Abstract
The aim of the paper was to develop satranidazole-containing mucoadhesive gel for the treatment of periodontitis. Different mucoadhesive gels were prepared, using various gelling agents like sodium carboxymethylcellulose (SCMC), poloxamer 407, hydroxyethylcellulose, hydroxypropylcellulose, hydroxypropylmethylcellulose, and the mucoadhesive polymer carbopol 934P. The selected formulations (based on the mucoadhesive force) were studied for different mechanical properties, such as mucoadhesive strength, hardness, compressibility, adhesiveness, and cohesiveness through Texture Profile Analyzer. In vitro satranidazole release from the prepared formulations was also determined and compared with marketed preparation of metronidazole (Metrogyl® gel). The formulation SC30 (containing SCMC 3% w/v) showed maximum mucoadhesive strength (167.72 ± 3.76 g) and adhesiveness (−46.23 ± 0.34 N mm), with low hardness (9.81 ± 0.04 N) and compressibility (40.05 ± 0.48 N mm) and moderate cohesiveness (0.87 ± 0.01). SC30 formulation exhibited long-term release. Thus, SC30 gel was evaluated for its clinical effectiveness along with marketed metronidazole gel. At the end of the study (42 days of clinical studies), both formulations were found to significantly reduce the probing depth, plaque index, gingival index, calculus criteria, and bleeding index. However, the SC30 gel was more effective in reducing the above parameters than marketed metronidazole gel. This study confirmed the acceptability and effectiveness of satranidazole gel for treatment of periodontitis.
Key words: mucoadhesive gel, periodontitis, satranidazole, texture profile analysis
INTRODUCTION
Periodontitis (pyorrhea) is a plaque-induced chronic inflammatory condition leading to the loss of tooth-supporting structures, namely periodontal ligament and alveolar bone. The current microbiological treatment of periodontitis is through either the mechanical cleaning of the teeth with systemic antibiotics or a localized delivery system incorporating an antibiotic. The use of systemic antibiotics raises a number of issues, like bacterial resistance to administered antibiotic and unpleasant or toxic side effects. Large doses must be taken in order to achieve sufficient concentrations in the gingival crevicular fluid of the periodontal pockets; this brings with it the associated side effects of antibiotics and problems regarding antibiotic resistance (1).
Because of these considerations, a variety of specialized local delivery systems (i.e., intrapocket devices) were designed to maintain the antibiotic in the gingival crevicular fluid at a concentration higher than that achieved by systemic administration (2). With respect to solid devices, semisolid (gel) formulations have some advantages, such as relatively faster release of the incorporated drug (particularly with respect to fibers or microparticles); easy preparation; easier administration and a higher biocompatibility and mucoadhesivity, allowing adhesion to the mucosa in the dental pocket and rapid elimination through normal catabolic pathways, decreasing the risk of irritative or allergic host reactions at the application site.
The current practice for the treatment of gingivitis and periodontitis involve the removal of plaque by scaling and root planning, along with application of metronidazole gel directly on the gums several times. The metronidazole (MZ) gel is a very bitter formulation and, thus, reduces patient compliance (3). Satranidazole (SZ) is a 5-nitroimidazole substituted at the 2-position and has been found to be more active against aerobic, microaerophilic, and anaerobic bacteria than MZ. The MIC90 of SZ was found to be fourfold lower than MZ against 50 clinical isolates of anaerobes (4). The literature review indicates that SZ, though more effective than MZ, has not been focused on for the treatment of periodontal disease as yet. It was, therefore, proposed to prepare a mucoadhesive gel of SZ that adheres with gums for a prolonged period of time, reduces the dosing frequency, and lowers the bitterness of the periodontal gel.
The mucoadhesive gels were prepared for administration to the periodontal pocket by using different gelling agents like sodium carboxymethylcellulose (SCMC), poloxamer 407 (PL407), hydroxyethylcellulose (HEC), hydroxypropylcellulose (HPC), hydroxypropylmethylcellulose (HPMC), and the mucoadhesive polymer carbopol 934P.
MATERIALS AND METHODS
Materials
SZ was obtained as a kind gift sample from Alkem, Mumbai, India, and PL407, SCMC, Carbopol 934P, HPMC K15, HPC, and HEC were obtained from BASF India; S.D. fine-chem., India; Loba Chemie, Mumbai, India; Dow Chemicals, France; Aqualon Pharmaceutical, USA; and Hercules, Hong Kong, respectively. Metrogyl® gel (1% w/v) was obtained from Lekar Pharma, Daman, India.
Method
Preparation of SZ Mucoadhesive Gel
The method of Varshosaz et al. (5) was followed to prepare the gels of the SZ drug. Weighed CB 934P was dissolved in 50 ml of McIlvaine buffer pH 6.6. The SZ drug was also dissolved in about 25 ml of McIlvaine buffer pH 6.6. This solution of SZ was slowly added in the solution of CB 934P with stirring. Then, the gelling agent (NaCMC, HPC, HEC, and HPMC) was added slowly under continuous magnetic stirring at 100 rpm. The volume was made up to 100 ml with McIlvaine buffer pH 6.6. The prepared gel was kept for 24 h at room temperature for complete polymer dissolution. The concentration of SZ (0.25% w/v) was kept constant in all the batches.
