Abstract
Chitosan-based carriers have important potential applications for the administration of drugs. In the present study, topical gel formulations of terbinafine hydrochloride (T-HCl) were prepared using different types of chitosan at different molecular weight, and the antifungal inhibitory activity was evaluated to suggest an effective formulation for the treatment of fungal infections. The characteristics of gel formulations were determined with viscosity measurements and texture profile analysis. Stability studies were performed at different temperatures during 3 months. The ex vivo permeation properties were studied through rat skin by using Franz diffusion cells. The antifungal inhibitory activity of formulations on Candida species and filamentous fungi was also examined with agar-cup method. The microbiological assay was found suitable for determination of in vitro antifungal activity of T-HCl. A marketed product was used to compare the results. The antifungal activity of T-HCl significantly increased when it was introduced into the chitosan gels. A higher drug release and the highest zone of inhibition were obtained from gels prepared with the lowest molecular weight chitosan (Protasan UP CL 213) compared to that of other chitosan gels and marketed product. These results indicated the advantages of the suggested formulations for topical antifungal therapy against Candida species and filamentous fungi.
Key words: antifungal activity, chitosan gel, ex vivo permeation, terbinafine hydrochloride, topical delivery
INTRODUCTION
The stratum corneum is the target organ of antimycotic drug treatment. Topical therapy is often preferred to oral drug administration in the treatment of cutaneous superficial fungal infections. In fact, the required concentration for antimycotic activity at the skin target site may be more easily achieved after topical dosing, if good drug release and penetration are ensured. To attain the same local drug concentration, a higher oral dose generally needs to be administered, hence increasing the risk of adverse effects. Usually, topical administration results in much lower and often undetectable systemic levels, thus reducing the possible toxicity of the drug. A topical formulation, intended to treat a cutaneous disease, must reach the target site at the required concentration to achieve its therapeutic action. To this end, the role of the vehicle is a very important parameter to obtain an efficient release (1, 2).
Topical products are important classes of drug delivery systems, and their use in therapy is becoming more widespread. The therapeutic efficacy of a topical formulation depends on both the nature of the vehicle and the physicochemical properties of the active substance. It is well known that vehicles used in the topically applied formulations can greatly influence the rate and extent of drug permeation into and across the skin (3). Gel base formulations prepared by using different polymers are also employed to optimize the topical formulation of drugs. A higher extent of permeation was observed with gels than the other vehicles such as creams and ointments. The vast majority of drugs incorporated in gels do not pass through the skin, but act locally mainly on the skin surface or in the whole epidermis. Gels have good viscosity, satisfactory bioadhesion, and lack irritating or sensitizing actions (4–6).
Chitosan has been previously examined as an excipient in the pharmaceutical industry for different pharmaceutical dosage forms and has been shown to have superior characteristics. It is a natural poly-(aminosaccharide), possessing structural characteristics similar to glycosaminoglycans, is easily bioabsorbable, and currently is in use for the release of several drugs. Chitosan is a hydrophilic biopolymer obtained by alkaline deacetylation of chitin, a major component of arthropod shells, and has favorable properties such as nontoxicity, bioadhesivity, biocompatability, and biodegradability. Moreover, chitosan itself possesses antimicrobial activity (7–10).
Terbinafine hydrochloride (T-HCl; (E)-N-(6,6-dimethyl-2-hepten-4-yn-yl)-N-methyl-1-naphthalenemethanamine hydrochloride) is a new potent antifungal agent of the allylamine class which selectively inhibits fungal squalene epoxidase. It has a broad-spectrum activity against yeast, fungi, molds, and dermatophytes and is indicated for both oral and topical treatment of mycoses. It is typically administered orally (250 or 500 mg per day) or topically (1% cream, applied twice daily) (11–13).
Candida species are opportunistic fungus species invading mucus membrane and deep tissues. Although T-HCl is mostly used in the treatment of dermatophyte infections, it has also been shown that it demonstrated a high in vitro activity against Aspergillus and other filamentous fungi. Both Candida species and filamentous fungi are the most common cause of human opportunistic fungal infections (14). The safety profile and efficacy of the antifungal agent are important to decide oral or topical antifungal administration. Lower drug requirement and higher affectivity for many fungal or yeast skin infections are the main advantages of T-HCl (12, 15).
There are few literatures about topical delivery of T-HCl. The antifungal activity of chitosan gels as topical delivery systems against Aspergillus and other filamentous fungi was first described in this study. For this purpose, T-HCl containing chitosan gels were prepared by using different types of chitosan at different molecular weights. The ex vivo permeation properties and in vitro antifungal activity on Candida species and filamentous fungi were evaluated to suggest a topical formulation with improved activity for the treatment of fungal infections. A marketed product was used as a reference to assess the possibility of experimental formulation in clinical use.
MATERIALS AND METHODS
Materials
Chitosans used in this study are given in Table I. T-HCl and marketed product (Terbisil®cream 1%; P) were kindly supplied by Santa Farma Ilaç San. A.Ş., Turkey. All other solvents and reagents were of analytical grade.
Table I.
The Codes of the Chitosan Formulations Used in This Study
| Type of chitosan | Molecular weight of chitosan (Da) | Formula code |
|---|---|---|
| Protasan UP CL 213a | 150–400 | G1 |
| Chitosan 448869b | 50,000–90,000 | G2 |
| Chitosan C3646b | 90,000–190,000 | G3 |
| Chitosan 448877b | 190,000–310,000 | G4 |
aNovaMatrix, Norway
bSigma-Aldrich, St. Louis, MO, USA
Preparation of Chitosan Gels
The gel formulations were prepared using different type of chitosans with different molecular weights at 3% (w/v) concentration in diluted lactic acid solution (1%). The mixture was prepared by agitating at approximately 600 rpm with aid of mechanical stirrer to get a smooth dispersion. T-HCl was incorporated into the formulations at 1% (w/v) concentration. P was also used to compare the results.
