Abstract
The purpose of the study was to prepare and evaluate the anti-inflammatory activity of cyclodextrin (CD) complex of curcumin for the treatment of inflammatory bowel disease (IBD) in colitis-induced rat model. Inclusion complexes of curcumin were prepared by common solvent and kneading methods. These complexes were further evaluated for increase in solubility of poorly soluble curcumin. The inclusion complexes were characterized for enhancement in solubility, in vitro dissolution, surface morphology, infrared, differential scanning calorimetry, and X-ray studies. Solubility, phase solubility, and in vitro dissolution studies showed that curcumin has higher affinity for hydroxypropyl-β-CD (HPβCD) than other CDs. HPβCD complex of curcumin was further investigated for its antiangiogenic and anti-inflammatory activity using chick embryo and rat colitis model. HPβCD complex of curcumin proved to be a potent angioinhibitory compound, as demonstrated by inhibition of angiogenesis in chorioallantoic membrane assay. Curcumin- and HPβCD-treated rats showed a faster weight gain compared to dextran sulfate solution (DSS) controls. Whole colon length appeared to be significantly longer in HPβCD-treated rats than pure curcumin and DSS controls. An additional finding in the DSS-treated rats was the predominance of eosinophils in the chronic cell infiltrate. Decreased mast cell numbers in the mucosa of the colon of CD of curcumin- and pure-curcumin-treated rats was observed. This study concluded that the degree of colitis caused by administration of DSS was significantly attenuated by CD of curcumin. Being a nontoxic natural dietary product, curcumin could be useful in the therapeutic strategy for IBD patients.
Key words: antiangiogenesis, curcumin, cyclodextrin, inflammatory bowel disease, solubility
INTRODUCTION
Inflammatory bowel disease (IBD) includes ulcerative colitis and Crohn’s disease and is associated with chronic relapsing inflammation of the intestinal tract of unknown etiology (1). Histologically, mucosal accumulation of leukocytes is a characteristic feature of IBD and activation of T cells and monocytes/macrophages has been regarded as a crucial factor in its pathogenesis. Treatment of IBD consists of drugs such as 5-aminosalicyclic acid, corticosteroids, azathioprine, mercaptopurines, and cyclosporine (1,2). However, use of these drugs is sometimes limited by their toxicity. There is an increasing need for alternative drugs that may be equally or more effective but less toxic and inexpensive.
Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1, 6-heptadiene-3, 5-dione], a low-molecular-weight polyphenol derived from the rhizomes of turmeric (Curcuma longa Linn.), is a yellow pigment widely used as a coloring agent and spice in many foods. It has been used in Ayurvedic and Chinese medicine for centuries. Interest in this dietary polyphenol has grown in recent years due to its vast array of beneficial pharmacological effects including antioxidant, anti-inflammatory, anticarcinogenic (3–6), hypocholesterolemic (7), antibacterial (8), wound healing, antispasmodic, anticoagulant, antitumor (9), and hepatoprotective (10) activities. The protective effect of curcumin on 2,4,6-trinitrobenzene sulfonic-acid-induced colitis in mice has been reported, where curcumin reduced significantly the degree of neutrophil infiltration, lipid peroxidation, and serine protease activity (11). Curcumin is also a potent free radical scavenger having superoxide anions, singlet oxygen, hydroxyl radical scavenging, and lipid peroxidation inhibitory activities (12).
Despite the promising biological effects of curcumin, its poor oral bioavailability in both rodents and humans (13,14) has restricted its use in the management of human ailments. It is well known that many drugs show bioavailability problems due to their low water solubility, slow dissolution rate, and instability in the gastrointestinal tract. Poor oral absorption due to its extremely low aqueous solubility or extensive presystemic metabolism may be responsible for the unfavorable pharmacokinetics of this molecule. In rodents, curcumin undergoes avid metabolism by conjugation and reduction and its absorption after oral dosing is characterized by poor systemic bioavailability (13,15,16).
Cyclodextrins (CDs) are cyclic oligosaccharides with a hydrophilic outer surface and lipophilic central cavity. Hydrophilic drug/cyclodextrin complexes are formed by inclusion of lipophilic drug or lipophilic drug moiety in the central cyclodextrin cavity. The lipophilic cavity thus protects the lipophilic guest molecule from aqueous environment, while the polar outer surface of the CD molecule provides the solubilizing effect. The polarity inside the cavity is suggested to be similar to that of a 40% solution of ethanol in water (17).Cyclodextrins have been frequently used as solubilizing and stabilizing agents in pharmaceutical preparations (18). Commonly used cyclodextrins are β-CD, γ-CD, and derivatives such as hydroxypropyl-β-CD (HPβCD) and methyl β-CD (MβCD).
Angiogenesis is a strictly controlled process in normal human body and regulated by a variety of endogenous angiogenic and angiostatic factors (19). However, on short notice, the microvascular system appears capable of responding with rapid capillary growth to physiological demands such as ovulation, as well as to pathologic conditions such as wounds, chronic inflammation, certain immune reactions, and tumors.
In the present study, we have developed a novel formulation approach for treating experimental colitis in rat model. Curcumin CD complexes with β-cyclodextrin (βCD) and its derivatives HPβCD and MβCD and gamma cyclodextrin (γCD) were prepared and evaluated for increasing solubility of curcumin. Optimized CD complex were evaluated for its effect on angiogenesis by chick embryo chorioallantoic membrane (CAM) method. Further, a modified pulsatile capsule was designed to deliver curcumin CD complex to terminal ileum and colon. Modified pulsatile capsule was designed by filling the optimized formulation of curcumin CD complex into hard gelatin capsule followed by coating with pH-dependent acrylic polymers. The developed delivery system was evaluated on synthetic dextran sulfate solution (DSS)-induced experimental colitis model for its potential as a new therapeutic approach for treatment of IBD.
MATERIALS AND METHODS
Materials
Curcumin was obtained as a gift sample from Natural Remedies Pvt. Ltd., Bangalore, India. Gift samples of βCD, γCD, HPβCD, and MβCD were obtained from Degussa, Mumbai, India. Methanol and Tween-80 were purchased from Thomas Baker, Mumbai, India. Eudragit S 100, Eudragit L100, Eudragit RL 100, and Eudragit RS 100 were obtained from Rohm GmbH, Degussa India Pvt. Ltd., Mumbai, India. Empty porcine hard gelatin capsule # 09 was obtained as a gift sample from TORPAC Inc., USA. All other chemicals used were of analytical grade.
Phase Solubility Analysis
The phase solubility study was carried out by the method reported by Higuchi and Connors (20). Different concentrations of cyclodextrin solutions such as 0, 2, 6, 8, 10, and 20 mM were prepared in distilled water and filled in screw-capped bottles. Excess curcumin was added to these solutions to attain saturation. Each bottle was capped and shaken for 72 h in a constant temperature water bath at 30 ± 2°C. Following equilibrium, these solutions were filtered using 0.45-µm nylon disk filter, diluted, and assayed for the total dissolved curcumin content by UV analysis at 425 nm (Shimadzu 1700 UV Visible spectrophotometer). Each sample was determined in triplicate and the samples were protected from light. CD solutions of βCD, γCD, HPβCD, MβCD, and γCD were evaluated. The phase solubility diagram was constructed by plotting concentrations of dissolved curcumin against cyclodextrin concentration. The binding constant, Ks, was calculated from the slope of phase solubility plot (20); 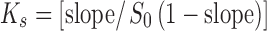 , where S0 is solubility of curcumin in the absence of cyclodextrin.
