Abstract
The purpose of this study was to examine the feasibility of using irreversible electroporation (IRE) as a non-chemical method for eliminating microorganisms of liquid drugs. The studied drug was a topical ophthalmic medication, a pharmaceutical field in which the problem of microbial contamination has not yet been adequately solved, especially in the case of eye drops prescribed for chronic use. Commercially available Hylo-Comod® preservative-free eye drop solution was subjected to contamination with Escherichia coli bacteria (106 colony forming units/mL). Electroporation parameters for bacterial control were investigated by comparing the effects of electrical fields of 5.4, 7.2, and 10 kV/cm, delivered as 100-µs square pulses at 1 Hz in sequences of 10 pulses, 20 pulses, or 20 pulses delivered as four sets of five pulses with 1-min intervals between each set. Microorganism survival after treatment was determined by pour plate counting. Effects of the treatment parameters on temperature and pH were recorded. Bacterial survival was lowest (0.14% ± 0.03%) after application of 20 pulses delivered as four separate sets. With that application mode, the solution remained at pH 7.5 and the temperature rose to 35.6° ± 0.2°C. Because IRE can be efficiently delivered under conditions that avoid the potentially deleterious effects of electrical pulses on temperature and pH, it appears to be a feasible method for bacterial control of drugs in solution. The principles established in this study can be applied to any drug in solution and optimized individually according to the solution's composition.
Key words: contamination, eye drops, irreversible electroporation, microorganisms, preservatives, sterilization of drugs
Contamination of liquid drugs can have substantial detrimental effects on the health of patients using drugs (1,2), necessitating the addition of preservatives in many pharmaceutical preparations. A particularly significant problem is the presence of preservatives in pediatric vaccination and the possible association with neurodevelopmental disorders such as autism (3,4). While this association is highly controversial, eliminating the need for preservatives in the vaccination will serve to allay the apprehension among parents and may increase the use of vaccinations. We focus here on the contamination of topical ophthalmic medications. The problem of infections engendered by microorganisms in eye drops has not yet been adequately solved and is especially troublesome when eye drops are used chronically for many years, as in glaucoma patients. In addition, patients who suffer from dry eyes and do not use the more expensive single-unit dose preparations are exposed to similar risks of infection, as are contact lens users. The problem of contamination can also arise in cases of acute eye drop treatment spanning days or weeks.
The prevalence of bacteria in anti-hypertensive glaucoma drops in the community setting has been documented in a number of studies. Geyer et al. found bacteria in more than 28% of in-use topical medications (bottle tips and drops) of 109 treated glaucoma patients (5). The contamination rate was significantly related to the time since the container was first opened; bacteria were detected in 40% of eye drops from bottles that had been opened more than 8 weeks earlier compared to 19% in bottles in use for less time. Similar findings were reported by Schein et al. (6) in drops used by patients suffering from ocular surface diseases. Lower rates (12.8% and 12.9%) have also been reported (7,8). The high contamination rate is not surprising given the way in which eye drop containers are handled by patients. More than half of all elderly patients in one study were found to touch the eyelid or conjunctiva with the container, undoubtedly causing the solution to become infected by flora of the skin and conjunctiva (9). In contrast, a much lower contamination rate (2.3%) was measured in drops used by medical personnel in a clinic (10).
Growth of microorganisms in ophthalmic medications can be reduced to some extent by adding preservatives to the solution, typically benzalkonium chloride (BAK). However, since the contamination rates cited above were found in eye drops that contain preservatives, their presence obviously does not solve the problem. Moreover, all preservatives have considerable side effects, particularly when the medications are used on a chronic basis. BAK, which is used in most topical ophthalmic preparations, harms the surface of the eye and probably accounts for the finding that well over half of treated glaucoma patients suffer from symptoms and signs of dry eyes (11). This compound can actually be used to induce inflammation when producing a dry eye model in rabbits (12). Not only does BAK damage the superficial eye tissues but its chronic administration apparently also harms the trabecular meshwork and thus may counteract the anti-hypertensive ocular drugs in which it helps control the bacterial load (13). Newer preservatives might be less injurious to the eyes than BAK, but they too are not free of complications (14), and they have not been in use long enough for their possible effects to be precisely determined. As expected, preservative-free medications seem to produce the least complications (15). One possible way to overcome the problem might be through the use of eye drops packaged in single-unit dose containers. These, however, are expensive and are not generally used for the glaucoma drugs financed by health maintenance organizations. Furthermore, many elderly patients find the containers difficult or impossible to manipulate properly (9).
