Abstract
Upstream ORFs (uORFs) are translational control elements found predominantly in transcripts of key regulatory genes. No mammalian genetic model exists to experimentally validate the physiological relevance of uORF-regulated translation initiation. We report that mice deficient for the CCAAT/enhancer-binding protein β (C/EBPβ) uORF initiation codon fail to initiate translation of the autoantagonistic LIP (liver inhibitory protein) C/EBPβ isoform. C/EBPβΔuORF mice show hyperactivation of acute-phase response genes, persistent repression of E2F-regulated genes, delayed and blunted S-phase entry of hepatocytes after partial hepatectomy, and impaired osteoclast differentiation. These data and the widespread prevalence of uORFs in mammalian transcriptomes suggest a comprehensive role of uORF-regulated translation in (patho)physiology.
Keywords: Upstream ORF, translational control, C/EBPβ, liver regeneration, cell cycle
Translational cis-regulatory upstream ORFs (uORFs) are found in the 5′ mRNA regions of numerous eukaryotic transcripts and are considered to regulate protein expression by controlling translation reinitiation at downstream initiation codons or by activating the nonsense-mediated mRNA decay pathway (Morris and Geballe 2000). The frequency of conserved uORFs (Iacono et al. 2005; Calvo et al. 2009) and their predominant prevalence in transcripts of key regulatory genes of growth, differentiation, and proliferation (Kozak 1987) suggest an important function of uORF-mediated translational control in mammals. Nevertheless, an experimental genetic model to examine the physiological relevance of uORF-regulated translation has not been established.
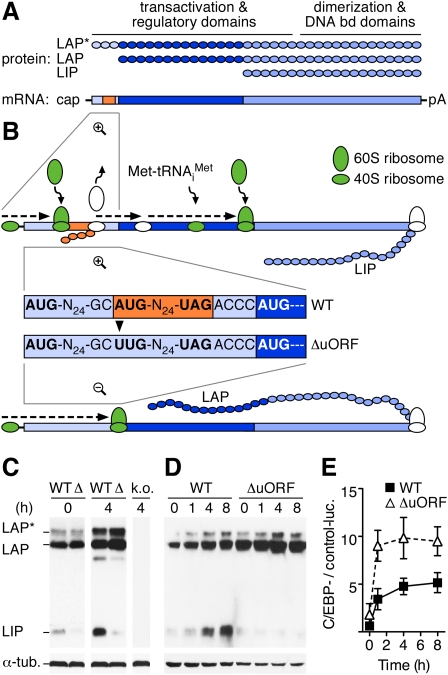
The transcription factor CCAAT/enhancer-binding protein β (C/EBPβ) exerts important functions in many physiological processes, including metabolism, innate immunity, liver development, and regeneration (Tanaka et al. 1995; Greenbaum et al. 1998; Ramji and Foka 2002). The C/EBPβ gene lacks introns, yet three N-terminal different isoforms (termed LAP* [liver-activating protein*], LAP, and LIP [liver inhibitory protein]) are translated from three consecutive in-frame AUG codons in a single transcript (Fig. 1A; Descombes and Schibler 1991). The truncated isoform LIP is devoid of N-terminal trans-activating domains but retains DNA-binding capacity and acts as a competitive inhibitor of the LAP and LAP* isoforms. Previous mutational analysis and tissue culture experiments suggested that translation of the conserved out-of-frame C/EBPβ uORF restrains initiation of LAP and causes resumption of ribosomal scanning and reinitiation at the downstream LIP start site (Fig. 1B; Raught et al. 1996; Lincoln et al. 1998; Calkhoven et al. 2000).
Figure 1.
