Abstract
Limited aqueous solubility of exemestane leads to high variability in absorption after oral administration. To improve the solubility and bioavailability of exemestane, the self-microemulsifying drug delivery system (SMEDDS) was developed. SMEDDS comprises of isotropic mixture of natural or synthetic oil, surfactant, and cosurfactant, which, upon dilution with aqueous media, spontaneously form fine o/w microemulsion with less than 100 nm in droplet size. Solubility of exemestane were determined in various vehicles. Ternary phase diagrams were plotted to identify the efficient self-emulsification region. Dilution studies, droplet size, and zeta potential of the formulations were investigated. The release of exemestane from SMEDDS capsules was studied using USP dissolution apparatus in different dissolution media and compared the release of exemestane from a conventional tablet. Oral pharmacokinetic study was performed in female Wistar rats (n = 8) at the dose of 30 mg kg−1. The absorption of exemestane from SMEDDS form resulted in about 2.9-fold increase in bioavailability compared with the suspension. Our studies illustrated the potential use of SMEDDS for the delivery of hydrophobic compounds, such as exemestane by the oral route.
Key words: bioavailability enhancement, exemestane, microemulsion, SMEDDS
INTRODUCTION
Oral route has been the major route of drug delivery for the chronic treatment of many diseases. Nearly 40% of new drug candidates exhibit low water solubility and hence high intra- and inter-subject variability and lack of dose proportionality. The formulation of such poorly water-soluble drugs is one of the most challenging tasks to the formulation experts. An enhancement in the solubility and dissolution rate can improve the oral bioavailability of such drugs, which further improves the therapeutic efficacy and patient compliance (1–4).
Exemestane (androsta-1,4 diene-3,17-dione-6-methylene) is a novel, very potent, orally active, selective, and irreversible steroidal aromatase inhibitor used in the adjuvant treatment of hormonally responsive breast cancer in postmenopausal women. It acts as a false substrate for the aromatase enzyme and is processed to an intermediate that binds irreversibly to the active site of the enzyme causing its inactivation, an effect also known as suicide inhibition (5,6).
Due to the absence of intravenous formulation, determination of absolute bioavailability in human was not possible. Preclinical data in animals (rats and dogs) when exemestane was administered via IV route (formulated in polypropylene glycol and saline 50:50 v/v) indicated that the absolute bioavailability was about 5%. Limited aqueous solubility and high lipophilicity limits the therapeutic outcome for all treatments requiring exemestane. It would be desirable to extend the therapeutic potential of exemestane by increasing the bioavailability of the drug and/or by reducing inter-patient variability in plasma concentration. This could be useful in enabling a reduction in the daily dose of exemestane required to achieve the same level of bioavailability seen with a conventional formulation. This would increase predictability of the treatment and increase uniformity of treatment in patient population (7–11).
Among the lipid-based systens, self-microemulsifying drug delivery system (SMEDDS) is a promising technology to improve the rate and extent of the absorbtion of poorly water-soluble drugs. The clinical usefulness of the SMEDDS is evident from the commercially available fromulation containing cyclosporine A, ritonavir and saquinavir. SMEDDS is pre-concentrate mixture of surfactants, co-surfactants, and lipophilic phase, which creates fine droplets of emulsion (5–100 nm), when diluted with water or the body fluids in the aqueous lumen of the gut (12–19).
In this study, exemestane SMEDDS formulations containing oil, surfactant, and co-surfactant were developed successfully, and the physicochemical characteristics were evaluated in vitro and in vivo. The solubility of exemestane in various excipients were determined. Ternary phase diagram is composed of oil; surfactant and co-surfactant were constructed to identify the area of microemulsion region. Droplet size, zeta potential, dilution, thermodynamic, accelerated, and long-term stability were investigated in detail. The morphology and droplet size/distribution of exemestane microemulsion were observed by transmittance electron microscope photograph. The release profile of exemestane SMEDDS from capsules were evaluated using USP dissolution apparatus in water, 0.1 N HCl, phosphate buffer (pH 6.8), water containing 0.5% w/v sodium dodecyl sulfate (SDS) and compared the release of exemestane from a conventional tablet. The pharmacokinetic study was performed by oral administration of 30 mg kg−1 exemestane (SMEDDS and plain drug suspension). The oral bioavailability of exemestane in SMEDDS formulation was significantly higher than the plain drug suspension. Our study indicates that SMEDDS formulations consisting of Capryol 90, Transcutol P, and Cremophore ELP are optimal. SMEDDS show good potential to improve oral bioavailability for the delivery of exemestane.
MATERIAL AND METHODS
Exemestane was obtained from Dabur Research Foundation (Ghaziabad, India). Cremophore ELP (polyoxyl 35 hydrogenated castor oil) was obtained from BASF (India). Labrasol (caprylocaproyl macrogol-8 glycerides), Labrafil M1944 (oleoyl macrogol glycerides), Labrafil M2125, (linoleoyl macrogoglycerides), Lauroglycol FCC (propylene glycol laurate), Plureol Oleique (polyglyceryl oleate), Capryol 90 (propylene glycol monocaprylate), Transcutol P (diethylene glycol monoethyl ethyl ether) were obtained from Gattefosse (Saint Priest, France). Soyabean oil, corn oil, oleic acid, castor oil, cotton seed oil, olive oil, iso-propyl myristate were obtained from Loba Chem. All other analytical grade chemicals and solvents were purchased from Qualigens Fine Chemicals (Mumbai, India).
HPLC Analysis of Exemestane In Vitro
High-performance liquid chromatography (HPLC) analysis of exemestane in SMEDDS formulation was done by validated HPLC method for the determination of exemestane in SMEDDS formulation (37).
Solubility Studies
Solubility of exemestane in various oils, surfactants, and co-surfactants was measured. An excess amount of exemestane was introduced into 2 mL of each vehicle, and mixture was kept in sealed vials. Vortex mixer (Heidolph Multi Reax) was used to facilitate the solubilization. Sealed vials were stirred in a water bath (Julabo SW 23) at 37°C for 72 h. After standing for 72 h, each vial was centrifuged at 5,000 rpm for 10 min using a centrifuge (Eppendorf Centrifuge 5810). Undissolved exemestane was removed by filtering with a membrane filter (0.45 µm). The concentration of exemestane in the supernatants was determined by HPLC analysis.
Preparation of Ternary Phase Diagram
Ternary diagrams of surfactants, co-surfactants, and oil were plotted; each of them representing an apex of the triangle. Ternary mixtures with varying compositions of surfactant, co-surfactant, and oil were prepared. Various combinations of surfactant, co-surfactant, and oil were prepared. The percentage of surfactant, co-surfactant, oil, and water used herein was decided on the basis of the requirements for the spontaneously emulsifying systems (21). The compositions were evaluated for microemulsion formation by diluting one part of pre-concentrate with deionized water (1:20). After equilibrium, the time of self-microemulsification, dispersibility, appearance, and flow ability was observed and scored according to the five grading system shown in Table I (17,21).