Clearly, then, it is important to find a non-chemical, practical method of bacterial control in liquid fluids in their delivery containers. Accordingly, the aim of this study was to examine the feasibility of using irreversible electroporation (IRE) as a method for controlling bacterial contamination in liquid drugs. Electroporation is a physical phenomenon in which a cell membrane becomes permeabilized by application of short (microsecond-scale) electrical pulses across the living cell. The mechanism presumably operates by forming nanoscale defects in the cell membrane. The overall effect of the electrical pulses is a function of various pulse parameters such as pulse length, pulse amplitude, and number of pulse repeats. These parameters determine whether the cell membrane will remain intact or will become permeabilized, either reversibly (reversible electroporation) or irreversibly (IRE). The nature of both reversible and irreversible electroporation, and their uses, most widely in the food industry, are well documented and have been comprehensively reviewed in the scientific literature (16–26).
We postulated that IRE can be used as a means of bacterial control in fluid drug containers, either for the whole volume or during the passage of fluid into and out of the container. The issues to be addressed when treating drugs by IRE are different from those documented in the case of foods. With drugs, the volume of the solvent is significantly smaller, its ionic content is proportionately much larger (25), and the solution conditions after IRE [in particular temperature and pH (25,26)] should remain unchanged to avoid their potentially undesirable effects on the drug. These issues are addressed in this preliminary study on the use of IRE in bacterial control in liquid drugs. We first studied the effects of IRE in a liquid ophthalmic preparation and at a volume typical of eye drop containers. We next investigated what are the IRE pulse modes capable of maximal reduction in bacterial contents of the solution in these small containers without substantially affecting its temperature. Finally, we examined the effects of the IRE pulses on the pH of a small volume of solution.
METHODS
Experimental Protocol
The procedure developed here was intended for domestic use by patients; thus, it was necessary to investigate the possibility of using much weaker electric fields than those routinely used for IRE in the food industry. Our earlier studies (27,28) showed that this can be accomplished using larger number of pulses delivered in groups. In the present study, therefore, we gradually increased the electrical field strength while also varying the numbers of pulses and their modes of delivery. Before applying the different sets of pulses, we employed our previously developed first-order mathematical analysis of the temperature increase during IRE (27) in order to eliminate a priori any parameters that might produce an undesirable increase in temperature. Effects of the treatment parameters on temperature and pH were monitored. The end point of each experiment was the measurement of cell viability, temperature, and pH. All experiments were repeated five times.
Pharmaceutical Preparation
The preparation used in this study as a model for a preservative-free liquid solution was a commercially available sodium hyaluronate 0.1% eye drop solution (Hylo-Comod®; Ursapharm, Arzneimittel GmbH, Industriestrasse, D-66129,Saarbrucken, Germany).
Microorganisms
Escherichia coli tetracycline-stable bacteria were kindly provided by the laboratory of Dr. Y. Tzfati, Alexander Silberman Institute of Life Sciences, Hebrew University, Jerusalem. Microorganisms were cultured by transferring the organisms from Luria–Bertani (LB) agar plates to 500 mL of LB Miller broth (Sigma-Aldrich, Israel), which was agitated in a temperature-controlled incubator at 37°C until a concentration of 108 colony forming unit (cfu)/mL was obtained.
Electroporation
After adding 5 µL of the culture preparation to 1 mL of eye drops, we diluted the drops tenfold to a final concentration of 106 cfu/mL. We then placed 80-µL aliquots of the diluted drops in a 1-mm gap electroporation cuvette (model 610; BTX, San-Diego, CA, USA) and subjected them to electroporation by a BTX ECM830 square-wave electroporator. To establish the parameters for bacterial control at 1 Hz, we compared the effects of electrical fields of 5.4, 7.2, and, 10 kV delivered as 100-µs unipolar rectangular pulses at 1-s intervals. Three sequences of delivery of 10 kV/cm were examined: (a) 10 pulses, (b) 20 pulses, or (c) 20 pulses delivered as four sets of five pulse sequences with 1-min intervals between sets.