Viscosity Measurements
Viscosity measurements of gels were performed on a Brookfield digital viscometer (Model DV-III) at 25 ± 1°C. The spindle (number 27) was rotated at 10–200 rpm.
Texture Profile Analysis
The mechanical properties of the gels were determined using software-controlled penetrometer, TA.XT Plus texture analyzer (Stable Micro System, UK) equipped with a 5-kg load cell. In brief, a defined mass of each formulation (10 g) was transferred into a beaker and was kept in the ultrasonic water bath to remove air bubbles for 20 min. After this, temperature of each sample was allowed to equilibrate to 20 ± 1°C by storage in an oven for 24 h. In texture profile analysis (TPA), the Perspex probe of 10 mm diameter was compressed twice into each gel sample at a defined rate of 2 mm s−1 to a depth of 15 mm. A delay period was 15 s between the two compressions. At least five replicate analyses were performed for each formulations at ambient temperature (ca. 20°C) using a fresh sample in each case. Data collection and calculation were performed using the Texture Exponent 3.0.5.0 software package of the instrument. From the resultant force–time plot, mechanical parameters such as hardness, compressibility, adhesiveness, cohesiveness, and elasticity were defined (16).
Drug Content Studies
Drug content of the gel formulations was determined by dissolving 1 g gel in 50 ml of pH 5.8 phosphate buffer. These solutions were quantitatively transferred to volumetric flasks and appropriate dilutions were made with the same buffer solution. The resulting solutions were then filtered through 0.45 μm membrane filters before subjecting the solutions to spectrophotometric analysis (UV 1208 Shimadzu Spectrophotometer) for T-HCl at 222 nm (17). Drug content was calculated from the linear regression equation obtained from a standard curve previously calculated. Samples from drug-free gel were used as blank solution during analyses.
Stability Studies
The prepared hydrogels were subjected to stability studies in screw capped glass tubes at three different temperatures (4°C, 25°C, and 40°C) and evaluated periodically for percent drug content, pH (Hanna HI 221 pH meter), and cosmetic qualities such as texture, appearance, and the feeling upon application macroscopically for 3 months.
Ex Vivo Permeation Studies
Preparation of Skin Samples
Wistar Albino rats were used for the study which was approved by the Ege University Faculty of Pharmacy Animal Ethics Committee (2007/12-1). The animals were sacrificed by overdose inhalation of chloroform. Hair on the dorsal side of the animals was removed with shaving razor in the direction of tail to head without damaging the skin. The shaven part of skin was separated from animals, and hypodermis including blood vessels was removed using surgical blade no. 23. The excised skin was washed with distilled water and subsequently used.
Permeation Studies
Drug permeation through excised rat skin was studied for 8 h using Franz diffusion cells. The surface area of the receptor compartment of assembly was 1.76 cm2. The receptor compartments were filled 20 ml of a phosphate buffer (pH 5.8) and were kept at 37 ± 0.5°C. Uniform mixing of the receptor medium was provided by magnetic stirring. The different gel preparations were applied at an amount equivalent to 1% of drug on the membrane in donor compartment ensuring an intimate contact with the rat skin. A 0.7-ml aliquot was periodically withdrawn at suitable time intervals from the sampling arm of receptor chamber. Fresh diffusion media were simultaneously replaced in the receptor chamber. The samples were analyzed spectrophotometrically at 222 nm against blank solution prepared under the same experimental conditions without T-HCl. Three replicates of each experiment were performed.
Antifungal Activity Studies
Test Microorganisms
Pathogenic and opportunistic filamentous fungi and Candida species were used for the determination of in vitro antifungal inhibitory activity of the T-HCl formulations. These species were Candida albicans ATCC 10231, C. albicans CDC-B 385, Candida krusei ATCC 6258, Candida parapsilosis ATCC 90018, Candida tropicalis RSSK 665, Candida glabrata, Candida keyfr, Candida guillermondi, Candida dubliniensis CD36, Aspergillus fumigatus NRRL 2999 and Aspergillus flavus, Aspergillus ochraceus, Aspergillus sydowi, Aspergillus wentii, Aspergillus terreus, Penicillium chrysogenum, Penicillium expansum, Penicillium hirsutum, Alternaria alternata, and Cladosporium herbarum isolates of Ege University Hospital air (EUHI).
Fungal Inoculum
Stock inoculum suspensions for filamentous fungi were prepared from 5–7-day-old cultures grown on potato dextrose agar. Number of spores was determined with Thoma’s hemocytometer. One hundred microliters of the spore suspension (2 × 103 spore per milliliter) was inoculated onto surface of the plates and spread (18). For Candida species, overnight cultures containing 107 cfu/ml of microorganisms were used and diluted with distilled water to obtain turbidity equivalent to a McFarland 0.5 turbidity standard (19).
Plate Diffusion Method
Potato dextrose agar (Merck) and Sabouraud’s dextrose agar (Merck) were used for plate diffusion method for filamentous fungi and Candida species, respectively. Twenty milliliters medium was poured into assay plates (90 mm diameter) and allowed to cool down on a leveled surface. Plates were kept for 24 h at 37°C to dry the plates.