Table I.
Visual Assessment of Efficiency of Self-Microemulsification
| Grade | Dispersibility | Time of self-microemulsification (min) |
|---|---|---|
| I | Rapid forming microemulsion which is clear or slightly bluish in appearance | <1 |
| II | Rapid forming, slightly less clear emulsion which has a bluish white appearance | <2 |
| III | Bright white emulsion (similar to milk in appearance) | <3 |
| IV | Dull, grayish white emulsion with a slightly oily appearance that is slow to emulsify | >3 |
| V | Poor or minimal emulsification with large oil droplets present on the surface | >3 |
The microemulsion regions in the diagrams were plotted, and the wider region indicated the better self-microemulsification efficiency.
Preparation of Exemestane SMEDDS
Once the microemulsion region was identified, SMEDDS formulation at desired component ratios was prepared with exemestane. Twenty-five milligrams of exemestane was added in the oily phase in small increment with continuous stirring. The surfactant system was prepared by mixing separately the chosen surfactant and co-surfactant in their determined ratios. Exemestane containing oil solution was added in the surfactant system solution with continuous stirring and vortex mixing. The stirring is continued until the homogenous mixture is formed. Finally, the mixture was kept at 25°C, then 1.0 g of the SMEDDS pre-concentrate mixture (containing 25 mg exemestane) were filled into hard gelatin capsules “size 00EL” for dissolution testing and stability studies (Table II).
Table II.
Result of Solubility Studies of Exemestane in Various Vehicles
| Sample number | Vehicle | Function in SMEDDS | Solubility (mg mL−1) |
|---|---|---|---|
| 1 | Corn oil | Oil | 9.6 ± 0.3 |
| 2 | Soyabean oil | Oil | 11.4 ± 0.4 |
| 3 | Castor oil | Oil | 30.4 ± 0.7 |
| 4 | Cotton seed oil | Oil | 11.7 ± 1.2 |
| 5 | Olive oil | Oil | 10.0 ± 1.4 |
| 6 | Oleic acid | Oil | 23.9 ± 1.5 |
| 7 | Iso propyl myristate (IPM) | Oil | 10.3 ± 0.8 |
| 8 | Labrafil M 2125 | Oil | 22.7 ± 1.1 |
| 9 | Labrafil M 1944 | Oil | 19.7 ± 0.2 |
| 11 | Capryol 90 | Oil | 88.7 ± 0.4 |
| 12 | Lauroglycol FCC | Co-surfactant | 31.2 ± 1.9 |
| 13 | Plureol oleique | Co-surfactant | 16.0 ± 1.8 |
| 14 | Transcutol P | Surfactant | 37.1 ± 3.7 |
| 15 | Cremophore ELP | Surfactant | 12.3 ± 2.6 |
| 16 | Labrasol | Surfactant | 37.2 ± 3.1 |
Data are mean ± SD, n = 3
SMEDDS self-microemulsifying drug delivery system
The Effect of Drug on the Phase Diagram
The objective of this experiment was to investigate the effects of exemestane on the self-emulsifying performance of the SMEDDS. Exemestane (25 mg) was added into the boundary formulations of the self-emulsifying formulation of the ternary phase diagram. The self-emulsifying performance was visually assessed after 100 times dilution with deionized water.
Effect of Dilution and pH of the Aqueous Phase on Ternary Phase Diagram of the Selected System
Dilution and pH of the vehicle have considerable effect on the phase separation of the spontaneously emulsifying systems (17,22–24). In view of this, selected exemestane self-microemulsifying drug delivery system (EXM SMEDDS) were diluted (20 times and 100 times) with various diluents (i.e., deionized water, 0.1 N HCl and phosphate buffer). The diluted microemulsions were stored for 8 h at room temperature and observed for any signs of phase separation or drug precipitation.
Droplet Size Determination
SMEDDS formulations (1 mL) were diluted with 20 mL deionized water in a beaker with constant stirring on a magnetic stirrer. The droplet size distribution and zeta potential of the resultant microemulsions were determined by dynamic light scattering with particle size apparatus (Malven Zetasizer 3000HS). After equilibrium, the droplet size was recorded.
Zeta potential
The emulsion stability is directly related to the magnitude of the surface charge (25–27). The zeta potential of the diluted SMEDDS formulation was measured using a (Malven Zetasizer 3000HS). The SMEDDS were diluted with a ratio of 1:20 v/v with distilled water and mixed for 1 min using a magnetic stirrer.
Characterization and Evaluation of the EXM SMEDDS Formulation
Percentage Transmittance (λmax, 560 nm)
A total of 1 mL of SMEDDS formulation was diluted 100 times with deionized water. Percentage transmittance were measured spectrophotometrically (Perkin Elmer Lamda 35 UV Spectrophotometer) at 560 nm using deionized water as a blank.
Thermodynamic Stability Studies
The objective of thermodynamic stability is to evaluate the phase separation and effect of temperature variation on SMEDDS formulation. EXMME3 was diluted with deionized water (1:20) and centrifuged (Eppendorf Centrifuge 5810) at 15,000 rpm for 15 min, and formulation was observed visually for phase separation. Formulations that did not show any sign of phase separation after centrifugation were subjected to freeze thaw cycle. In a freeze thaw study, exemestane SMEDDS was diluted with deionized water (1:20) and two freeze thaw cycle between (−20°C and +25°C) with storage at each temperature for not less than 4 h were done for formulations.
Transmission Electron Microscopy
Transmission electron microscope (TEM) (Philips CM12 Electron Microscope, Eindhoven, The Netherlands) was used as a visualizing aid for the observation of morphology of droplets. Exemestane SMEDDS (EXMME3) was diluted with water (1/100). A drop of the diluted microemulsion was directly deposited on the holey film grid to observe the morphology of formulations.
In Vitro Dissolution
To understand the characteristics of drug release from SMEDDS, an in vitro release was carried out. When SMEDDS encountered aqueous media, the drug existed in the system in different forms including a free molecular form or mixed in the micelles or in the microemulsion droplets. Dissolution studies were performed for the SMEDDS form and conventional tablet form. The dissolution test was performed in USP type II dissolution apparatus II (Distek) according to United State Pharmacopoeia (USP 30) dissolution procedure. Exemestane SMEDDS (EXMME3) hard gelatin capsule/tablet was put into a sinker. This sinker was loaded with 900 mL of water, 0.5% w/v SDS in water, simulated gastric fluid without enzymes (pH 1.2) and phosphate buffer pH (6.8) at 37 ± 0.5°C with paddle speed of 50 rpm. Each sample (2 mL) was withdrawn at 5, 15, 30, and 45 min with replacement by an equal volume of temperature-equilibrated media. Concentration of exemestane was determined by HPLC method.
