Abstract
Aims
Sphingosine kinase 1 (SPHK1), its product sphingosine-1-phosphate (S1P), and S1P receptor subtypes have been suggested to play protective roles for cardiomyocytes in animal models of ischaemic preconditioning and cardiac ischaemia/reperfusion injury. To get more insight into roles for SPHK1 in vivo, we have generated SPHK1-transgenic (TG) mice and analysed the cardiac phenotype.
Methods and results
SPHK1-TG mice overexpressed SPHK1 in diverse tissues, with a nearly 20-fold increase in enzymatic activity. The TG mice grew normally with normal blood chemistry, cell counts, heart rate, and blood pressure. Unexpectedly, TG mice with high but not low expression levels of SPHK1 developed progressive myocardial degeneration and fibrosis, with upregulation of embryonic genes, elevated RhoA and Rac1 activity, stimulation of Smad3 phosphorylation, and increased levels of oxidative stress markers. Treatment of juvenile TG mice with pitavastatin, an established inhibitor of the Rho family G proteins, or deletion of S1P3, a major myocardial S1P receptor subtype that couples to Rho GTPases and transactivates Smad signalling, both inhibited cardiac fibrosis with concomitant inhibition of SPHK1-dependent Smad-3 phosphorylation. In addition, the anti-oxidant N-2-mercaptopropyonylglycine, which reduces reactive oxygen species (ROS), also inhibited cardiac fibrosis. In in vivo ischaemia/reperfusion injury, the size of myocardial infarct was 30% decreased in SPHK1-TG mice compared with wild-type mice.
Conclusion
These results suggest that chronic activation of SPHK1-S1P signalling results in both pathological cardiac remodelling through ROS mediated by S1P3 and favourable cardioprotective effects.
Keywords: Sphingosine kinase-1 transgenic mouse, Cardiac fibrosis, Ischemia/reperfusion injury, S1P3, Reactive oxygen species
1. Introduction
Sphingosine kinases (SPHKs) catalyse phosphorylation of sphingosine to produce the pleiotropic lysophospholipid mediator sphingosine-1-phosphate (S1P), which has attracted much attention because of its diverse effects in a variety of cell types.1,2 These include stimulation of cell proliferation, inhibition of apoptosis, and regulation of cell shape and cell motility, among others. SPHK1 and SPHK2, the two isozymes thus far identified, are ubiquitously but differentially expressed during embryogenesis and in adult tissues.1 Previous studies3,4 have demonstrated that either SPHK1 or SPHK2 single knockout (KO) mice are phenotypically normal, whereas SPHK1/SPHK2 double KO mice are embryonic lethal with undetectable S1P levels.5 These observations indicate that S1P is required for mammalian embryogenesis, and is produced exclusively by SPHK1 and SPHK2 in vivo at least during the embryonic period.
Most of the S1P effects are mediated through members of the G protein-coupled S1P receptor family, which include ubiquitously expressed subtypes, S1P1, S1P2, and S1P3.6,7 The receptor repertoire of heterotrimeric G protein coupling to downstream signalling pathways, including the Rho family small GTPases, is responsible for diverse S1P actions.6,7 For example, S1P1 mediates S1P-directed cell migration and other biological effects through activation of the Rho family small GTPase Rac1 via Gi, whereas S1P2 mediates inhibition of cell migration through activation of Rho and resultant Rac inhibition via G12/13.6 S1P3 couples to Gi and G12/13, mediating activation of both Rac and Rho as well as activation of phospholipase C via Gq.6 In the cardiovascular system, S1P3 is abundantly expressed on cardiomyocytes, cardiac fibroblasts, vascular endothelial, and smooth muscle cells, whereas S1P1 and S1P2 are mainly expressed on vascular endothelial and smooth muscle cells, respectively.6–12 S1P3 KO mice are phenotypically normal,13 however, S1P3 deletion abrogates negative chronotropic and hypertensive effects after intravenous administration of S1P in vivo.14 Recent investigations in the cardiovascular system have suggested the involvement of the SPHK1-S1P signalling system in the protection of myocardium from ischaemia/reperfusion (I/R) injury. The protective effect of S1P from I/R injury was abrogated by deletion of S1P3.15 Deletion of both S1P2 and S1P3, but not either alone, resulted in aggravation of myocardial infarction due to ischaemia/reperfusion (IR) injury.16 Deletion of SPHK1 sensitized the myocardium to I/R injury.17 However, the effects of chronic activation of the SPHK1-S1P signalling system in the heart in vivo have not yet been addressed thus far.
In order to evaluate the effects of endogenous overproduction of S1P on the cardiovascular system in vivo, we generated SPHK1-transgenic (TG) mice that overexpress functional SPHK1 isoform in diverse tissues with more than 10-fold increases in enzymatic activity. We found that SPHK1a TG mice showed 100% occurrence of cardiac fibrosis, which is associated with cardiomyocyte degeneration, but without cardiac hypertrophy or hypertension. The development of cardiac fibrosis mechanistically involved activation of the S1P3-Rho family small G protein signalling pathway and increased reactive oxygen species (ROS). SPHK1a TG mice also exhibited attenuation of myocardial infarct due to I/R injury compared with wild-type (WT) mice, indicating that SPHK1a overexpression has both the favourable and unfavourable effects on the heart.