Petri dishes containing 20 ml medium were seeded with 100 µl of the fungal inoculum. The plates were dried at room temperature for 15 min. Wells, each 1 cm in diameter, were cut out of the agar, and 100 mg of the gel formulation was placed into each well. Chitosan gels without T-HCl were used to compare the inhibition zones as well. The fungal plates were incubated at 27 ± 0.1°C for 3 days. The plates of Candida species were incubated at 37 ± 0.1°C for 24 h. The results were recorded by measuring the zones of growth inhibition surrounding the wells (20). All determinations were made in triplicate for each test microorganism. For positive control, dimethyl sulfoxide (DMSO) solution of T-HCl (1%) was used.
Statistical Analysis
All the data were statistically analyzed by analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test. p value of less than 0.05 was considered as evidence of a significant difference (21, 22).
RESULTS AND DISCUSSION
In Vitro Evaluations
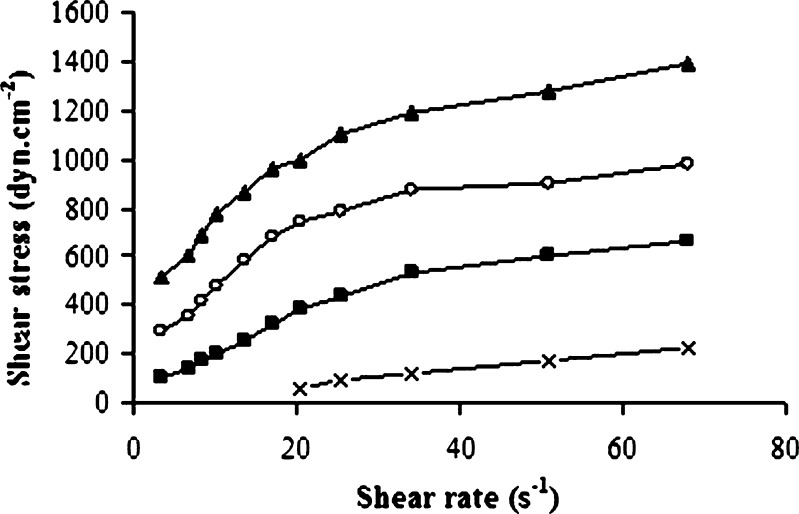
The performance of topical products depends to a great extent on their viscosity. Viscosity measurements realized by Brookfield digital viscometer showed that the viscosity of gels was increased significantly with increasing molecular weight of chitosan (G1 < G2 < G3 < G4). Flow curves of chitosan gels were shown in Fig. 1. The lowest viscosity was found for G1 in the range of 300–350 cps. Chitosans exhibited pseudoplastic behavior, i.e., a decrease of viscosity with increasing shear rate, which indicates a gel structure. Incorporation of T-HCl into the gels did not affect the viscosity. The flow curves of chitosan gels were adjusted to the Herschel–Bulkley mathematical model, a power law modification, in which the shear stress (τ) is related to the shear rate (γ) by the Eq. (23):
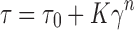 |
where τ0 is the yield stress, K is a factor related to the apparent viscosity of the dispersions, and n is the flow index (n = 1, Newtonian flow; n > 1, dilatant flow; n < 1, pseudoplastic flow). The n value of gels was found lower than 1, indicating that the flow type was pseudoplastic.
Fig. 1.
Flow curves of chitosan gels (multiplication symbol G1, square G2, circle G3, triangle G4)
Traditionally, flow rheometry has been used to characterize the rheological properties of topical pharmaceutical formulations. However, more recently, TPA has been used for this purpose because the parameters defined by this technique have direct relevance to both the nonclinical and clinical properties of such systems (24). The obtained data from TPA using chitosan gel formulations could be transformed into the results of the viscosity measurements. In doing so, it was possible to use TPA to characterize the flow behavior of pharmaceutical gels.
The mechanical properties of formulations were summarized in Table II. The hardness expresses the applicability of the gels to the desired site. The gels should have low hardness value to be administered to the skin. The compressibility expresses taking the prepared gel from the container and the simplicity of the spreadability on the application site. The compressibility value should be low to take the prepared gel from the container and to be easily spread on the skin. The hardness and compressibility of the gels raised when the viscosity of gel formulations increased as it was showed in our viscosity measurements as well. The highest compressibility was obtained with marketed product. It was evident that the gel formulations prepared with chitosans showed suitable compressibility values regarding applicability to the skin. The cohesiveness increases the performance of the product at the application site. The high value of cohesiveness provides full structural recovery following gel application. In our study, all gel formulations were showed high cohesiveness. In this study, the adhesiveness of gel decrement was affected by chitosan used. When all test results were compared, G1 was observed to be more elastic than the other gel formulations. All the formulations were found to be easily spreadable and nondripping in nature.
Table II.
