A. Applications of thermo-reversible Pluronic 127 gels in pharmaceutical formulations. J Pharm Pharmaceut Sci. 2006;9:339–358. [PubMed] [Google Scholar]