Abstract
We have recently demonstrated that coprecipitation of cimetidine (C) and piroxicam (P) at a mole ratio of 1:1 results in the transformation of the crystalline forms of both drugs to an amorphous state. In this study, coprecipitates and physical mixtures of cimetidine and piroxicam were further investigated at C/P mole ratios of 1:10, 1:5, 1:4, 1:2, 10:1, 20:1, 30:1, 40:1, and 52.5:1, the latter being the composition of a clinically used dosage. The physicochemical properties of these samples were examined using X-ray diffraction and Fourier transform infrared spectroscopy. Additionally, dissolution of piroxicam in the samples at C/P mole ratios of 10:1, 20:1, 30:1, 40:1, and 52.5:1 was investigated at pH 1.2 and pH 4. In coprecipitates with C/P mole ratios of 10:1, 20:1, 30:1, and 40:1, crystalline forms of both drugs were transformed to amorphous states. A mixture of an amorphous state and cimetidine crystalline form A was observed for the coprecipitate with a C/P mole ratio of 52.5:1. For the coprecipitates with C/P mole ratios of 1:2, 1:4, 1:5, and 1:10, cimetidine form A was transformed to form C, whereas piroxicam form II was modified to form I. It is interesting that small molecules, instead of polymers or solvents, can cause such crystal structure transformations. The dissolution of piroxicam at pH 4 is lower than that at pH 1.2. Additionally, the coprecipitates and physical mixtures with C/P mole ratios of 10:1, 20:1, 30:1, 40:1, and 52.5:1 demonstrate substantially higher dissolution of piroxicam compared to that of drug alone.
Key words: cimetidine, dissolution, FTIR, piroxicam, XRD
INTRODUCTION
Piroxicam is a nonsteroidal anti-inflammatory drug (NSAID) generally used to alleviate pain, inflammation, and stiffness as a result of arthritis. In addition, it exhibits chemopreventive and antitumor effects (1,2). The use of NSAIDs can cause gastroenteropathy (3). Cimetidine, a H2-receptor antagonist, is a widely used antiulcer drug (4). It also exhibits antitumor activity on gastrointestinal cancers (5,6). In addition, concomitant use of cimetidine can reduce gastric ulcers caused by NSAIDs and also enhance the anti-inflammatory activity of the NSAIDs (7).
According to some studies, the coadministration of cimetidine and piroxicam does not impair the efficacy of piroxicam or cause any adverse effects (8) but can increase the plasma concentrations of piroxicam (9). Recent studies have demonstrated that coprecipitation of cimetidine and piroxicam at a 1:1 mole ratio can transform the crystalline structures of both drugs to an amorphous state (manuscript in preparation). Therefore, other mole ratios of coprecipitates or simple physical mixtures of cimetidine and piroxicam were systematically investigated in this study. X-ray powder diffraction (XRD) measurements and Fourier transform infrared spectroscopy (FTIR) were performed to explore the crystalline structure of the drugs in the coprecipitates and to investigate the interaction between cimetidine and piroxicam. According to the Biopharmaceutics Classification System, piroxicam is classified as a class II drug exhibiting low solubility and high permeability (10,11). Cimetidine is assigned as a class III drug (10,12), a drug with high solubility and poor permeability. A combination of these two drugs may lead changes in the dissolution profile of piroxicam. Therefore, the dissolution of piroxicam in coprecipitates and physical mixtures of cimetidine and piroxicam was also carried out to investigate the possible improvement in the dissolution of piroxicam.
MATERIALS AND METHODS
Materials
Piroxicam was purchased from Vertex Chemicals (Hong Kong) and was found to correspond to form II (13,14); piroxicam form I was obtained from Sigma (St. Louis, MO, USA). Cimetidine (A polymorph) was purchased from Sigma (St. Louis, MO, USA). Cimetidine form C was prepared according to the previously described method by dissolving the drug in hot water (15). Other reagents were purchased from Aldrich (St. Louis, MO, USA) and Fisher Scientific (Fairlawn, NJ, USA).
Preparation of Cimetidine–Piroxicam Coprecipitates and Physical Mixtures
Coprecipitates and physical mixtures of C/P at mole ratios 1:10, 1:5, 1:4, 1:2, 10:1, 20:1, 30:1, 40:1, and 52.5:1, the latter being the composition of a clinically used dosage, were prepared by coprecipitation and physical mixing. In the preparation of coprecipitates, generally, a solution of cimetidine (2 g) in methanol (20 mL) was added to a stirred solution of an appropriate amount of piroxicam in acetonitrile (100 mL) at room temperature. The resulting mixture was stirred for about 2 h and then evaporated under reduced pressured at 40°C. Any residual solvent was removed under vacuum for 2 days. The residual solid samples were pulverized using a mortar and pestle, and the 0.05–0.25-mm particle size fractions were obtained by sieving.
The physical mixtures were prepared by manual mixing the appropriate amounts of the 0.05–0.25-mm particle size fractions of cimetidine and piroxicam.
X-ray Powder Diffraction Measurements
X-ray diffraction patterns were obtained using a Philips PW 3710 diffractometer with Cu-Kα radiation, collimated by a 0.08° divergence slit and a 0.2° receiving slit and scanned at a rate of 2.4°/min over the 2θ range of 5.0–60.0°.
Fourier Transform Infrared Spectroscopy
FTIR spectra were recorded on a PerkinElmer Spectrum One spectrometer in a transmission mode. Samples were prepared as KBr discs. All measurements were performed using 32 scans and 4 cm−1 resolution.
Dissolution Studies
The dissolution media consisted of 900 mL simulated gastric fluid TS prepared without pepsin as described in USP27 (16) at pH 1.2 and also at a pH 4.0 maintained at 37.0 ± 0.5°C. The dissolution medium at pH 1.2 was prepared by dissolving 2 g NaCl in 7 mL HCl and adding water to 1,000 mL. The buffered medium at pH 4.0 consisted of 0.1 M KH2PO4, using either KOH or phosphoric acid to adjust the pH to 4.0. For dissolution studies, the samples were tested with the dispersed amounts method (17) by placing 10 mg of piroxicam or its equivalent in coprecipitates or in physical mixtures on the surface of the dissolution medium. Aliquots (5 mL) were withdrawn at 2, 4, 6, 8, 10, 15, 20, 30, 40, and 60 min and replaced with 5 mL of fresh dissolution medium. The amounts of piroxicam in dissolution media pH 1.2 and pH 4.0 were determined using UV spectroscopy at 344 and 356 nm, respectively. The piroxicam concentration was calculated with reference to a calibration curve and expressed as percent drug released. The dissolution tests were performed at least in triplicate. The cumulative percentages of piroxicam dissolved were calculated. Univariate ANOVA with Dunnett’s test (two-sided) using SPSS 10.0 was applied to investigate the differences of percent dissolved at each time point among the various samples.