Genetic ablation of cis-regulatory translational control by the C/EBPβ uORF. (A) Three protein isoforms (LAP* [38 kDa], LAP [35 kDa], and LIP [20 kDa]) are translated from consecutive in-frame initiation codons in the same transcript (Descombes and Schibler 1991). The C/EBPβ mRNA contains a conserved cis-regulatory small uORF (30 base pairs [bp], orange) terminating 4 bp upstream of the LAP initiation site in a different reading frame. (bd) Binding; (pA) poly(A) tail. (B) Translation of the uORF serves to strip ribosomes from their initiating Met-tRNAiMet (green to white) and prevents initiation at the proximate LAP initiation codon. Upon reloading of ribosomes with the ternary eIF2–GTP–Met-tRNAiMet complex (white to green), translation reinitiation from the downstream AUG codon generates LIP. In C/EBPβΔuORF mice, an A-to-U point mutation was designed to abrogate ribosomal initiation at the uORF start codon without changing the amino acid sequence of the C/EBPβ isoforms. Most ribosomes will thus initiate at the LAP AUG instead. (Display of LAP* translation was omitted for simplicity. For details on alternative start site selection, see Supplemental Fig. S1.) (C) Upon i.p. injection of LPS, LIP is strongly induced in C/EBPβWT (WT) but not in C/EBPβΔuORF livers (Δ). (h) Hours of LPS treatment; (α-tub.) α-tubulin; (k.o.) lysate of C/EBPβ knockout mouse. (D) In MEFs, LPS induces LIP expression in C/EBPβWT but not in C/EBPβΔuORF cells. (E) Representative luciferase reporter assay (n = 3) demonstrating increased luciferase reporter activity (luc.) in C/EBPβΔuORF (open triangles) as compared with C/EBPβWT (black squares) MEFs at indicated times after LPS treatment. Error bars show SEM.
Results and Discussion
To determine the physiological importance of uORF-mediated translational control, recombinant mice were generated by introduction of an ATG-to-TTG point mutation at the C/EBPβ uORF translational initiation site (Fig. 1B; Supplemental Fig. S2). The C/EBPβΔuORF mutation was designed to abrogate uORF initiation without altering the amino acid sequence of C/EBPβ. A C/EBPβ wild-type knock-in control strain (C/EBPβWT) was generated and analyzed in parallel to exclude potential artifacts caused by the gene targeting approach. Throughout the experiments, no differences were detected between C/EBPβWT and parental wild-type mice. Offspring of heterozygous C/EBPβΔuORF matings showed the expected Mendelian ratio (Supplemental Table S1). Homozygous C/EBPβΔuORF mice showed normal weight gain and no overt developmental defects or premature death (Supplemental Fig. S3). In contrast to C/EBPβ knockout animals, homozygous C/EBPβΔuORF females were fertile, gave birth to normal size litters (eight out of eight females tested), and showed intact mammary gland development and function (Robinson et al. 1998; Seagroves et al. 1998; Supplemental Figs. S3A, S4).
Bacterial lipopolysaccharide (LPS) is a major inducer of acute-phase response mediated by C/EBPβ in the liver (Poli 1998), and has been shown to enhance expression of the truncated C/EBPβ isoform LIP (Timchenko et al. 2005). In livers of C/EBPβWT mice, LIP was strongly induced after LPS administration, whereas C/EBPβΔuORF mice failed to express high levels of LIP (Fig. 1C). Likewise, lung, spleen, and white adipose tissue of C/EBPβΔuORF mice displayed reduced expression of LIP after LPS treatment as compared with C/EBPβWT tissues (Supplemental Fig. S5). Failure to induce LIP was also observed in C/EBPβΔuORF mouse embryo fibroblasts (MEFs) (Fig. 1D) and was associated with superactivation of a C/EBP-responsive luciferase reporter (Fig. 1E), suggesting a lack of trans-repressive function of LIP. Hence, genetic ablation of the C/EBPβ uORF in mice abolishes the inducible expression of LIP and validates the functional importance of the C/EBPβ uORF as a translational cis-regulatory element in the animal.