Immediately after electroporation, we measured both the temperature and the pH of the solution. Temperature was measured in the cuvette using a Neoptix Reflex® signal conditioner with a 0.7-mm probe covered with polyimide (Neoptix, Québec,Canada). The pH was measured with pH indicator paper (Neutralit®, pH 5.0–10.0; Special indicator, pH 8.2– 10.0; and Acilit®, pH 0.5–5.0; Merck, Darmstadt, Germany).
Microorganism Viability Test
Live bacteria were counted by the pour plate method. After electroporation, the solution was diluted tenfold in Dulbecco's phosphate-buffered saline (Biological Industries, Kibbutz Beit Ha-Emek, Israel) to eliminate effects of the eye drop contents on cell growth (29). Samples (100 µL) of each of the tested solutions were plated in duplicate on LB–Miller agar plates supplemented with tetracycline and incubated at 37°C for 18 h. Cells were counted in an MRC colony counter model 570 (MRC, Holon, Israel).
RESULTS
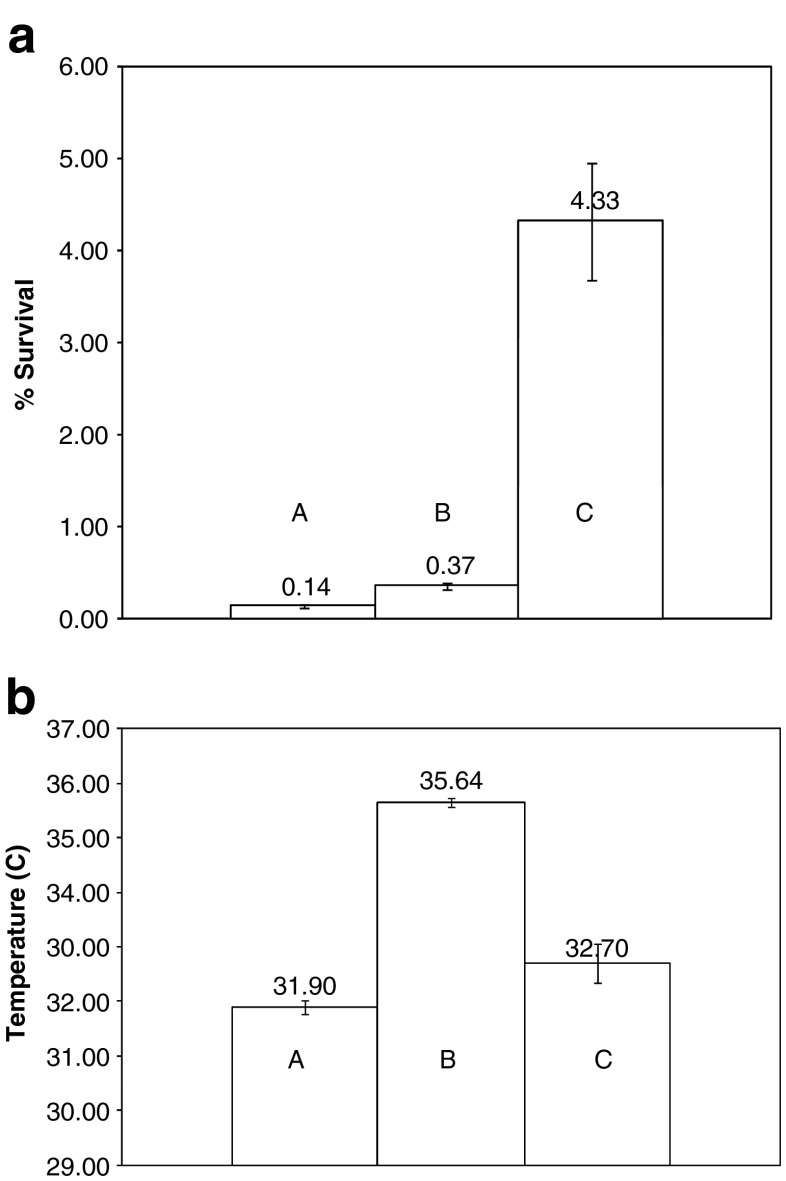
Figure 1a, b shows the effects of the number of IRE pulses, as well as the sequence in which they are delivered, on microorganism survival and solution temperature, respectively. The results are for an electrical field of 10 kV/cm and the pulse sequences are (a), (b), and (c) as described in “Experimental Protocol”. Doubling the number of pulses from 10 to 20 reduced the percentage of microorganism survival by more than tenfold, from 4.33 ± 1.26% to 0.37 ± 0.07% (Fig. 1a). Doubling the number of pulses increased the sample temperature by only 3°C from 32.7 ± 0.7°C to 35.6 ± 0.2°C (Fig. 1b). Altering the delivery sequence of the 20 pulses from continuous (b) to four discrete groups of five (c) resulted in an additional threefold decrease in microorganism survival, from 0.37 ± 0.07% to 0.14 ± 0.03% (Fig. 1a). However, there was less increase in temperature than when ten pulses were delivered continuously (Fig. 1b). In all experiments, samples maintained the same pH (7.5) after treatment as in the untreated control.