Preparation of SZ mucoadhesive gel with PL407
PL407 possesses a reverse thermal gelling property, and therefore, the gel containing PL407 was prepared by a modification of cold method of Varshosaz et al. (5).
CB 934P was dissolved in 50 ml and SZ in about 25 ml of McIlvaine buffer pH 6.6 separately. The solution of SZ was added to the solution of CB 934P slowly with continuous stirring at 100 rpm. It was then cooled by placing it in an ice bath. Weighed amount of PL407 was then added slowly with continuous stirring (100 rpm) at 5°C temperature. The volume was made up to 100 ml with McIlvaine buffer pH 6.6. The prepared gel was kept for 24 h at room temperature for complete polymer dissolution. The final concentration of SZ in the gel was 0.25% w/v.
Different formulations of SZ mucoadhesive gels are given in Table I. The prepared SZ formulations were abbreviated as SC20 (2% SCMC), SC30 (3% SCMC), HPMC30 (3% HPMC), HPMC50 (5% HPMC), HPC50 (5% HPC), HPC150 (15% HPC), HEC20 (2% HEC), HEC30 (3% HEC), and PL300 (30% PL).
Table I.
Formulation of SZ (0.25% w/v) Mucoadhesive Gels
| Batch code | Carbopol 934P (g) | SZ (g) | SCMC (g) | HPMC (g) | HPC (g) | HEC (g) | PL407 (g) |
|---|---|---|---|---|---|---|---|
| SC20 | 1 | 0.25 | 2 | – | – | – | – |
| SC30 | 1 | 0.25 | 3 | – | – | – | – |
| HPMC30 | 1 | 0.25 | – | 3 | – | – | – |
| HPMC50 | 1 | 0.25 | – | 5 | – | – | – |
| HPC50 | 1 | 0.25 | – | – | 5 | – | – |
| HPC150 | 1 | 0.25 | – | – | 15 | – | – |
| HEC20 | 1 | 0.25 | – | – | – | 2 | – |
| HEC30 | 1 | 0.25 | – | – | – | 3 | – |
| PL300 | 1 | 0.25 | – | – | – | – | 30 |
Characterization
Mucoadhesiveness by Modified Physical Balance Method
The method developed by Choi et al. (6) and Yong et al. (7) was slightly modified to study the mucoadhesive character of the prepared gels. The modified apparatus was used in the study. This apparatus comprised of a two-arm balance, one side of which contained two glass plates and the other side contained a container. One of the two glass plates was attached permanently to the base of the stage, and the other was attached to the arm of the balance by a thick strong thread. The membrane used for mucoadhesive testing was fresh rabbit intestinal membrane (rabbit intestine was collected from anesthetized rabbits). Fresh rabbit intestine was glued to the upper side of the lower plate and another was glued to the lower side of the upper plate by using cyanoacrylate adhesive. The weighed gel (0.1 g) was placed on the rabbit intestine glued to the upper side of the lower plate. Then, the upper plate was placed over the lower plate and 50 g preload force (or contact pressure) was applied for 5 min (preload time). After removal of the preload force, the water was kept in a bottle at some height and was siphoned into the container at a rate of 10 ml per minute till the plates were detached from each other. The dropping rate of the water was controlled with an on–off switch, the same as that in an infusion bottle. The weight of the water (g) required for the detachment of the glass plates was considered as the mucoadhesion force of the applied gel.
Texture Profile Analysis
Texture profile analysis (TPA) may be applied for the mechanical characterization of pharmaceutical gels and a semisolid system (8). The mechanical properties of selected formulations (based on mucoadhesiveness) were examined using texture profile analyzer (Fig. 1). Formulations HPMC30 and HPMC50 were not considered for studies with TPA due to their low mucoadhesiveness, as determined by the modified physical balance method (Fig. 2). The selected formulations were transferred into McCartney (30-ml volume, grade 2 clear glass) bottles to a fixed height, taking care to avoid the introduction of air into the samples. TPA was performed using a Stable Micro Systems Texture Analyser (TA-XT2® Texture Analyzer, Haslemere, Surrey, UK) (Fig. 2). In TPA mode, the hemispherical analytical probe (diameter 1 cm) was twice compressed into each sample at a defined rate (2 mm s−1) to a depth of 15 mm. The trigger force was 3 g. A delay period (15 s) was allowed between the end of the first and the beginning of the second compression, and all analyses were performed at least in quadruplicate. From the resultant force–time plots, the following mechanical parameters were derived (8):
Mucoadhesive strength (stickiness: by using rabbit intestinal membrane)
Hardness (the force required to attain a given deformation)
Compressibility (the work required to deform the sample during the first compression of the probes)
Adhesiveness (the work required to overcome the attractive forces between the surface of the sample and the surface of the probe)
Cohesiveness (the ratio of the area under the force–time curve produced on the second compression cycle to that on the first compression cycle, where successive compressions are separated by a defined recovery period)
Fig. 1.
TA.XT2 plus texture profile analyzer
Fig. 2.