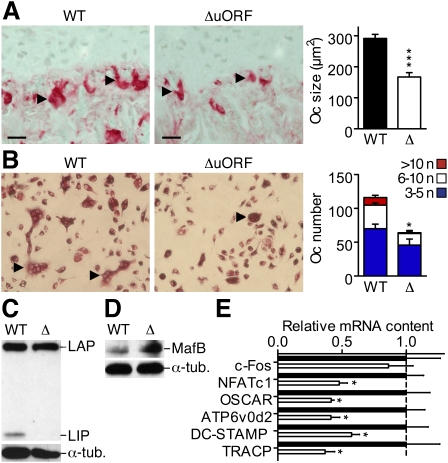
Recently, we showed that the long and truncated C/EBPβ isoforms opposingly regulate the differentiation of bone-resorbing osteoclasts (Smink et al. 2009). C/EBPβLIP mice that express LIP only and not the long C/EBPβ isoforms showed strongly enhanced osteoclast differentiation. Ectopic expression of LAP inhibited osteoclast differentiation (Smink et al. 2009), suggesting that an increase of the LAP/LIP isoform ratio in C/EBPβΔuORF mice would also inhibit osteoclastogenesis. In tibiae of C/EBPβΔuORF mice, we observed a reduction in osteoclast size and number (Fig. 2A), which was accompanied by an increase in thickness of bone trabeculae and bone volume (Supplemental Fig. S6). Bone marrow cell cultures from C/EBPβΔuORF mice formed fewer and smaller osteoclasts as compared with C/EBPβWT and failed to express LIP (Fig. 2B,C). C/EBPβΔuORF osteoclasts showed increased expression of the transcription factor MafB (Fig. 2D), a previously identified target of LAP and a repressor of osteoclastogenesis (Smink et al. 2009). MafB inhibits a number of osteoclastic genes, including Nfatc1, Oscar, Atp6v0d2, DC-STAMP, and TRACP (Kim et al. 2007, 2008; Smink et al. 2009). Transcript levels of these osteoclast markers were found to be reduced in C/EBPβΔuORF osteoclasts (Fig. 2E), while expression of the MafB-independent c-Fos gene (Kim et al. 2007) was not affected. These data suggest that the abrogation of C/EBPβ uORF-mediated translational control, and the resulting increase in the LAP/LIP ratio, constrains osteoclast differentiation by enhancing the expression of MafB.
Figure 2.
The C/EBPβΔuORF mutation impairs osteoclast differentiation. (A) Tibia sections showing tartrate-resistant acid phosphatase (TRACP)-stained osteoclasts (red staining, light-green counterstain) in C/EBPβΔuORF as compared with C/EBPβWT mice. (Arrowheads) Multinucleated osteoclasts; bars, 50 μm. The bar graph displays average osteoclast sizes as determined from six mice per genotype at 8 wk of age. (B) TRACP staining (red) showing osteoclast differentiation of bone marrow-derived precursors of C/EBPβΔuORF and C/EBPβWT mice after 6 d in culture with M-CSF and RANK-L (n = 6). (Arrowheads) Multinucleated osteoclasts. The bar graph displays the differential quantification of osteoclasts by the number of nuclei (n) per cell. (C) Immunoblot analysis showing LIP expression in C/EBPβWT but not in C/EBPβΔuORF osteoclasts at day 2 of culture. (D) Immunoblot analysis showing increased MafB protein in C/EBPβΔuORF as compared with C/EBPβWT osteoclasts. (E) Real-time PCR analysis showing decreased expression of MafB-regulated osteoclast markers in C/EBPβΔuORF (open bars) as compared with C/EBPβWT (black bars) osteoclasts. Normalized to Gapdh (glyceraldehyde-3-phosphate-dehydrogenase) and presented relative to C/EBPβWT (set to 1, dashed line). Error bars show SEM; (*) P < 0.05; (***) P < 0.001.
Since C/EBPβ is an important regulator of liver regeneration, acute-phase response, and interleukin-6 (IL-6) expression (Screpanti et al. 1995; Greenbaum et al. 1998; Poli 1998), we implemented partial hepatectomy (PH) to analyze the consequences of the C/EBPβΔuORF mutation in this physiological context. C/EBPβΔuORF mice failed to induce expression of the truncated LIP isoform throughout the 72-h observation period after PH (Fig. 3A), while LIP was strongly induced in a two-wave kinetic in regenerating livers of C/EBPβWT animals, suggesting consecutive functions of LIP in the course of liver regeneration. After PH, IL-6 serum levels of C/EBPβΔuORF mice rose higher as compared with control animals (Fig. 3B), reaching a 4.7-fold difference after 3 h (1254 ± 265 vs. 263 ± 49 pg/mL, n = 6, P < 0.01) and a 3.3-fold difference at the peak of wild-type expression 6 h after surgery (1578 ± 132 vs. 472 ± 93 pg/mL, n = 6, P < 0.01). IL-6 signaling is known to rapidly confer activating phosphomodifications to both C/EBPβ and STAT3 transcription factors (Akira 1997), resulting in synergistic induction of type I acute-phase response genes (Alonzi et al. 2001). Real-time PCR analysis of known acute-phase response C/EBPβ target genes revealed consistently increased transcription of serum amyloid A1 (Saa1), α-1 antitrypsin (Aat), haptoglobin (Hp), and hemopexin (Hpx), ranging from 1.2-fold to 8.0-fold in hepatectomized C/EBPβΔuORF as compared with C/EBPβWT mice (Fig. 3C). Maxima of enhanced expression of Saa1, Aat, and Hp in C/EBPβΔuORF mice correlated with the peak of LAP expression at 6 h after surgery (Fig. 3A). Together with the superactivation of the C/EBP-responsive reporter construct in C/EBPβΔuORF MEFs, these data suggest that uORF-mediated induction of LIP serves to restrict the trans-activation of early acute-phase response genes.