, where S0 is solubility of curcumin in the absence of cyclodextrin.
The Gibbs free energies of transfer of drug from aqueous solution to the cavity of cyclodextrin have been calculated from the following equation 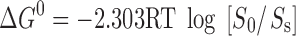 , where Ss and S0 are the solubility of drug in the presence and in the absence of cyclodextrin, respectively.
, where Ss and S0 are the solubility of drug in the presence and in the absence of cyclodextrin, respectively.
Preparation of Inclusion Complexes
The binary systems of curcumin–HPβCD and curcumin–MβCD were prepared by kneading and common solvent evaporation methods. The physical mixtures were prepared for the purpose of comparison.
Preparation of Inclusion Complexes by Kneading Method
Curcumin and cyclodextrin (HPβCD and MβCD) in the proportion of 1:1 and 1:2 molar concentrations were mixed in a mortar for 1 h with small quantity of alcohol; distilled water was added intermittently to get slurry-like consistency. The paste was dried in a hot air oven at a temperature of 45°C for 24 h. Dried complex was pulverized into fine powder and sifted with sieve # 80.
Preparation of Inclusion Complexes by Common Solvent Evaporation Method
Curcumin and cyclodextrin (HPβCD and MβCD) in ratio of 1:1 and 1:2 were dissolved in alcohol to get a clear solution. The preparation was allowed to evaporate overnight at room temperature. The cyclodextrin complexes so prepared were pulverized and sifted with sieve # 80.
Initial Characterization of Inclusion Complexes
The samples of pure curcumin, curcumin–HPβCD, and curcumin–MβCD were subjected to initial characterization immediately after their preparation as follows.
Percentage Yield
The efficiency of the process is determined by the yield. It is calculated using the following equation: 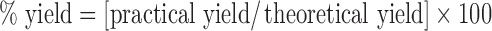 .
.
Drug Content
The amount of curcumin present in CD complex containing 10 mg of the drug was determined by dissolving in 10 ml of methanol and after suitable dilution with methanol UV absorbance was measured at 425 nm.
Solubility Studies of Cyclodextrin Complexes
Excess of CD complexes were dispersed in 25 ml of distilled water in screw-capped bottles to get a supersaturated solution. These bottles were shaken continuously for 2 h at ambient temperature until equilibrium was attained. Supersaturated solution was filtered through a 0.22-µm nylon filter and further diluted with methanol and absorbance was measured at 425 nm. Solubility studies were also performed for pure drug.
Dissolution Rate Study
In vitro dissolution studies were performed for pure curcumin and the inclusion CD complexes in order to select the CD complex having maximum solubility. Dissolution study was performed using US Pharmacopeia 24 Type II dissolution test apparatus (Electrolab TDT-06P, India). CD complexes equivalent to 25 mg pure curcumin were placed in dissolution vessels containing 500 ml of simulated intestinal fluid and polyethylene glycol (PEG) 400 maintained at 37 ± 0.1°C and stirred at 75 rpm. Samples were collected periodically and replaced with a fresh dissolution medium. A sample of 5 ml was withdrawn and filtered through Whatman filter paper # 1. Suitable dilutions were further made and absorbance read at 425 nm against blank. Dissolution studies were also carried out for pure curcumin.
Infrared Studies
Instrument used was Shimadzu Fourier transform infrared (FTIR)-8700 spectrophotometer. In this study, potassium bromide disk method was employed (Spectra Lab., India). Both pure drug and cyclodextrin complexes were subjected to infrared (IR) studies. Sample was intimately mixed with dry powdered potassium bromide. The mixture was then compressed into transparent disk under high pressure using special dies. The disk was placed in IR spectrophotometer using sample holder and spectrum was scanned over wavenumber range of 4,000–400 cm−1.
Scanning Electron Microscope Studies
Surface morphology of CD complex, physical mixture, CD and pure curcumin samples were examined by scanning electron microscope (SEM; AKASHI SX-40, Japan). Samples were dispersed onto carbon tabs (double-adhesive carbon-coated tape) adhered to aluminum stubs. Further, these sample stubs were coated with a thin layer (30 Å) of gold by employing Polaron-E 3000 sputter coater. Samples were subsequently examined by SEM and photographed under various magnifications with direct data capture of the images onto a computer.
DSC Studies
Differential scanning calorimetric studies of pure drug, CD, and CD complexes were performed by using differential scanning calorimeter (DSC; Mettler Toledo Star system). Samples were weighed (5.00–8.00 ± 0.5 mg) and placed in sealed aluminum pans. The coolant was liquid nitrogen. The samples were scanned at 10°C/min from 20°C to 300°C and thermograms were recorded.
X-ray Diffraction Studies
X-ray diffraction patterns of pure curcumin, CD, and CD complexes were determined using a diffractometer (SMART & SAINT Bruker, 2000) equipped with a rotating target X-ray tube and a wide-angle goniometer. The X-ray source was Kα radiation from a copper target with graphite monochromater. The X-ray tube was operated at a potential of 50 kV and a current of 150 mA. The range (2θ) of scans was from 0° to 70° at a speed of 2° per minute at increments of 0.02°.
Angiostatic Activity by Chick Embryo Chorioallantoic Membrane Method
The angiostatic activity of CD complex of curcumin was demonstrated using chick chorioallantoic membrane (21). Fertilized domestic 5-day-old chick embryos were purchased from poultry farm (GKVK Veterinary Sciences, Hebbal, Bangalore, India) and were incubated at 37 ± 1°C under 60% relative humidity. A small, 0.5–0.6-cm2, opening was made through the shell at the end of the egg directly over embryonic blood vessels using a 4.0-mm scalpel blade, as previously determined by candling. The shell membrane was removed slowly. CD complex of curcumin weighing about 15 ± 0.2 mg containing curcumin (5 mg) and pure curcumin 5 mg/ml (ethanol–PBS 1:9 v/v), respectively, was transferred directly on the CAM surface and the shell was closed using parafilm. Photographs were taken by using 4.2-megapixel camera (Cannon Cybershot Model V3X); after 48 h of incubation, the vasculature was observed using a dissecting microscope at magnification of ×10 and six eggs were tested in each group and the experiment was performed in triplicate to ensure reproducibility.