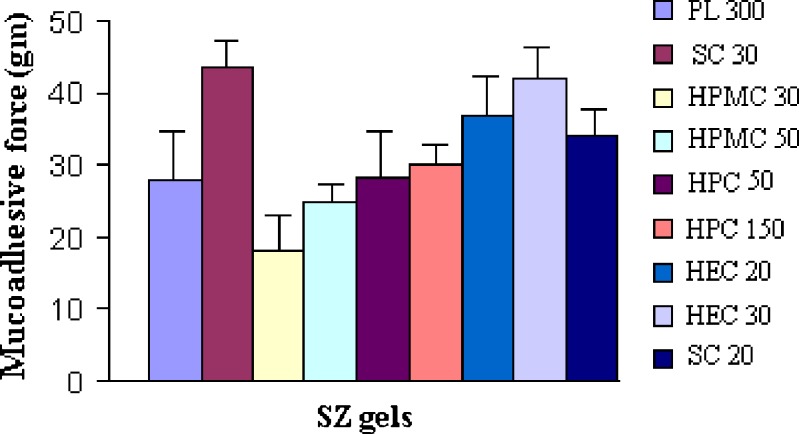
Mucoadhesiveness determined by modified physical balance method
Determination of SZ Solubility
SZ solubility (Cs) in oversaturation condition was obtained by dispersing 500 mg of the drug in 50 ml of McIlvaine buffer pH 6.6. The suspension was stirred under constant magnetic stirring (100 rpm), at 37 ± 0.5°C for 24 h (sufficient time for equilibration), filtered through a syringe filter (0.45 µm), and then assayed spectrophotometrically at 319 nm (UV-spectrophotometric method was developed in McIlvaine buffer pH 6.6). The method validation parameters were as: R2 = 0.9998, accuracy = 99.88 ± 2.03, precision = 2.06, range = 5 to 35 µg/ml, linearity = 5 to 40 µg/ml, LOD (Limit of Detection) = 0.846 µg/ml, and LOQ (Limit of Quantification) = 2.564 µg/ml). The experimental value of solubility was the average of three replicates.
In-vitro Release Study
Accurately weighed gel equivalent to 2.5 mg of the drug was taken in the center of a hollow cylindrical dialysis membrane (9, 10). This membrane was then folded and hermetically sealed from both ends. This “dialysis bag” was then hanged with the help of a wire in a 100-ml beaker. Dissolution medium was McIlvaine buffer pH 6.6. The entire system was kept at 37°C with continuous magnetic stirring at 100 rpm. Sampling of 1 ml was done at time intervals of 5, 10, 15, 30, 60, 120, 180, 240, 300, 360, and 420 min. The media volume was maintained by adding equal volumes of fresh media, and the concentration of SZ was measured spectrophotometrically. Sink conditions were maintained for release studies (C1 < Cs × 0.2) (11).
- C1
The final concentration of SZ in the medium after the complete release of the drug in the McIlvaine buffer pH 6.6
- Cs
Saturation solubility of SZ in the McIlvaine buffer pH 6.6
In-vitro Kinetic Release Model
The dissolution profile of all the batches was fitted to zero-order, first-order, and Higuchi equations to ascertain the kinetic modeling of drug release.
Clinical Evaluation
A total of ten patients of both sexes more than 21 years old who had been diagnosed as suffering from generalized or chronic periodontitis (with a pocket depth of 5 to 8 mm in at least two nonadjacent sites in different quadrants of the mouth) were considered for the clinical evaluation (single-blind study design). The ten selected patients were divided into two equal groups, A and B, and each group contained ten experimental sites. Group A and group B were treated with scaling and root planning, followed by application of SZ gel and metrogyl gel, respectively. A scaling was performed to remove soft and calcified plaque accumulation by means of an ultrasonic instrument. The patients were receiving outpatient treatment in the Faculty of Dental Sciences, Institute of Medical Science, Banaras Hindu University, Varanasi, India. The patients received complete periodontal examination, including oral hygiene standards, gingival health recordings, and probing measurements. The experimental sites were completely dried using an air syringe, and then the sites were isolated with cotton rolls to prevent contamination from saliva. The gel formulations were stored at 4°C (before application).
Thereafter, all the experimental sites were treated subgingivally, twice daily, till last follow-up day with selected SZ-containing best mucoadhesive periodontal gel, or Metrogyl® gel (1% w/v), according to a randomization list. SZ-containing gels were slowly delivered to the bottom of the periodontal pocket by using a disposable syringe equipped with a 23-gage blunt needle. The gels were injected into the pockets until the gel overflowed from the gingival margin. Patients were instructed not to brush in the area where the periodontal dressing had been placed, not to chew hard or sticky food, not to floss on the treated site, and not to probe the area with the tongue, a finger, or a toothpick. Each patient was also told to report immediately if the material was dislodged before the scheduled recall visit or if discomfort, a burning sensation, or any other allergic reaction occurred.
The clinical parameters, like probing depth (PD), plaque index (PI), gingival index (GI), calculus criteria, and bleeding index (BI), were measured and scored before treatment (baseline data) and on each 21st and 42nd follow-up day after treatment initiation (Table II).
Table II.