In Vivo Study
LC-MS analysis of exemestane in rat plasma
The concentration of exemestane in wistar rat plasma was determined by validated liquid chromatography–mass spectrometry (LC-MS) method developed in house (20).
Animal and Dosing Procedure
Eight healthy female Wistar rats (supplied by the Animal Facility, Dabur Research Foundation, Sahibabad, Ghaziabad, India) weighing 200 ± 10 g were used in the study. All rats were dosed following an overnight fast; food was returned after 4 h after dosing. Rats were divided in two groups at random. First group was administered exemestane suspension (0.25% w/v CMC Na) because exemestane was virtually insoluble in water, and the second group was administered exemestane SMEDDS formulation. The amount of exemestane in each one of these formulations was adjusted to contain 30 mg kg−1 body weights. Blood samples (approximately 0.5 mL) was collected from retro-orbital plexus of rat in tube containing saturated solution of di-sodium EDTA at pre-dose and 0.25, 0.5, 0.75, 1.0, 2.0, 4.0, 6.0, 8.0, 24.0, 48.0, and 72.0 h post-dose. During collection, blood sample has been mixed thoroughly with di-sodium EDTA solution in order to prevent blood clotting. Plasma was separated by centrifugation of the blood at 5,000 rpm in cooling centrifuge for 5 min and stored frozen at −20°C until analysis. The experimental procedures were approved by the intuitional animal ethical committee and were in compliance with the National Institutes of health Guide for Care and Use of Laboratory Animals.
Data Analysis
The pharmacokinetic parameters were performed by a non-compartmental analysis using WinNonlin 3.3® pharmacokinetic software (Pharsight Mountain View, CA USA). All values are expressed as the mean ± SD. Statistical analysis was performed with GraphPad InStat software (version 3.00, GraphPad Software, San Diego, CA, USA) using one-way analysis of variance (ANOVA) followed by Tukey–Kramer multiple comparison test. Difference with p < 0.05 was considered statistically significant.
RESULTS
Solubility Studies of Exemestane in Various Excipients
The self-emulsifying formulation consist of one or more surfactants and drug dissolved in oil. The pre-concentrate mixture should be clear, monophasic liquid at ambient temperature, and should have good solvent properties to allow presentation of the drug in solution. The objective of solubility studies is to identify the suitable oil, which has good solubilizing capacity for exemestane. The concentration of exemestane in various excipients at 37°C was determined by HPLC, and solubility results are presented in Table II.
Phase Diagram Study of Exemestane SMEDDS (EXM SMEDDS)
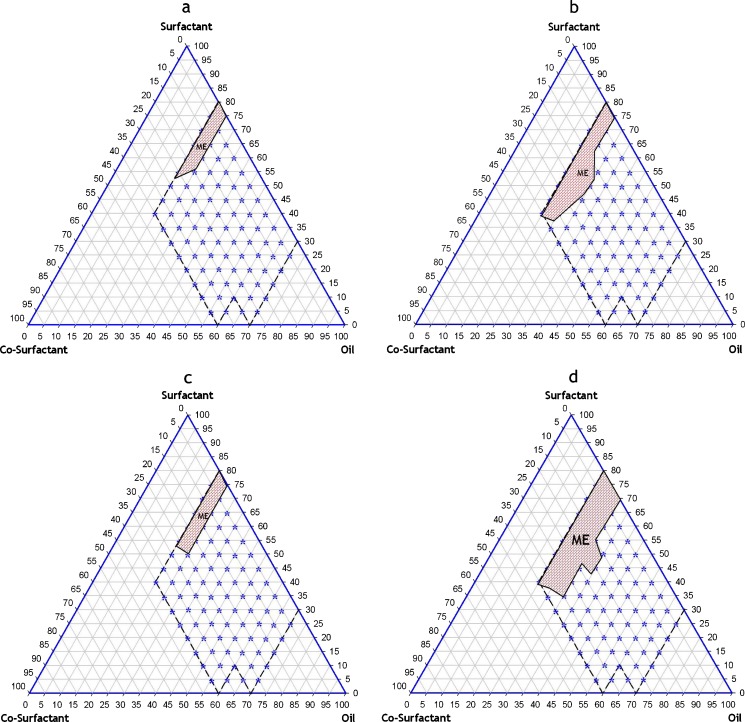
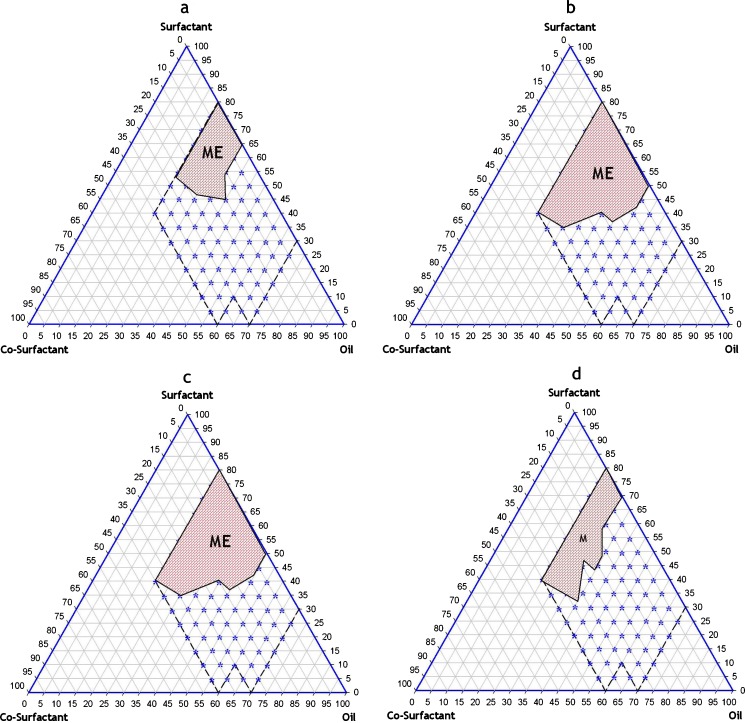
Based on the results of solubility studies, eight potential different combinations of surfactant, co-surfactant, and oil (Table III) were used for the phase diagram study for EXM SMEDDS. Corresponding ternary phase diagram of each combination are presented in Fig. 1a–d and Fig. 2a–d. The outer parallelogram indicates the area that was explored for location area of microemulsification region. No distinct conversion from water-in-oil (w/o) to (o/w) microemulsion was observed. The translucent and low viscosity microemulsion area is presented in the phase diagram (marked as ME; shaded area in the phase diagram). Quantitative unit compositions of the selected EXM SMEDDS are presented in Table IV.