Fig. 1.
a Post-IRE survival of microorganisms as a function of the number and sequence of electroporation pulses for an electrical field of 10 kV/cm. 10 kV 20 pulses (four sets × 5), 10 kV 20 pulses (one set), 10 kV ten pulses (one set). Error bars represent 1 standard deviation. b Post-IRE temperature of the solution as a function of the number and sequence of electroporation pulses for an electrical field of 10 kV/cm. 10 kV 20 pulses (four sets × 5), 10 kV 20 pulses (one set), 10 kV ten pulses (one set). Error bars represent 1 standard deviation.
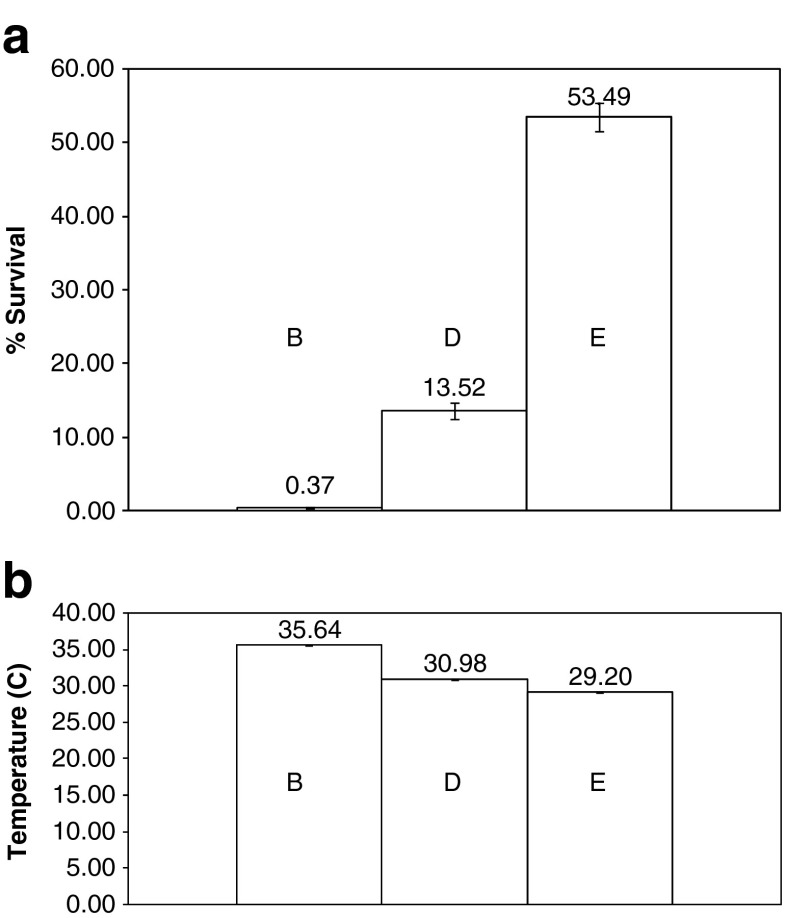
Figure 2a, b shows the effects of field strength on microorganism survival and solution temperature, respectively. Shown are the results for a sequence of 20 pulses of 5.4, 7.2, and 10 kV/cm, all delivered at a frequency of 1 Hz. Increasing the field strength from 5.4 to 10 kV/cm caused a reduction of more than 100-fold in microorganism viability, i.e., from 53.49 ± 3% to 0.37 ± 0.07%. The difference in temperature between the highest and lowest field was about 6°C, and the highest temperature did not exceed 35.6 ± 0.2°C. In all experiments, samples maintained the same pH (7.5) after treatment as in the untreated control.