Scoring of the Clinical Parameters
| Scale | GI | BI | PI | Calculus Index |
|---|---|---|---|---|
| 0 | Normal gingival | No bleeding within 30 s of probing | Absence of dental plaque | Absence of calculus |
| 1 | Mild inflammation, slight color change, and edema | Bleeding within a few seconds of probing | Supragingival calculus extends only slightly less than 1/3rd of the gingival half of facial or lingual surface of the tooth | Supragingival calculus extending only slightly below the free gingival margin (not more than 1 mm) |
| 2 | Moderate inflammation, marked redness, edema, glazing, bleeding on probing | Immediate bleeding on probing | Dental plaque covering more than 1/3rd but less than 2/3rd of the gingival half of the facial or lingual surface of the tooth | Moderate amount of supragingival and subgingival calculus |
| 3 | Severe inflammation, marked redness, edema, spontaneous bleeding | Bleeding along gingival sulcus with the slightest touch | Dental plaque covering 2/3rd or more of the gingival half of the facial or gingival surface of the tooth | Abundance of supragingival and subgingival calculus |
The studies were carried out in the Faculty of Dental Sciences, Institute of Medical Science, Banaras Hindu University, Varanasi, India. The necessary clearance was taken for conducting the above studies from the Institutional Human Ethical Committee, Institute of Medical Science, Banaras Hindu University, Varanasi, India.
For statistical comparisons between treatments, the subject was considered as the statistical unit. The t test was used to evaluate mean differences in clinical recordings within and between treatment modalities. The level of significance was set at 5%.
RESULTS AND DISCUSSION
The amount of gelling agents was selected on the basis of optimum quantity required for gel preparation, which has been reported in various literatures [HEC and SCMC (8, 12), HPMC (13), PL (14) and HPC (15)]. McIlvaine buffer pH 6.6 was composed of potassium dihydrogen orthophosphate and citric acid in water and was used to simulate the gingival fluid environment (16).
The results of mucoadhesive strength measurement by modified physical balance method are shown in Fig. 2. It was found that, as the total concentration of polymers increased, the mucoadhesive strength (MS) was found to increase. MS characterization was done mainly to select the polymer proportion and total polymer concentration for the prepared gels. The mucoadhesive material must come in close contact with the tissue for mucoadhesion to occur. Carbopol resins, due to their chemical nature, are high-molecular-weight polymers that readily swell in water; the swelling exposes a large adhesive surface for maximum contact with the mucin (the glycoprotein predominant in the mucous layer) and, thus, provides excellent mucoadhesiveness. Mucoadhesion of batch SC30 (containing SCMC) was maximized followed by HEC30 (containing HEC).
TPA has been used to characterize the mechanical properties of pharmaceutical gels and semisolid systems. This simple and rapid technique could provide information related to the gel mechanical parameters, such as MS, hardness, adhesiveness, compressibility, and cohesiveness.
The results of mechanical properties of the SZ gels are given in Table III. The MS (stickiness) of selected gels was studied by TPA. The formulation SC30 (167.72 ± 3.76 g) showed maximum MS followed by HEC30 (154.76 ± 2.11 g) and HEC20 (153.96 ± 1.24 g). The formulation containing SCMC required proportionally greater vertical force to break the mucoadhesive bond than HEC-containing formulations. These results are in accordance with the finding of Smart et al. (17). Mucoadhesive properties of polymeric gels were dependent both on the initial time of contact between mucin and each formulation, and on the concentration of each polymeric component. The MS of gels prepared with HPC and PL300 polymers was quite low in comparison to gels of SCMC and HEC (Table III). The adhesive characteristic is an important parameter in the design of an oral gel, since a desirable gel contact and retention at the mucosal surface will ensure better clinical efficacy.
Table III.
Mechanical Properties of SZ Containing Mucoadhesive Gels
| Batch | MS (g) (±SD) | Hardness (N) (±SD) | Adhesiveness (N mm) (±SD) | Compressibility (N mm) (±SD) | Cohesiveness (±SD) |
|---|---|---|---|---|---|
| HEC20 | 153.96 ± 1.24 | 9.44 ± 0.04 | −37.75 ± 0.11 | 31.62 ± 0.76 | 0.92 ± 0.01 |
| HEC30 | 154.76 ± 2.11 | 23.28 ± 0.01 | −39.05 ± 0.17 | 114.65 ± 0.20 | 0.81 ± 0.02 |
| HPC50 | 65.67 ± 1.79 | 13.69 ± 0.02 | −1.05 ± 0.22 | 19.72 ± 0.23 | 0.82 ± 0.01 |
| HPC150 | 92.54 ± 2.65 | 5.34 ± 0.01 | −2.56 ± 0.26 | 67.75 ± 0.40 | 0.81 ± 0.06 |
| PL300 | 92.39 ± 2.30 | −0.44 ± 0.03 | −2.27 ± 0.12 | 17.42 ± 0.08 | 0.89 ± 0.02 |
| SC20 | 138.66 ± 2.04 | 6.81 ± 0.02 | −31.30 ± 0.03 | 34.44 ± 0.36 | 1.01 ± 0.01 |
| SC30 | 167.72 ± 3.76 | 9.81 ± 0.04 | −46.23 ± 0.34 | 40.05 ± 0.48 | 0.87 ± 0.01 |
The adhesiveness of the formulations was in the following order:
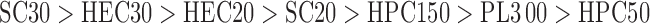 |
The HEC30 formulation showed maximum hardness (23.28 ± 0.01 N), followed by HPC50, SC30, HEC20, HPC150, and PL300. The compressibility of gels decreased in the following manner: HEC30 > HPC150 > SC30 > SC20 > HEC20 > HPC50 > PL300. As the concentration of SCMC was increased from 2% w/v to 3% w/v, the hardness increased approximately 1.5 times (6.81 ± 0.02 N to 9.81 ± 0.04 N), and compressibility also increased nearly 1.15 times (34.44 ± 0.36 N to 40.05 ± 0.48 N). In HEC gel, as the concentration of HEC was increased from 2% w/v to 3% w/v, hardness increased nearly 2.5 times from 9.44 ± 0.04 N to 23.28 ± 0.01 N, and compressibility increased nearly four times from 31.62 ± 0.76 to 114.65 ± 0.20 N mm, which was not suitable from a mechanical point of view (Table III). The above finding suggests that, with increase in concentration of HEC and SCMC in the formulation, the product hardness and compressibility increased. Each of these parameters describes the resistance of each formulation to compression and, therefore, reflects alternations in product viscosity.