Table III.
Oil, Surfactant and Co-surfactant Combinations used in EXM SMEDDS
| Combination | Oil | Surfactant | Co-surfactant |
|---|---|---|---|
| I | Castor oil | Cremophore ELP | Lauroglycol FCC |
| II | Castor oil | Cremophore ELP | Transcutol P |
| III | Castor oil | Labrasol | Lauroglycol FCC |
| IV | Castor oil | Labrasol | Transcutol P |
| V | Capryol 90 | Cremophore ELP | Lauroglycol FCC |
| VI | Capryol 90 | Cremophore ELP | Transcutol P |
| VII | Capryol 90 | Labrasol | Lauroglycol FCC |
| VIII | Capryol 90 | Labrasol | Transcutol P |
EXM SMEDDS exemestane self-microemulsifying drug delivery system
Fig. 1.
Ternary phase diagram of pre-concentrate mixture of exemestane (a) Castor oil + Cremophore ELP + Lauroglycol FCC (EXMME1) (b), Castor oil + Cremophore ELP + Transcutol P (EXMME2) (c), Castor oil + Labrasol + Lauroglycol FCC (EXMME3) (d), Castor oil + Labrasol + Transcutol P (EXMME4)
Fig. 2.
a Capryol 90 + Cremophore ELP + Lauroglycol FCC (EXMME5) (b), Capryol 90 + Cremophore ELP + Transcutol P (EXMME6) (c), Capryol 90 + Labrasol + Lauroglycol FCC (EXMME7) (d), Capryol 90 + Labrasol + Transcutol P (EXMME8)
Table IV.
Composition and Assessment of Exemestane SMEDD Formulation
| SMEDDS formulation | EXMME1 | EXMME2 | EXMME3 | EXMME4 |
|---|---|---|---|---|
| Exemestane (mg) | 25 | 25 | 25 | 25 |
| Capryol 90 (mg) | – | 400 | 500 | 350 |
| Castor oil (mg) | 350 | – | – | – |
| Transcutol P (mg) | 215 | – | 70 | 325 |
| Cremophore ELP (mg) | – | 450 | 430 | – |
| Labrasol (mg) | 435 | – | – | 325 |
| Lauroglycol FCC (mg) | – | 150 | – | – |
| Assay (By HPLC)* | 100.1% ± 2.1% | 99.4 ± 1.4% | 102.3 ± 0.9% | 100.3 ± 1.6% |
| Deionized water (1:20) | ||||
| Grade | II | II | II | II |
| Mean droplet size (nm) ± SD# | 65.6 ± 9.7 | 22.9 ± 6.9 | 28.5 ± 9.2 | 57.9 ± 14.2 |
| Mean zeta potential (mV) ± SD# | –7.2 ± 6.5 | –10.9 ± 4.3 | –9.7 ± 3.2 | –5.6 ± 3.8 |
*Data are mean ± SD (assay)
#Data are mean ± SD (droplet size and zeta potential)
SMEDDS self-microemulsifying drug delivery system
The Effect of Drug on the Phase Diagram
In these experiments, no significant difference was observed in self-emulsifying performance, when compared with the corresponding placebo formulation.
Droplet Size
The mean droplet sizes of the diluted SMEDDS pre-concentrates were very low, and all were found to be in the nanometric range (<100 nm). The mean droplet sizes of the formulations were 65.6, 22.9, 28.5 and 57.9 nm for EXMME1, EXMME2, EXMME3, and EXMME4, respectively. EXMME2 was found to have the smallest mean droplet size of 22.9 nm. Droplets size results of SMEDDS formulation were reported in Table IV.
Zeta Potential
The zeta potential results of the diluted SMEDDS formulations are shown in Table IV. Miner differences in the zeta potential were observed among all the tested exemestane SMEDDS formulations.
Dilution Studies
The influence of dilution (i.e., 1:20 and 1:100) with various diluents (i.e., deionized water, 0.1 N HCl and phosphate buffer, pH 6.8) was evaluated; larger dilutions may better mimic conditions in the stomach following oral administration of SMEDDS (pre-concentrate). Dilution with all the diluents no change in the visual clarity was observed even after 8 h at room temperature for EXMME2 and EXMME3 formulations. While EXMME4 formulation turned hazy after 8 h of dilution. Observation of dilution studies is shown in Table V.
Table V.
Visual Assessment of Exemestane SMEDDS Formulation
| After dilution with water | ||||
| Dilution | 1:20 | 1:100 | ||
| Time (h) | 0.5 | 8 | 0.5 | 8 |
| EXMME1 | II | II | II | II |
| EXMME2 | II | II | II | II |
| EXMME3 | I | II | I | II |
| EXMME4 | II | IV | II | IV |
| After dilution with 0.1 N HCl | ||||
| Time (h) | 0.5 | 8 | 0.5 | 8 |
| EXMME1 | II | II | II | II |
| EXMME2 | II | II | II | II |
| EXMME3 | I | II | II | II |
| EXMME4 | II | III | II | III |
| After dilution with pH 6.8 phosphate buffer | ||||
| Time (h) | 0.5 | 8 | 0.5 | 8 |
| EXMME1 | II | III | II | III |
| EXMME2 | II | II | II | II |
| EXMME3 | II | II | II | II |
| EXMME4 | II | IV | II | IV |
SMEDDS self-microemulsifying drug delivery system
Selection of Optimized Formulation
Formulation EXMME2 and EXMME3 were found to have the smallest mean droplet size (22.9 and 28.5 nm, respectively). Small differences have been observed in the absolute zeta potential of all the tested EXM SMEDDS formulations. No significant changes observed after dilution with various diluents. Highest oil solubilization capacity is the desirable attributes for any SMEDDS formulation. EXMME3 offer the highest oil solubilization capacity than EXMME2 formulations. This becomes the basis for selection EXMME3 formulation for further characterization.
Characterization
Percentage Transmittance (λmax560 nm)
Percentage transmittance of SMEDDS after dilution for 100 times with deionized water was 99.6%. Transmittance value of SMEDDS formulation was proximity to 100%. It indicates that clear microemulsion was formed when SMEDDS was diluted 100 times with deionized water.
Thermodynamic Stability Studies
Thermodynamic stability study was designed to identify and avoid the metastable systems. In thermodynamic stability studies, formulation selected was subjected to stress tests like centrifugation and freeze–thaw test. The SMEDDS formulation is found to be stable under these stressed conditions. Since exemestane SMEDDS formulation was found to be stable in these conditions, metastable formation is thus avoided and frequent test need not be performed during storage.