Formulation of Pulsatile Capsules for Colon-Specific Delivery
Selected CD formulations of curcumin based on particle size, drug entrapment, drug content, and in vitro release were chosen for colon-specific delivery by developing pulsatile capsules. The formulations of pulsatile capsules were earlier optimized. Around 80 mg of formulation was filled into the capsule manually. Capsules were coated with Eudragit RS100, RL100, S100, and L100 in optimum concentrations. The polymers were dissolved in acetone by mixing for 20 min to which specified quantities of talc and PEG 6000 were added and dispersed to obtain the coating dispersion. The various parameters of coating conditions were standardized such as dipping time (5 s), coating solution concentration, temperature (25 ± 1°C), and drying time (5 min) at 50°C air. The coating process was carried until a desired weight was gained. The capsules were coated with two coats; the first coat was with pH-independent polymer (Eudragit RS100 and Eudragit RL100 in the ratio of 6:2) and the second coat was with pH-dependent polymer (Eudragit S100 and Eudragit L100 in the ratio of 60:40). PEG 6000 was used as plasticizer in both the coatings. Capsule was placed in between the arms of the forceps and dipped into the coating solution for 5 s and dried by hot air blower at 40°C for 5 min. This alternative dipping and drying was repeated until the desired weight was gained by the capsule. The filled capsules were subjected for further study for site-specific delivery of curcumin in terminal ileum.
In Vivo Study
All experimental procedures were in accordance with guidelines for animal welfare set out by the Medical Research Council of India. Animal Ethics Committee approval was obtained. The 24 male Sprague Dawley rats, aged 6–7 weeks (175–205 g), were obtained from the Indian Institute of Science, Bangalore, India. They all received the standard laboratory diet and were not fasted prior to the experiments, with free access to drinking water except during the induction of colitis. Rats were housed (two per cage) and kept in a regulated environment (temperature, 22 ± 1°C; humidity, 50 ± 5%; night/day cycle, 12 h). The rats were randomized into four groups (n = 6). Rats in groups 1, 2, and 3 received 5% DSS solution (molecular weight 40,000; Sigma Chemicals, India) in drinking water for 9 days. The last groups of control animal received plain drinking water. After 9 days, rats in group 1 received CD complex of curcumin, 100-mg/kg body weight in pulsatile capsule (22); rats in group 2 received pure curcumin (100-mg/kg body weight in pulsatile capsule) and rats in group 3 served as DSS control. The clinical symptoms recorded in DSS-treated and control rats were body weight, stool consistency, fecal bleeding, and diarrhea.
The experiment was terminated at the end of 90 days and all animals were killed by cervical dislocation after overnight fasting. The colon was slit open longitudinally and cleaned of fecal material with a gentle jet of water. Then, colon was stored in formalin (10%) solution and subjected for histopathological examination.
Assessment of Inflammation: Symptoms and Inflammatory Score
Clinical assessment of inflammation included weekly monitoring of body weight and general health condition. The macroscopic appearance of colon (inflammatory score), based on the degree of inflammation and the presence of edema and/or ulcerations, stool consistency (diarrhea score), and visible fecal blood, was scored separately on a scale of 0–3 (Table I) (23).
Table I.
Assessment of Inflammation by Means of Clinical and Macroscopic Score
| Scorea | Diarrhea score | Visible fecal blood | Inflammatory score |
|---|---|---|---|
| 0 | Normal pellets | Normal | Normal |
| 1 | Slightly loose feces | Slightly bloody | Slight inflammation |
| 2 | Loose feces | Bloody | Moderate inflammation and/or edema |
| 3 | Watery diarrhea | Blood in whole colon | Heavy inflammation and/or ulcerations and/or edema |
aScore reflects degree of diarrhea, visible fecal blood, and inflammatory score on the day of termination and is characterized on a scale of 0–3. Inflammatory score reflects macroscopical appearance of the colon (inflammatory score) judged by degree of inflammation and presence of ulcerations and/or edema in the tissue.
The colon was excised totally, opened longitudinally, and cleaned of fecal material with a gentle jet of water. The distal colon, proximal colon, and ileum were studied. Each specimen was sliced down the antimesenteric border to give two symmetrical halves, stretched, and fixed in 10% buffered formalin for 4 h followed by rinsing in 70% alcohol. The formalin-fixed sections were embedded in paraffin blocks and sections were cut, dewaxed, and stained with hematoxylin and eosin. The severity of inflammation was assessed first macroscopically. The freshly opened colon was examined under a magnifying glass by two independent observers blinded to the procedure. The extent of mucosal damage was assessed using the macroscopic inflammatory scoring system (24).
Histopathological Study
Tissue sections (5 μm) of distal and proximal colon were stained with hematoxylin–eosin to address the degree of inflammation and with Masson’s trichrome to visualize the connective tissue. The stained tissue was analyzed independently and in a blinded fashion with a standard microscope (Nikon charge-coupled device with digital display at ×40 and ×100). The pathophysiology of the tissue was characterized and the lesions were scored as described by Vilaseca et al. (23) by the presence of ulcerations, inflammatory cells (neutrophils, macrophages, lymphocytes, and plasma cells), signs of edema, crypt loss, surface epithelial cell hyperplasia, goblet cell reduction, and signs of epithelial regeneration (25).
RESULTS AND DISCUSSION
Phase Solubility Studies
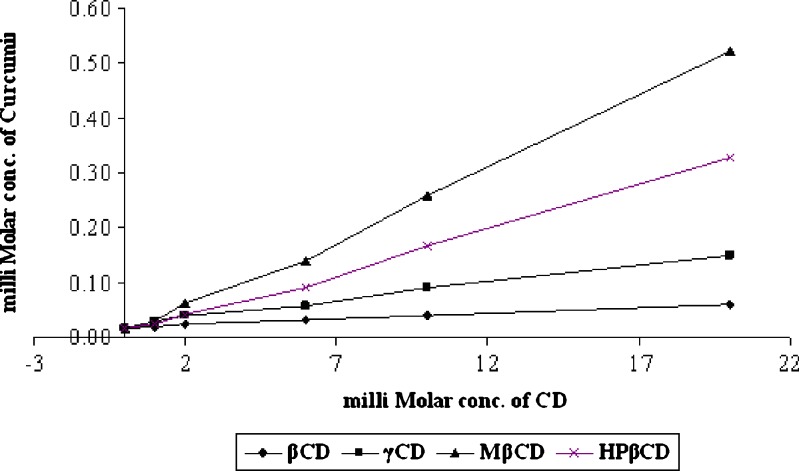
The phase solubility diagrams for the complex formation between curcumin and βCD/γCD/MβCD/HPβCD are presented in Fig. 1. These plots show that aqueous solubility of the curcumin increases linearly as a function of cyclodextrin concentration up to 20 mM. It is clearly observed that the solubility diagram of curcumin in the presence of βCD, γCD, MβCD, and HPβCD can be classified as AL type according to Higuchi and Conners. This may attributed to the formation of soluble 1:1 curcumin–CD inclusion complexes. The cavity size of MβCD and HPβCD seems to be optimal for entrapment of the curcumin molecules and provides the greatest solubilization effect. Stability constants obtained for curcumin are in the rank order of HPβCD (424 M−1) > MβCD (401 M−1) > γCD (154 M−1) > βCD (134 M−1) as shown in Table II, which indicates the formation of adequately stable CD complexes.
Fig. 1.
Phase solubility diagram of curcumin–CD complexes
Table II.