2. Methods
2.1. Generation of SPHK1 TG mice and S1P3 knock-out (KO)/SPHK1 TG bigenic mice
We generated SPHK1 TG mice that overexpress SPHK1a in a variety of tissues under the universal CAG promoter (see Supplementary material online for details). S1P3 knock-out (KO) mice in C57BL/6 genetic background13 were mated with SPHK1 TG mice. All experiments using mice were approved by and performed according to the Guidelines for the care and Use of laboratory Animals in Kanazawa University, which strictly conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.2. Measurements of SPHK activity and levels of S1P, dihydrosphingosine-1-phosphate (DH-S1P), sphingosine, and ceramide
SPHK activity in homogenates of the heart and other tissues was measured by using sphingosine and [γ-32P]ATP as substrates.5 Labelled lipids were analysed as described.8 The contents of S1P, DH-S1P, sphingosine, and ceramide in the heart, serum, and plasma were quantified as detailed in the Supplementary material online.
2.3. Experimental I/R injury
The heart was exposed and ischaemia was achieved by ligating the left anterior descending coronary artery (LAD). After 30 min occlusion, reperfusion was achieved. After 24 h of reperfusion the chest was re-opened and the LAD was occluded with the same suture. To estimate the ischaemic area at risk (AAR), Evan's blue (3%) was injected into the left ventricle, circulated, and uniformly distributed throughout the myocardium except AAR.16 The hearts were excised and transversely sliced. The heart sections were incubated with a 2% triphenyl tetrazolium chloride (TTC) for 10 min at 37°C to stain viable myocardium. TTC-non-stained area (infarct area) was determined and expressed as infarct area/AAR (%).
2.4. Immunohistochemistry
Serial horizontal sections of formalin-fixed, paraffin-embedded hearts at the level of the maximal diameter were subjected to anti-SPHK1a immunohistochemistry using a rabbit polyclonal anti-SPHK1a antibody as described in Supplementary material online.18 Aceton-fixed, fresh-frozen sections were also examined by indirect immunofluorescence by using a goat anti-desmin polyclonal antibody and a rabbit anti-SPHK1a antibody.
2.5. Determination of the activities of Rac1 and RhoA in cardiac tissue
Determinations of GTP-bound, active forms of Rac1 and RhoA in mouse hearts was performed by pull-down assay techniques as described previously.9 Data are expressed as a percentage of the control value in the basal unstimulated state (=100%).
2.6. Detection of oxidative stress markers
The content of malondialdehyde, a major lipid peroxidation product, in heart tissues and urinary excretion of 8-hydroxydeoxyguanosine (8-OHdG), a stable marker of oxidative DNA damage, were determined by a spectrophotometrical method using BIOXYTECH MDA-586 assay kit (Oxis Research, Portland, OR, USA) and ELISA using an 8-OHdG ELISA assay kit (Nikken SEIL Corporation, Shizuoka, Japan).
2.7. Statistics
All data are shown as means ± SEM. ANOVA (analysis of variance) was followed by Dunnette's test to determine the statistical significance of differences between mean values. Unpaired t-test was performed for the comparison between two groups in Figures 1B, 3E, 4B, 6B, and 6C. For all statistical comparisons, P < 0.05 was considered significant.
Figure 1.
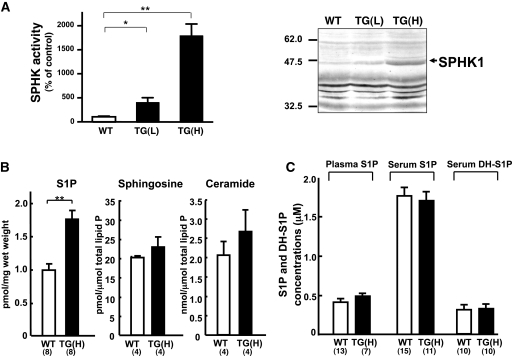
Functional expression of SPHK1a in transgenic mice. (A) Functional expression of SPHK1a protein in TG(L) and TG(H) heart tissue. Cytosolic fractions of the heart homogenates (n = 3) were subjected to in vitro SPHK assay. Left: The results are expressed as % of the control value obtained from WT mice. *P < 0.05, **P < 0.01. Right: western blot analysis of SPHK1a protein (indicated by an arrowhead) in the heart. (B) S1P, sphingosine, and ceramide contents in the heart of WT and TG(H) mice. Data represent the mean ± SE obtained from the numbers of animals shown in parenthesis at the bottom. **P < 0.01. (C) S1P and dihydro-S1P (DH-S1P) levels in plasma and serum of WT and TG(H) mice.
Figure 3.