Mechanical Properties of Formulations
| Codes | Hardness (N) ± SD | Compressibility (N mm) ± SD | Adhesiveness (N mm) ± SD | Cohesiveness ± SD | Elasticity ± SD |
|---|---|---|---|---|---|
| G1 | 0.010 ± 0.000 | 0.019 ± 0.001 | 0.008 ± 0.003 | 0.819 ± 0.018 | 1.089 ± 0.024 |
| G2 | 0.023 ± 0.003 | 0.021 ± 0.002 | 0.024 ± 0.003 | 0.894 ± 0.004 | 0.976 ± 0.003 |
| G3 | 0.025 ± 0.002 | 0.026 ± 0.001 | 0.032 ± 0.005 | 0.912 ± 0.012 | 1.007 ± 0.008 |
| G4 | 0.028 ± 0.002 | 0.034 ± 0.001 | 0.054 ± 0.003 | 0.923 ± 0.007 | 1.021 ± 0.018 |
| P | 0.146 ± 0.009 | 0.692 ± 0.025 | 0.740 ± 0.029 | 0.952 ± 0.011 | 0.995 ± 0.002 |
Each value represents the mean ± SD (n = 5)
SD standard deviation
When the prepared T-HCl hydrogel formulations were subjected to stability studies at three different temperatures (4°C, 25°C, and 40°C), it was found that all the formulations should be stored 3 months without any apparent change in the product’s characteristics. The drug content was found to be in the range of 88.8–92.4%. pH remained unchanged after 3 months at all the temperatures. The pH value of formulations was found as 3.28, 5.30, 4.96, 5.26, and 4.67 for G1, G2, G3, G4, and P, respectively, at 25°C (Table III).
Table III.
Stability Evaluations of Formulations at 25°C
| Codes | No of days | Drug content (%) | pH | Viscosity (cP) | Macroscopic aspect |
|---|---|---|---|---|---|
| G1 | 30 | 88.9 ± 0.36 | 3.22 ± 0.09 | 300 ± 20 | Homogeneous |
| 60 | 89.0 ± 0.41 | 3.34 ± 0.06 | 320 ± 15 | Viscous | |
| 90 | 88.8 ± 0.52 | 3.28 ± 0.06 | 350 ± 40 | White | |
| G2 | 30 | 89.6 ± 0.38 | 5.25 ± 0.04 | 1,800 ± 150 | Homogeneous |
| 60 | 91.2 ± 0.43 | 5.35 ± 0.02 | 2,000 ± 100 | Viscous | |
| 90 | 90.4 ± 0.39 | 5.30 ± 0.08 | 2,000 ± 120 | White | |
| G3 | 30 | 88.9 ± 0.52 | 4.94 ± 0.06 | 4,000 ± 220 | Homogeneous |
| 60 | 89.4 ± 0.45 | 4.98 ± 0.05 | 4,100 ± 200 | Viscous | |
| 90 | 89.1 ± 0.61 | 4.96 ± 0.03 | 4,200 ± 250 | White | |
| G4 | 30 | 91.8 ± 0.49 | 5.23 ± 0.04 | 7,800 ± 350 | Homogeneous |
| 60 | 93.2 ± 0.44 | 5.29 ± 0.07 | 8,000 ± 320 | Viscous | |
| 90 | 92.3 ± 0.67 | 5.26 ± 0.06 | 8,000 ± 400 | White | |
| P | 30 | 93.5 ± 0.36 | 4.65 ± 0.08 | 19,500 ± 300 | Homogeneous |
| 60 | 92.9 ± 0.39 | 4.68 ± 0.05 | 20,000 ± 100 | Compact | |
| 90 | 92.4 ± 0.56 | 4.67 ± 0.03 | 19,800 ± 380 | White |
Each value represents the mean ± SD (n = 3)
The gels obtained were transparent and nongritty. The presence of T-HCl gave the preparation a white color. No macroscopically physical changes were observed during storage. This showed that the prepared formulations were stable at the studied temperatures during 3 months. Our results were in accordance with the results in the literature which emphasized the properties of different chitosan gels both at different temperatures and in time remained unchanged for 3 months (23, 25).
Ex Vivo Permeation Studies
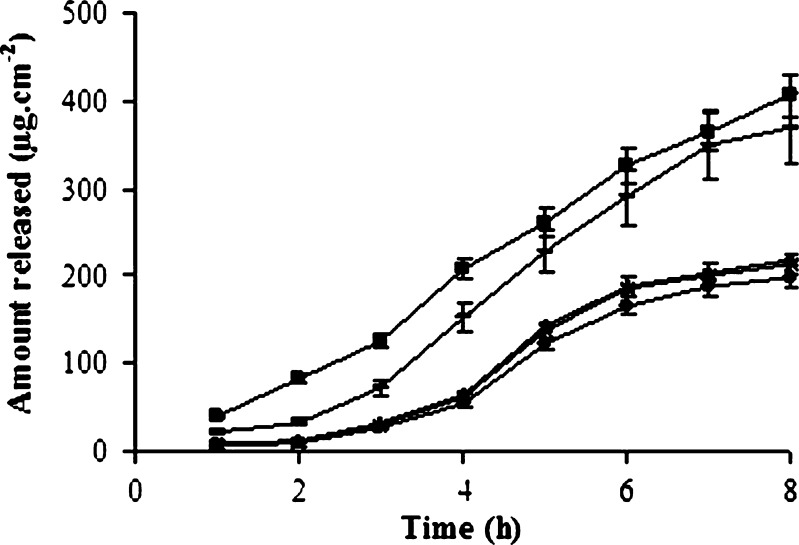
Drug permeation through excised rat skin was studied using Franz diffusion cells for 8 h. It was observed that the release of T-HCl from G1 was higher than from G2, G3, G4, and P. The amount released from G1 was found as 406.36 μg/cm2. It was observed that nearly 81% of the total amount was released at the end of 8 h. The lowest drug release was observed with G4. The amount of release from G2, G3, G4, and P was found as 219.31, 213.14, 197.64, and 369.88 μg/cm2, respectively. No significant difference in drug permeation was obtained between different chitosans (p > 0.05). The release from G1 and P was similar and this was almost 46% more in comparison to G2–G3–G4. Differences in release were observed due to the different viscosity of the gels. It has been shown that the viscosity increases with the increasing molecular weight of chitosan (8, 26). Higher viscosity of the gels resulted in a decrease in release. Because of the loose structure of the gels prepared with low molecular weight chitosan, the amount of released drug was increased (27). No lag time was observed in release from gels. The results of permeation studies are shown in Fig. 2 and Table IV.