RESULTS AND DISCUSSION
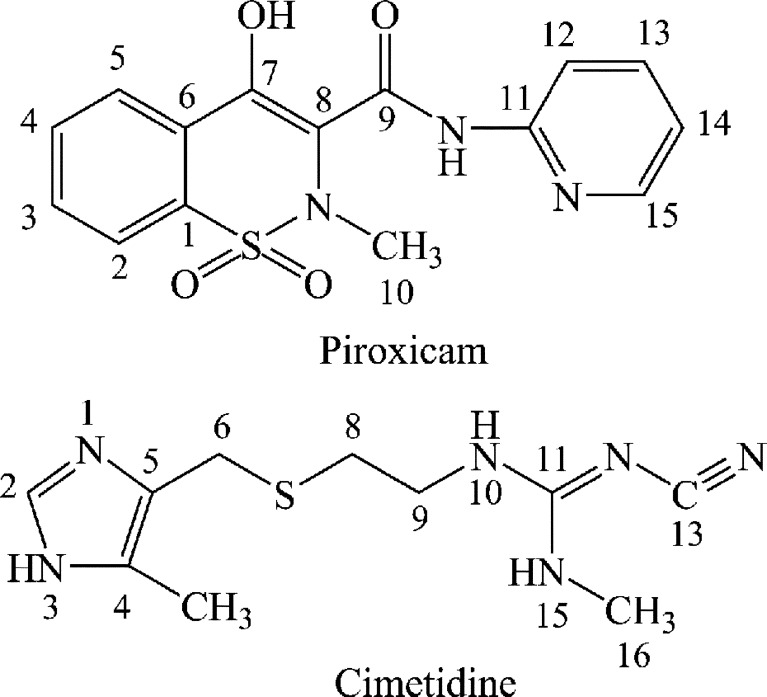
The molecular structures of cimetidine and piroxicam are presented in Fig. 1. The polymorphic forms of cimetidine and piroxicam are designated by comparing their IR, 13C CP/MAS spectra, and XRD patterns with those reported in the literature (13). The starting material of cimetidine used here is form C2 (15). However, due to the inconsistency of nomenclature, this form is also designated form A according to its IR spectrum (18), XRD profile (19), and 13C CP/MAS spectra (20). This crystalline form is specified in this study as cimetidine A. The polymorphic form of piroxicam used as starting material here is form II according to its IR spectra, XRD pattern (13), and 13C CP/MAS spectra (21).
Fig. 1.
Structures of piroxicam and cimetidine
X-ray Diffraction Measurements
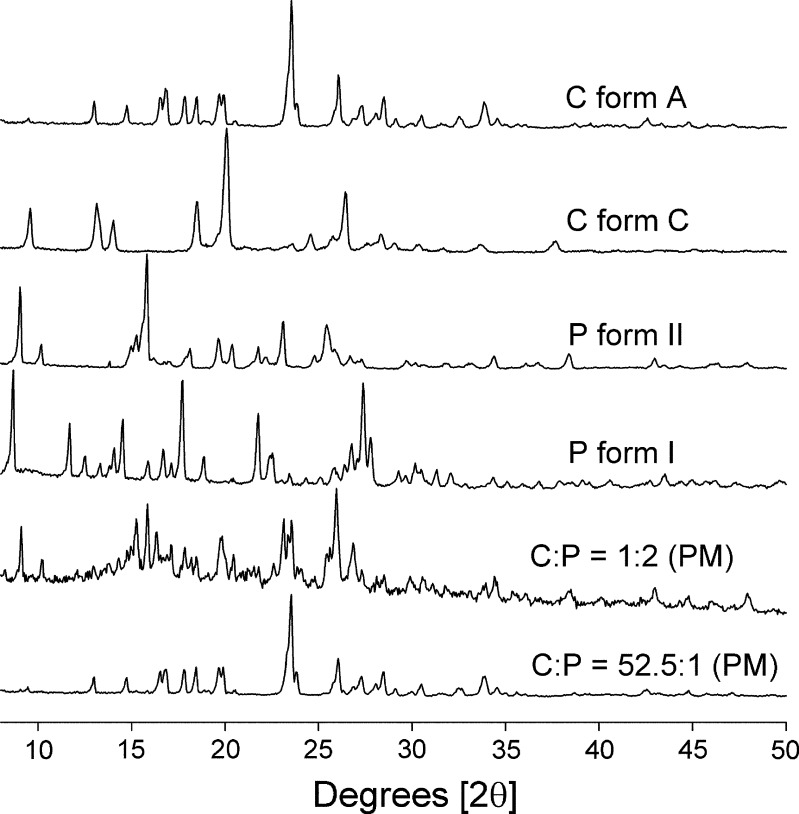
The XRD patterns of cimetidine forms A and C, piroxicam forms I and II, and physical mixtures at mole ratios of C/P 1:2 and 52.5:1 are shown in Fig. 2. Typically, the XRD patterns of the physical mixtures should show the same features that are present in cimetidine form A and piroxicam form II if there is no interaction between these two drugs. However, for mixtures containing high proportions of cimetidine, the characteristic cimetidine peaks should be prominent; and as expected, for C/P at 52.5:1 mole ratio, only the cimetidine peaks are prominent. With physical mixtures containing high proportions of piroxicam, the major peak shown in the XRD pattern is the piroxicam peak as illustrated at C/P mole ratio of 1:2.
Fig. 2.