Cohesiveness is a parameter related to the structural reformation following successive shearing stress during application. In the case of cohesiveness, the reverse was observed. As the concentration of SCMC and HEC was increased from 2% w/v to 3% w/v, the cohesiveness decreased from 0.92 ± 0.01 to 0.81 ± 0.02 and from 1.01 ± 0.01 to 0.87 ± 0.01, respectively. Maximum cohesiveness was in SC30, and minimum cohesiveness was in HPC50. Product cohesiveness has been reported to describe spatial aspects of structural information following product compression (18). As the polymer concentration was increased, the mass of suspended solids increased. Subsequently, the semisolid nature of the product increased, which, in turn, decreased the product cohesiveness. Decreased product cohesiveness associated with increased concentration of HEC and SCMC is a function of product viscosity, as the viscoelastic properties of these formulations are affected by this cohesiveness.
However, ideally, formulations designed for buccal drug delivery should have low hardness and compressibility, yet high MS, adhesiveness, and cohesiveness. Low gel hardness and compressibility will ensure that minimum work is required for gel removal from the container and administration onto the oral mucosal epithelium, while high gel adhesiveness and cohesiveness will ensure prolonged adhesion of the gel onto the oral mucosa and a complete structural recovery of the gel following application (8, 12). The SC30 gel formulation has comparatively better mechanical properties than other prepared gel formulations.
In vitro release profiles give important information on the efficiency of a delivery system proposed for controlled release of drugs. The choice of an appropriate in vitro model has to take into account the need to resemble the in vivo behavior as strictly as possible. In this way, the bioavailability parameters may be reliably predicted from the in vitro studies. Among the different experimental protocols proposed for the determinations of drug release profiles, the dialysis method was chosen for its simple experimental procedure and high degree of reproducibility (19). It should be pointed out that the dialysis method was criticized because of its low in vivo predictivity in the case of intravenously or orally administered delivery systems, where biological sink conditions are predominant (20). Nevertheless, in our opinion, the dialysis technique could reproduce the situation of a formulation applied into the periodontal pocket; in this case, the carrier is presumably surrounded by a stagnant layer, causing a slow diffusion of the drug (i.e., nonsink conditions).
The saturation solubility (Cs) of SZ in McIlvaine buffer pH 6.6 was 416.54 µg/ml at 37 ± 0.5°C. Sink conditions were maintained for release study: C1 < Cs × 0.2 (11). C1 is the final concentration of SZ after complete release of the drug in dissolution medium. Therefore, the final concentration of SZ after the complete release in dissolution media was maintained at less than 83.308 µg/ml, in compliance with the sink condition.
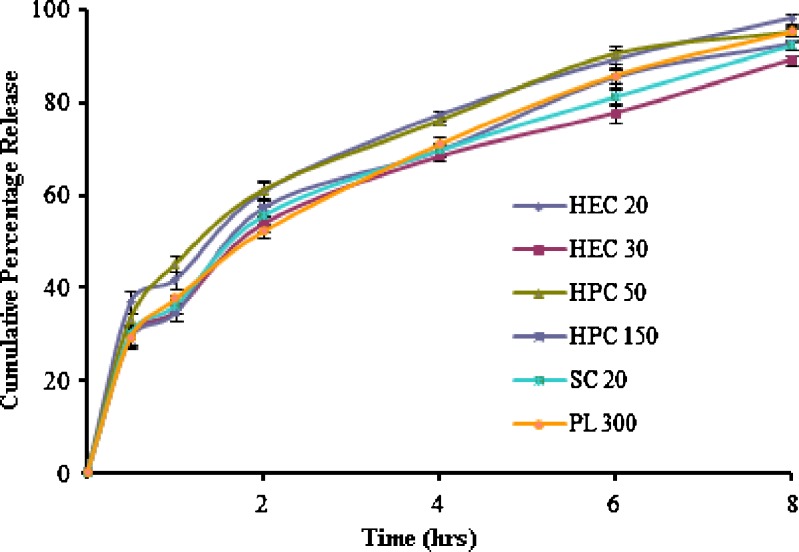
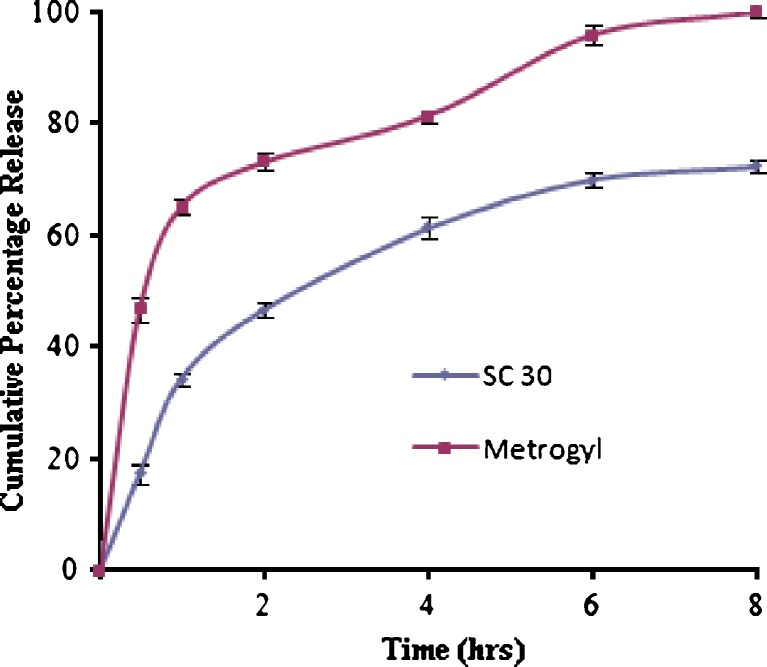