Figure 3.
The C/EBPβΔuORF mutation causes superinduction of C/EBPβ target genes. (A) Induction of LIP in C/EBPβWT livers upon PH is abolished in C/EBPβΔuORF animals. (B) ELISA showing elevated average levels of serum IL-6 at 3 h and 6 h after PH in C/EBPβΔuORF (open triangles) as compared with C/EBPβWT (black squares) animals (n = 6; [**] P < 0.01). (C) Real-time PCR analysis demonstrating elevated mRNA contents of acute-phase response genes in C/EBPβΔuORF (open bars) as compared with C/EBPβWT (black bars) livers at indicated times after PH (n = 6; [*] P < 0.05; [**] P < 0.01). Error bars show SEM.
In an in vitro proliferation assay, reduced expansion of C/EBPβΔuORF MEF cultures became evident at day 3 (Fig. 4A) and resulted in significantly lower cell numbers at day 5 of the experiment (9.2 ± 0.2 vs. 11.9 ± 0.5 × 105 per well, n = 5, P < 0.01) as compared with C/EBPβWT MEFs. To examine whether the C/EBPβΔuORF mutation also affected cell proliferation in mice, we compared liver regeneration properties of C/EBPβΔuORF, C/EBPβWT, and C/EBPβLIP animals (C/EBPβ expression control in Supplemental Fig. S7). Hepatocytes in regenerating livers of C/EBPβΔuORF mice entered the cell cycle later and at lower frequency as compared with C/EBPβWT animals (Fig. 4B,C). S-phase labeling of liver cells by 5-Bromo-2-deoxy-uridine (BrdU) revealed a 9.3-fold reduction in the proportion of BrdU-positive C/EBPβΔuORF hepatocytes at 36 h (1.2% ± 0.5% vs. 11.6% ± 2.8%, n = 8, P < 0.01) and a 1.9-fold reduction at 48 h after surgery (28.9% ± 4.0% vs. 54.9% ± 3.8%, n = 7, P < 0.01). At the same times, regenerating C/EBPβLIP livers contained similar numbers of BrdU-positive hepatocytes as compared with C/EBPβWT (36 h: 11.5% ± 0.4%, n = 5; 48 h: 56.9% ± 2.1%, n = 4). Virtually no BrdU incorporation was observed in hepatocytes of sham-operated animals at 48 h after PH (n = 3) (data not shown). Transcript levels of cyclin A1 (CcnA1), CcnA2, CcnB1, CcnE1, CcnE2, and proliferating cell nuclear antigen (Pcna) were induced at 36 h after PH in C/EBPβWT and C/EBPβLIP livers, but remained significantly lower in C/EBPβΔuORF animals (Fig. 4D). Twelve hours later, the expression of the cyclins and Pcna were similar in the three genotypes (data not shown), suggesting that re-entry of C/EBPβΔuORF hepatocytes into the cell cycle was impaired but not abolished by the compromised induction of LIP. Similar recovery of liver weight in C/EBPβWT and C/EBPβΔuORF mice, accompanied by an increased hepatocyte volume in C/EBPβΔuORF livers (Supplemental Fig. S8), suggested that enhanced hepatocyte hypertrophy compensated for the blunted S-phase entry to restore adequate liver/body weight ratios.
Figure 4.