Stability Constant (Ks) for Cyclodextrin Complexes of Curcumin
| Type of cyclodextrin | Stability constant (Ks; M−1) |
|---|---|
| β CD | 134.002 (r = 0.991) |
| γ CD | 153.019 (r = 0.992) |
| HP β CD | 424.119 (r = 0.998) |
| M β CD | 401.817 (r = 0.997) |
Gibbs’ free energy is a thermodynamic function. The change in Gibbs’ free energy (∆G0) is the net energy available to do useful work and is a measure of the “free energy” (Table III). ∆G0 gives criteria for spontaneity at constant pressure and temperature (26). If ∆G0 is negative, the process is spontaneous. As ∆G0 becomes more negative, the reaction becomes more favorable. In the present case, the reaction consists of the solubility of the drug in cyclodextrin solution. ∆G0 is related to the equilibrium constant of a reaction K, by the relation ∆G0 = −2.3.3RT log K where K is calculated from (S0/Ss). It was observed that ∆G0 values obtained were negative which increased with increasing CD concentration in all the different types of CDs evaluated in this study. This indicates that CD solutions offer a more favorable environment than water for curcumin. Moreover, the interaction between curcumin and CD increased with increasing CD concentration.
Table III.
Gibb’s Free Energy of Curcumin–CD Complex in Water
| Concentration of cyclodextrin (mM) | Gibb’s free energy −∆transG0/cal mol−1 | |||
|---|---|---|---|---|
| βCD | γCD | HP-βCD | M-βCD | |
| 1 | 364.3 | 438.5 | 678.9 | 548.6 |
| 2 | 499.7 | 587.4 | 764.6 | 723.8 |
| 6 | 642.8 | 712.6 | 965.8 | 856.3 |
| 10 | 982.6 | 942.4 | 1,457.0 | 1,168.4 |
| 20 | 1,140.2 | 1,192.6 | 1,641.2 | 1,327.5 |
It has been reported that the driving force for inclusion complexation between cyclodextrin and a guest (curcumin) may include van der Waals interaction, hydrogen bonding, and hydrophobic interaction, resulting in release of high-energy water molecules from the cavity of cyclodextrins and release of strain energy in the ring of cyclodextrin. Stability constant (Ks) value between 200 and 5,000 M−1 is considered as most suitable for the improvement of solubility and stability of poorly soluble drug (26). The (Ks) values of different complexes are shown in Table II. All the values are within the range 200–5,000 M−1. Thus, it may be concluded that the binary complexes are capable of improving the solubility and stability of curcumin.
Drug Content in the Binary Mixtures
The actual drug content in each binary mixture was determined. The results are reported in Table IV. As can be seen, kneading method products showed a good agreement between theoretical and actual drug content.
Table IV.
Evaluation Studies of Cyclodextrin Complexes of Curcumin
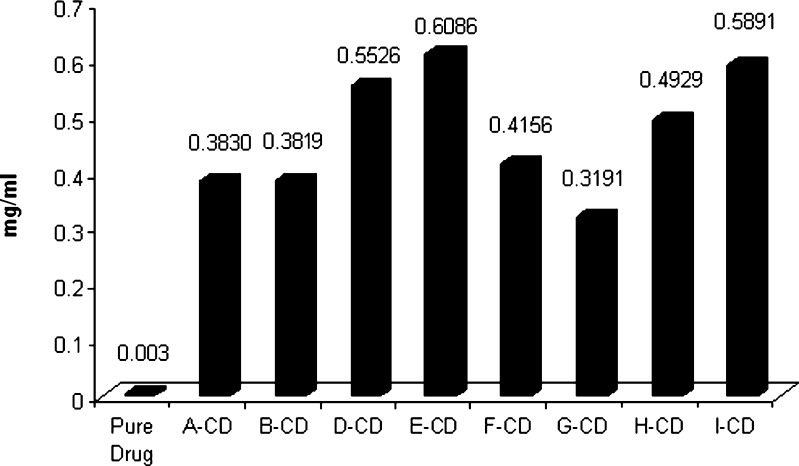
| Code | Name of complex | Complexing agent used | Total drug (%)a | Solubility (mg/ml)a | |
|---|---|---|---|---|---|
| Ratio | Method | ||||
| Pure drug | Not applicable | 0.0030 | |||
| A-CD | (1:1) | CS | HP-βCD | 99.47 | 0.3830 |
| B-CD | (1:2) | CS | HP-βCD | 93.68 | 0.3819 |
| D-CD | (1:1) | KN | HP-βCD | 99.21 | 0.5526 |
| E-CD | (1:2) | KN | HP-βCD | 97.63 | 0.6086 |
| F-CD | (1:1) | CS | M-βCD | 99.21 | 0.4156 |
| G-CD | (1:2) | CS | M-βCD | 96.05 | 0.3191 |
| H-CD | (1:1) | KN | M-βCD | 95.79 | 0.4929 |
| I-CD | (1:2) | KN | M-βCD | 97.37 | 0.5891 |
CS common solvent evaporation method, KN kneading method
a±SD = average of three readings
Solubility Studies
MβCD and HPβCD cyclodextrin complexes prepared by kneading and solvent evaporation methods showed a maximum increase in solubility and were selected for further evaluations. Maximum solubility was observed in MβCD and HPβCD complex prepared by kneading method, where an increase in solubility by 190- and 202-folds, respectively, was observed. Results are shown in Table IV and Fig. 2. These complexes were further evaluated by in vitro dissolution study.
Fig. 2.
Solubility profile of pure curcumin and its cyclodextrin complexes
Dissolution Studies
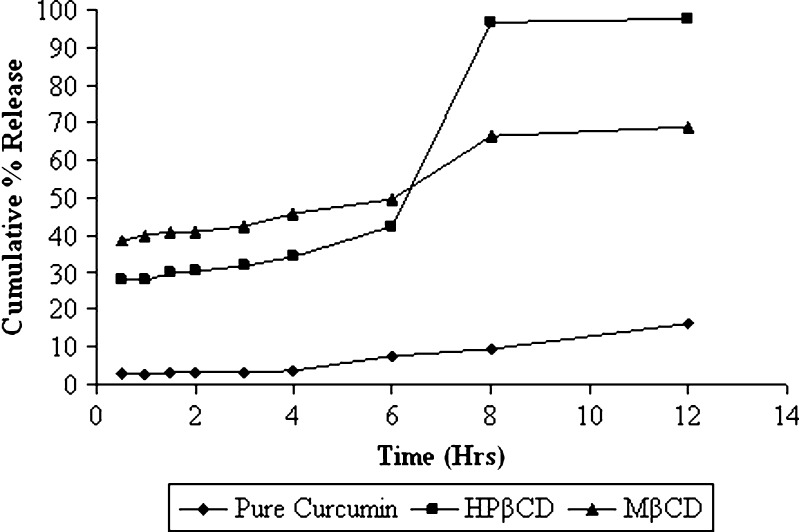
Dissolution profiles of the pure curcumin and the selected MβCD and HPβCD curcumin cyclodextrin complexes are shown in Fig. 3. The rate of dissolution was found to increase in cyclodextrin complexes as shown by time taken for 50% release. It was evident that both complexes exhibited faster dissolution rates than that of pure curcumin. A remarkable increase in dissolution rate was obtained for the HPβCD inclusion complexes. At the end of 1 h, pure curcumin, HPβCD complex, and MβCD complex released 2.8%, 27.94%, and 39.47%, respectively. At the end of 12 h, 16.12%, 97.82%, and 68.75% of curcumin was released from pure curcumin, HPβCD complex, and MβCD complex, respectively. Increased solubility with these CD derivatives has been reported to be attributed to the fact that during chemical manipulation there is conversion to amorphous mixture of isomeric derivatives (27). The improvement of dissolution rate obtained with inclusion complexes could also be due to drug wettability and formation of readily soluble complexes in the dissolution medium. Further, HPβCD complexes of curcumin had enhanced solubility when compared to MβCD and was selected for further evaluations.