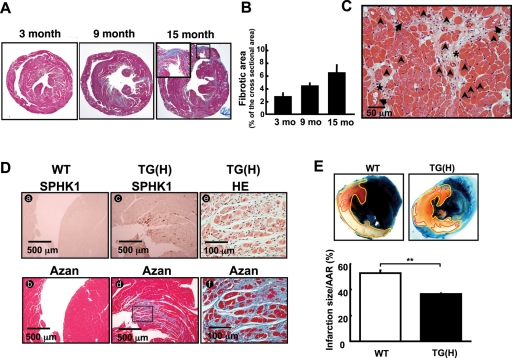
Development of cardiac fibrosis in TG(H) mice. (A) Representative photographs of Azan-stained horizontal sections of the heart from 3, 9, and 15-month-old TG(H) mice. Inset: magnification of a region of transmural fibrosis. (B) Quantified data of cardiac fibrosis. Four or five mice in each group were analysed. (C) HE-stained section of a TG(H) mouse heart, showing vacuolar changes (arrows) and mild coagulative necrosis (arrowheads) of cardiomyocytes around the fibrotic area (asterisks). Cardiac fibrosis was not observed in TG(L) mice until the age of 9 months. (D) Comparison of immunohistochemical staining of the SPHK1 protein (brown colour, a and c) and fibrosis in Azan staining (blue colour, d and f) and HE staining (e) in a TG(H) mouse heart. (E) Myocardial infarct in WT and TG(H) mice after ischaemia/reperfusion (I/R). Upper: representative photomicrographs of ventricles. Blue areas, non-ischaemic tissue; white areas circumscribed with the red line, infarcted area; red areas circumscribed with the yellow line except white area, salvaged tissues. The red and white areas are the area at risk (AAR). Lower: quantified infarct areas corrected for AAR. **P < 0.01.
Figure 4.
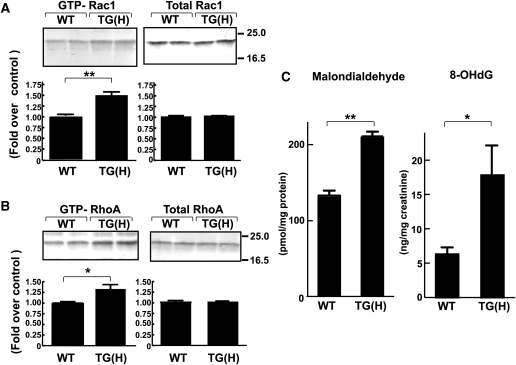
TG(H) heart tissue showed increased activities in Rac and RhoA small GTPases and increased levels of oxidative markers. The amounts of GTP-bound, active forms of Rac (A) and RhoA (B) in the heart of WT and TG(H) mice were determined by pull-down assay technique and normalized by total GTPase amounts. *P < 0.05, **P < 0.01. Total Rac and RhoA protein levels were not different from WT control. (C) TG(H) mice showed increased levels of oxidative stress markers. Malondialdehyde content in the heart tissue (left) and urinary secretion of 8-OHdG (normalized by creatinine concentration, right) were quantified and compared between TG(H) and WT mice. Data represent the mean ± SE obtained from 5 to 9 mice. *P < 0.05, **P < 0.01.
Figure 6.
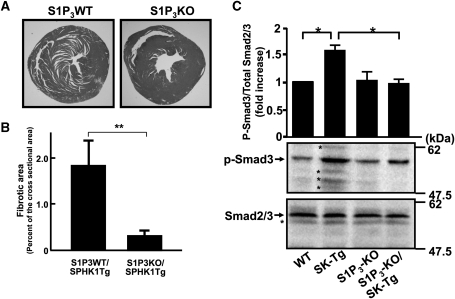
Deletion of the S1P3 receptor reduced the development of cardiac fibrosis and stimulated Smad3 phosphorylation in TG(H) mice. (A) Representative photographs of Azan stained horizontal sections of the heart obtained from WT/TG(H) or S1P3KO/TG(H) mice. (B) Quantitative analysis of the fibrotic area expressed as a percentage of the total cross sectional area. Littermate S1P3WT/TG(H) and S1P3KO/TG(H) mice (n = 5) at the age of 16 weeks were subjected to analysis. (C) Western blot analysis of Smad3 phosphorylation. The upper and lower panels show phosphorylated Smad3 (p-Smad3) and total Smad2/3, respectively. The bands indicated by the asterisks on the blots most likely represent non-specific or degradation products of total Smad2/3 and p-Smad3. *P < 0.05.
3. Results
3.1. Expression and activity of SPHK1, and tissue and blood levels of S1P and related lipids in SPHK1 TG mice
We established two independent lines with different transgene expression levels as evaluated by northern blot analysis (see Supplementary material online, Figure S1). One of the TG lines, designated as TG(H), showed widespread and high expression levels of the transgene transcript in a variety of organs including the heart, brain, kidney, stomach, and other tissues. The other TG lineage, TG(L), showed a moderate or low level of transgene expression in the heart, testis, and other tissues. TG(H) and TG(L) mice were both fertile and apparently normal, and grew comparably with WT mice. Measurements of sphingosine kinase enzymatic activity and western blot analysis in the cardiac extracts confirmed functional overexpression of SPHK1 in the heart of both TG(H) and TG(L) mice (Figure 1A). SPHK activity in the cytosolic fraction of the heart was 4- and 18-fold greater in TG(L) and TG(H) when compared with that in WT controls, respectively (Figure 1A, lower). The heart tissues of TG(L) and TG(H) showed moderately and strongly elevated SPHK1 protein expression levels, respectively, which appeared comparable to respective SPHK activities, as compared with the WT heart (Figure 1A, upper). Unexpectedly, however, the S1P content in the heart tissue was elevated by only 1.6-fold in TG(H), but not in TG(L), when compared with WT (Figure 1B). We also measured the sphingosine and ceramide contents in the heart and found no difference between TG(H) and WT littermates (Figure 1B). S1P concentrations in the plasma or serum were not significantly different between WT and TG(H) mice (Figure 1C). Dihydro-S1P level in the serum were also similar between WT and TG(H) mice.