Fig. 2.
Permeation profiles of T-HCl (square G1, triangle G2; multiplication symbol G3, circle G4, dash P)
Table IV.
Results of Permeation Studies
| Formulations | Amount of drug released (μg/cm2) | Jss (μg/cm2/h) | r 2 |
|---|---|---|---|
| G1 | 406.36 | 55.1 ± 0.12 | 0.992 |
| G2 | 219.31 | 43.1 ± 0.06 | 0.963 |
| G3 | 213.14 | 42.7 ± 0.04 | 0.990 |
| G4 | 197.64 | 42.0 ± 0.03 | 0.979 |
| P | 369.88 | 53.0 ± 0.02 | 0.994 |
Chitosan is a weak base and is insoluble in water and organic solvent. However, it is soluble in dilute aqueous acidic solution (pH <6.5), which can convert glucosamine units into soluble form R-NH+3. It gets precipitated in alkaline solution or with polyanions and forms gel at lower pH (28, 29). In this study, the significantly lower pH value of G1 than that of other gels resulted in an increase at the release of T-HCl. The low release rate at higher pH could also be attributed to the binding of cationic T-HCl to the polymer (15).
Antifungal Activity Studies
The antifungal activity of the drug against Candida species and filamentous fungi was determined in terms of mean diameter of zone inhibition. The results were recorded by measuring the zones of growth inhibition surrounding the wells. In DMSO, 1% (w/v) T-HCl solution was used as positive control. In a previous study, T-HCl was found to be effective against Aspergillus species (30–32). In this study, inhibition zone of T-HCl obtained for filamentous fungi has been detected to be higher than those of Candida species. Inhibition zone diameters obtained for Candida species and filamentous fungi are given in Tables V and VI, respectively. The inhibition zones of G1 and control were found similar. Although no significant difference in antifungal activity was obtained between G2, G3, and G4, a significantly higher antifungal activity against all microorganisms was observed with G1 (p < 0.05). It was thought that because of the low viscosity of G1, T-HCl easily penetrated into the microorganism, thus resulting in an increase in the inhibition zone. pH is also an effective factor on the antimicrobial efficacy of chitosan (33). The lower pH values resulted in higher antimicrobial activity as it was detected in this study.
Table V.
Results of Antifungal Activity Studies on Candida Species
| Inhibition zone (mm)a ± SD | ||||||
|---|---|---|---|---|---|---|
| Microorganisms | G1 | G2 | G3 | G4 | P | T-HCl |
| Candida albicans ATCC 10231 | 41 ± 0.5 | 36 ± 0.4 | 36 ± 0.5 | 35 ± 0.3 | 21 ± 0.4 | 35 ± 0.5 |
| Candida albicans CDC-B 385 | 40 ± 0.4 | 28 ± 0.3 | 29 ± 0.5 | 28 ± 0.5 | 18 ± 0.3 | 34 ± 0.5 |
| Candida krusei ATCC 6258 | 31 ± 0.7 | 19 ± 0.4 | 20 ± 0.5 | 18 ± 0.2 | 12 ± 0.2 | 27 ± 0.4 |
| Candida parapsilosis ATCC 90018 | 59 ± 0.5 | 50 ± 0.7 | 45 ± 0.3 | 45 ± 0.5 | 37 ± 0.6 | 53 ± 0.8 |
| Candida tropicalis RSSK 665 | 37 ± 0.3 | 27 ± 0.5 | 21 ± 0.6 | 20 ± 0.4 | 11 ± 0.5 | 35 ± 0.6 |
| Candida glabrata (clinical isolate) | 35 ± 0.5 | 18 ± 0.3 | 17 ± 0.4 | 16 ± 0.4 | 11 ± 0.5 | 33 ± 0.5 |
| Candida keyfr (clinical isolate) | 36 ± 0.5 | 26 ± 0.4 | 26 ± 0.4 | 24 ± 0.5 | 12 ± 0.3 | 33 ± 0.5 |
| Candida guillermondi (clinical isolate) | 45 ± 0.6 | 33 ± 0.7 | 33 ± 0.5 | 33 ± 0.8 | 15 ± 0.4 | 43 ± 0.7 |
| Candida dubliniensis CD36 | 49 ± 0.8 | 38 ± 0.3 | 40 ± 0.8 | 35 ± 0.5 | 23 ± 0.5 | 43 ± 0.7 |
aEach value represents the mean ± SD (n = 3)
Table VI.