Fig. 3.
Dissolution profiles of pure curcumin and its complexes
Infrared Studies
The FTIR spectra of curcumin and cyclodextrin were determined. The prominent peaks in curcumin are as follows: (1) 3,595 cm−1 to phenolic (OH) vibrations; (2) 3,075 cm−1 to aromatic C–H stretching vibrations; (3) 1,600 cm−1 to the stretching vibration of benzene ring skeleton; (4) 1,510 cm−1 to the mixed (C=O) and (C=C) vibration; (5) 1,425 cm−1 to the olefinic C–H in-plane bending vibration (δC–H); (6) 1,280 cm−1 to the Ar–O stretching vibration. In the FTIR spectra of the physical mixtures, there are no changes. The broad absorption bands at 3,410 cm−1 arise from the stretching mode of OH groups. The bands in the range of 3,079–3,000 cm−1 can be attributed to aromatic C–H stretching vibration which may indicate intercalation of curcumin in CD complex.
SEM Studies
Figure 4 illustrates SEM of pure curcumin, HPβCD, physical mixture, and HPβCD complex of curcumin at different magnifications. Curcumin was observed to be irregular in shape whereas HPβCD was seen as spherical particles. In the physical mixture, the characteristic curcumin particle, when mixed with cyclodextrin, was found to adhere onto the surface of cyclodextrin. SEM of HPβCD curcumin complex shows aggregation into irregularly shaped amorphous particles in which the original morphology of both the components had disappeared.
Fig. 4.
SEM of curcumin and its cyclodextrin complexes: a pure curcumin; b HPΒCD; c physical mixture; and d HPΒCD complex of curcumin
DSC Studies
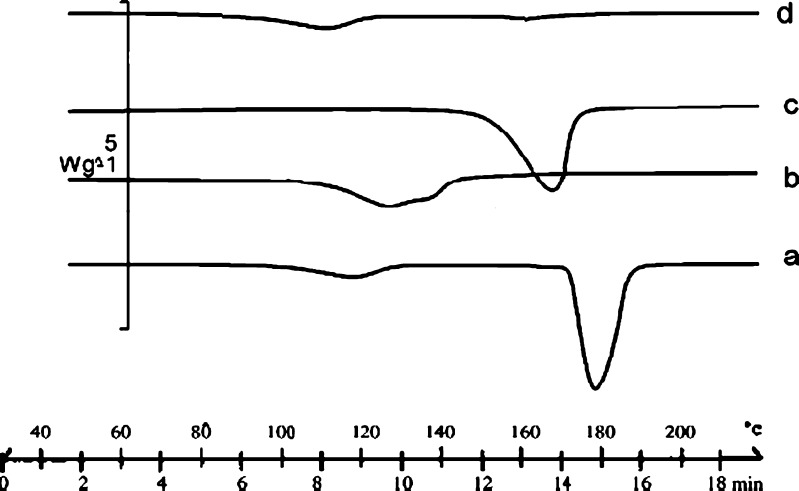
It has been reported that DSC is a very useful tool in the investigation of thermal properties of CD complexes and can provide both qualitative and quantitative information about the physicochemical state of the drug inside the CD complexes. In general, complexation results in the absence of endothermic peak or shifting to different temperature, indicating a change in the crystal lattice, melting, boiling, or sublimation points.
The DSC thermograms of pure curcumin, HPβCD, physical mixture, and HPβCD curcumin complex are represented in Fig. 5. DSC thermograms of curcumin showed one endothermic peak at 180°C corresponding to the melting point of curcumin. Physical mixture of HPβCD and curcumin showed persistence of endothermic peak of both the constituents. Here, the drug endothermic peak shifted to 165.29°C with reduced intensity. A very broad endothermic peak was obtained at 122.75°C corresponding to dehydration process.
Fig. 5.
DSC of curcumin and its cyclodextrin complexes: (a) pure curcumin; (b) HPΒCD; (c) physical mixture; and (d) HPΒCD complex of curcumin
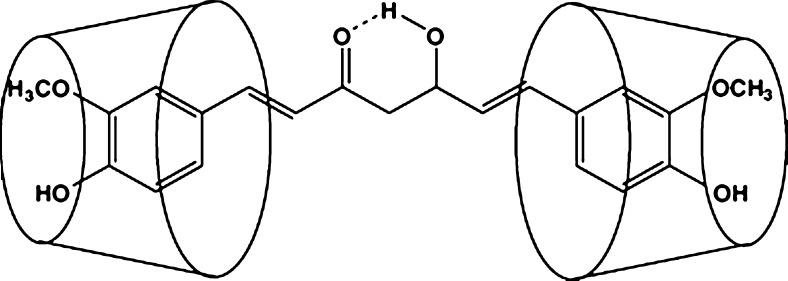
In case of HPβCD drug complexes, all the prominent peaks of curcumin had disappeared in the inclusion complex; the disappearance of the thermal features of the drug indicated that the drug may have been included within the cyclodextrin cavity replacing water molecules (28) and a true complex was formed at 1:2 M in the solid state (27), which is shown in Fig. 6. The complete disappearance of curcumin endothermic peak at 180°C in this system may indicate the formation of a true complex. This phenomenon is thus indicative of a stronger interaction between curcumin and HPβCD.
Fig. 6.