XRD patterns of cimetidine (C) forms A and C, piroxicam (P) forms I and II, and physical mixtures (PM) of C/P at 1:2 and 52.5:1 mole ratios
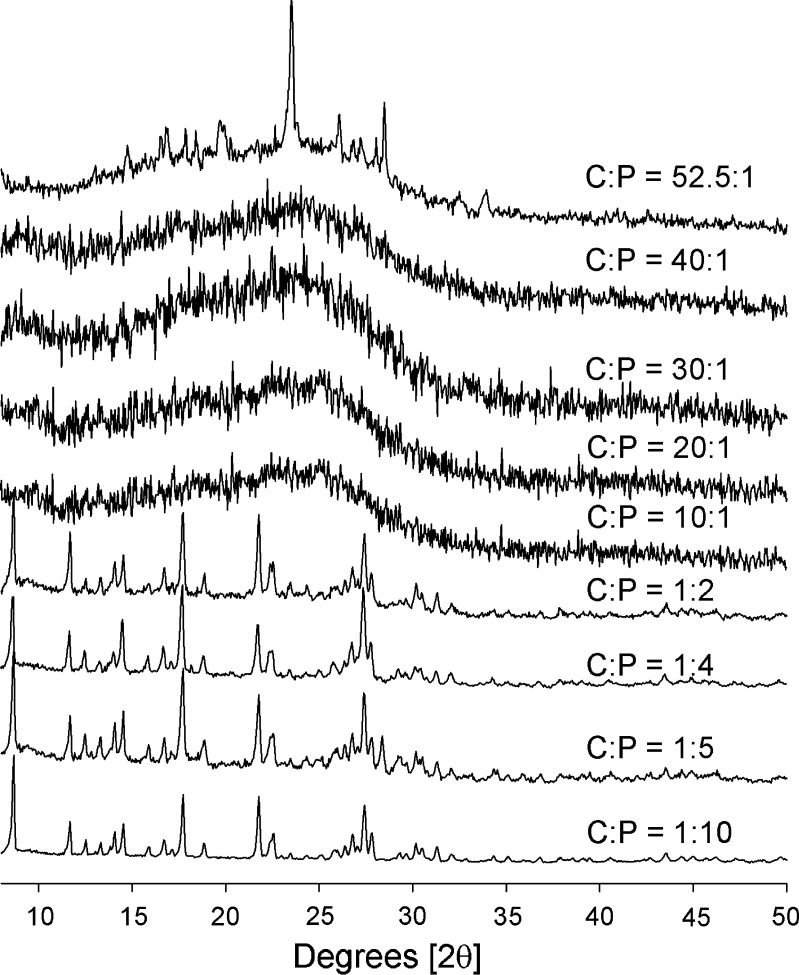
The XRD patterns of coprecipitates of C/P at mole ratios of 1:10, 1:5, 1:4, 1:2, 10:1, 20:1, 30:1, 40:1, and 52.5:1 are presented in Fig. 3. The coprecipitates of C/P at mole ratios of 10:1, 20:1, 30:1, and 40:1 do not show any peak in their XRD profiles. This indicates that both cimetidine and piroxicam in these coprecipitates are in an amorphous state. The XRD profile of C/P at mole ratio of 52.5:1 showed a halo pattern, which is a significant pattern of amorphous forms of compounds (19) (Fig. 3). The fact that the characteristic peak of cimetidine A was observed in this XRD profile (Figs. 2, 3) probably suggests that piroxicam in this coprecipitate is in an amorphous form, whereas cimetidine is in its crystalline A form or possibly in a mixture with its amorphous state.
Fig. 3.
XRD patterns of coprecipitates of cimetidine/piroxicam (C/P) at 1:10, 1:5, 1:4, 1:2, 10:1, 20:1, 30:1, 40:1, and 52.5:1 mole ratios
As shown in Fig. 3, when high proportion of piroxicam are present in the coprecipitates (C/P at mole ratios of 1:2, 1:4, 1:5, and 1:10), cimetidine peaks are barely evident in the XRD analyses. Interestingly, the characteristic peak of piroxicam form I is prominent in these XRD profiles (Figs. 2, 3). As a result of coprecipitation of cimetidine and piroxicam at these mole compositions, the piroxicam starting material form II is apparently transformed to form I.
Fourier Transform Infrared Spectroscopy
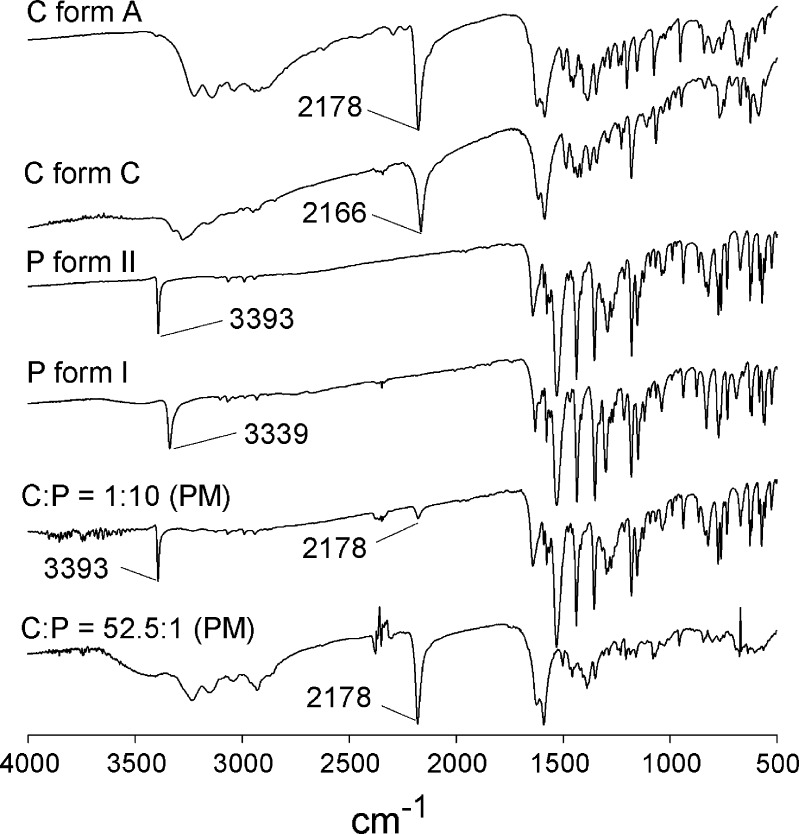
The FTIR spectra of cimetidine forms A and C, piroxicam forms I and II, and physical mixtures of C/P at mole ratios of 1:10 and 52.5:1 are shown in Fig. 4. The FTIR spectra show prominent N–H stretching peaks for piroxicam froms I and II at 3,339 and 3,393 cm1, respectively (13,22). In addition, the FTIR spectra of cimetidine forms A and C display major peaks for C≡N stretching at 2,178 and 2,166 cm−1, respectively (18). The N–H stretching band of piroxicam at 3,393 cm−1 and the C≡N band of cimetidine at 2,178 cm−1 should both be detected in the FTIR spectra of the physical mixtures provided there is no interaction between these two drugs. According to the FTIR investigation, these two prominent peaks are observed for most physical mixtures as illustrated in Fig. 4 for C/P at mole ratio of 1:10. However, depending on the proportion of each drug in the mixtures, as for C/P at 52.5:1 mole ratio, N–H peak at 3,393 cm−1 cannot be observed, and only the C≡N band of cimetidine can be detected at 2,178 cm−1.
Fig. 4.