Results of Antifungal Activity Studies on Filamentous Fungi
| Inhibition zone (mm)a ± SD | ||||||
|---|---|---|---|---|---|---|
| Microorganisms | G1 | G2 | G3 | G4 | P | T-HCl |
| Aspergillus flavus EUHI | 77 ± 0.8 | 60 ± 0.5 | 61 ± 0.7 | 57 ± 0.5 | 45 ± 0.4 | 73 ± 0.8 |
| Aspergillus fumigatus NRRL 2999 | 57 ± 0.5 | 31 ± 0.6 | 39 ± 0.5 | 33 ± 0.3 | 28 ± 0.3 | 51 ± 0.7 |
| Aspergillus ochraceus EUHI | 77 ± 0.7 | 60 ± 0.5 | 59 ± 0.6 | 57 ± 0.6 | 47 ± 0.5 | 77 ± 0.6 |
| Aspergillus sydowii EUHI | 75 ± 0.6 | 63 ± 0.8 | 72 ± 0.9 | 65 ± 0.8 | 54 ± 0.5 | 75 ± 0.7 |
| Aspergillus wentii EUHI | 71 ± 0.8 | 46 ± 0.5 | 50 ± 0.7 | 47 ± 0.5 | 35 ± 0.3 | 67 ± 0.6 |
| Aspergillus terreus EUHI | 74 ± 0.5 | 56 ± 0.8 | 66 ± 0.6 | 54 ± 0.6 | 43 ± 0.4 | 71 ± 0.6 |
| Penicillium chrysogenum EUHI | 64 ± 0.6 | 44 ± 0.4 | 55 ± 0.5 | 42 ± 0.5 | 30 ± 0.4 | 60 ± 0.5 |
| Penicillium expansum EUHI | 64 ± 0.5 | 45 ± 0.5 | 53 ± 0.6 | 44 ± 0.4 | 31 ± 0.4 | 60 ± 0.5 |
| Penicillium hirsutum EUHI | 70 ± 0.7 | 51 ± 0.5 | 63 ± 0.8 | 51 ± 0.6 | 38 ± 0.5 | 68 ± 0.8 |
| Alternaria alternata EUHI | 61 ± 0.5 | 42 ± 0.6 | 51 ± 0.5 | 39 ± 0.4 | 36 ± 0.5 | 57 ± 0.7 |
| Cladosporium herbarum EUHI | 73 ± 0.8 | 62 ± 0.8 | 70 ± 0.8 | 60 ± 0.6 | 48 ± 0.5 | 70 ± 0.8 |
aEach value represents the mean ± SD (n = 3)
According to the results, it was concluded that even the release results of G1 and P were found similar, the antifungal activity of T-HCl increased when it was introduced into the chitosan gels. It was attributed to the antifungal and antimicrobial effects of chitosan itself (34, 35). Chitosan gels provided drug concentration above its minimum inhibition concentration (MIC) and were expected to remain more on the skin due to their bioadhesive properties. An ionic interaction between the cations due to the amino groups of chitosan and anionic parts of bacteria cell wall has been proposed as the mechanism for the antimicrobial activity of chitosan (36).
All formulations were found to be more effective against Candida species and filamentous fungi compared to P. The antifungal activity results obtained from inhibition zones were comparable to the concentration of T-HCl determined spectrophotometrically. Therefore, it can be said that the amount of drug permeated through rat skin inhibited the growth of microorganism in vitro.
MIC of the T-HCl was found to be 0.05–1.56 and 0.03–4 μg/ml for filamentous fungi and Candida species, according to the microbiological studies (37, 38). This value was taken into account when evaluating the results. The amount of T-HCl released from the formulations was found to be above the MIC value for Candida species and filamentous fungi. The antimicrobial activity of T-HCl was also determined in correlation with release, and it was indicated that the released amounts of T-HCl from the formulations were in the range of those recommended for the antimicrobial effect.
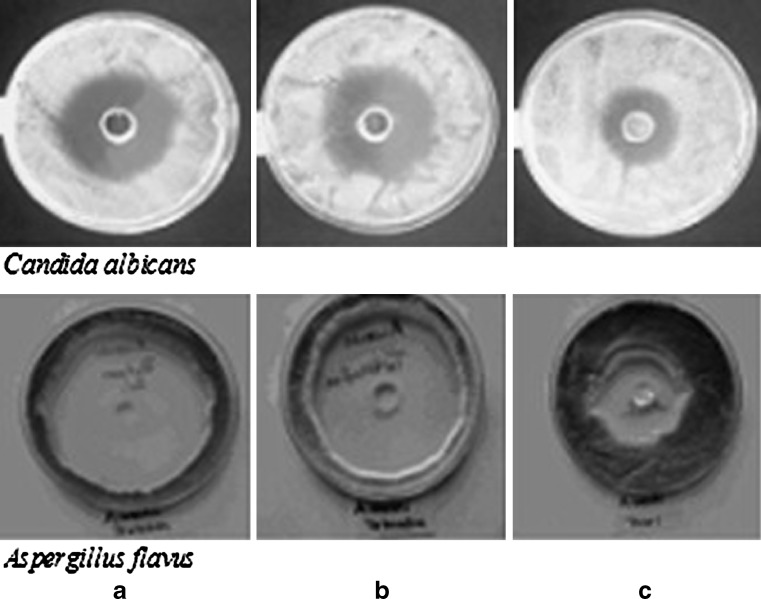
Inhibition zones for C. albicans and A. flavus which are the most significant pathogenic species were presented in Fig. 3. The results of variance analysis clearly showed that microorganism type and diameter of zone were the important variables which affected the determination of antifungal activity of T-HCl. The interactions between these variables were found statistically significant (p < 0.05).
Fig. 3.
Inhibition zones of a G1, b 1% (w/v) T-HCl solution, and c P formulation
CONCLUSION
In the present study, the chitosan gel formulations of T-HCl were prepared. The properties of the formulations were investigated at different temperatures during 3 months, and no significant change was observed in tested properties. The mechanical properties of the gels were also determined using TPA, and the results were evaluated in relevance with viscosity measurements. Gel formulations prepared with chitosan were found more easily applicable to the skin when compared to marketed product.