Figures 3 and 4 show the release profiles of SZ-containing gels and Metrogyl® gel, determined by using the dialysis method at 37°C. The HEC20, HEC30, HPC50, HPC150, SC20, and PL300 gels showed burst released (approximately 30–40% of the drug released within 30 min), and almost complete drug release (90–100%) was obtained within 8 h. These gels were unable to give prolonged action and maintain the therapeutic action for longer periods of time. On the other hand, the drug release from the SC30 gel was slow in comparison to the other prepared gels, as well as Metrogyl® gel. The Metrogyl® gel showed a burst release; approximately 46% release was observed within 30 min. In contrast, SC30 gel released nearly 20% within the same amount of time and also showed long-term controlled release for more than 8 h. Thus, SC30 gel appears to be more suitable for obtaining a long-term release kinetic, assuring a constant and prolonged concentration at the application site.
Fig. 3.
Release profiles of SZ containing mucoadhesive gels
Fig. 4.
Release profiles of SZ containing mucoadhesive gels
The regression coefficient (R2) was used as an indicator of the best fit for each of the models considered (Table IV). It was clear from R2 values that the best linearity was found in Higuchi's equation plot, indicating the release of drug from the gel as a square root of time-dependent process based on Fickian diffusion (21). Diffusion is related to transport of drug from the gel matrix into the surrounding in-vitro medium, and it depends on the drug concentration. As gradient varies, the drug is released and the distance for diffusion increases. This could explain why the drug diffuses at a comparatively slower rate as the distance for diffusion increases. This is referred to as the square-root kinetics or the Higuchi's kinetics model. These results are in agreement with the finding of Esposito (14).
Table IV.
Kinetic Release of SZ Mucoadhesive Gels and Metrogyl® Gel
| Gel | Zero order | First order | Higuchi |
|---|---|---|---|
| R 2 | R 2 | R 2 | |
| HEC20 | 0.8496 | 0.9599 | 0.9895 |
| HEC30 | 0.8688 | 0.9824 | 0.9868 |
| HPC50 | 0.8499 | 0.9943 | 0.9809 |
| HPC150 | 0.8731 | 0.9909 | 0.9864 |
| PL300 | 0.8943 | 0.9791 | 0.9972 |
| SC20 | 0.8680 | 0.9816 | 0.9895 |
| SC30 | 0.8761 | 0.9709 | 0.9821 |
| Metrogyl® gel | 0.8680 | 0.9249 | 0.9249 |
The ten selected patients were divided randomly into two equal groups, A and B, and each group contained ten experimental sites. Group A and group B were treated with scaling and root planning, followed by SZ gel and metrogyl gel, respectively. At the end of the 21st- and 42nd-day observation intervals, all the sites healed uneventfully. Neither complications nor allergic reactions that could be related to the experimental treatment modalities were observed. Both gel preparations appeared to be safe and easy to use in clinical handling (gels were fluid enough to allow subgingival placement using a simple syringe, requiring only a few seconds to completely fill the periodontal pocket). Table V shows the mean values for PD, PI, GI, calculus criteria (CAL), and BI scores measured at baseline (before treatment) and at 21 and 42 days following treatment.
Table V.