FTIR spectra of cimetidine (C) forms A and C, piroxicam (P) forms I and II, and physical mixtures (PM) of C/P at 1:10 and 52.5:1 mole ratios
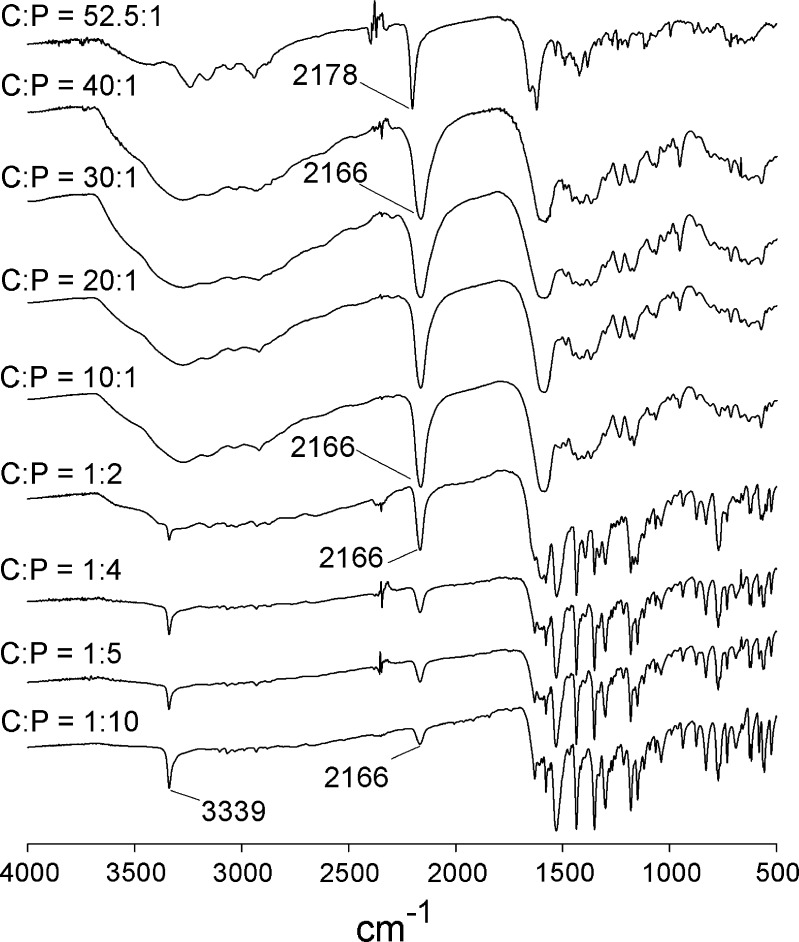
The FTIR spectra of the coprecipitates of C/P at mole ratios of 1:10, 1:5, 1:4, 1:2, 10:1, 20:1, 30:1, 40:1, and 52.5:1 are shown in Fig. 5. The N–H stretching bands of piroxicam at 3,393 cm−1 were not evident in the spectra of coprecipitates at mole ratios of 10:1, 20:1, 30:1, and 40:1. According to XRD analysis (Fig. 3), these coprecipitates are in an amorphous state. In accordance with the wavenumber of the C≡N band, cimetidine in these amorphous forms shows characteristics of cimetidine form C. For the coprecipitate of C/P at 52.5:1, the C≡N stretching band of cimetidine remains at 2,178 cm−1, suggesting that the crystalline structure of cimetidine stays in the original polymorphic form A. This conclusion is reinforced by the XRD studies which indicate that this coprecipitate is a mixture of an amorphous material and cimetidine form A. These studies suggest that the C/P 52.5:1 coprecipitate may contain piroxicam in an amorphous form, but cimetidine is in its crystalline A structure. However, since the proportion of piroxicam in this coprecipitate is very low, piroxicam features are hardly observable either from XRD or FTIR measurements. Therefore, it cannot be conclusively established that the entire portion of piroxicam is in an amorphous state or whether the drug exists in a mixture of crystalline and amorphous forms.
Fig. 5.
FTIR spectra of coprecipitates of cimetidine/piroxicam (C/P) at 1:10, 1:5, 1:4, 1:2, 10:1, 20:1, 30:1, 40:1, and 52.5:1 mole ratios
Due to the high proportion of piroxicam in the coprecipitates at mole ratios of 1:10, 1:5, 1:4, and 1:2, the characteristic peaks of piroxicam are the major features observed in these spectra (Fig. 5). The N–H stretching band of piroxicam at 3,393 cm−1 is shifted to 3,339 cm−1 in these coprecipitates. This indicates that the polymorphic form II of the original piroxicam is converted to form I. These FTIR analyses correlate well with the XRD results. Due to the strong stretching band of C≡N, this cimetidine peak is still observed in the spectra of these coprecipitates. However, at these high proportions of piroxicam, e.g., C/P at 1:10 mole ratio, this peak is low in intensity compared to those at the lower amounts of piroxicam, e.g., C/P at 1:2 mole ratio. Additionally, in coprecipitates of these all compositions (except 52.5:1), the C≡N stretching band of starting material cimetidine form A at 2,178 cm−1 is shifted to 2,166 cm−1 (Fig. 5). As shown in Fig. 3, at high proportions of piroxicam, cimetidine features cannot be observed in the XRD patterns of these coprecipitates. However, FTIR analysis can clarify that the starting material of cimetidine form A has probably changed to form C during the coprecipitation process.
The mechanisms of polymorphic transformation in molecular crystals are not entirely understood. Polymorphic conversions in this study took place in heterogeneous system comprising two organic compounds, cimetidine and piroxicam. According to the previous investigations, piroxicam form I is more stable than form II (13) and cimetidine form A is more stable than form C (23). This study, in most coprecipitates, piroxicam form II is converted to form I and cimetidine form A is transformed to form C; thus, the polymorphic conversion of both drugs is not entirely the thermodynamic polymorphs.