Cell proliferation defect in C/EBPβΔuORF mice. (A) In vitro proliferation assay demonstrating reduced expansion of C/EBPβΔuORF (open triangles) as compared with C/EBPβWT (black squares) MEF cultures (n = 5 independent embryos per genotype; [**] P < 0.01). (B) Quantification of BrdU-labeled hepatocyte nuclei (2-h pulse-labeling) in liver sections showing a reduced proportion of hepatocytes in S phase in C/EBPβΔuORF (open bars) as compared with C/EBPβWT (black bars) and C/EBPβLIP (gray bars) livers at 36 and 48 h after PH (n = 8, [***] P < 0.001; n = 7, [*] P < 0.05 vs. wild type, respectively). (C) BrdU immunofluorescence stainings of C/EBPβWT, C/EBPβΔuORF, and C/EBPβLIP liver sections 36 h after PH. Bars, 100 μm. (D) Real-time PCR analysis showing reduced mRNA contents of CcnA1, CcnA2, CcnB1, CcnE1, CcnE2, and Pcna in C/EBPβΔuORF (open bars) as compared with C/EBPβWT (black bars) andC/EBPβLIP (gray bars) livers at indicated times after PH. (n = 6, [*] P < 0.05, [**] P < 0.01 vs. wild type). (n.d.) Not determined. Error bars show SEM.
To further characterize the altered dynamics of cell cycle entry in regenerating C/EBPβΔuORF livers, we performed a genome-wide microarray expression analysis at 36 h after PH. A total number of 546 underrepresented transcripts (392 annotated genes) and 266 overrepresented transcripts (161 annotated genes) were identified in regenerating C/EBPβΔuORF as compared with C/EBPβWT livers (Fig. 5A; Supplemental Table S2). Comparison of all deregulated transcripts to a database of cell cycle-associated genes (http://www.geneontology.org) resulted in 191 matches, of which 99% (189 matches) grouped to the underrepresented fraction (Fig. 5A; Supplemental Table S3). The microarray analysis results were validated for a selection of transcripts on the mRNA (Fig. 4D) and/or the protein level (Supplemental Fig. S9). The high proportion of underrepresented cell cycle genes at 36 h after PH verified the immunohistochemically detected reduction in hepatocyte S-phase entry in C/EBPβΔuORF mice on a transcriptional level, and implied a regulatory function of the C/EBPβ LAP/LIP isoform ratio.
Figure 5.
The C/EBPβΔuORF mutation causes repression of E2F target genes. (A) Graphic representation of a genome-wide microarray expression analysis comparing transcript levels in C/EBPβWT and C/EBPβΔuORF liver at 36 h after PH. (B) Representative ChIP assay on C/EBPβWT liver chromatin showing the association of E2F3, C/EBPα, and C/EBPβ to indicated gene promoters in regenerating liver at 36 h after PH (n = 2). (C) Luciferase reporter assay demonstrating the repressive function of long (black bars), but not of truncated (open bars) C/EBPα and C/EBPβ isoforms on the pGL3TATAbasic-6xE2F reporter construct (n = 3). (luc) Luciferase activity; (p42 and p30) long and truncated C/EBPα isoforms. (D) Luciferase reporter assay with constant, intermediately repressive C/EBPα p42 expression (luciferase activity set to 0.5) showing the corepressive function of LAP* and LAP (black bars) and the derepressive function of LIP (open bars) on the same E2F-responsive reporter construct as used in C (n = 3). Error bars show SEM.
C/EBP transcription factors are known to affect the expression of cell cycle regulatory genes controlled by E2F transcription factors (Sebastian and Johnson 2006; Nerlov 2007). Full-length C/EBPα (p42), but not the N-terminally truncated isoform (p30), acts as a cell cycle inhibitor by repressing E2F target genes (Slomiany et al. 2000; Porse et al. 2001; Iakova et al. 2003). For C/EBPβ, isoform-specific data on E2F coregulation is scarce and suggested a corepressive function of LAP (Sebastian et al. 2005). A comparison of deregulated cell cycle genes in regenerating C/EBPβΔuORF liver to previously identified E2F targets (Ishida et al. 2001; Ren et al. 2002; Bracken et al. 2004) revealed that at least 42% of them were known E2F target genes. Chromatin immunoprecipitation (ChIP) analysis performed 36 h after PH showed that both E2F3 and C/EBPβ were associated with promoters of underrepresented E2F target genes in regenerating liver (E2F1, Rbl1 [retinoblastoma-like 1], CcnA2, CcnE1, Cdc2 [cell division cycle-associated 2], Cdc25, Mcm3 [minichromosome maintenance-deficient 3], Mcm6, and Plk1 [Polo-like kinase 1]) (Fig. 5B). At the same time, C/EBPα showed little or no association with these E2F target gene promoters. Furthermore, transient down-regulation of transcript and protein levels of C/EBPα after PH (Supplemental Fig. S10) suggested a predominant role for C/EBPβ in the coregulation of many E2F target genes in cycling hepatocytes. To examine the effect of individual C/EBPβ isoforms on E2F coregulation, we used an E2F-responsive luciferase reporter construct that has been employed previously to address the mechanism of C/EBP-mediated E2F repression (Porse et al. 2001). Luciferase activity induced by ectopic expression of the transcription factors E2F1 and DP1 (dimerization partner 1, conferring full activity to E2F1) was proportionally repressed by increasing amounts of coexpressed p42, LAP*, or LAP, but remained unaffected by coexpression of p30 or LIP (Fig. 5C). Importantly, the inhibition of E2F activity by p42 was effectively relieved by coexpression of LIP, whereas increasing amounts of LAP* or LAP further repressed reporter activity (Fig. 5D).