Statistically Comparative Analysis of Clinical Parameters
| Parameters | Baseline mean (±SD) | 21st day | 42nd day | ||||
|---|---|---|---|---|---|---|---|
| Mean (±SD) | Change (±SD) | P | Mean (±SD) | Change (±SD) | P | ||
| PD | |||||||
| Group A | 4.66 ± 0.05 (100%) | 3.5 ± 0.22 (75.10%) | 1.16 (±0.45) | <0.01 | 1.5 ± 0.10 (32.19%) | 3.16 (±0.45) | <0.01 |
| Group B | 4.5 ± 0.08 (100%) | 4.0 ± 0.19 (88.89%) | 0.5 (±0.22) | <0.01 | 2.3 ± 0.33 (51.11%) | 2.20 (±0.22) | <0.01 |
| PI | |||||||
| Group A | 2.73 ± 0.10 (100%) | 1.65 ± 0.17 (60.44%) | 1.08 (±0.13) | <0.01 | 0.75 ± 0.20 (27.47%) | 1.98 (±0.13) | <0.01 |
| Group B | 2.48 ± 0.14 (100%) | 1.96 ± 0.26 (79.03%) | 0.52 (±0.15) | <0.01 | 1.00 ± 0.21 (40.32%) | 1.48 (±0.15) | <0.01 |
| GI | |||||||
| Group A | 2.10 ± 0.03 (100%) | 1.16 ± 0.31 (55.24%) | 0.94 (±0.31) | <0.01 | 1.00 ± 0.16 (47.62%) | 1.10 (±0.31) | <0.01 |
| Group B | 2.05 ± 0.06 (100%) | 1.50 ± 0.37 (73.17%) | 0.55 (±0.24) | <0.01 | 1.25 ± 0.35 (60.98%) | 0.80 (±0.24) | <0.01 |
| Calculus | |||||||
| Group A | 1.79 ± 0.11 (100%) | 1.00 ± 0.24 (55.87%) | 0.79 (±0.23) | <0.01 | 0.75 ± 0.27 (41.90%) | 1.04 (±0.23) | <0.01 |
| Group B | 1.89 ± 0.09 (100%) | 1.20 ± 0.33 (63.49%) | 0.69 (±0.16) | <0.01 | 1.00 ± 0.14 (52.91%) | 0.89 (±0.16) | <0.01 |
| BI | |||||||
| Group A | 2.16 ± 0.21 (100%) | 1.39 ± 0.27 (64.35%) | 0.77 (±0.26) | <0.01 | 0.56 ± 0.33 (25.93%) | 1.60 (±0.26) | <0.01 |
| Group B | 2.0 ± 0.13 (100%) | 1.67 ± 0.18 (83.50%) | 0.33 (±0.29) | <0.01 | 0.88 ± 0.25 (44.00%) | 1.12 (±0.29) | <0.01 |
t Test; P < 0.05 is significant. Group A: Site treated with SC30 gel. Group B: Site treated with Metrogyl® gel
At the end of the last follow-up period (after the 42nd day), the percentage reduction in PD, PI, GI, CAL, and BI from the baseline were about 68%, 73%, 52%, 58%, and 74%, respectively, in patients treated with SC30 gel, whereas, in the Metrogyl® gel case, the percentage reduction in PD, PI, GI, CAL, and BI from the baseline were about 49%, 60%, 39%, 47%, and 56%, respectively, which shows that the formulation containing SC30 gel has a significantly better treatment mode than Metrogyl® gel (Table V).
The acceptability of the experimental material was observed in terms of the subject's taste and comfort. Good biological acceptability was seen as no evidence of burning sensation, dryness/soreness, ulcer formation, or staining of teeth being found. Thus, the gel formulation was effective in reducing gingival inflammation, reducing pocket depth, and increasing clinical attachment. It also controlled the localized infection and prevented new lesion formation. The mucoadhesive gel drug delivery system used in the present study is simple and easy to use. Also, the fact that the experimental drug delivery system is mucoadhesive allows better retention and reduction in frequency of administration. The system is biologically accepted without any side effects.
CONCLUSION
SC30 gel in a concentration of 3% w/v shows the desired balance of mechanical properties (mucoadhesiveness, hardness, adhesiveness, compressibility, and cohesiveness). The clinical effectiveness of the prepared formulation and marketed MZ formulation were observed in ten patients with periodontitis. The clinical parameters, like BI, GI, BI, and pocket depth, assessed during the treatment indicate more effectiveness of the prepared mucoadhesive SZ gel (SC30) in comparison to MZ gel (Metrogyl®). However, it is necessary to conduct more studies, like microbiological studies, long-term clinical trials with large numbers of patients, comparative studies with other modes of treatment, and formulations to assess its therapeutic efficacy. This mucoadhesive SZ gel is likely to be well received by dental professionals in the future because of its better effectiveness and less bitterness than the marketed MZ gel (Metrogyl® gel).
Acknowledgement
We acknowledge the help of the University Grant Commission (UGC), India, for providing the financial support.