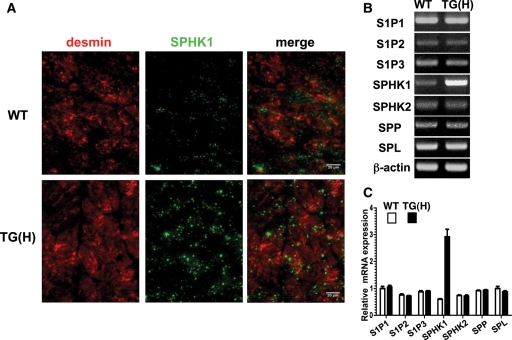
Immunofluorescent staining using anti-SPHK1 and anti-desmin antibodies demonstrated that SPHK1 was expressed in cardiomyocytes and other types of cells (Figure 2A, upper panels). SPHK1 showed two expression patterns in WT cardiomyocytes, which included a faint homogenous pattern and a sparsely speckled pattern. These two patterns of staining by anti-SPHK1 antibody was not at all observed in the heart of SPHK1-knockout mice (see Supplementary material online, Figure S2), indicating that the employed antibody specifically detected SPHK1 protein. In cardiomyocytes of TG(H) mice, SPHK1 expression was markedly elevated in both of these two patterns (Figure 2A, lower panels). SPHK1 was also expressed in blood vessels of WT and TG(H) hearts, with similar expression levels (data not shown). RT–PCR analysis of a panel of key molecules of the S1P signalling system demonstrated that mRNA expression levels of S1P1, S1P2, and S1P3 receptors and the enzymes, S1P phosphohydrolase 1 (SPP1), S1P lyase (SPL), and SPHK2, were not significantly different between WT and TG(H) hearts (Figure 2B and C). Neither the heart rate, systemic blood pressure at resting, nor heart weight to body weight ratio were different between TG(H) mice and their WT littermates (Supplementary material online, Table S1). TG(L) mice also showed normal values of these parameters.
Figure 2.
Expression of SPHK1 protein and related molecules in heart. (A) Immunofluorescent analysis of desmin (red) and SPHK1a (green) in cardiac tissues of WT (upper panel) and TG(H) (lower panel) mice. (B) Representative results of RT–PCR analysis performed in triplicate of the S1P1, S1P2, and S1P3 receptors, SPHK1a, SPHK2, SPP, SPL. β-Actin was adopted as an internal control. (C) Quantification of the results in (B) expressed as relative mRNA expression levels, which were normalized to β-actin.
3.2. TG(H) mice develop cardiac fibrosis and are protected from I/R injury of myocardium
We found that both male and female TG(H) mice developed both interstitial and perivascular cardiac fibrosis, which were evident by 3 months and gradually progressed with aging (Figure 3A and B). At 3 months of age none of the WT mice showed detectable pathological fibrosis in the heart. In the aged population (18 months or older), a small population of control WT mice (13%) also showed slight fibrosis, contrasting with the extensive fibrosis observed in 100% of TG(H) mice. TG(L) mice showed virtually no cardiac fibrosis until at least 9 months of age and were not different from WT mice. Fibrosis was observed in both the left and right ventricles of TG(H) hearts, and its distribution pattern was focal and scattered in nature. In most advanced cases fibrosis was transmural, in part, with marked thinning of the ventricular wall (Figure 3A, right). Inspections with higher magnification revealed degenerative changes, which included intracellular small vacuoles and mild coagulative necrosis of cardiomyocytes (Figure 3C). These changes were evident especially around the focal fibrotic lesions of the myocardium. Immunohistochemical analysis revealed scattered foci of extremely strong SPHK1 overexpression in the myocardium of TG(H) mice (Figure 3Dc). The reason for this non-uniform expression pattern of the SPHK1 transgene product is unknown at present. High magnification inspection of serial sections processed for anti-SPHK1a immunohistochemistry, Azan-, and HE-staining revealed that SPHK1a was expressed throughout the cytoplasm of affected cardiomyocytes with signs of mild degenerative changes, which were surrounded by collagen fibres, myocardial cells showing vacuolar degeneration, and a small numbers of necrotic cells (Figure 3Dd–f). Thus, strong focal overexpression of SPHK1 protein was closely associated with degenerative changes and fibrosis. We also observed occasional calcification within the fibrotic areas. TUNEL-positive apoptotic cells were restricted to calcified regions (data not shown). We did not observe inflammatory cell infiltration in the heart nor did we find vessel abnormalities in the coronary vessels. We also found that TG(H) mice had normal blood biochemistry (see Supplementary material online, Table S1) and blood cell counts including lymphocyte count (see Supplementary material online, Figure S3).
Echocardiographic analysis of 3, 6, and 12-month-old TG(H) mice revealed that most TG(H) mice showed normal left-ventricular size with no thickness of the posterior wall or the interventricular septum, and they maintained the normal %FS (Supplementary material online, Table S2). However, we did observe one 9-month-old male mouse with dilated left ventricle and reduced %FS, which are echocardiographic signs reminiscent of human dilated cardiomyopathy.