Transmittance Electron Microscope
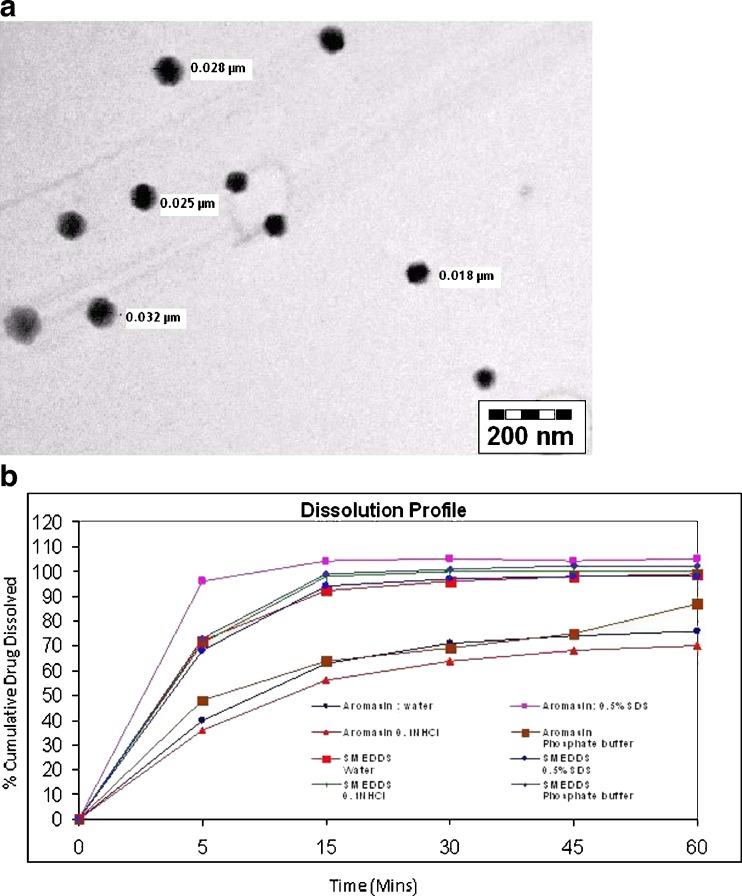
The morphology of microemulsion was examined with a transmission electron microscope. The droplet on the microemulsion appears dark with the bright surroundings. TEM photographs Fig. 3a further conformed that the globules are spherical in shape.
Fig. 3.
a TEM photograph of optimized SMEDDS formulation EXMME3. b Comparative dissolution profile of EXMME3and Aromasin®
In Vitro Dissolution
Dissolution from Aromasin® tablets was improved with increasing amount of surfactant in the media. Exemestane from SMEDDS was completely and rapidly dissolved regardless of the fluid condition. The percentage of exemestane released from the EXMME3 was significantly higher (p < 0.05) than that from the conventional marketed formulation of exemestane (Aromasin® tablets 25 mg). Dissolution profile of Aromasin® and exemestane SMEDDS formulation were evaluated in water, 0.5% w/v SDS in water, simulated gastric fluid without enzymes (pH 1.2), and pH 6.8 phosphate buffer. Comparative dissolution profiles of EXMME3 and Aromasin® in different media are shown in Fig. 3b. Dissolution profile of EXMME3 in various dissolution media showed that 100% (approximate) of exemestane was released within 15 min irrespective of media composition.
In Vivo Pharmacokinetic Studies of Exemestane SMEDDS Formulation
Pharmacokinetic parameters in plasma were obtained from the pooled concentration–time data with statistical moment algorithm using WinNonlin 3.3® program package. The AUC0→72 from time 0 to 72 (T72) was calculated using the linear trapezoidal method, and 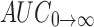 was calculated by dividing the concentration of 72 h data point (C72) by the eliminating rate constant (k) as follows:
was calculated by dividing the concentration of 72 h data point (C72) by the eliminating rate constant (k) as follows:
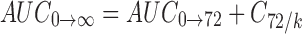 |
The area under the first moment curve (AUMC) was calculated as follows:
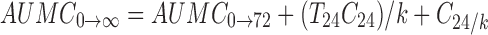 |
The relative bioavailability (Fr) was calculated as follows:
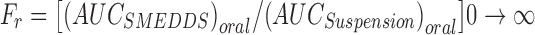 |
Thus, the relative bioavailability (Fr) at 72 h was calculated as follows:
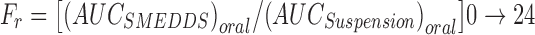 |
The mean residence time (MRT) was determined by dividing 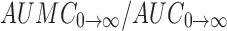
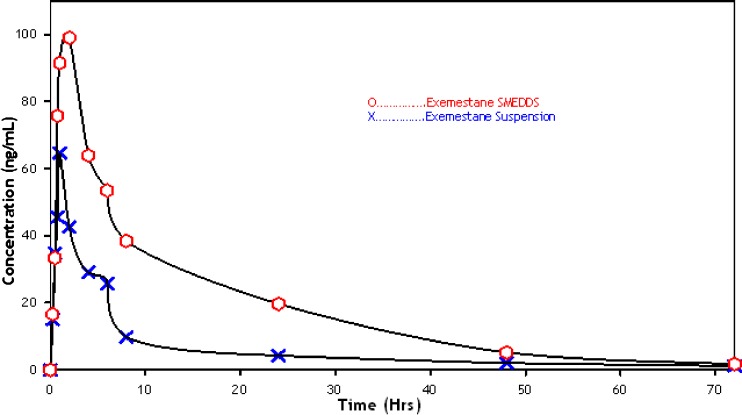
Figure 4 shows the plasma concentration–time curve in female Wistar rats after a single oral dose of EXMME3 as compared to exemestane suspension. At all the indicated time points, the exemestane plasma concentrations in rats treated with SMEDDS were significantly higher than those treated with suspension formulation.
Fig. 4.
Plasma concentration profile of exemestane (SMEDDS vs suspension) formulation after oral administration in female Wistar rats (n = 8; dose 30 mg kg−1)
Plasma pharmacokinetic parameters of exemestane after oral administration of the two formulations to female Wistar rats are shown in Table VI.
Table VI.
Pharmacokinetic Parameters of Exemestane SMEDDS and Suspension Formulation After Single Dose Administration
| Pharmacokinetic Parameters | Exemestane suspension | EXMME3 | Ratio (SMEDDS/suspension) |
|---|---|---|---|
| AUC (0–t) (ng h/mL) | 471.95 ± 48.42 | 1,356.12 ± 191.88* | 2.87 |
| AUC (0–∞) (ng h/mL) | 473.00 ± 47.97 | 1,357.04 ± 191.79* | 2.87 |
| MRT (h) | 17.54 | 17.53* | – |
| C max (ng/mL) | 64.67 ± 8.01 | 99.03 ± 13.03* | 1.53 |
| T max (h) | 1 | 2 | NA |
| Relative bioavailability (%) | – | – | 287.32 |
SMEDDS self-microemulsifying drug delivery system
*p < 0.05 when compared with suspension formulation using one-way ANOVA followed by Turkey Krammer multiple comparison test
As can be seen from the above table, the test preparation shows the increased AUC and Cmax values, which are about 2.9 and 1.5 times, respectively, as high as those in the reference formulation. Accordingly, it can be identified that the exemestane of the SMEDDS formulation is significantly increased in comparison with that of the reference formulation (exemestane suspension).