The proposed structure of cyclodextrin curcumin inclusion complex
X-ray Diffraction
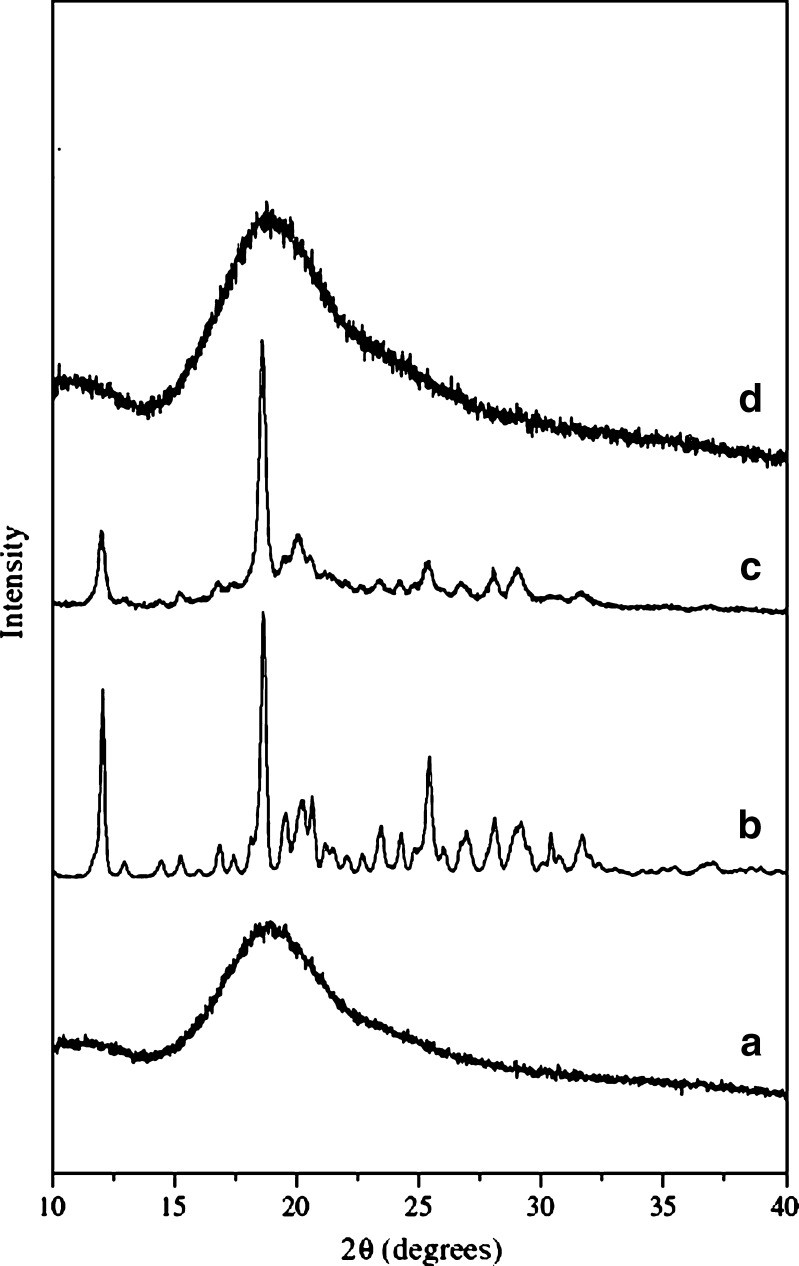
The X-ray diffraction patterns of pure curcumin, HPβCD, physical mixture, and HPβCD–curcumin complex are shown in Fig. 7. Curcumin shows the characteristic peaks, which are displayed in the wide-angle regions pointing to its crystalline nature. HPβCD–curcumin complex did not contain any peaks associated with crystals of the drug, suggesting that the drug may have formed an inclusion complex with CD; thus, there is reduction in the number of peaks. Reduction in the number of peaks also indicates that the complex may have been converted from crystalline into amorphous form. X-ray diffraction study only allows differentiation between crystalline data and amorphous material. In case of the physical mixture, the characteristic peaks of curcumin were visible; however, there was a reduction in the intensity of the peaks.
Fig. 7.
X-ray diffraction patterns of curcumin and its cyclodextrin complexes: (a) HPΒCD; (b) pure curcumin; (c) physical mixture; and (d) HPΒCD complex of curcumin
Angiostatic Activity by Chick Embryo Chorioallantoic Membrane Study
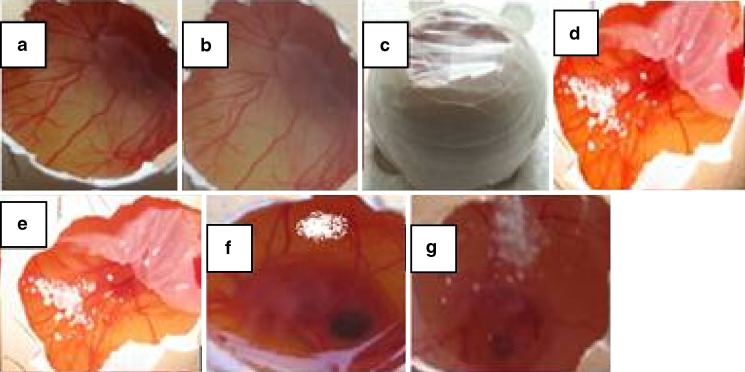
The term angiogenesis generally refers to the development of new blood vessels from preexisting ones. However, in pathological conditions such as inflammatory diseases, tumor growth, and tumor metastasis, a chronic “unregulated” angiogenic state often exacerbates the disease. Thus, it was hypothesized that there is a link between angiogenesis and inflammation. Angiogenesis inhibition is defined as a zone of avascularity, 2–6 mm in diameter, which is a direct result of occlusion, disruption, degeneration, and regression. Curcumin has potent antiangiogenic activity as demonstrated by experiments using chick chorioallantoic membrane assay (21,22). The CAM studies provide a rapid method for investigating the release of curcumin in a biological system and penetration of curcumin through the cell membrane and a comparative evaluation of the antiangiogenic activity of the formulation. Figure 8 shows CAM with control (a and b), which shows a normal capillary network structure. The CAM treated with curcumin (d and f) and CD complex of curcumin (f and g) showed vascular regression and zones, which are devoid of a capillary network and which clearly indicate the angiostatic activity of CD complex of curcumin and pure curcumin.
Fig. 8.
Antiangiogenic activity of cyclodextrin complexes of curcumin. Representative photographs of CAM: a visualization of vascularity and embryo in control at 0 h; b control after 48 h; c sealing of egg; d effect of pure curcumin at 0 h; e effect of curcumin after 48 h; f effect of curcumin cyclodextrin complex at 0 h; g effect of curcumin cyclodextrin complex after 48 h
Assessment of Inflammation: Symptoms and Inflammatory Score
The focus of this study was to characterize the molecular events, histopathology, and role of inflammation in dysplasia in DSS model of rat colitis and draw analogy to ulcerative-colitis-associated dysplasia in the human with the intent to use this model for further molecular and inflammatory research. DSS is thought to exert its action initially by effects on tight junctions, with a subsequent immunological activation and alterations in luminal flora and mucous composition. A predominance of cecal injury has been noted or distal injury in the more severe models (29). Oral administration of DSS in drinking water resulted in extensive hemorrhagic and ulcerative damage to the distal colon as observed up to 9 days. Macroscopic examination of the distal colon and rectum from DSS-treated rats revealed the presence of multiple mucosal erosions and ulcerations. The colon and rectum showed evidence of mucosal congestion, erosions, and hemorrhagic ulcerations evaluated at 10, 15, and 25 days as shown in the damage score.