Concerning the polymorphic conformation of cimetidine, molecular conformation of cimetidine form C was initially proposed to lie between the extended and bent structures using 13C CP-MAS solid-state NMR (24). Afterward, with the use of high-resolution synchrotron powder diffraction, the small crystal structure of cimetidine form C can be measurable. Thus, the conformation of cimetidine form C was reinvestigated and suggested as an extended structure (25). According to the crystal structure of cimetidine form A, its conformation is in a “horseshoe” structure in which the guanidinium proton (N15–H) internally hydrogen bonds to the imidazole nitrogen (N1) (24). This intramolecular hydrogen bond is probably disrupted when cimetidine interacts with piroxicam molecule. Thus, the folded structure of cimetidine form A is changed to the extended structure of cimetidine form C. This phenomenon can occur as the result of the stronger interactions between cimetidine and piroxicam than that within the cimetidine molecule. In addition, cimetidine and piroxicam contain various groups of proton donors and acceptors that can interact to each other. Recently, we performed 13C CP-MAS solid-state NMR experiment and molecular dynamic simulations to study the interaction between cimetidine and piroxicam in an amorphous coprecipitate at 1:1 mole ratio. According to the radial distribution functions (RDFs) analyses following MD simulations for detailed investigating the interaction of specific groups, the imidazole nitrogen (N1) forms hydrogen bond with H–O at C7 of piroxicam (data not shown, manuscript in preparation). This reinforces the break of internal hydrogen bond within cimetidine form A molecule and supports the extended conformation of cimetidine form C. The specific groups of cimetidine and piroxicam that interact to each other at different mole ratios are beyond the scope of this work. However, according to the results of this present experiment as well as the recent RDFs analyses, we believe that the imidazole residue of cimetidine forms intermolecular hydrogen bonds with piroxicam. This interaction will disrupt the internal hydrogen bond in the cimetidine molecule, the folded structure of form A is converted to extended conformation of cimetidine form C. This may give reasons for the transformation of cimetidine in coprecipitates of C/P at mole ratios of 1:2, 1:4, 1:5, and 1:10 to the polymorphic form C. Piroxicam and cimetidine in the coprecipitate of C/P at mole ratios of 10:1, 20:1, 30:1, and 40:1 also interact to each other and cause the transformation of cimetidine form A to an amorphous form. In reference to the FTIR analyses, this amorphous cimetidine shows some characteristic of cimetidine form C (C≡N stretching at 2,166 cm−1). In the preparation of amorphous material, some polymorphic memory of the original crystal structure may be retained in the amorphous form (14). Thus, for these coprecipitates, the amorphous cimetidine is probably produced from cimetidine form C obtained from the transformation of form A. At C/P mole ratio of 52.5:1, most of cimetidine molecules probably did not interact with piroxicam, and most cimetidine structure remains in the folded conformation of polymorphic form A.
Regarding the polymorphic structure of piroxicam, various intermolecular and intramolecular hydrogen bonds have been reported to exist in piroxicam forms I and II. The hydrogen bond network in forms I and II causes the arrangement of piroxicam molecule as dimers and infinite sheets, respectively (14,26). For coprecipitate at C/P mole ratio of 1:2, 1:4, 1:5, and 1:10, the interaction between piroxicam and cimetidine possibly disrupts the infinite chain of piroxicam molecules and generates a dimer arrangement of form I. At the lower proportion of piroxicam (C/P at mole ratio of 10:1, 20:1, 30:1, and 40:1), the intermolecular interaction between cimetidine and piroxicam also changes the hydrogen bond pattern of piroxicam form II. For these coprecipitates, piroxicam dimers of form I were not produced but the interaction between both drugs at these specific ratios yields alternatively an amorphous form.
Dissolution Studies