It was observed that the release of T-HCl from G1 was higher than from the G2, G3, G4, and P. The amount released from G1 was found as 406.36 μg/cm2. The inhibition zone values of the gels were compared to control and P using ANOVA followed by Tukey’s multiple comparisons test. Although the release amounts of G1 and P were found similar, it was found that the chitosan gels, especially G1, were significantly more effective than marketed cream against Candida species and filamentous fungi. These results suggested that the vehicle is an important factor in determining the efficacy of antifungal agents on skin. The incorporation of drug into chitosan gels exhibited better antimicrobial activity against tested microorganisms. The increased antifungal activity of T-HCl when it was introduced into the chitosan gels was attributed to the antifungal and antimicrobial effects of chitosan itself. Protasan UP CL 213 gel formulation demonstrated at about twofold increase at inhibition zones compared to marketed product. The results of this study showed that the chitosan gels of T-HCl could be suggested as successful topical delivery systems for the treatment of fungal infections.
Acknowledgments
We would like to express our grateful acknowledgments to Prof. Dr. Sevda Şenel for her guidance and help throughout this work.
References
- 1.Alberti I, Kalia YN, Naik A, Bonny JD, Guy RH. Effect of ethanol and isopropyl myristate on the availability of topical terbinafine in human stratum corneum, in vivo. Int J Pharm. 2001;219:11–19. doi: 10.1016/S0378-5173(01)00616-0. [DOI] [PubMed] [Google Scholar]
- 2.Shishu G, Aggarwal N. Preparation of hydrogels of griseofulvin for dermal application. Int J Pharm. 2006;326:20–24. doi: 10.1016/j.ijpharm.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Csoka I, Csanyi E, Zapantis G, Nagy E, Feher-Kiss A, Horvath G, Blazso G, Eros I. In vitro and in vivo percutaneous absorption of topical dosage forms: case studies. Int J Pharm. 2005;291:11–19. doi: 10.1016/j.ijpharm.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 4.Valenta C, Auner BG. The use of polymers for dermal and transdermal delivery. Eur J Pharm Biopharm. 2004;58:279–289. doi: 10.1016/j.ejpb.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Tas C, Ozkan Y, Savaser A, Baykara T. In vitro release studies of chlorpheniramine maleate from gels prepared by different cellulose derivatives. Il Farmaco. 2003;58:605–611. doi: 10.1016/S0014-827X(03)00080-6. [DOI] [PubMed] [Google Scholar]
- 6.Wang YY, Hong CT, Chiu WT, Fang JY. In vitro and in vivo evaluations of topically applied capsaicin and nonivamide from hydrogels. Int J Pharm. 2001;224:89–104. doi: 10.1016/S0378-5173(01)00755-4. [DOI] [PubMed] [Google Scholar]
- 7.Fakhry A, Schneider GB, Zaharias R, Senel S. Chitosan supports the initial attachment and spreading of osteoblast preferentially over fibroblasts. Biomaterials. 2004;25:2075–2079. doi: 10.1016/j.biomaterials.2003.08.068. [DOI] [PubMed] [Google Scholar]
- 8.Ikinci G, Senel S, Akıncıbay H, Kas S, Ercis S, Wilson CG, Hıncal AA. Effect of chitosan on a peridontal pathogen Prophyromonas gingivalis. Int J Pharm. 2002;235:121–127. doi: 10.1016/S0378-5173(01)00974-7. [DOI] [PubMed] [Google Scholar]
- 9.Jumaa M, Furkert FH, Müler BW. A new lipid emulsion formulation with high antimicrobial efficacy using chitosan. Eur J Pharm Biopharm. 2002;53:115–123. doi: 10.1016/S0939-6411(01)00191-6. [DOI] [PubMed] [Google Scholar]
- 10.No HK, Park NY, Lee SH, Meyers SP. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol. 2002;74:65–72. doi: 10.1016/S0168-1605(01)00717-6. [DOI] [PubMed] [Google Scholar]
- 11.Alberti I, Kalia YN, Naik A, Bonny JD, Guy RH. In vivo assessment of enhanced topical delivery of terbinafine to human stratum corneum. J Control Release. 2001;71:319–327. doi: 10.1016/S0168-3659(01)00244-9. [DOI] [PubMed] [Google Scholar]
- 12.Balfour JA, Faulds D. Terbinafine: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial mycoses. Drugs. 1992;43:259–284. doi: 10.2165/00003495-199243020-00010. [DOI] [PubMed] [Google Scholar]
- 13.Kazakov PV, Golosov SN. A simple method for obtaining terbiınafine hydrochloride. Pharm Chem J. 2004;38:34–36. doi: 10.1023/B:PHAC.0000038420.81588.50. [DOI] [Google Scholar]
- 14.Gené J, Azón-Masoliver A, Guarro J, De Febrer G, Martínez A, Grau C, Ortoneda M, Ballester F. Cutaneous infection caused by Aspergillus ustus, an emerging opportunistic fungus in immunosuppressed patients. J Clin Microbiology. 2001;39:1134–1136. doi: 10.1128/JCM.39.3.1134-1136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sen M, Uzun C, Güven O. Controlled release of terbinafine hydrochloride from pH sensitive poly(acrylamide/maleic acid) hydrogels. Int J Pharm. 2000;203:149–157. doi: 10.1016/S0378-5173(00)00449-X. [DOI] [PubMed] [Google Scholar]
- 16.Karavana (Hızarcıoglu) S, Guneri P, Ertan G. Benzydamine hydrochloride buccal bioadhesive gels designed for oral ulcers: preparation, rheological, textural, mucoadhesive and release properties. Pharm Dev Tech. 2009; in press doi:10.1080/10837450902882351. [DOI] [PubMed]
- 17.Sen M, Yakar A. Controlled release of antifungal drug terbinafine hydrochloride from poly(N-vinyl 2-pyrrolidone/itaconic acid) hydrogels. Int J Pharm. 2001;228:33–41. doi: 10.1016/S0378-5173(01)00804-3. [DOI] [PubMed] [Google Scholar]
- 18.NCCLS, National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. NCCLS document M38-A (ISBN 1-56238-470-8). Wayne: NCCLS; 2002.