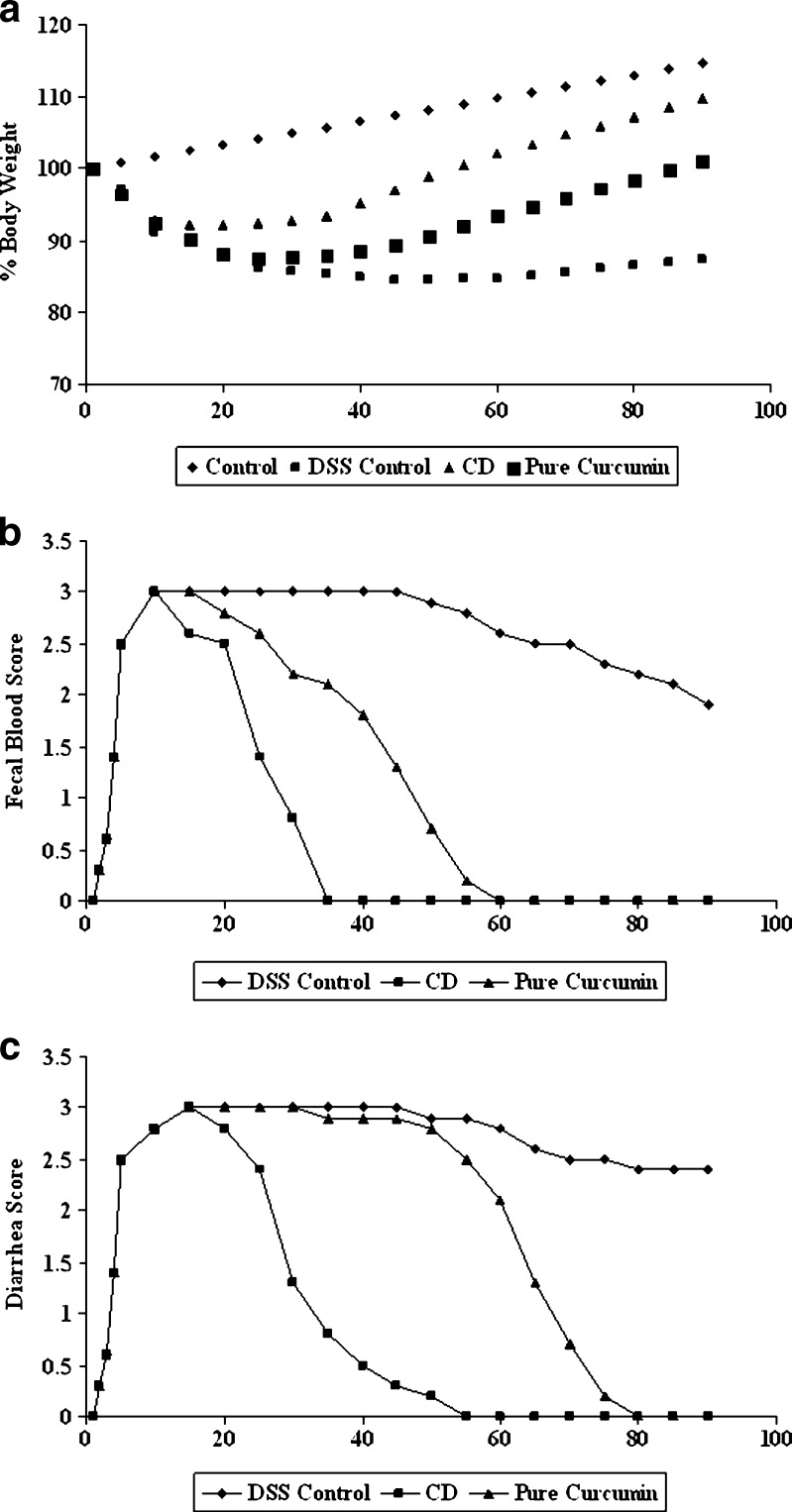
Treatment of rat with curcumin and CD complex of curcumin resulted in a significant decrease in the extent and severity of the injury of the large intestine as evidenced by disease activity index (Fig. 9) as well as histopathological assessment (Fig. 10). The observed inflammatory changes of the large intestine was associated with an increase in weight of the colon as well as a significant increase in body weight as compared to DSS control rat (Fig. 9a). In contrast, no significant increase in the weight of the colon of DSS-treated rat was found. Colon weight-to-length ratio, a marker of tissue edema, was significantly higher in DSS-treated rats than in curcumin and cyclodextrin complexes of curcumin-treated rats (P < 0.05). Moreover, treatment with curcumin and its CD complex also significantly reduced the loss in body weight, which correlated well with the recovery of the colonic injury. During the 90-day period, rats receiving CD complex of curcumin showed more reduced level of disease activity index compared to pure curcumin. These observations indicate that CD complex of curcumin suppressed the development of DSS colitis in rats.
Fig. 9.
Effect of curcumin and its cyclodextrin complexes on inflammatory markers during colitis. a Effect on body weight. Body weight change was calculated by dividing body weight on specified day by body weight at day 0 (starting body weight) and expressed in percentage; b diarrhea score by necropsy; c visible fecal blood score by necropsy
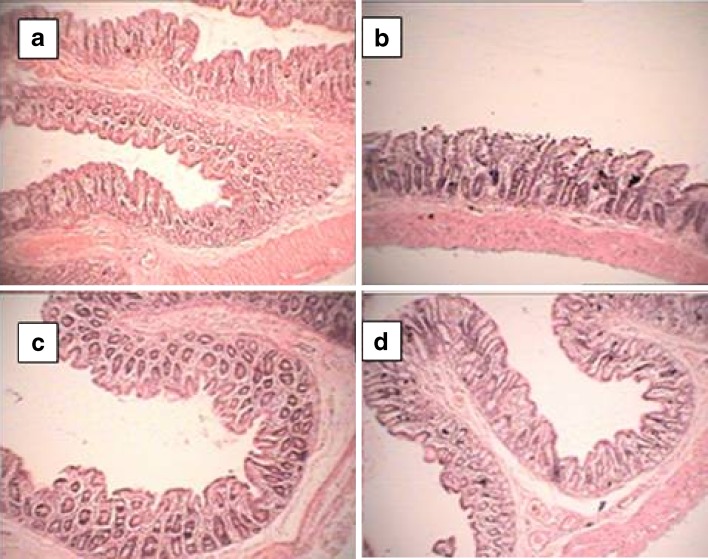
Fig. 10.
Effect of curcumin and its CD complex on colon injury. a Colonic mucosa of control rat did not show any histological modifications; b DSS-induced mucosal injury associated with transmucosal necrosis, edema, and submucosal infiltration of inflammatory cells; c effect of CD complex of curcumin showing normalization of disturbances in morphology associated with DSS treatment; d effect of pure curcumin showing normalization of disturbances in morphology associated with DSS treatment. Original magnification ×100
Histopathological Study
No obvious macroscopic inflammation was observed at surface of curcumin and CD complex of curcumin-treated groups (Fig. 10). A mild microscopic increase in chronic inflammation was observed in DSS control rats. DSS-induced colitis is characterized by histological findings such as edema, transmucosal necrosis, infiltration of inflammatory cells into both the mucosa and the submucosa, destruction of epithelial cells, and mucosal thickening. At day 90, CD complex of curcumin-treated rats showed minimal changes in the surface epithelium; no infiltration of inflammatory cells to the mucosa was observed. The changes were more pronounced in both DSS control and pure curcumin control, with loss of crypts and reduction of goblet cells, signs of surface epithelial regeneration, and focal ulcerations. The number of neutrophils was slightly increased and remained elevated during the inflammation in DSS and pure curcumin. We also found decreased eosinophils and mast cell numbers in the mucosa of the proximal colon of CD complex of curcumin and pure-curcumin-treated rats.