Since cimetidine exhibits high aqueous solubility, only the dissolution of piroxicam was examined. Furthermore, this study was performed to investigate the possible improvement in the dissolution of piroxicam when cimetidine is incorporated in the preparations. Due to the higher proportion of cimetidine compared to that of piroxicam in clinically used dosage, only the coprecipitates and physical mixtures at higher mole ratios of cimetidine to piroxicam (C/P at mole ratios of 10:1, 20:1, 30:1, 40:1, and 52.5:1) were examined. Due to the fact that the administration of cimetidine can elevate the gastric pH to 3.5–5.0 (27,28), the dissolution experiments were also performed at pH 4.0.
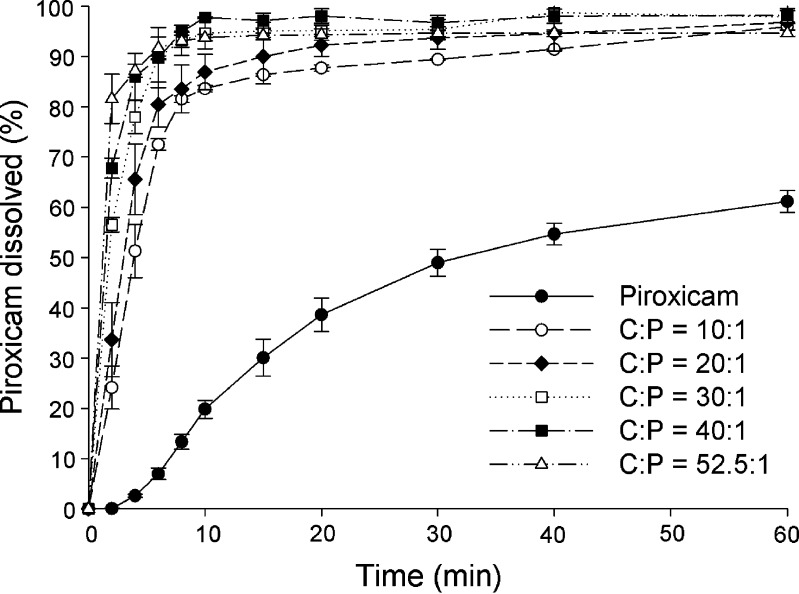
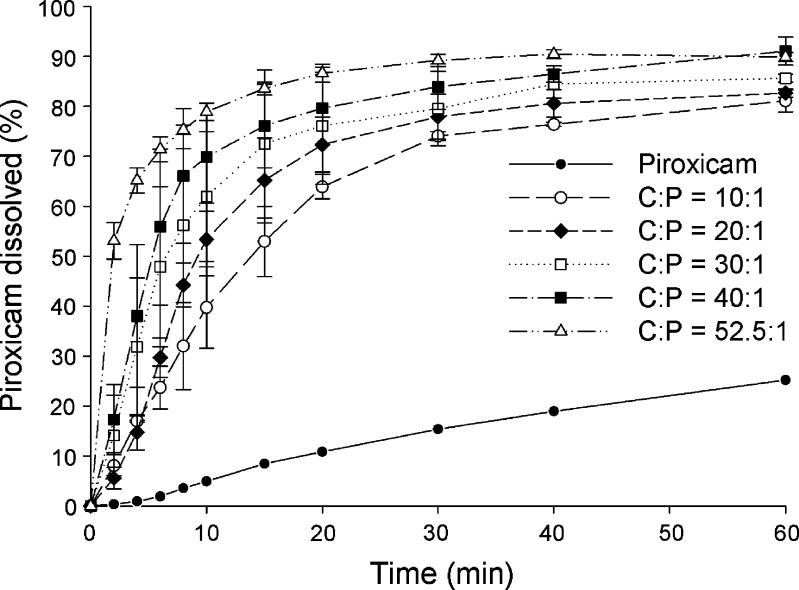
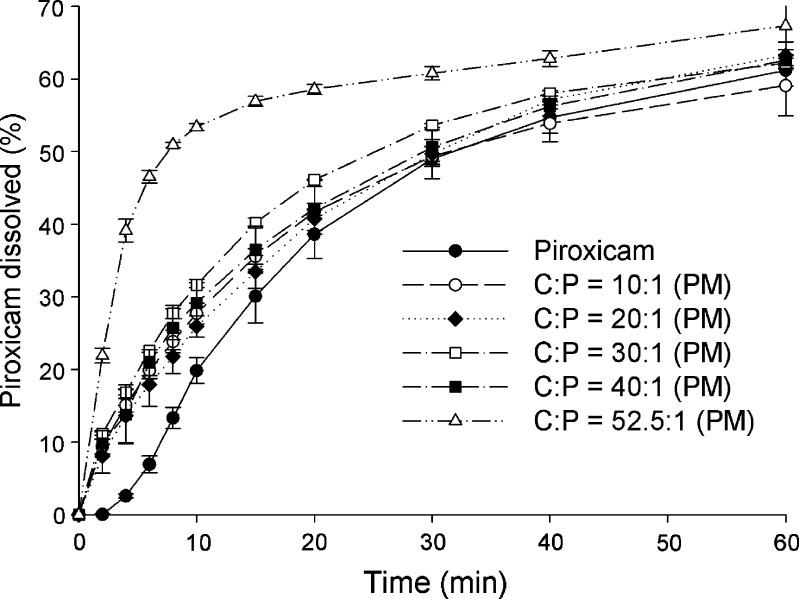
The dissolution profiles for piroxicam form II and coprecipitates at C/P mole ratios of 10:1, 20:1, 30:1, 40:1, and 52.5:1 at pH 1.2 and pH 4.0 are presented in Figs. 6 and 7, respectively. Dissolution profiles for piroxicam form II and physical mixtures of the same compositions at pH 1.2 and pH 4.0 are shown in Figs. 8 and 9, respectively. The dissolution data show that the dissolution of piroxicam pure drug is higher at pH 1.2 compared to that at pH 4.0. Piroxicam (Fig. 1) is an amphoteric compound, with a weakly acidic 7-OH proton (pKa = 5.07) and a weakly basic pyridyl nitrogen (pKa = 2.33) (29). Accordingly, the pyridyl nitrogen is protonated (about 92%) at pH 1.2, whereas the pyridyl nitrogen is barely protonated at pH 4.0. The uncharged proportion calculated for the 7-OH proton of piroxicam at pH 4.0 is about 91%. Consequently, piroxicam exhibits higher dissolution at pH 1.2 than at pH 4.0, which correlates well with its ionic forms at these two pH values.
Fig. 6.
Dissolution profiles of piroxicam form II and coprecipitates of cimetidine/piroxicam (C/P) at 1:1, 10:1, 20:1, 30:1, 40:1, and 52.5:1 mole ratios at pH 1.2 (mean ± SD, n = 3)
Fig. 7.
Dissolution profiles of piroxicam form II and coprecipitates of cimetidine/piroxicam (C/P) at 1:1, 10:1, 20:1, 30:1, 40:1, and 52.5:1 mole ratios at pH 4.0 (mean ± SD, n = 3)
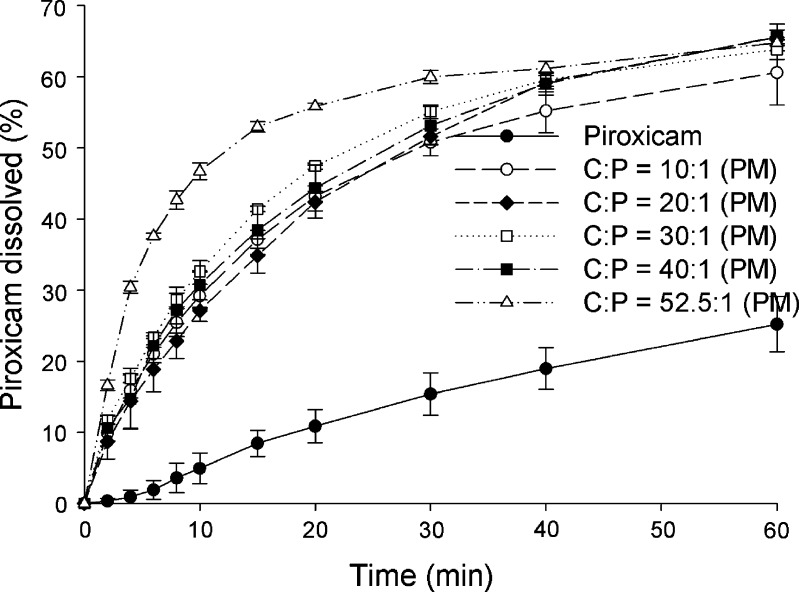
Fig. 8.
Dissolution profiles of piroxicam form II and physical mixtures (PM) of cimetidine/piroxicam (C/P) at 1:1, 10:1, 20:1, 30:1, 40:1, and 52.5:1 mole ratios at pH 1.2 (mean ± SD, n = 3)
Fig. 9.
Dissolution profiles of piroxicam form II and physical mixtures (PM) of cimetidine/piroxicam (C/P) at 1:1, 10:1, 20:1, 30:1, 40:1, and 52.5:1 mole ratios at pH 4.0 (mean ± SD, n = 3)
A one-way ANOVA test with Dunnett’s post test was used for pairwise comparisons of different samples against a reference. Generally an amorphous drug will exhibit higher solubility than its crystalline forms. The coprecipitates at C/P mole ratios of 10:1, 20:1, 30:1, 40:1, and 52.5:1 demonstrate significant higher piroxicam dissolution than the pure piroxicam form II (p < 0.01) at all the same time points in both pH 1.2 and 4.0. When using the coprecipitate C/P at mole ratio of 52.5:1 as a reference, at pH 1.2, the percents piroxicam dissolved from the coprecipitates at C/P mole ratios of 30:1, 40:1, and the reference are not significantly different after 4 min (p > 0.01), and significant differences are not observed for any of the coprecipitates from the reference at the time points after 8 min (p > 0.01). At pH 4.0, the percents piroxicam dissolved from the coprecipitates at C/P mole ratios of 30:1, 40:1, and the reference are not significantly different after 6 min (p > 0.01), and the dissolution profiles of all, except that of mole ratio of 10:1, are parallel at time point after 15 min (p > 0.01). At this pH, significant differences between the coprecipitate at mole ratio of 10:1 and the reference are observed at all the same time points (p < 0.001). On the whole, the use of higher proportions of cimetidine results in an increase in the dissolution of piroxicam to some extent. As mentioned above, the coprecipitate at mole ratio of 52.5:1 contains a mixture of amorphous and crystalline forms of the drugs. However, this coprecipitate displays comparable dissolution of piroxicam to the other amorphous coprecipitates with high mole ratios of cimetidine. In addition, the percents piroxicam dissolved from this coprecipitate are significantly higher than those of all physical mixtures at all the same time points (p < 0.001). This may suggest that piroxicam in this coprecipitate is in an amorphous form.