Fig. 2.
a Microorganism survival as a function of electrical field strength. 10 kV 20 pulses (one set), 7.2 kV 20 pulses (one set), 5.4 kV 20 pulses(one set). Error bars represent 1 standard deviation. b Solution temperature as a function of electrical field strength. 10 kV 20 pulses (one set), 7.2 kV 20 pulses (one set), 5.4 kV 20 pulses(one set). Error bars represent 1 standard deviation.
Discussion
The results of this feasibility study demonstrate the ability of irreversible electroporation pulses, applied at certain electrical parameters, to reduce the bacterial contamination of topical ophthalmic preparations to acceptable levels without the need for chemical preservatives and without substantially raising the temperature or changing the pH of the solution. We anticipate that one possible application of the concept of microorganism control by IRE will be as a system for use by patients at home.
By plotting the effects of electrical fields against the efficacy of the bacterial control process, we found that the effect is not linear and that by increasing the electrical field strength, it is possible to increase the efficacy of the electrical pulse. For example, increasing the electrical field strength from 5.4 to 10 kV/cm decreased the microorganism survival by two orders of magnitude.
Our results further showed that the number of pulses and their sequence of delivery strongly affect the efficacy of IRE in bacterial control. Increasing the number of pulses by a factor of 2 decreases microorganism survival by one order of magnitude. As an example, survival decreases from 4.33 ± 1.26% to 0.37 ± 0.07% when the number of pulses is increased from 10 to 20. In a previous study using eukaryotic cells (27), our group showed that the efficacy of IRE can be significantly increased by using the same number of pulses but delivering them in groups separated by certain time intervals. In the present work, we tested this concept for the control of microbial contaminants. IRE with 20 pulses of 10 kV/cm, delivered at a frequency of 1 Hz, increased microorganism destruction such that bacterial survival was reduced to 0.37 ± 0.07%. When these 20 pulses were delivered in four groups of five pulses with 1-min intervals between them, microorganism survival was further decreased to 0.14 ± 0.03%, i.e., by a factor of almost 3. We found that by investing the same amount of electrical energy and without changing any device settings, we achieved a significantly higher degree of inactivation. To the best of our knowledge, this is the first report of such effective microbial inactivation not only in the pharmaceutical field but also in water and in food. It is therefore of considerable importance because it also suggests a way to significantly increase the energetic efficacy of IRE systems for bacterial control in the food and water industries where such efficacy is a crucial requirement for process acceptability.
Our results can be explained by invoking current electroporation theory (16), which suggests that the formation of pores is a stochastic process that is dependent on the electrical field. Therefore, increasing the number of pulses increases the probability of pore formation by IRE, and increasing the field strength increases the numbers and sizes of the pores that form. Electroporation-induced formation of pores in the cell membrane is a dynamic process in which pores form, increase in size, and then decrease. The time taken for pore formation is measured in microseconds, whereas for pore closure, it is orders of magnitude longer. Therefore, it is possible that IRE can be made more efficient by increasing the electrical field strength and by optimizing the choice of delivery and of the numbers and sequences of pulses in order to achieve the desired level of reduction in bacterial load without increasing the temperature or changing the pH. Knowing that bacterial control of this type is possible and that many parameters can be exploited to optimize performance makes IRE a promising method for the bringing the bacterial load of drug solutions to acceptable levels under a variety of conditions.
If the temperature rises during the delivery of IRE pulses, it could become high enough to induce undesirable chemical changes in the drugs. The increase in temperature after 20 sequential pulses, for example, was from 26°C to 35.6 ± 0.2°C. This suggests that if a harmful increase in temperature is to be avoided, there is an upper limit to the number of pulses that can be delivered safely. It was therefore encouraging to find that the most significant decrease in microbial load was achievable by delivering the 20 pulses in four separate sets of five pulses each and that the temperature increase (from 26°C to 32.7 ± 0.7°C) in that case was the lowest obtained in this study. This finding points to new ways of designing optimal IRE protocols that can avoid any undue increase in temperature.