Liver regeneration defects observed in C/EBPβ knockout mice have been attributed to a lack of coactivating C/EBPβ function on E2F target genes (Greenbaum et al. 1998; Wang et al. 2007). This interpretation contrasts data showing E2F-repressive functions of LAP in vitro (Sebastian et al. 2005) and LAP-mediated retardation of hepatocyte cell cycle entry after PH in mice (Luedde et al. 2004). Our observations of impaired cell cycle entry in C/EBPβΔuORF livers and the rescue of this phenotype in C/EBPβLIP mice suggest that long C/EBPβ isoforms are dispensable for accurate hepatocyte S-phase entry. The data presented here imply a model in which uORF-mediated induction of LIP is required to overcome repression of E2F targets by long C/EBPα and C/EBPβ isoforms to facilitate rapid cell cycle entry during liver regeneration.
The analysis of the C/EBPβΔuORF mice proves the physiological relevance of uORF-mediated translational control in mammals. We note that low amounts of LIP can be detected in C/EBPβΔuORF mice, which might originate from leaky ribosomal scanning over both the LAP* and LAP start codons (Supplemental Fig. S1) or from partial proteolytic cleavage (Baer and Johnson 2000). Nevertheless, the lack of a functional C/EBPβ uORF start codon results in the inability to induce LIP expression under inflammatory conditions, as well as during differentiation and regeneration processes. Aberrant protein expression caused by defective translational control is increasingly recognized as a pathophysiological mechanism in the etiology of human diseases (Scheper et al. 2007). Specifically, mutations affecting uORF-mediated translational control have been connected to the development of diseases such as hereditary thrombocythemia (Wiestner et al. 1998), familial cutaneous melanoma (Liu et al. 1999), or Marie Unna hereditary hypotrichosis (Wen et al. 2009). The high prevalence of uORFs in human transcripts (35%–49%) implies a comprehensive, yet underestimated, cis-regulatory function in adjusting protein expression (Iacono et al. 2005; Calvo et al. 2009). Future studies will have to address to what extent aberrant uORF-mediated translational control accounts for the development of disease, and how it can be targeted by therapeutic intervention.
Materials and methods
Generation of C/EBPβΔuORF and C/EBPβWT mice
Mutant (C/EBPβΔuORF) and control (C/EBPβWT) mice were generated by homologous recombination according to standard protocols (Supplemental Fig. S2). The neomycin cassette was removed by crossing C/EBPβΔuORF and C/EBPβWT mice to the Cre-deleter strain (Schwenk et al. 1995), and the new strains were kept in a 129Ola × C57Bl/6 background. Female and male mice showed the same phenotype and were analyzed as one group. Mice were provided with standard mouse diet and water ad libitum on a 12-h light–dark cycle. All procedures and animal experiments were conducted in compliance with protocols approved by the Institutional Animal Care and Use Committee.
Additional methods can be found in the Supplemental Material.
Acknowledgments
We thank C. Birchmeier for providing the pTV-flox targeting vector; K. Helin, S. Gaubatz, and E. Kowenz-Leutz for kind donation of luciferase reporter or expression plasmids; J. Lausen and O. Pless for continuous scientific discussion; M. Borowiak, B. von Eyss, and U. Ziebold for valuable technical advice; M. Huska and M. Andrade for help with microarray data analysis; and N. Burbach, R. Zarmstorff, and S. Schmidt for excellent assistance. This work was supported by the Deutsche Krebshilfe (grant 107968 to K.W. and A.L.).
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.557910.
Supplemental material is available at http://www.genesdev.org.