CONCLUSION
The ability to increase the solubility of curcumin by CD increased in the order HPβCD > MβCD > βCD > γCD. Curcumin molecules with bulky side groups on the phenyl moiety seemed to fit better into the HPβCD cavity than into the cavities of MβCD. Curcumin seems to be better included in HPβCD with very significant increase in solubility when compared to pure drug.
Antiangiogenic drug therapy is a promising development in anti-inflammatory and anticancer treatments. HPβCD complexes of curcumin exhibited antiangiogenic properties which was found to be more that of pure drug. In in vivo studies using rat colitis model, HPβCD-treated group showed a faster weight gain compared to DSS control. An additional finding in the DSS-treated rats was the predominance of eosinophils in the chronic cell infiltrate. Decreased mast cell numbers were observed in the mucosa of the colon of HPβCD of curcumin- and pure-curcumin-treated rats. This effect of curcumin may be associated with inhibition of nuclear factor κB activation and blockade of infiltration of inflammatory cells including CD4 and CD8 T cells. This study has demonstrated that the degree of colitis caused by administration of DSS was significantly attenuated by HPβCD of curcumin. Being a nontoxic natural dietary product, curcumin could be useful in the therapeutic strategy for IBD patients.
Acknowledgements
The authors would like to thank Prof. B.G. Shivananda, Principal, Al-Ameen College of Pharmacy, for his kind support and encouragement. This study was supported by SRF grant to Vivek Yadav from Indian Council of Medical Research, India
References
- 1.Podolsky DK. Inflammatory bowel disease. N Eng J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer SB. Inflammatory bowel disease. N Eng J Med. 1996;334:841–848. doi: 10.1056/NEJM199603283341307. [DOI] [PubMed] [Google Scholar]
- 3.Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Antitumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-J. [DOI] [PubMed] [Google Scholar]
- 4.Gescher AJ, Sharma RA, Steward WP. Cancer chemoprevention by dietary constituents: a tale of failure and promise. Lancet Oncol. 2001;2:371–379. doi: 10.1016/S1470-2045(00)00392-2. [DOI] [PubMed] [Google Scholar]
- 5.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005;41:1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, et al. Pharmacodynamic and pharmacokinetic study of oral curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- 7.Rao DS, Sekhara NC, Satyanarayana MN, Srinivasan M. Effect of curcumin on serum and liver cholesterol levels in the rat. J Nutrition. 1970;100:1307–1315. doi: 10.1093/jn/100.11.1307. [DOI] [PubMed] [Google Scholar]
- 8.Ramaprasad C, Sirsi M. Indian medicinal plants: Curcuma longa; in vitro antibacterial activity of curcumin and the essential oil. J Sci Ind Res. 1956;15C:239–241. [Google Scholar]
- 9.Ammon HPT, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 10.Subramanian L, Selvam R. Prevention of CCI4-induced hepatotoxicity by aqueous extract of turmeric. Nutra Res. 1999;19:429–441. doi: 10.1016/S0271-5317(99)00011-1. [DOI] [Google Scholar]
- 11.Ukil A, Maity S, Karmakar S, Datta N, Vedasiromoni JR, Das PK. Curcumin, the major component of food flavor turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br J Pharmacol. 2005;139(2):209–218. doi: 10.1038/sj.bjp.0705241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tonnesen HH, Greenhill JV. Studies on curcumin and curcuminoids XXII. Curcumin as a reducing agent and as a radical scavenger. Int J Pharm. 1992;87:79–87. doi: 10.1016/0378-5173(92)90230-Y. [DOI] [Google Scholar]
- 13.Wahlstrom B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol. 1978;43:86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 14.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27:486–494. [PubMed] [Google Scholar]
- 15.Holder GM, Plummer JL, Ryan AJ. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica. 1978;8:761–768. doi: 10.3109/00498257809069589. [DOI] [PubMed] [Google Scholar]
- 16.Ireson CR, Orr S, Jones DL, Verschoyle R, Lim CK, Luo JL, et al. Characterization of metabolites of the chemopreventive agent curcumin in humans and rat hepatocytes and in rat plasma and evaluation of their ability to inhibit cyclooxygenase-2 expression. Cancer Res. 2001;61:1058–1064. [PubMed] [Google Scholar]
- 17.Fromming KH, Szejtli J. Cyclodextrins in pharmacy. Dordrecht: Kluwer Academic; 1994. [Google Scholar]
- 18.Loftsson T, Masson M, Brewster ME. Self-association of cyclodextrins and cyclodextrin complexes. J Pharm Sci. 2005;93:1091–1099. doi: 10.1002/jps.20047. [DOI] [PubMed] [Google Scholar]
- 19.Folkman J, Klagsbrun M. Angiogenetic factors. Science. 1987;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 20.Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Chem Instrum. 1965;4:117–212. [Google Scholar]
- 21.Presta M, Belleri M, Vacca A, Ribatti D. Anti-angiogenic activity of the purine analog 6-thioguanine. Leukemia. 2002;16:1490–1499. doi: 10.1038/sj.leu.2402646. [DOI] [PubMed] [Google Scholar]
- 22.Anupama EG, Belakavadi M, Venkatesh DA, Marme D, Salimatha BP. Molecular mechanisms of anti-angiogenic effect of curcumin. Biochem and Biophys Res Com. 2002;297:934–942. doi: 10.1016/S0006-291X(02)02306-9. [DOI] [PubMed] [Google Scholar]
- 23.Vilaseca J, Salas A, Guarner F, Rodinguez R, Martinez M, Malagelada JR. Dietary fish oil reduces progression of chronic inflammatory lesions in rat model of granulomatous colitis. Gut. 1990;31:539–544. doi: 10.1136/gut.31.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace JL, Mac Naughton WK, Morris GP, Beck PL. Inhibition of leucotriene synthesis markedly accelerates healing in rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29–36. doi: 10.1016/0016-5085(89)90760-9. [DOI] [PubMed] [Google Scholar]
- 25.Skaper SD, Pollock M, Facci L. Mast cells differentially express and release active high molecular weight neurotrophins. Mol Brain Res. 2001;97:177–185. doi: 10.1016/S0169-328X(01)00314-X. [DOI] [PubMed] [Google Scholar]
- 26.Patel RP, Patel MM. Preparation and evaluation of inclusion complex of lipid lowering drug lovastatin with β-cyclodextrin. Dhaka Univ J Pharm Sci. 2007;6(1):25–36. doi: 10.3329/dujps.v6i1.340. [DOI] [Google Scholar]
- 27.Baglole KN, Boland PG, Wagner BD. Fluorescence enhancement of curcumin upon inclusion into parent and modified cyclodextrins. J Photochem Photobiol. 2005;173:230–237. doi: 10.1016/j.jphotochem.2005.04.002. [DOI] [Google Scholar]
- 28.Loftsson T, Brewster ME. Pharmaceutical application of cyclodextrin. Drug solubilization and stabilization. J Pharm Sci. 1996;85:1017–1025. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- 29.Cooper HS, Murthy S, Kido K, Yoshitake H, Flanigan A. Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis- associated neoplasia in the human: a study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis. 2000;21:757–768. doi: 10.1093/carcin/21.4.757. [DOI] [PubMed] [Google Scholar]