Sale and Hamilton (22) demonstrated successful inactivation of microorganisms with IRE as long as 40 years ago. Subsequent studies showed that IRE can be used to destroy various microorganisms in different liquid solutions (25,26) and fluid type (26). Microbial inactivation for specific pathogens can be predicted by models (26) based on principles of chemical reaction kinetics (30–32). These models are still at an empirical stage because the nature of the processes that occur on the membrane during and after application of strong electric pulses is not yet fully understood (16). It is thought that the pores formed during electroporation cause the release of intracellular content, changes in intracellular homeostatic conditions, and osmotic swelling followed by cell death (26). Electroporation is reversible under certain conditions because of the ability of pores to reseal (33). A possible explanation for our findings might be related to the mathematical expression derived by Saulis (34) and previously observed experimental work (33–35). According to those studies, the moderate increase in temperature (35.6 ± 0.2°C) that was produced after we applied the 20 consecutive electrical pulses should facilitate pore sealing, while the smaller temperature increase we obtained when we delivered the 20 pulses in four sets should stabilize the pores, thereby increasing the cell death rate (26). The dependence of pore sealing rate on temperature might explain, at least in part, the threefold differences in rates of microorganism survival in our experiment when we applied 20 consecutive pulses (and obtained a final temperature of 35.6 ± 0.2°C) compared to application of the 20 pulses in four groups 1 min apart (final temperature 31.9 ± 0.3°C). The observed differences might be further explained in terms of the asymmetric distribution of large and small pores relative to the anode/cathode. Smaller pores, which seal more readily, occur mostly at the anode side, and larger pores mostly at the cathode side (36). Application of all 20 pulses in one set might trigger such asymmetry. When the pulses were applied in several sets, however, the microorganisms may have had the opportunity to mix and change the polarization site so that the distribution of large and small pores was symmetrical, causing more large pores to appear and thus leading to an increased rate of leakage.
During IRE treatment, the electric potential is applied on one of the electrodes and the second electrode is grounded. During the pulsed voltage application, a current flows in the liquid solution and both oxidation and reduction reactions occur on the electrodes. The electrochemical aspects, as well as theoretical ways of determining the maximum allowable pulse time, are critically dependent on the electrode material, as discussed elsewhere (18,26). Here, we applied a pulse length of 100 µs and immediately afterward removed the sample from the treatment chamber in order to avoid metal release or any additional reactions related to the electrodes. In all of our experiments, the pH of each treated sample (7.5) was the same as that of the untreated control. However, if the drug in the solution is affected by pH, it will be important to design optimal IRE electroporation protocols that will avoid such changes. One possible measure in that case might be to use alternating-current IRE, as recently described (37).
Conclusions and Future Research Directions
The results of this study support the feasibility of using IRE for bacterial control of drugs as an alternative to the use of chemical preservatives. The dose responses studied showed that it is possible to find electroporation parameters that can reduce the bacterial load of liquid drugs sufficiently without producing a substantial rise in temperature or change in pH.
Acceptable reduction in microbial contamination of eye drops and other fluid medications by IRE is expected to be applied at the application site, especially at patients’ homes, using a device which will deliver the necessary pulse to the solution as often as required. Electricity can be supplied to the device from the mains or, since the power consumption is low, from batteries. The IRE pulse can be actuated on a predetermined time schedule, manually by the user or automatically when the container is removed from the device.
Thus, the medication can be kept continuously with acceptable bacterial load after the container is opened. Consequently, bacterial control by IRE seems to be potentially superior to control by preservatives in efficacy as well as in eliminating the preservatives' side effects. Furthermore, it may extend the usage time of eye drops after the bottle is opened.
The present work is only preliminary and much research remains to be done to establish whether all drugs can be thus treated by IRE as the current may decompose drug molecules, especially charged ones. Furthermore, the applicability of the method and the optimal parameters for each specific combination of the various solutions, drugs, containers, and specific microorganisms such as pseudomonas and fungi species should be separately evaluated. The results should be compared to traditional bacterial control methods and the long-term effects of this novel procedure should be determined.
Acknowledgment
This study is supported by the Israel Science Foundation grant 403/06 (AG and BR).