References
- Akira S. IL-6-regulated transcription factors. Int J Biochem Cell Biol. 1997;29:1401–1418. doi: 10.1016/s1357-2725(97)00063-0. [DOI] [PubMed] [Google Scholar]
- Alonzi T, Maritano D, Gorgoni B, Rizzuto G, Libert C, Poli V. Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene activation in the liver. Mol Cell Biol. 2001;21:1621–1632. doi: 10.1128/MCB.21.5.1621-1632.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer M, Johnson PF. Generation of truncated C/EBPβ isoforms by in vitro proteolysis. J Biol Chem. 2000;275:26582–26590. doi: 10.1074/jbc.M004268200. [DOI] [PubMed] [Google Scholar]
- Bracken AP, Ciro M, Cocito A, Helin K. E2F target genes: Unraveling the biology. Trends Biochem Sci. 2004;29:409–417. doi: 10.1016/j.tibs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Calkhoven CF, Muller C, Leutz A. Translational control of C/EBPα and C/EBPβ isoform expression. Genes & Dev. 2000;14:1920–1932. [PMC free article] [PubMed] [Google Scholar]
- Calvo SE, Pagliarini DJ, Mootha VK. Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans. Proc Natl Acad Sci. 2009;106:7507–7512. doi: 10.1073/pnas.0810916106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descombes P, Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991;67:569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Greenbaum LE, Li W, Cressman DE, Peng Y, Ciliberto G, Poli V, Taub R. CCAAT enhancer-binding protein β is required for normal hepatocyte proliferation in mice after partial hepatectomy. J Clin Invest. 1998;102:996–1007. doi: 10.1172/JCI3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono M, Mignone F, Pesole G. uAUG and uORFs in human and rodent 5′untranslated mRNAs. Gene. 2005;349:97–105. doi: 10.1016/j.gene.2004.11.041. [DOI] [PubMed] [Google Scholar]
- Iakova P, Awad SS, Timchenko NA. Aging reduces proliferative capacities of liver by switching pathways of C/EBPα growth arrest. Cell. 2003;113:495–506. doi: 10.1016/s0092-8674(03)00318-0. [DOI] [PubMed] [Google Scholar]
- Ishida S, Huang E, Zuzan H, Spang R, Leone G, West M, Nevins JR. Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol Cell Biol. 2001;21:4684–4699. doi: 10.1128/MCB.21.14.4684-4699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Kim JH, Lee J, Jin HM, Kook H, Kim KK, Lee SY, Kim N. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood. 2007;109:3253–3259. doi: 10.1182/blood-2006-09-048249. [DOI] [PubMed] [Google Scholar]
- Kim K, Lee SH, Ha Kim J, Choi Y, Kim N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP) Mol Endocrinol. 2008;22:176–185. doi: 10.1210/me.2007-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln AJ, Monczak Y, Williams SC, Johnson PF. Inhibition of CCAAT/enhancer-binding protein α and β translation by upstream open reading frames. J Biol Chem. 1998;273:9552–9560. doi: 10.1074/jbc.273.16.9552. [DOI] [PubMed] [Google Scholar]
- Liu L, Dilworth D, Gao L, Monzon J, Summers A, Lassam N, Hogg D. Mutation of the CDKN2A 5′ UTR creates an aberrant initiation codon and predisposes to melanoma. Nat Genet. 1999;21:128–132. doi: 10.1038/5082. [DOI] [PubMed] [Google Scholar]
- Luedde T, Duderstadt M, Streetz KL, Tacke F, Kubicka S, Manns MP, Trautwein C. C/EBP β isoforms LIP and LAP modulate progression of the cell cycle in the regenerating mouse liver. Hepatology. 2004;40:356–365. doi: 10.1002/hep.20333. [DOI] [PubMed] [Google Scholar]
- Morris DR, Geballe AP. Upstream open reading frames as regulators of mRNA translation. Mol Cell Biol. 2000;20:8635–8642. doi: 10.1128/mcb.20.23.8635-8642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerlov C. The C/EBP family of transcription factors: A paradigm for interaction between gene expression and proliferation control. Trends Cell Biol. 2007;17:318–324. doi: 10.1016/j.tcb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- Porse BT, Pedersen TA, Xu X, Lindberg B, Wewer UM, Friis-Hansen L, Nerlov C. E2F repression by C/EBPα is required for adipogenesis and granulopoiesis in vivo. Cell. 2001;107:247–258. doi: 10.1016/s0092-8674(01)00516-5. [DOI] [PubMed] [Google Scholar]