Cmax of the SMEDDS formulation 99.03 ng mL−1 was significant (p < 0.05) as compared to the suspension formulation 64.67 ng mL−1. Tmax of both solution SMEDDS and suspension was 1 and 2 h, respectively. AUC is an important parameter in evaluating bioavailability of drug from dosage form, as it represents the total integrated area under the blood concentration time profile and represents the total amount of drug reaching the systemic circulation after oral administration. 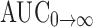 for SMEDDS formulation was higher (1,357.04 ng h mL−1) than the suspension formulation 473.00 ng h mL−1. Statistically,
for SMEDDS formulation was higher (1,357.04 ng h mL−1) than the suspension formulation 473.00 ng h mL−1. Statistically, 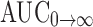 of the SMEDDS formulation was significantly higher (p < 0.05) as compared to suspension formulation. Higher amount of drug concentration in blood indicated better systemic absorption of exemestane from SMEDDS formulation as compared to the suspension formulation. In the current study, EXMSMEDDS formulation demonstrates almost similar MRT (17.53 h) as compared to the suspension formulation MRT (17.54 h). Statistically, MRT of SMEDDS formulation was not significantly different (p > 0.05) from the suspension formulation; therefore, no prolonged action was found in the SMEDDS formulations.
of the SMEDDS formulation was significantly higher (p < 0.05) as compared to suspension formulation. Higher amount of drug concentration in blood indicated better systemic absorption of exemestane from SMEDDS formulation as compared to the suspension formulation. In the current study, EXMSMEDDS formulation demonstrates almost similar MRT (17.53 h) as compared to the suspension formulation MRT (17.54 h). Statistically, MRT of SMEDDS formulation was not significantly different (p > 0.05) from the suspension formulation; therefore, no prolonged action was found in the SMEDDS formulations.
DISCUSSION
Poor water solubility and consequent restricted absorption is a major limitation with many drugs despite their good therapeutic efficacy. SMEDDS provides an opportunity for the improvement in the in vitro and in vivo performance of poorly water soluble drugs and thus serve as an ideal carrier for the delivery of drugs belonging to BCS classes II and IV. The current study was performed to define the role of self-emulsifying formulations to enhance the bioavailability of anti-cancer drug exemestane.
SMEDDS represent a possible alternative to the more traditional oral formulations for lipophilic compounds. It has isotropic mixture of oil, surfactants, and co-surfactants. This mixture should be clear, monophasic liquid at ambient room temperature. SMEDDS self-emulsifies rapidly in the aqueous contents of the stomach under gentle digestive motility in the gastrointestinal tract to present the drug in solution in small droplets of oil (<100 nm) (4). It is considered that the excipients in SMEDDS increase the dissolution and permeability of drug by significantly decreasing droplet size and restraining the secretion of drug efflux transporter P-gp (21).
Absolute bioavailability of exemestane could not be determined in human due to the absence of suitable intravenous formulation. The use of SMEDDS for the delivery of exemestane could improve its solubility and permeability through the mucous membranes significantly. In the present work, we have prepared the exemestane SMEDDS formulations and assessed the droplet size, zeta potential, robustness to dilution, dissolution in vitro and in vivo oral bioavailability (conducted in Wistar rats). Suspensions of drugs (exemestane) in traditional form were used as a control in the present study.
Excipients chosen to formulate oral SMEDDS formulations in our study were of definite regulatory status. The choice of excipients to prepare SMEDDS depends on its drug dissolving capacity. Medium chain triglycerides (MCT) are commonly used in the SMEDDS formulation (15,28,29). MCT are medium-chain (6–12 carbons) fatty acid esters of glycerol. MCT passively diffuse from the GI tract to the portal system (longer fatty acids are absorbed into the lymphatic system) without requirement for modification like long chain fatty acids or very long chain fatty acids do. In addition, MCT do not require bile salts for digestion (30). MCT possess higher ester content per gram than long chain triglycerides (LCT) so drugs have higher solubility in MCT than LCT (31,32).
Exemestane possess the highest solubility in Capryol 90 (88.7 mg/mL)followed by castor oil (30.4 mg/mL); hence, we selected Capryol 90 and castor oil as oil phase for EXT SMEDDS formulation. The selection of surfactant and co-surfactant in this study was governed by their emulsification efficiency rather than their ability to solubilize drug. The efficiency of self-microemulsification is much more related to the hydrophilic–lipophilic balance (HLB) value of the surfactant. Surfactants with HLB value >10 are greatly superior at providing fine, uniform microemulsion droplets. Safety is the main determining factor in choosing a surfactant. Non-ionic surfactants are less toxic and less affected by pH and ionic strength than ionic surfactants. Surfactants increase the permeability by interfering with the lipid bilayer of the epithelial cell membrane (4,33). Two high-value surfactants Cremophore ELP (12.3 mg/mL) and Labrasol (37.2 mg/mL) were chosen as surfactant for EXM SMEDDS in this study.
Co-surfactant increases the interfacial fluidity by penetrating the surfactant film and consequently creating a disordered film due to the void space among surfactant molecules. Self-microemulsification efficiency of SMEDDS formulation changes with the chain length of co-surfactant. A lipophilic, nonvolatile co-surfactant is less likely to migrate to the capsule shell than solvent such as ethanol and also more likely to be retained by the oil phase upon dilution with aqueous media, thus avoiding precipitation (13,15).
Transcutol P (37.1 mg/mL) and Lauroglycol FCC (31.2 mg/mL) solubilize exemestane in good amount. Hence, these two co-surfactants were chosen for EXM SMEDDS formulation. Results of solubility of exemestane in various excipients are presented in Table II.
Introduction of SMEDDS pre-concentrate into the aqueous media under gentle agitation results in the formation of fine oil-in-water microemulsion. Since the free energy required to form an emulsion is very low, the formation is thermodynamically spontaneous (22,34,35). Surfactants form a layer around the emulsion droplets and reduce the interfacial energy as well as provide a mechanical barrier to coalescence (4,17,18,31). These combination were presented in Table III (exemestane SMEDDS). The visual test was conducted to observe the apparent spontaneity of microemulsion formation (Table I).