Consistent with the pathological changes in TG(H) mouse hearts, they showed well established molecular signs of cardiac remodelling, i.e. marked upregulation of the embryonic genes, including atrial natriuretic peptide (ANP) [3.12- (3 months (m)) and 3.12-fold (9 m) increases in TG(H) mice compared with WT mice], β-myosin heavy chain (β-MCH) (2.14- and 5.55-fold increases) and, to a lesser extent, brain natriuretic peptide (BNP) (1.13- and 1.72-fold increases) and skeletal muscle (SKM) α-actin (1.05- and 1.46-fold increases), as evaluated by northern blot analysis (see Supplementary material online, Figure S4). The mRNA expression levels of profibrotic factor TGF-β1 and fibrosis-marker genes type I and III collagens, fibronectin and α-smooth muscle actin were not different between SPHK1 TG(H) and WT hearts (data not shown). The expression levels of collagen type I α1 and α2 proteins were elevated in the SPHK1-TG heart compared with WT heart (see Supplementary material online, Figure S5).
We evaluated the effect of SPHK1 overexpression on I/R injury. Cardiomyocyte death in hearts (white area in Figure 3E) exposed to 30 min of coronary occlusion and followed by 24 h of reperfusion was 30% reduced in TG(H) mice compared with WT mice. The ischaemic AAR (white and red areas) was not different between the two mouse groups. Thus, TG(H) mice are protected from myocardial I/R injury.
3.3. Elevated Rac1 and RhoA activities and accumulation of oxidative stress markers in the heart of TG(H) mice
We and others have demonstrated in various types of cultured cells that S1P regulates Rac1 and Rho, which are implicated in ischaemic and non-ischaemic cardiac remodelling,19,20 in receptor subtype-specific manners.6 TG(H) heart tissues showed a 1.5-fold increase in the amount of GTP-bound, active form of Rac1 when compared with heart tissues obtained from WT littermates (Figure 4A, left). In contrast, the total amount of Rac1 protein in the heart was similar between WT and TG(H) mice (Figure 4A, right). The amount of GTP-bound RhoA in the TG(H) heart tissues was 1.3-fold greater compared with the WT (Figure 6B, left). The total amount of RhoA protein was similar between TG(H) and WT heart (Figure 4B, right).
Rac1 is involved in the generation of ROS in the heart,20 which in concert with other mechanisms, contributes to cardiac remodelling. Indeed, TG(H) heart tissues showed a 1.5-fold increase in the content of malondialdehyde, which is a major lipid peroxide and a useful marker for tissue oxidative stress, when compared with WT littermates (Figure 4C, left). Another oxidative stress marker, 8-OHdG, which is excised from oxidized DNA and secreted in the urine, showed a more than two-fold increase over control (Figure 4C, right). These results suggest that the hearts in TG(H) mice are exposed to increased oxidative stress when compared with those of WT littermates.
3.4. Successful prevention of cardiac fibrosis in TG(H) mice by the HMG CoA reductase inhibitor pitavastatin and the anti-oxidant N-2-mercaptopropyonylglycine
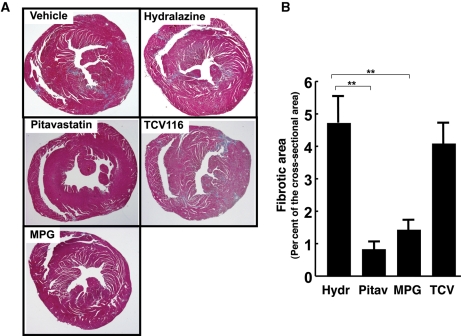
In order to gain insight into the pathophysiology of SPHK1-mediated cardiac remodelling, we tested whether pitavastatin, which prevents membrane recruitment and activation of the Rho family GTPases,21 could exert a beneficial effect on TG(H) hearts. The administration of pitavastatin beginning at the age of 6 weeks after birth markedly reduced the extent of cardiac fibrosis evaluated at 12 week old, when compared with vehicle control (Figure 5A and B). We also examined the effect of the anti-oxidant N-2-mercaptopropyonylglycine (MPG),22 and found that MPG was effective in inhibiting development of cardiac fibrosis (Figure 5A and B). In contrast, the angiotensin II type 1 receptor (AT1) antagonist candesartan, which effectively inhibits pressure overload-induced cardiac hypertrophy and fibrosis, and another anti-hypertensive hydralazine, failed to inhibit fibrosis at doses that effectively and similarly lowered blood pressure (Figure 5A and B).
Figure 5.
Preventive effects of an HMG CoA reductase inhibitor (pitavastatin) and an anti-oxidant (MPG), but not an angiotensin II type 1 receptor antagonist (TCV116), against development of cardiac fibrosis. (A) Representative photographs of Azan stained horizontal sections of the heart obtained from TG(H) mice that received pitavastatin, MPG, TCV-116, hydralazine, or vehicle. (B) Quantitative analysis of fibrotic area expressed as the percentage of the total cross sectional area. TG(H) mice at the age of 6 weeks were randomly assigned to either of the treatment groups, each consisting of 5–7 mice. Medical intervention was continued until 12 weeks old. Hydralazine, which was employed as a control for TCV116 that causes a decrease in blood pressure, did not have any effect on fibrosis by itself. **P < 0.01.
3.5. S1P3 deletion partially inhibits cardiac fibrosis in TG(H) mice
S1P3 is the major S1P receptor subtype expressed in rodent cardiomyocytes and fibroblasts.8,10 Hence, we evaluated whether S1P3 signalling was involved in cardiac fibrosis induced by SPHK1a overexpression, by generating S1P3KO/TG(H) mice. Deletion of S1P313 resulted in a substantial reduction in the area of cardiac fibrosis when compared with S1P3WT(+/+)/TG(H) littermates (Figure 6A and B), indicating that S1P3 receptor signalling is at least partially involved in SPHK1-dependent cardiac remodelling and fibrosis. We examined activation of Smad3, which is a downstream effector of TGF-β and transactivated by S1P3,23,24 in the heart. In the background of S1P3+/+, Smad3 phosphorylation in TG(H) heart was 50% increased compared with WT heart (Figure 6C). Deletion of S1P3 abolished an increase in Smad3 phosphorylation in TG(H) heart.