When choosing the physical mixture at mole ratio of 52.5:1 as a reference, the percents pure drug dissolved are lower at all the same time points at both pH 1.2 and 4.0 (p < 0.001). The percents piroxicam dissolved from all physical mixtures are significantly lower than the reference at all the same times point before 40 min (p < 0.01). This suggests that cimetidine can increase the dissolution of piroxicam, even though there is no interaction between these two drugs. This dissolution enhancement of piroxicam in the physical mixtures is probably due to the change of microenvironment pH surrounding piroxicam molecules. As the above-mentioned, the pKa of the weakly acidic 7-OH proton of amphoteric piroxicam is 5.07 (29). Meanwhile, cimetidine is a basic compound with a pKa reported as 6.80 (30) and 6.93 (31), and this drug is soluble in water (32). The solubility of cimetidine may be able to cause change of pH especially in the microenvironment. In order to prove this effect, various solutions of cimetidine were prepared and their pHs were measured. The pH values obtained from the cimetidine solutions at the concentrations of 0.50, 0.10, and 0.01 mM are 8.37, 7.52, and 7.12, respectively. The increase in the microenvironment pH caused by dissolved cimetidine may trigger the deprotonation of the 7-OH of piroxicam. This ionic form of piroxicam is more soluble than the nonionic form, thus, resulting in higher dissolution of the drug. In addition, the higher amount of cimetidine can produce higher pH and cause more effect to the microenvironmental pH changes. The dissolution of piroxicam from the physical mixture at mole ratio of 52.5:1 is, therefore, higher than those from the other mixtures.
Taken as a whole, the highest dissolution of piroxicam in the physical mixtures is obtained at the C/P mole ratio of 52.5:1 which is the composition used in clinical dosage. Thus, it is beneficial to combine these two drugs in the same dosage forms. Due to the fact that amorphous states present in coprecipitates are less stable than crystalline states of physical mixtures (33), the use of a physical mixture in the drug formulation may be more appropriate. This is despite the fact that percentages increase in piroxicam dissolution from the physical mixture of cimetidine and piroxicam is slightly lower compared to percentages increase when using the coprecipitate (p < 0.001).
CONCLUSIONS
Generally, polymers or various solvents are used to induce the change of crystal structures. In this study, it is noteworthy that the coprecipitation of cimetidine and piroxicam results in the transformation of both original polymorphic forms. For the coprecipitates at C/P mole ratios of 10:1, 20:1, 30:1, and 40:1, both cimetidine and piroxicam were transformed to amorphous forms. At the C/P mole ratio of 52:1, piroxicam is possibly modified to an amorphous form while the original polymorphic form A of cimetidine can still be detected by XRD and FTIR analyses. For coprecipitates at C/P mole ratios of 1:10, 1:5, 1:4, and 1:2, the starting material form II of piroxicam is converted to form I, whereas the original cimetidine form A is transformed to form C. The dissolution of piroxicam in most coprecipitates is much higher than that of the pure drug. The high piroxicam dissolution is obtained both for the coprecipitate and the physical mixture at the C/P mole ratio of 52.5:1 which is the mole ratio used in clinical dosage. Therefore, it may be advantageous to combine these two drugs in the same formulation for clinical use. In addition, as demonstrated in this study, the dissolution of piroxicam decreases when the pH increases from 1.2 to 4.0. This may be a disadvantage when piroxicam is administered with some specific drugs such as antacid, H2-antagonists or proton-pump inhibitors that are used to reduce gastrointestinal side effects of piroxicam. These drugs can raise the gastric pH (34) and possibly lower piroxicam dissolution. This study clearly demonstrates that cimetidine can increase the solubility of piroxicam, and that the coadministration of both drugs in the same formulation will not cause a piroxicam dissolution setback but in fact can result in an improvement of piroxicam solubility.
Acknowledgements
This work was supported by Prince of Songkla University.
References
- 1.Morre DJ, Morre DM. tNOX, an alternative target to COX-2 to explain the anticancer activities of non-steroidal anti-inflammatory drugs (NSAIDS) Mol Cell Biochem. 2006;283:159–167. doi: 10.1007/s11010-006-2568-z. [DOI] [PubMed] [Google Scholar]
- 2.Knottenbelt C, Chambers G, Gault E, Argyle DJ. The in vitro effects of piroxicam and meloxicam on canine cell lines. J Small Anim Pract. 2006;47:14–20. doi: 10.1111/j.1748-5827.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- 3.Semble EL, Wu WC. Antiinflammatory drugs and gastric mucosal damage. Semin Arthritis Rheum. 1987;16:271–286. doi: 10.1016/0049-0172(87)90005-9. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein W, Isselbacher KJ. Drug therapy: cimetidine. N Engl J Med. 1978;299:992–996. doi: 10.1056/NEJM197811022991806. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi HK, Watanabe T, Yokoyama A, Iwagaki H, Yoshino T, Tanaka N, Nishibori M. Cimetidine induces interleukin-18 production through H2-agonist activity in monocytes. Mol Pharmacol. 2006;70:450–453. doi: 10.1124/mol.106.025890. [DOI] [PubMed] [Google Scholar]
- 6.Lefranc F, Yeaton P, Brotchi J, Kiss R. Cimetidine, an unexpected anti-tumor agent, and its potential for the treatment of glioblastoma (review) Int J Oncol. 2006;28:1021–1030. [PubMed] [Google Scholar]
- 7.Maciel HP, Cardoso LG, Ferreira LR, Perazzo FF, Carvalho JC. Anti-inflammatory and ulcerogenic effects of indomethacin and tenoxicam in combination with cimetidine. Inflammopharmacology. 2004;12:203–210. doi: 10.1163/1568560041352266. [DOI] [PubMed] [Google Scholar]