References
- 1.Jimenez L. Microbial diversity in pharmaceutical product recalls and environments. PDA J Pharm Sci Technol. 2007;61(5):383–399. [PubMed] [Google Scholar]
- 2.Nichols RL, Smith JW. Editorial. Bacterial contamination of an anesthetic agent. N Engl J Med. 1995;333(3):184–185. doi: 10.1056/NEJM199507203330310. [DOI] [PubMed] [Google Scholar]
- 3.Young HA, Geier DA, Geier MR. Thimerosal exposure in infants and neurodevelopmental disorders: an assessment of computerized medical records in the Vaccine Safety Datalink. J Neurol Sci. 2008;271:110–118. doi: 10.1016/j.jns.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Geier DA, Sykes LK, Geier MR. A review of thimerosal (merthiolate) and its ethylmercury breakdown product: specific historical considerations regarding safety and effectiveness. J Toxicol Environ Health, Part B. 2007;10:575–596. doi: 10.1080/10937400701389875. [DOI] [PubMed] [Google Scholar]
- 5.Geyer O, Bottone EJ, Podos SM, Schumer RA, Asbell PA. Microbial contamination of medications used to treat glaucoma. Br J Ophthalmol. 1995;79(4):376–379. doi: 10.1136/bjo.79.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schein OD, Hibberd PL, Starck T, Baker AS, Kenyon KR. Microbial contamination of in-use ocular medications. Arch Ophthalmol. 1992;110(1):82–85. doi: 10.1001/archopht.1992.01080130084030. [DOI] [PubMed] [Google Scholar]
- 7.Porges Y, Rothkoff L, Glick J, Cohen S. Sterility of glaucoma medications among chronic users in the community. J Ocul Pharmacol Ther. 2004;20(2):123–128. doi: 10.1089/108076804773710795. [DOI] [PubMed] [Google Scholar]
- 8.Hovding G, Sjursen H. Bacterial contamination of drops and dropper tips of in -use multidose eye-drop bottles. Acta Ophthalmol. 1982;60(2):213–222. doi: 10.1111/j.1755-3768.1982.tb08375.x. [DOI] [PubMed] [Google Scholar]
- 9.Dietlein TS, Jordan JF, Lüke C, Schild A, Dinslage S, Krieglstein GK. Self-application of single-use eyedrop containers in an elderly population: comparisons with standard eyedrop bottle and with younger patients. Acta Ophthalmol. 2008;86:856–859. doi: 10.1111/j.1755-3768.2007.01155.x. [DOI] [PubMed] [Google Scholar]
- 10.Stevens JD, Matheson MM. Survey of the contamination of eye drops of hospital inpatients and recommendations for the changing of current practice in eye drop dispensing. Br J Ophthalmol. 1992;76(1):36–38. doi: 10.1136/bjo.76.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–355. doi: 10.1097/IJG.0b013e31815c5f4f. [DOI] [PubMed] [Google Scholar]
- 12.Xiong C, et al. A rabbit dry eye model induced by topical medication of a preservative benzalkonium chloride. Invest Ophthalmol Vis Sci. 2008;49(5):1850–1856. doi: 10.1167/iovs.07-0720. [DOI] [PubMed] [Google Scholar]
- 13.Baudouin C, et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology. 1999;106(3):556–563. doi: 10.1016/S0161-6420(99)90116-1. [DOI] [PubMed] [Google Scholar]
- 14.Labbé A, et al. Comparison of toxicological profiles of benzalkonium chloride and polyquaternium-1: an experimental study. J Ocul Pharmacol Ther. 2006;22(4):267–278. doi: 10.1089/jop.2006.22.267. [DOI] [PubMed] [Google Scholar]
- 15.Liang H, et al. Conjunctival and corneal reactions in rabbits following short-and repeated exposure to preservative-free tafluprost, commercially available latanoprost and 0.02% benzalkonium chloride. Br J Ophthalmol. 2008;92(9):1275–1282. doi: 10.1136/bjo.2008.138768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weather JC, Chimadzev YA. Theory of electroporation: a review. Biolectrochem Bioenerg. 1996;41(2):135–160. doi: 10.1016/S0302-4598(96)05062-3. [DOI] [Google Scholar]
- 17.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electrical fields. EMBO J. 1980;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller GW. Report on the investigations into the purification of the Ohio River water at Louisville Kentucky. New York: D. Van Nostrand; 1898. [Google Scholar]