4. Discussion
The present study demonstrates for the first time that chronic increased SPHK1 activity can induce cardiac fibrosis in vivo. Despite constitutive high enzymatic activity of SPHK1 in a variety of tissues including platelets, which have been believed to be one of major sources of plasma S1P,1 SPHK1 TG(H) mice showed normal plasma and serum S1P levels, and only moderate increases in heart S1P level (Figure 1B and C). These results strongly suggest the existence of a feedback regulation mechanism for homeostasis of blood and tissue S1P levels in vivo. In the heart tissue, the levels of ceramide and sphingosine, the precursors of S1P, were not significantly different between WT and TG(H) mice, suggesting an increased turnover of sphingolipid metabolism in the transgenic heart, in which increased production of S1P by SPHK1 overexpression is balanced by increased degradation of S1P. Since mRNA expression levels of the S1P degrading enzymes, SPL and SPP1, were similar between WT and TG(H) mice (Figure 2B and C), it is possible that these enzymes have a substantial intrinsic activity, or, alternatively, might be functionally upregulated by an excess amount of S1P, resulting in a homeostatically maintained S1P level that allows survival of mice despite increased SPHK activity. Particularly, it is an interesting possibility that SPL might play a major role in homeostasis of tissue and blood S1P levels in the face of increased SPHK activity, because a recent study25 showed that deletion of SPL resulted in marked increases in tissue and blood S1P levels.
Chronic overexpression of SPHK1 resulted in the development of degenerative changes and interstitial fibrosis in the myocardium in the naïve heart, which advances with age (Figure 3). Fibrosis was observed in 100% of the TG(H) hearts but not at all in TG(L) heart, indicating that cardiac fibrosis in SPHK1 TG mice was gene dose-dependent. Comparable myocardial changes were not observed in WT heart at the same age throughout the observation period of 15 months, indicating that this cardiac fibrosis is not associated with normal aging. Despite degeneration and fibrosis, the cardiac function in most of TG(H) hearts was maintained at least until 12 months after birth (see Supplementary material online, Table S2), and the lifespan of the TG(H) mice were not shorter than the WT littermates (N.T., unpublished observation). However, we cannot exclude the possibility that TG(H) mice might develop diastolic dysfunction due to cardiac fibrosis because echocardiography is not optimal to detect diastolic dysfunction.26 We observed that one 9-month-old TG(H) mouse showed marked ventricular dilation reminiscent of human dilated cardiomyopathy. The ventricular dilation may have represented a decompensated stage of cardiac remodelling under sustained oxidative and possibly additional stresses in this SPHK1 TG(H) mice.
The development of SPHK1-mediated cardiac fibrosis was strongly inhibited in S1P3 KO mice (Figure 6). It is likely that S1P locally produced by overexpressed SPHK1 acts in a paracrine fashion on S1P3, which is abundantly expressed in cardiomyocytes and fibroblasts among S1P receptors, and mediates cardiac fibrosis.10,14 The Smads, the major downstream signalling molecules of TGF-β, constitute the signalling pathways to induce tissue fibrosis through activating multiple downstream pathways.19,24 Deletion of S1P3 abolished stimulation of Smad3 phosphorylation in the heart of SPHK1 TG mice (Figure 6C). It was recently demonstrated in several other cell types including fibroblasts, keratinocytes, and mesangial cells that the Smad signalling pathway is transactivated by S1P receptors including S1P3.23,24 Particularly, S1P3 stimulation in fibroblasts induced differentiation into myofibroblasts via transactivation of Smad3.23 The present observations together with these previous findings suggest the possibility that stimulation of Smad3 in SPHK1-TG heart may be brought about by transactivation of TGF-β signalling pathway by S1P-S1P3 and involved in cardiac fibrosis. Further study is required to substantiate the role of the Smad pathway in S1P-S1P3 mediated cardiac fibrosis. Our observation that TGF-β1 mRNA level in the heart was not increased in SPHK1-TG mice compared with WT mice lends the support to the notion that Smad3 may be transactivated in SPHK1-TG heart. It is possible that degradation process of collagens might be suppressed via S1P3 receptor-mediated Smad transactivation in the heart of SPHK1-TG mice as suggested in mesangial cells,24 which is consistent with our observations that there was no increase in the mRNA levels of collagens and fibronectin in TG(H) heart.