Contributor Information
M. K. Rawat, Email: bitsmanoj@gmail.com
S. Singh, Phone: +91-542-2315871, FAX: +91-542-2368428, Email: sanjays@bhu.ac.in, Email: drsanjaysingh@rediffmail.com
References
- 1.Winkelhoff AJV, Rodenburg JP, Goene RJ, Abbas F, Winkel EG, DeGraaff J. Metronidazole plus amoxydillin in the treatment of Actinobacillus actinomycetemcomitans associated periodontitis. Clin Periodontol. 1989;16:128–131. doi: 10.1111/j.1600-051X.1989.tb01626.x. [DOI] [PubMed] [Google Scholar]
- 2.Kornman KS. Controlled-release local delivery antimicrobials in periodontics: prospect for the future. J Periodontal. 2005;64:782–791. doi: 10.1902/jop.1993.64.8s.782. [DOI] [PubMed] [Google Scholar]
- 3.Szejtli J, Szente L. Elimination of bitter, disgusting tastes of drugs and foods by cyclodextrins. Eur J Pharm Biopharm. 2005;61:115–125. doi: 10.1016/j.ejpb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Gowrishankar R, Phadke RP, Oza SD, Tulwalker S. Satranidazole: experimental evaluation of activity against anaerobic bacteria in vitro and in animal models of anaerobic infection. J Antimicrob Chemother. 1985;4:463–470. doi: 10.1093/jac/15.4.463. [DOI] [PubMed] [Google Scholar]
- 5.Varshosaz J, Tavakoli N, Saidan S. Development and physical characterization of a periodontal bioadhesive gel of metronidazole. Drug Delivery. 2002;9:127–133. doi: 10.1080/10426500290095601. [DOI] [PubMed] [Google Scholar]
- 6.Choi HG, Oh YK, Kim CK. In-situ gelling and mucoadhesive liquid suppository containing acetaminophen: enhanced bioavailability. Int J Pharm. 1998;165:23–32. doi: 10.1016/S0378-5173(97)00385-2. [DOI] [Google Scholar]
- 7.Yong CS, Choi JS, Quan QZ, Rhee JD, Kim CK, Lim SJ, Kim KM, Choi HG. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac sodium. Int J Pharm. 2001;226:195–205. doi: 10.1016/S0378-5173(01)00809-2. [DOI] [PubMed] [Google Scholar]
- 8.Jones DS, Woolfson AD, Brown AF, O'Neill MJ. Mucoadhesive, syringeable drug delivery system for controlled application of metronidazole to the periodontal pocket: in vitro release kinetics, syringeability, mechanical and mucoadhesive properties. J Control Rel. 1997;49:71–79. doi: 10.1016/S0168-3659(97)00060-6. [DOI] [Google Scholar]
- 9.Bilensoy E, Rouf MA, Vural I, Sen M, Hıncal AA. Mucoadhesive, thermosensitive, prolonged-release vaginal gel for clotrimazole: β-cyclodextrin complex. AAPS PharmSciTech. 2007;7(2), article 38. [DOI] [PMC free article] [PubMed]
- 10.Verger ML, Fluckiger L, Kim Y, Hoffman M, Maincent P. Preparation and characterization of nanoparticles containing an antihypertensive agent. Eur J Pharm Biopharm. 1998;46:137–143. doi: 10.1016/S0939-6411(98)00015-0. [DOI] [PubMed] [Google Scholar]
- 11.Moneghini M, Volinovich D, Princivalle F. Formulation and evaluation of vinypyrrolidone/vinylacetate copolymer microspheres with carbamazepine. Pharm Dev Technol. 2000;5(3):347–353. doi: 10.1081/PDT-100100550. [DOI] [PubMed] [Google Scholar]
- 12.Jones DS, Woolfson DA, Brown AF. Textute, viscoelastic and mucoadhesive properties of the pharmaceutical gels composed of cellulose polymers. Int J Pharm. 1997;151:223–233. doi: 10.1016/S0378-5173(97)04904-1. [DOI] [Google Scholar]
- 13.Charoo AN, Shamsher AAA, Kolhi K, Pillai K, Rahman Z. Improvement in bioavailability of transdermally applied flurbiprofen using tulsi (Ocimum sanctum) and turpentine oil. Colloids Surf B Biointerfaces. 2008;65:300–307. doi: 10.1016/j.colsurfb.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Esposito E, Carotta V, Scabbia A, Trombelli L, Antona DP, Menegetti E, Nastruzi C. Comparative analysis of tetracycline containing dental gels: poloxamer and mono glycerides based formulations. Int J Pharm. 1996;142:9–23. doi: 10.1016/0378-5173(96)04649-2. [DOI] [Google Scholar]
- 15.Ishida M, Nambu N, Nagai T. Mucosal dosage form of lidocaine for toothache using hydroxypropyl cellulose and carbopol. Chem Pharm Bull. 1982;30:980–984. doi: 10.1248/cpb.30.980. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi K, Soeda W, Watanabe T. Gingival crevicular pH in experimental gingivitis and occlusal trauma in man. J Periodontol. 1998;69:1036–1043. doi: 10.1902/jop.1998.69.9.1036. [DOI] [PubMed] [Google Scholar]
- 17.Smart JD, Kellaway IW, Worthington HEC. An in vitro investigation of mucosa-adhesive materials for use in controlled drug delivery. J Pharm Pharmacol. 1984;36:295–299. doi: 10.1111/j.2042-7158.1984.tb04377.x. [DOI] [PubMed] [Google Scholar]
- 18.Gavin PA, Sean PG, David SJ. Rheological characterization of primary and binary interactive bioadhesive gels composed of cellulose derivatives designed as ophthalmic viscosurgical devices. Biomaterials. 2005;26(5):571–580. doi: 10.1016/j.biomaterials.2004.02.062. [DOI] [PubMed] [Google Scholar]
- 19.Nastruzzi C, Pastesini C, Cortesi R, Esposito E, Gambari R, Menegatti E. Production and in vitro evaluation of gelatin microspheres containing an antitumor tetra-amidine. J Microencapsul. 1994;11:249–260. doi: 10.3109/02652049409040454. [DOI] [PubMed] [Google Scholar]
- 20.Washington C. Drug release from microdisperse systems: a critical review. Int J Pharm. 1990;58:1–12. doi: 10.1016/0378-5173(90)90280-H. [DOI] [Google Scholar]
- 21.Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–133. doi: 10.1016/S0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]