Exemestane ternary phase diagrams (Figs. 1a–d and Figs. 2a–d) demonstrate that a large microemulsion region was formed with combination 6 (EXMME3), which comprised of Capryol 90, Cremophore ELP, and Transcutol P. Phase diagrams showed that a bigger microemulsion area was achieved when using MCT (Capryol 90) instead of LCT (Castor oil). This is due to the differences in polarity between the lipids, as more hydrophobic LCT is more difficult to emulsify. In general, when using LCT, a higher concentration of Cremophore ELP was required to form microemulsions compared with MCT. Various other investigators have also documented that the use of high concentration of surfactants is necessary in these combinations to achieve fast and efficient self-emulsification (29,31,36).
In this study of SMEDDS pre-concentrate containing Capryol 90 as oil, Cremophore ELP as surfactant, and Transcutol P as a co-surfactant, the mean droplet size was smaller than the other combinations (Table IV). The droplet size of all the studied exemestane SMEDDS formulations was noted to be less than 100 nm. Droplet size of the EXMME1 was significantly greater than the EXME2 and EXMME3 formulations. This is because chain length of the oil plays a role in the ease of emulsification, stabilization of the emulsions, as well as the emulsion droplet size. EXMME2 and EXMME3 formulations have a relatively shorter triglycerides chain, which is the reason behind the smaller mean droplet size observed in EXMME2 (mean, 22.9 ± 6.9 nm) and EXMME3 (mean, 28.5 ± 9.2 nm) formulations (27,29). EXMME4 formulation showed higher mean droplet size (mean, 57.9 ± 14.2 nm) than EXMME2 and EXMME3 because it comprised of labrasol (32.5% w/w) and Capryol 90 (35% w/w), with labrasol showing instability at high concentration and phase separation if kept for few hours at room temperature (21).
Dissolution profile of SMEDDS formulation (EXMME3) was significantly higher than the marketed formulations [Exemestane (Aromasin®)]. Regardless of the media compositions, exemestane exhibited complete and rapid release in the dissolution medium from SMEDDS formulations. Thus, an improvement in exemestane and absorption and consequently its bioavailability due to enhanced dissolution could be anticipated (27).
In vivo testing of the prepared SMEDDS formulation was done in healthy female Wistar rats. The mean exemestane plasma concentration–time profile following oral administration of EXMME3 and plain drug suspension are shown in Fig. 4, and comparative results of pharmacokinetic parameters are presented in Table VI. The mean AUC (o to t) and AUC (o to infinity) of exemestane SMEDDS were 2.87 times higher than the plain drug suspension of exemestane; thus, bioavailability-enhancing capacity of our SMEDDS formulation of exemestane could be successfully proven.
CONCLUSION
Bioavailability is a vital issue in the development of clinically useful drug formulations to decrease the dose and consequently the toxic effects. SMEDDS formulations are important pharmaceutical tools used for the improvement in the in vitro and in vivo bioavailability of poorly water-soluble drugs. SMEDDS serve as an ideal carrier for the delivery of drugs belonging to BCS classes II and IV. The current study aimed to physicochemically characterize the self-emulsifying formulation of exemestane.
The optimum formulation of EXM SMEDDS consisted of Capryol 90, Cremophore ELP, and Transcutol P, which had sufficient drug loading, rapid self-emulsification in aqueous media, and could produce small mean droplet size in the range of microemulsion. The average droplet size of the optimal formulation is within 100 nm and shows Gaussian distribution. Release of exemestane from SMEDDS capsules was significantly higher than the conventional marketed formulation of exemestane (Aromasin®) in various dissolution media, i.e., water, 0.5% w/v SDS in water, simulated gastric fluid without enzymes (pH 1.2), and pH 6.8 phosphate buffer. After oral administration of exemestane (30 mg kg−1) to eight female Wistar rats, this formulation showed superior absorption profile than the suspension of drug solution. The relative bioavailability of EXM SMEDDS was enhanced by 287.32%.
It can be concluded that exemestane SMEDDS formulations offer more predictable and more extensive drug release/absorption than the corresponding conventional formulations. The present exploratory work successfully illustrates the potential utility of SMEDDS for the delivery of poor water-soluble compounds.
Contributor Information
Ajeet K. Singh, Phone: +91-989-9200511, FAX: +91-120-4378400, Email: ajeetrj@rediffmail.com
Akash Chaurasiya, Email: akashchaurasiya@rediffmail.com.
Anshumali Awasthi, Email: awasthi.anshumali@dabur.com.
Gautam Mishra, Email: mishra.gautam@dabur.com.
Dinesh Asati, Email: dineshasati@rediffmail.com.
Roop K. Khar, Email: roopkhar@hotmail.com
Rama Mukherjee, Email: rama.mukherjee@gmail.com.
References
- 1.Robinson JR. Introduction: semi-solid formulations for oral drug delivery. Buletin Technique Gatefosse. 1996;89:3–11. [Google Scholar]
- 2.Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44:235–49. doi: 10.1016/S1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 3.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25. doi: 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- 4.Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58:173–82. doi: 10.1016/j.biopha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Lonning PE. Pharmacological profiles of exemestane and formestane, steroidal aromatase inhibitors used for the treatment of post-menopausal breast cancer. Breast Can Res Treat. 1998;49:S45–52. doi: 10.1023/A:1006048722559. [DOI] [PubMed] [Google Scholar]
- 6.Weippl KS, Goss PE. Prevention of breast cancer using SERMs and aromatase inhibitors. J Mammary Gland Biol Neoplasia. 2003;8:5–18. doi: 10.1023/A:1025727103811. [DOI] [PubMed] [Google Scholar]
- 7.Dowsett M. Theoretical considerations for the ideal aromatase inhibitors. Breast Can Res Treat. 1998;49:S39–44. doi: 10.1023/A:1006088405721. [DOI] [PubMed] [Google Scholar]
- 8.Physician Desk Reference, 60th edn. Thomson Healthcare, Montvale, NJ, 2006: pp. 2600–2602.