- Ramji DP, Foka P. CCAAT/enhancer-binding proteins: Structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raught B, Gingras AC, James A, Medina D, Sonenberg N, Rosen JM. Expression of a translationally regulated, dominant-negative CCAAT/enhancer-binding protein β isoform and up-regulation of the eukaryotic translation initiation factor 2α are correlated with neoplastic transformation of mammary epithelial cells. Cancer Res. 1996;56:4382–4386. [PubMed] [Google Scholar]
- Ren B, Cam H, Takahashi Y, Volkert T, Terragni J, Young RA, Dynlacht BD. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes & Dev. 2002;16:245–256. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GW, Johnson PF, Hennighausen L, Sterneck E. The C/EBPβ transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes & Dev. 1998;12:1907–1916. doi: 10.1101/gad.12.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper GC, van der Knaap MS, Proud CG. Translation matters: Protein synthesis defects in inherited disease. Nat Rev Genet. 2007;8:711–723. doi: 10.1038/nrg2142. [DOI] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screpanti I, Romani L, Musiani P, Modesti A, Fattori E, Lazzaro D, Sellitto C, Scarpa S, Bellavia D, Lattanzio G, et al. Lymphoproliferative disorder and imbalanced T-helper response in C/EBP β-deficient mice. EMBO J. 1995;14:1932–1941. doi: 10.1002/j.1460-2075.1995.tb07185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves TN, Krnacik S, Raught B, Gay J, Burgess-Beusse B, Darlington GJ, Rosen JM. C/EBPβ, but not C/EBPα, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Genes & Dev. 1998;12:1917–1928. doi: 10.1101/gad.12.12.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian T, Johnson PF. Stop and go: Anti-proliferative and mitogenic functions of the transcription factor C/EBPβ. Cell Cycle. 2006;5:953–957. doi: 10.4161/cc.5.9.2733. [DOI] [PubMed] [Google Scholar]
- Sebastian T, Malik R, Thomas S, Sage J, Johnson PF. C/EBPβ cooperates with RB:E2F to implement Ras(V12)-induced cellular senescence. EMBO J. 2005;24:3301–3312. doi: 10.1038/sj.emboj.7600789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany BA, D'Arigo KL, Kelly MM, Kurtz DT. C/EBPα inhibits cell growth via direct repression of E2F-DP-mediated transcription. Mol Cell Biol. 2000;20:5986–5997. doi: 10.1128/mcb.20.16.5986-5997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smink JJ, Begay V, Schoenmaker T, Sterneck E, de Vries TJ, Leutz A. Transcription factor C/EBPβ isoform ratio regulates osteoclastogenesis through MafB. EMBO J. 2009;28:1769–1781. doi: 10.1038/emboj.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- Timchenko NA, Wang G-L, Timchenko LT. RNA CUG-binding Protein 1 increases translation of 20-kDa isoform of CCAAT/enhancer-binding protein β by interacting with the α and β subunits of eukaryotic initiation translation factor 2. J Biol Chem. 2005;280:20549–20557. doi: 10.1074/jbc.M409563200. [DOI] [PubMed] [Google Scholar]
- Wang H, Larris B, Peiris TH, Zhang L, Le Lay J, Gao Y, Greenbaum L. C/EBPβ activates E2F regulated genes in vivo via recruitment of the coactivator CBP/P300. J Biol Chem. 2007;282:24679–24688. doi: 10.1074/jbc.M705066200. [DOI] [PubMed] [Google Scholar]
- Wen Y, Liu Y, Xu Y, Zhao Y, Hua R, Wang K, Sun M, Li Y, Yang S, Zhang XJ, et al. Loss-of-function mutations of an inhibitory upstream ORF in the human hairless transcript cause Marie Unna hereditary hypotrichosis. Nat Genet. 2009;41:228–233. doi: 10.1038/ng.276. [DOI] [PubMed] [Google Scholar]
- Wiestner A, Schlemper RJ, van der Maas AP, Skoda RC. An activating splice donor mutation in the thrombopoietin gene causes hereditary thrombocythaemia. Nat Genet. 1998;18:49–52. doi: 10.1038/ng0198-49. [DOI] [PubMed] [Google Scholar]