- 8.Milligan PA, McGill PE, Howden CW, Kelman AW, Whiting B. The consequences of H2 receptor antagonist-piroxicam coadministration in patients with joint disorders. Eur J Clin Pharmacol. 1993;45:507–512. doi: 10.1007/BF00315306. [DOI] [PubMed] [Google Scholar]
- 9.Said SA, Foda AM. Influence of cimetidine on the pharmacokinetics of piroxicam in rat and man. Arzneimittelforschung. 1989;39:790–792. [PubMed] [Google Scholar]
- 10.Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- 11.Wilson WI, Peng Y, Augsburger LL. Generalization of a prototype intelligent hybrid system for hard gelatin capsule formulation development. AAPS PharmSciTech. 2005;6:E449–457. doi: 10.1208/pt060356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jantratid E, Prakongpan S, Amidon GL, Dressman JB. Feasibility of biowaiver extension to biopharmaceutics classification system class III drug products: cimetidine. Clin Pharmacokinet. 2006;45:385–399. doi: 10.2165/00003088-200645040-00004. [DOI] [PubMed] [Google Scholar]
- 13.Vrecer F, Vrbinc M, Meden A. Characterization of piroxicam crystal modifications. Int J Pharm. 2003;256:3–15. doi: 10.1016/S0378-5173(03)00057-7. [DOI] [PubMed] [Google Scholar]
- 14.Sheth AR, Bates S, Muller FX, Grant DJW. Polymorphism in piroxicam. Cryst Growth Des. 2004;4:1091–1098. doi: 10.1021/cg049876y. [DOI] [Google Scholar]
- 15.Baranska M, Proniewicz LM. FT-IR and FT-Raman spectra of cimetidine and its metallocomplexes. J Mol Struct. 1999;511–512:153–162. doi: 10.1016/S0022-2860(99)00154-4. [DOI] [Google Scholar]
- 16.US Pharmacopeia . US Pharmacopeia 27 and National Formulary 22 (USP27 and NF22) Rockville: US Pharmacopeia; 2004. [Google Scholar]
- 17.Kim KH, Frank MJ, Henderson NL. Application of differential scanning calorimetry to the study of solid drug dispersions. J Pharm Sci. 1985;74:283–289. doi: 10.1002/jps.2600740312. [DOI] [PubMed] [Google Scholar]
- 18.Hegedus B, Gorog S. The polymorphism of cimetidine. J Pharm Biomed Anal. 1985;3:303–313. doi: 10.1016/0731-7085(85)80037-6. [DOI] [PubMed] [Google Scholar]
- 19.Otsuka M, Kato F, Matsuda Y. Physicochemical stability of cimetidine amorphous forms estimated by isothermal microcalorimetry. AAPS PharmSciTech. 2002;3(4):30. doi: 10.1208/pt030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Middleton DA, Duff CSL, Berst F, Reid DG. A cross-polarization magic-angle spinning 13C NMR characterization of the stable solid-state forms of cimetidine. J Pharm Sci. 1997;86:1400–1402. doi: 10.1021/js970139g. [DOI] [PubMed] [Google Scholar]
- 21.Sheth AR, Bates S, Muller FX, Grant DJW. Local structure in amorphous phases of piroxicam from powder x-ray diffractometry. Cryst Growth Des. 2005;5:571–578. doi: 10.1021/cg049757i. [DOI] [Google Scholar]
- 22.Tantishaiyakul V, Permkam P, Suknuntha K. Use of DRIFTS and PLS for the determination of polymorphs of piroxicam alone and in combination with pharmaceutical excipients: a technical note. AAPS PharmSciTech. 2008;9:95–99. doi: 10.1208/s12249-007-9003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer-Brandl A. Polymorphic transitions of cimetidine during manufacture of solid dosage forms. Int J Pharm. 1996;140:195–206. doi: 10.1016/0378-5173(96)04587-5. [DOI] [Google Scholar]
- 24.Middleton DA, Duff CSL, Peng X, Reid DG, Saunders D. Molecular conformations of the polymorphic forms of cimetidine from 13C solid-state NMR distance and angle measurements. J Am Chem Soc. 2000;122:1161–1170. doi: 10.1021/ja993067z. [DOI] [Google Scholar]
- 25.H. Birkedal, A. Bauer-Brandl, and P. Pattison. The surprising polymorph C of cimetidine: synchrotron radiation to the rescue, XIXth European Crystallographic Meeting, Abstracts. Acta Cryst. A56 (Supplement), s337, Nancy, France, 2000.
- 26.Taddei P, Torreggiani A, Simoni R. Influence of environment on piroxicam polymorphism: vibrational spectroscopic study. Biopolymers. 2000;62:68–78. doi: 10.1002/1097-0282(2001)62:1<68::AID-BIP80>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 27.Thomson AB, Kirdeikis P, Zuk L. Comparison of 200 mg cimetidine with multiple doses of antacid on extent and duration of rise in gastric pH in volunteers. Digest Dis Sci. 1999;44:2051–2055. doi: 10.1023/A:1026678503604. [DOI] [PubMed] [Google Scholar]
- 28.Karlstadt RG, Hedrich DA, Asbel-Sethi NR, Palmer RH. Acid-suppression profile of two continuously infused intravenous doses of cimetidine. Clin Ther. 1993;15:97–106. [PubMed] [Google Scholar]
- 29.Avdeef A. Physicochemical profiling (solubility, permeability and charge state) Curr Top Med Chem. 2001;1:277–351. doi: 10.2174/1568026013395100. [DOI] [PubMed] [Google Scholar]
- 30.Gerk PM, Oo CY, Paxton EW, Moscow JA, McNamara PJ. Interactions between cimetidine, nitrofurantoin, and probenecid active transport into rat milk. J Pharmacol Exp Ther. 2001;296:175–180. [PubMed] [Google Scholar]
- 31.Avdeef A, Berger CM. pH-metric solubility: 3. dissolution titration template method for solubility determination. Eur J Pharm Sci. 2001;14:281–291. doi: 10.1016/S0928-0987(01)00190-7. [DOI] [PubMed] [Google Scholar]
- 32.Katrukha SP, Davydov SM, Selina EV, Kukes VG. Determination of cimetidine in blood plasma by high performance liquid chromatography. Pharm Chem J. 1988;22:85–87. doi: 10.1007/BF00759065. [DOI] [Google Scholar]
- 33.Grant DJW. Theory and origin of polymorphism. In: Brittain HG, editor. Polymorphism in Pharmaceutical Solids. New York: Marcel Dekker; 1999. pp. 1–33. [Google Scholar]
- 34.Maton PN. Review article: prevention of stress-related mucosal bleeding with proton-pump inhibitors. Aliment Pharm Therap. 2006;22:45–52. doi: 10.1111/j.1365-2036.2005.02713.x. [DOI] [PubMed] [Google Scholar]