- 9.Lombardi P. Exemestane: a new steroidal aromatase inhibitor of clinical relevance. Biochim Biophys Acta. 2002;1587:326–37. doi: 10.1016/s0925-4439(02)00096-0. [DOI] [PubMed] [Google Scholar]
- 10.Lonning PE. Exemestane: a review of its clinical efficacy and safety. Breast. 2001;10:198–208. doi: 10.1054/brst.2001.0293. [DOI] [PubMed] [Google Scholar]
- 11.Shetty YC, Chhakkarwar PN, Acharya SS, Rajadhayaksha VD. Exemestane: a milestone against breast cancer. J Postgrad Med. 2007;53:135–8. doi: 10.4103/0022-3859.32218. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh PK, Murthy RSR. Microemulsions: a potential drug delivery system. Curr Drug Del. 2006;3:167–80. doi: 10.2174/156720106776359168. [DOI] [PubMed] [Google Scholar]
- 13.Pouton CW. Lipid formulation for oral administration of drugs: non emulsifying, self- emulsifying and self- microemulsifying drug delivery systems. Eur J Pharm Sci. 2000;11:S93–8. doi: 10.1016/S0928-0987(00)00167-6. [DOI] [PubMed] [Google Scholar]
- 14.Pouton CW. Formulation of self-microemulsifying delivery system. Adv Drug Del Rev. 1997;25:47–58. doi: 10.1016/S0169-409X(96)00490-5. [DOI] [Google Scholar]
- 15.Constantinides PP. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm Res. 1995;12:1561–72. doi: 10.1023/A:1016268311867. [DOI] [PubMed] [Google Scholar]
- 16.Humberstone AJ, Charman WN. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Adv Drug Deliv. 1997;25:103–28. doi: 10.1016/S0169-409X(96)00494-2. [DOI] [Google Scholar]
- 17.Pouton CW. Self-emulsifying drug delivery systems: assessment of the efficiency of emulsification. Int J Pharm. 1985;27:335–48. doi: 10.1016/0378-5173(85)90081-X. [DOI] [Google Scholar]
- 18.Pouton CW. Effects of the inclusion of a model drug on the performance self emulsifying formulations. J Pharm Pharmacol. 1985;37:1P. [Google Scholar]
- 19.Pouton CW. Formulation of self-emulsifying drug delivery systems. Adv Drug Deliv Rev. 1997;25:47–58. doi: 10.1016/S0169-409X(96)00490-5. [DOI] [Google Scholar]
- 20.Singh A. Bioavailability enhancement of lipophilic anticancer drugs via self-microemulsifying drug delivery system (SMEDDS). Thesis, Hamdard University New Delhi, India, 2008.
- 21.Shen H, Zhong M. Preparation and evaluation of self-microemulsifying drug delivery systems (SMEDDS) containing atorvastatin. J Pharm Pharmacol. 2006;58:1183–91. doi: 10.1211/jpp.58.9.0004. [DOI] [PubMed] [Google Scholar]
- 22.Kim JY, Ku YS. Enhanced absorption of indomethacin after oral or rectal administration of a self-emulsifying system containing indomethacin to rats. Int J Pharm. 2000;194:81–9. doi: 10.1016/S0378-5173(99)00367-1. [DOI] [PubMed] [Google Scholar]
- 23.Kawakami K, Yoshikawa T, Moroto Y, Kanaoka E, Takahashi K, Nishihara Y, Masuda K. Microemulsion formulation for enhanced absorption of poorly soluble drugs: I. Prescription design. J Control Release. 2000;81:65–74. doi: 10.1016/S0168-3659(02)00049-4. [DOI] [PubMed] [Google Scholar]
- 24.Nagarsenker MS, Date AA. Design and evaluation of self-nanoemulsifying drug delivery (SNEDDS) for cefpodoxime proxetil. Int J Pharm. 2007;329:166–72. doi: 10.1016/j.ijpharm.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 25.Pongcharoenkiat N, Narsimhan G, Lyons RT, Hem SL. The effect of surface charge and partition coefficient on the chemical stability of solutes in o/w emulsions. J Pharm Sci. 2002;91:559–70. doi: 10.1002/jps.10064. [DOI] [PubMed] [Google Scholar]
- 26.Chansiri G, Lyons RT, Patel MV, Hem SL. Effect of surface charge on the stability of oil/water emulsions during steam sterilization. J Pharm Sci. 1999;88:454–8. doi: 10.1021/js980293i. [DOI] [PubMed] [Google Scholar]
- 27.Belmonte AA, Atef E. Formulation and in vitro and in vivo characterization of a phenytoin self-emulsifying drug delivery system (SEDDS) Eur J Pharm Sci. 2008;35:257–63. doi: 10.1016/j.ejps.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Charman SA, Charman WN, Rogge MC, Wilson TD, Dutko FJ, Pouton CW. Self-emulsifying drug delivery systems: formulation and biopharmaceutical evaluation of an investigational lipophilic compound. Pharm Res. 1992;9:87–93. doi: 10.1023/A:1018987928936. [DOI] [PubMed] [Google Scholar]
- 29.Shah NH, Carvajal MT, Patel CI, Infeld MH, Malick AW. Self-emulsifying drug delivery systems (SEDDS) with polyglycolized glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Int J Pharm. 1994;106:15–23. doi: 10.1016/0378-5173(94)90271-2. [DOI] [Google Scholar]
- 30.Martena B, Pfeuffer M, Schrezenmeir J. Medium-chain triglycerides. Int Dairy J. 2006;16:1374–82. doi: 10.1016/j.idairyj.2006.06.015. [DOI] [Google Scholar]
- 31.Khoo SM, Humberstone AJ, Porter CJH, Edwards GA, Charman WN. Formulation design and bioavailability assessment of lipidic self-emulsifying formulations of Halofantrine. Int J Pharm. 1998;167:155–64. doi: 10.1016/S0378-5173(98)00054-4. [DOI] [Google Scholar]
- 32.Cao Y, Marra AY, Anderson BD. Predictive relationships for the effects of triglyceride ester concentration and water uptake on solubility and partitioning of small molecules into lipid vehicles. J Pharm Sci. 2004;93:2768–79. doi: 10.1002/jps.20126. [DOI] [PubMed] [Google Scholar]
- 33.MacGregor KJ, Embleton JK, Lacy JE, Perry EA, Solomon LJ, Seager H, Pouton CW. Influence of lipolysis on drug absorption from the gastro intestinal tract. Adv Drug Deliv Rev. 1997;25:33–46. doi: 10.1016/S0169-409X(96)00489-9. [DOI] [Google Scholar]
- 34.Schulman JH, Montagne JB. Formation of microemulsions by amino alkyl alcohols. Ann N Y Acad Sci. 1961;92:366–71. doi: 10.1111/j.1749-6632.1961.tb44987.x. [DOI] [PubMed] [Google Scholar]
- 35.Craig DQM, Barker SA, Banning D, Booth SW. An investigation into the mechanisms of self-emulsification using particle size analysis and low frequency dielectric spectroscopy. Int J Pharm. 1995;114:103–10. doi: 10.1016/0378-5173(94)00222-Q. [DOI] [Google Scholar]
- 36.Kommuru TR, Gurley B, Khan MA, Reddy IK. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: formulation development and bioavailability assessment. Int J Pharm. 2001;212:233–46. doi: 10.1016/S0378-5173(00)00614-1. [DOI] [PubMed] [Google Scholar]
- 37.Singh A, Chaurasiya A, Singh M, Upadhyay S, Mukherjee R, Khar RK. Exemestane loaded self-microemulsifying drug delivery system (SMEDDS): development and optimization. AAPS PharmSciTech. 2008;9:628–34. doi: 10.1208/s12249-008-9080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]