As the molecular mechanisms for S1P-mediated cardiac fibrosis, we focused on Rho family GTPases, which contribute to cardiac fibrosis through more than a single mechanisms including ROS production, Rho kinase, and NF-κB activation19 and are activated by S1P receptors in a receptor subtype-specific manner.6 We detected a modest increase in the activities of Rac and Rho in the TG(H) heart when compared with WT littermates (Figure 4). Consistent with the notion that the Rho family GTPases are involved in the pathogenesis of cardiac fibrosis in the TG(H) heart, pitavastatin, which inhibits membrane targeting of both Rac1 and RhoA,21 strongly inhibited the development of fibrosis (Figure 5). Rac is an upstream regulator essential for ROS production, which is implicated in cardiac fibrosis and remodelling.19,20,27 The levels of oxidative stress markers were increased in TG(H) mice (Figure 4C). Moreover, the administration of the anti-oxidant MPG inhibited fibrosis (Figure 5). It is reported that SPHK is also involved in ROS production in vascular smooth muscles.8 The morphological and pathophysiological analyses of SPHK1-TG mice (see Supplementary material online, Table S1 and other data) suggested that cardiac remodelling in the TG(H) heart was not secondary to hypertension, ischaemic heart disease, or other metabolic disorders. These results collectively suggest that S1P3-mediated Rho GTPase activation, Smad activation, and ROS production in the heart in concert result in cardiac fibrosis when S1P level in the heart is elevated. However, we cannot unequivocally preclude the possibility that the effects of SPHK1 overexpression in other tissues in part contribute to the observed cardiac phenotype indirectly through humoral or other mechanisms.
Spiegel and her colleagues28 first proposed that SPHK1 and its product S1P act inside of cells to mediate mitogenic and anti-apoptotic effects. Recent studies29,30 showed in SPHK1−/−SPHK2+/− mice or SPHK1−/− mice that marked accumulation of sphingosine and dihydrosphingosine, but not a decreased S1P level, resulted in increased cell death in the deciduum of pregnant mice and reduced tumour size in the spontaneous intestinal polyps, respectively. In the latter report,30 deletion of the S1P2 or S1P3 receptor did not alter size of tumours. These observations suggested that intracellular accumulation of pro-apoptotic sphingosine, but not S1P receptor signalling, led to inhibition of cell proliferation or increased cell death. Our data rather suggest that cardiac fibrosis in SPHK1 TG mice is an S1P receptor-mediated event. Thus, the roles of sphingolipids in the regulation of cell survival and proliferation may be different, depending on cell types and the context of impairment of sphingolipid metabolism.
Recent investigations8 (for review) in the cardiovascular system have showed the cardioprotective role of the S1P signalling system in myocardial I/R injury. SPHK1 was suggested to be a key enzyme by the observation that deletion of SPHK1 aggravated I/R injury in an in vitro Langendorff apparatus.17 In vivo studies using S1P receptor-deleted mice15,16 showed that the cardioprotective effects of the S1P signalling system in ischaemia/reperfusion injury models were mediated via S1P3 or both S1P3 and S1P2. Consistent with these previous investigations, the present study showed that chronic overexpression of SPHK1 in the mouse heart is cardioprotective against myocardial infarction due to I/R injury in vivo (Figure 3E). Thus, SPHK1 overexpression has both the unfavourable fibrosis-promoting and favourable cardioprotective effects.
Immunofluorescent detection revealed two types of SPHK1 expression patterns, including a faintly homogenous pattern and a sparsely speckled pattern in both SPHK1-TG and WT mice (Figure 2A). The speckled expression pattern may suggest that SPHK1 is present not only diffusely in the cytoplasm but also in the subcellular locations, most likely in the subcellular organella. In addition, the SPHK1 TG(H) mouse heart showed focal regions of strong overexpression of SPHK1 in the myocardium (Figure 3D). At present, the reason for the latter expression pattern of the transgene product is not known. TG mice have been back-crossed to C57BL/6 mice more than nine times, which excludes the possibility of genetic chimerism of the TG mice adopted in the present study. Whatever the reason for the uneven overexpression of the transgene product in the myocardium, our observation that severe pathological changes occur in the regions with SPHK1 overexpression strongly suggests that locally expressed SPHK1 is causally related to cardiomyocyte degeneration and fibrosis in the TG(H) heart.
In summary, the present study demonstrated for the first time that transgenic overexpression of SPHK1a in heart tissue led to chronic, progressive myocardial degeneration and fibrosis in mice while it conferred a protective effect on the myocardium against I/R injury. The SPHK1-dependent cardiac fibrosis in TG(H) mice likely involves the S1P3-Rho family small G protein signalling pathway and downstream ROS production and subsequent TGF-β activation. These findings are consistent with the demonstration of other lysophospholipid receptors in fibrosis.31 The relevance of these observations in transgenic mice to human cardiac fibrosis of the ischaemic, hypertensive, and other origins deserves further investigation.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported in part by Grants-in Aid for Scientific Research from the Ministry of Education, Sciences, Sports, and Culture of Japan (Y.T. and N.T.) and the NIH (DA019674 and NS048478) (J.C.).
Supplementary Material
Acknowledgements
We would like to thank Dr R. Proia in National Institute of Diabetes and Digestive and Kidney Diseases in USA for providing SPHK1-KO mice. We also thank Ms Y. Ohta and C. Hirose for technical and secretarial assistance.
Conflict of interest: none declared.