- 19.NCCLS, National Committee for Clinical Laboratory Standards. Method for antifungal disk diffusion susceptibility testing of yeasts; proposed guideline. NCCLS document M44-P (ISBN 1-56238-488-0). Wayne: NCCLS; 2003.
- 20.Nesseem DI. Formulation and evaluation of itraconazole via liquid crystal for topical delivery system. J Pharm Biomed Analysis. 2001;26:387–399. doi: 10.1016/S0731-7085(01)00414-9. [DOI] [PubMed] [Google Scholar]
- 21.Dawson B, Trapp RG. Basic and clinical biostatistics. USA: McGraw Hill; 2001. pp. 161–182. [Google Scholar]
- 22.Daniel WW. Biostatistics; a foundation for analysis in the health sciences. New York: Wiley; 1983. pp. 459–483. [Google Scholar]
- 23.Anchisi C, Maccioni AM, Meloni MC. Physical properties of chitosan dispersions in glycolic acid. Il Farmaco. 2004;59:557–561. doi: 10.1016/j.farmac.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Jones D, Lawlor MS, Woolfson AD. Examination of the flow rheological and textural properties of polymer gels composed of poly(methylvinylether-comaleic anhydride) and poly(vinylpyrrolidone): rheological and mathematical interpretation of textural parameters. J Pharm Sci. 2002;91:2091–2101. doi: 10.1002/jps.10195. [DOI] [PubMed] [Google Scholar]
- 25.Ruel-Gariepy E, Chenite A, Chaput C, Guirguis S, Leroux JC. Characterization of thermosensitive chitosan gels for the sustained delivery of drugs. Int J Pharm. 2000;203:89–98. doi: 10.1016/S0378-5173(00)00428-2. [DOI] [PubMed] [Google Scholar]
- 26.Aksungur P, Sungur A, Ünal S, Iskit AB, Squier CA, Senel S. Chitosan delivery systems for the treatment of oral mucositis: in vitro and in vivo studies. J Control Release. 2004;98:269–279. doi: 10.1016/j.jconrel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Senel S, Ikinci G, Kas S, Yousefi-Rad A, Sargon MF, Hıncal AA. Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery. Int J Pharm. 2000;193:197–203. doi: 10.1016/S0378-5173(99)00334-8. [DOI] [PubMed] [Google Scholar]
- 28.Qin C, Li H, Xiao Q, Liu Y, Zhu J, Du Y. Water-solubility of chitosan and its antimicrobial activity. Carbonhydrate Polym. 2006;63:367–374. doi: 10.1016/j.carbpol.2005.09.023. [DOI] [Google Scholar]
- 29.Kumar MNVR, Muzzarelli RAA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104:6017–6084. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- 30.Cardoso SG, Schapoval EES. Microbiological assay for terbinafine hydrochloride in tablets and creams. Int J Pharm. 2000;203:109–113. doi: 10.1016/S0378-5173(00)00439-7. [DOI] [PubMed] [Google Scholar]
- 31.Gil-Lamaignere C, Müler FMC. Differential effects of the combination of caspofungin and terbinafine against Candida albicans, Candida dubliniensis and Candida kefyr. Int J Antimicrob Agents. 2004;23:520–523. doi: 10.1016/j.ijantimicag.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Karaarslan A, Arikan S, Ozcan M, Ozcan KM. In vitro activity of terbinafine and itraconazole against Aspergillus species isolated from otomycosis. Mycoses. 2003;47:284–287. doi: 10.1111/j.1439-0507.2004.00988.x. [DOI] [PubMed] [Google Scholar]
- 33.Lim SH, Hudson SM. Review of chitosan and its derivatives as microbial agents and their uses as textile chemicals. J Macromol Sci: Part C-Polym Rev. 2003;43:223–229. [Google Scholar]
- 34.Gupta KC, Ravi Kumar MNV. pH dependent hydrolysis and drug release behavior of chitosan/poly(ethylene glycol) polymer network microspheres. J Materials Sci: Materials in Medicine. 2001;12:753–759. doi: 10.1023/A:1017976014534. [DOI] [PubMed] [Google Scholar]
- 35.Kang IJ. A study on the effective application chitosan to drug delivery system. The 7th Asia-Pacific Chitin and Chitosan Symposium, Korea; 23/26 April. 2006, p. 273.
- 36.Bae K, Jun EJ, Lee SM, Paik DI, Kim JB. Effect of water-soluble reduced chitosan on Streptococcus mutans, plaque regrowth and biofilm vitality. Clin Oral Invest. 2006;10:102–107. doi: 10.1007/s00784-006-0038-3. [DOI] [PubMed] [Google Scholar]
- 37.Garg S, Naidu J, Singh SM, Nawange SR, Jhara N, Saxena M. In itro activity of terbinafine against Indian clinical isolates of Candida albicans and non-albicans using a macrodilution method. J Mycol Med. 2006;16:119–125. [Google Scholar]
- 38.Petrayni G, Meingassner JG, Mieth H. Antifungal activity of the allylamine derivative terbinafine in vitro. Antimicrob Agents Chemother. 1987;31:1365–1368. doi: 10.1128/aac.31.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]