- 19.Beattie JM, Lewis FC. The electric current (apart from the heat generated), a bacteriological agent in the sterilisation of milk and other fluids. J Hyg. 1925;24(2):123–137. doi: 10.1017/S0022172400008640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moses BD. Electrical pasteurization of milk. Agric Eng. 1938;19(12):525–526. [Google Scholar]
- 21.Sale AJ, Hammilton WA. Effect of high electric field on micro-organisms. 1. Killing of bacteria and yeast. 2 Mechanism of action of lethal effect. Biochim Biophys Acta. 1967;148(3):781–800. [Google Scholar]
- 22.Sale AJ, Hammilton WA. Effects of high electric fields on microorganisms. 3 Lysis of erythrocytes and protoplasts. Biochim Biophys Acta. 1968;163(1):37–43. doi: 10.1016/0005-2736(68)90030-8. [DOI] [PubMed] [Google Scholar]
- 23.Doevenspeck H. Influencing cells and cell walls by electrostatic impulses. Fleischwirtschaft. 1961;13(12):968–987. [Google Scholar]
- 24.Rubinsky B. Irreversible electroporation in medicine. Technol Cancer Res Treat. 2007;6(4):255–260. doi: 10.1177/153303460700600401. [DOI] [PubMed] [Google Scholar]
- 25.FDA. Kinetics of microbial inactivation for alternative food processing technologies. US Food and Drug Administration Center for Food Safety and Applied Nutrition; 2000.
- 26.Lelieved HLM, Notermans S, de Haan SWH. Food Preservation by pulsed electric fields. From research to application. Cambridge, England: World Publishing; 2007. [Google Scholar]
- 27.Miller L, Leor J, Rubinsky B. Cancer cells ablation with irreversible electroporation. Technol Cancer Res Treat. 2005;6:699–705. doi: 10.1177/153303460500400615. [DOI] [PubMed] [Google Scholar]
- 28.Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality clinical implications. Technol Cancer Res Treat. 2007;6(1):37–48. doi: 10.1177/153303460700600106. [DOI] [PubMed] [Google Scholar]
- 29.European Pharmacopoeia, 5th edition. Test for efficiency of antimicrobial preservations. 2005; Supplement 5.1.3.
- 30.Hulsheger H, Potel J, Niemann EG. Killing of bacteria with electric pulses of high field strength. Radiat Environ Biophys. 1981;20(1):53–65. doi: 10.1007/BF01323926. [DOI] [PubMed] [Google Scholar]
- 31.Peleg M. A model of microbial survival after exposure to pulse electic field. J Sci Food Agric. 1995;67(1):93–99. doi: 10.1002/jsfa.2740670115. [DOI] [Google Scholar]
- 32.van Boekel M. On the use of the Weibull model to describe thermal inactivation of microbial vegetative cells. Int J Food Microbiol. 2002;74(1-2):139–159. doi: 10.1016/S0168-1605(01)00742-5. [DOI] [PubMed] [Google Scholar]
- 33.Saulis G, Venslauskas MS, Naktinis J. Kinetics of pore resealing in cell membrane after electroporation. Bioelectrochem Bioenerg. 1991;26(1):1–13. doi: 10.1016/0302-4598(91)87029-G. [DOI] [Google Scholar]
- 34.Saulis G. Pore disappearance in a cell after electroporation. Theoretical simulation and comparison with experiments. Biophys J. 1997;73(3):1299–1309. doi: 10.1016/S0006-3495(97)78163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinosita K, Tsong TY. Voltage-induced pore formation and hemolyses of human erythrocytes. Biochim Biophys Acta. 1977;471(2):227–242. doi: 10.1016/0005-2736(77)90252-8. [DOI] [PubMed] [Google Scholar]
- 36.Tekle E, Astumian RD, Chock PB. Selective and asymmetric molecular transport across electroporated cell membranes. Proc Natl Acad Sci USA. 1994;91(24):11512–11516. doi: 10.1073/pnas.91.24.11512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziv R, Steinhardt Y, Pelled G, Gazit D, Rubinsky B. Micro-electroporation of mesenchymal stem cells with alternating electrical current pulses. Biomed Microdevices. 2009;11:95–101. doi: 10.1007/s10544-008-9213-4. [DOI] [PubMed] [Google Scholar]