References
- 1.Spiegel S, Milstien SJ. Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem. 2007;282:2125–2129. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- 2.Gardell SE, Dubin AE, Chun J. Emerging medicinal roles for lysophospholipid receptors. Trends in Molecular Med. 2006;12:65–75. doi: 10.1016/j.molmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 4.Kharel Y, Lee S, Snyder AH, Sheasley-O'neill SL, Morris MA, Setiady Y, et al. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J Biol Chem. 2005;280:36865–36872. doi: 10.1074/jbc.M506293200. [DOI] [PubMed] [Google Scholar]
- 5.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takuwa Y. Subtype-specific differential regulation of Rho family G proteins and cell migration by the Edg family sphingosine-1-phosphate receptors. Biochim Biophys Acta. 2002;1582:112–120. doi: 10.1016/s1388-1981(02)00145-2. [DOI] [PubMed] [Google Scholar]
- 7.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Ann Rev Biochemistry. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 8.Takuwa Y, Okamoto Y, Yoshioka K, Takuwa N. Sphingosine-1-phosphate signaling and biological activities in the cardiovascular system. Biophys Biochim Acta. 2008;1781:483–488. doi: 10.1016/j.bbalip.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Ryu Y, Takuwa N, Sugimoto N, Sakurada S, Usui S, Okamoto H, et al. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ Res. 2002;90:325–332. doi: 10.1161/hh0302.104455. [DOI] [PubMed] [Google Scholar]
- 10.Landeen LK, Aroonsakool N, Haga JH, Hu BS, Giles WR. Sphingosine-1-phosphate receptor expression in cardiac fibroblasts is modulated by in vitro culture conditions. Am J Physiol. 2007;292:H2698–H2711. doi: 10.1152/ajpheart.01065.2006. [DOI] [PubMed] [Google Scholar]
- 11.Robert P, Tsui P, Laville MP, Livi GP, Sarau HM, Bril A, et al. EDG1 receptor stimulation leads to cardiac hypertrophy in rat neonatal myocytes. J Mol Cell Cardiol. 2001;33:1589–1606. doi: 10.1006/jmcc.2001.1433. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol. 2007;293:H3150–H3158. doi: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
- 13.Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, Contos JJ, et al. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/EDG-3. J Biol Chem. 2001;276:33697–33704. doi: 10.1074/jbc.M104441200. [DOI] [PubMed] [Google Scholar]
- 14.Forrest M, Sun SY, Hajdu R, Bergstrom J, Card D, Doherty G, et al. Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J Pharmacol Exp Ther. 2004;309:758–768. doi: 10.1124/jpet.103.062828. [DOI] [PubMed] [Google Scholar]
- 15.Theilmeier G, Schmidt C, Herrmann J, Keul P, Schafers M, Herrgott I, et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–1409. doi: 10.1161/CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- 16.Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, et al. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol. 2007;292:H2944–H2951. doi: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- 17.Jin ZQ, Zhang J, Huang Y, Hoover HE, Vessey DA, Karliner JS. A sphingosine kinase 1 mutation sensitizes the myocardium to ischemia/reperfusion injury. Cardiovasc Res. 2007;76:41–50. doi: 10.1016/j.cardiores.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Murate T, Banno Y, T-Koizumi K, Watanabe K, Mori N, Wada A, et al. Cell type-specific localization of sphingosine kinase 1a in human tissues. J Histochem Cytochem. 2001;49:845–855. doi: 10.1177/002215540104900705. [DOI] [PubMed] [Google Scholar]
- 19.Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame. A dacade of hypertrophic signaling hits. Circ Res. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- 20.Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci USA. 2006;103:7432–7437. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laufs U, Kilter H, Konkol C, Wassmann S, Bohm M, Nickenig G. Impact of HMG CoA reductase inhibition on small GTPases in the heart. Cardiovasc Res. 2002;53:911–920. doi: 10.1016/s0008-6363(01)00540-5. [DOI] [PubMed] [Google Scholar]
- 22.Sun JZ, Tang XL, Park SW, Qiu Y, Turrens JF, Bolli R. Evidence for an essential role of reactive oxygen species in the genesis of late preconditioning against myocardial stunning in conscious pigs. J Clin Invest. 1996;97:562–576. doi: 10.1172/JCI118449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller CD, Rivera Gil P, Tölle M, van der Giet M, Chun J, et al. Immunomodulator FTY720 induces myofibroblast differentiation via the lysophospholipid receptor S1P3 and Smad3 signaling. Am J Pathol. 2007;170:281–292. doi: 10.2353/ajpath.2007.060485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xin C, Ren S, Kleuser B, Shabahang S, Eberhardt W, Radeke H, et al. Sphingosine 1-phosphate cross-activates the Smad signaling cascade and mimics transforming growth factor-β-induced cell response. J Biol Chem. 2004;279:35255–35262. doi: 10.1074/jbc.M312091200. [DOI] [PubMed] [Google Scholar]
- 25.Vogel P, Donoviel MS, Read R, Hansen GM, Hazlewood J, Anderson SJ, et al. Incomplete inhibition of sphingosine 1-phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PLoS ONE. 2009;4:e4112. doi: 10.1371/journal.pone.0004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouzounian M, Lee DS, Liu PP. Diastolic heart failure: mechanisms and controversies. Nat Clin Pract Cardiovasc Med. 2008;5:375–586. doi: 10.1038/ncpcardio1245. [DOI] [PubMed] [Google Scholar]
- 27.Sirker A, Zhang M, Murdoch C, Shah AM. Involvement of NADPH oxidase in cardiac remodeling and heart failure. Am J Nephrol. 2007;27:649–660. doi: 10.1159/000109148. [DOI] [PubMed] [Google Scholar]
- 28.Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, et al. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–558. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizugishi K, Li C, Olivera A, Bielawski J, Bielawska A, Deng CX, et al. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J Clin Invest. 2007;11:2993–3006. doi: 10.1172/JCI30674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohno M, Momoi M, Oo ML, Paik JH, Lee YM, Venkataraman K, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;9:7211–7223. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.