Abstract
Aims
Previous studies suggested that androgens augmented renal vascular responses to angiotensin II (Ang II). The protein kinase C (PKC)-CPI-17 pathway is involved in vascular constriction. We tested the hypothesis that this pathway may contribute to androgenic amplification of Ang II-renal vasoconstriction in the New Zealand genetically hypertensive (NZGH) rat.
Methods and results
NZGH underwent sham operation, castration, or castration with testosterone replacement at 5 weeks of age. When the rats were 16–17 weeks of age, mean arterial pressure (MAP) and renal vascular resistance (RVR) responses to intravenous Ang II infusion (20, 40, and 80 ng/kg/min) were recorded before and after treatment with a PKC inhibitor, chelerythrine. mRNA expression of PKC isoforms and CPI-17 protein expression were analysed in renal cortex. MAP and RVR responses to Ang II were enhanced in androgen-replete NZGH. The Ang II-induced increase in RVR was significantly lower in castrated NZGH (ranged from 100 ± 8% to 161 ± 9% of baseline) than in sham-operated NZGH (ranged between 123 ± 3% and 237 ± 19% of baseline). Testosterone treatment restored RVR responses to Ang II in castrated rats. Chelerythrine treatment markedly reduced the MAP and RVR responses to Ang II in each group and attenuated the differential MAP and RVR responses to Ang II amongst the three groups. PKCδ and PKCε mRNA levels were significantly reduced by castration and increased by testosterone treatment. In contrast, no significant differences in protein expression were detected for these PKC isoforms. Castration decreased while testosterone treatment increased CPI-17 and phospho-CPI-17 expression.
Conclusion
Collectively, these results suggest that androgens modulate renal vascular responses to Ang II in part via an effect on the PKC-CPI-17 signalling pathway.
Keywords: Androgen, Renal vascular resistance, Angiotensin II, Hypertension, Protein kinase C
1. Introduction
Animal and human studies have clearly shown gender differences in the development of hypertension.4 When compared with females hypertension development is exaggerated in male spontaneously hypertensive rat (SHR),10,27 in male Dahl salt-sensitive rats,3,9 and in deoxycorticosterone + salt (DOCA-salt) treated male rats.2,16 The relative contribution of androgens and estrogens to these gender differences remains controversial.4,25,27 While a great deal of research has focused on the effects of estrogen, several observations suggest that testosterone may also amplify hypertension development. First, castration reduced hypertension development in the SHR,7,20,27,37 the DOCA-salt model,2 and the Dahl salt-sensitive3 rats. Second, testosterone replacement in castrated hypertensive rats reversed this antihypertensive effect of castration.20,27,39 Third, treatment with androgen antagonists7,29 or treatments that reduced testosterone synthesis5 mimicked the effects of castration. Thus, androgens appear to amplify the hypertensive process. Available data in humans are conflicting.4,17,28 Since androgen concentrations decrease in males with ageing whereas cardiovascular disease increases, a protective effect of testosterone was suggested.17 However, interpretation of such correlations is problematic since stress and disease lower plasma testosterone,11 possibly as a compensatory mechanism.28 Indeed, direct administration of testosterone induced hypertension in humans.4 Thus, direct evidence implicates androgens in hypertension in humans. The mechanisms by which androgens amplify hypertension remain to be fully elucidated.
Chronic exposure to physiological concentrations of testosterone increases vascular resistance.38 These effects involve, at least in part, enhanced reactivity to constrictor agents. Testosterone increased vascular reactivity in coronary artery13 and aorta21 in animals and in men.19 Conversely, castration reduced constrictor responses in rat kidney,34–36 whereas chronic testosterone treatment reversed these deficits. Androgen receptor antagonist treatment mimicked the effects of castration.13 Thus, current data suggest that the long-term effects of testosterone increase vascular constrictor reactivity.
The cellular mechanisms contributing to androgen amplification of vascular reactivity remain to be fully elucidated. Nevertheless, protein kinase C (PKC) represents an attractive possibility since PKC is a key regulator of vascular tone.24,31 A major downstream effector of PKC in vascular smooth muscle is CPI-17.33 PKC phosphorylates CPI-17 at Thr-38,43 which in turn inhibits myosin light chain (MLC) phosphatase, increases MLC phosphorylation, and thereby augments vascular smooth muscle contraction.33 Increased PKC expression/activity was reported in hypertensive models, including the SHR,1,40 and DOCA-salt hypertensive rats.41 Nevertheless, the effect of sex hormones on PKC function in vascular smooth muscle is less clear. Some data showed sex-associated differences in PKC activity/expression in vascular smooth muscle.31 Phorbol ester-activated PKC induced greater vasoconstriction in male than in female rats.25 However, the involvement of PKC-CPI-17 signalling in androgenic potentiation of renal vascular responses has not been resolved.
Accordingly, we tested the hypothesis that androgens potentiate renal vascular resistance (RVR) responses, at least in part, via an effect on the PKC-CPI-17 signalling pathway. The specific aims were to test: (i) whether PKC inhibition affected androgen modulation of pressor and renal vascular responses to angiotensin II (Ang II) in vivo and (ii) whether manipulation of androgen status affected the expression of PKC isoforms or of their downstream effector, CPI-17.
2. Methods
2.1. Animals
Male New Zealand genetically hypertensive (NZGH) rats were obtained at 4 weeks of age and maintained on phytoestrogen-free diet (Harland Teklad 2016) and tap water. All protocols conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and approved by the Institutional Animal Care and Use Committee at the University of South Dakota. Each cohort of rats consisted of sham-operated, castrated, and castrated + testosterone groups.
2.2. Castration and testosterone replacement
At 5 weeks of age, rats were subjected to sham operation (Sham) or castration or castration + testosterone supplementation which we showed previously resulted in plasma testosterone concentrations similar to those of sham-operated NZGH.35
2.3. Haemodynamic assessment
At 16–17 weeks of age, a cohort of rats was anaesthetized and instrumented with femoral arterial catheters. After 48–72 h of recovery and training to the recording environment, mean arterial pressure (MAP) was measured from conscious rats in their home cages.34 On the recording day, after approximately 60 min of acclimatization, MAP and HR were recorded for 60 min. MAP and HR measurements were taken from periods when the rats were calm.
A separate cohort of rats was anaesthetized with Inactin (100 mg/kg, IP) and femoral artery and vein catheters were implanted for MAP measurement and Ang II infusion, respectively. A jugular vein catheter was inserted for infusion (3 mL/h) of saline or saline with chelerythrine, a PKC inhibitor, (Tocris, MO, USA). Left renal artery blood flow (RBF) was measured via an ultrasonic probe (Transonic) implanted on the left renal artery via a midline laparotomy. After stabilization (60 min), the rats received enalapril, an angiotensin-converting enzyme inhibitor, (1 mg/kg, IV) to inhibit endogenous Ang II production. After 25–30 min to allow stabilization of the response to enalapril, MAP and RBF were measured during cumulative intravenous Ang II infusions (20, 40, and 80 ng/kg/min) (20–30 min each dose). After recovery from Ang II (∼60 min), the rats were treated with chelerythrine (80 µg/kg/min infusion, IV). After 10–20 min of stabilization, MAP and RBF responses to Ang II were repeated during the chelerythrine infusion. Since there were significant differences in kidney weight amongst the groups (Sham: 1.22 ± 0.04 g; castrated: 1.07 ± 0.02 g; castrated + testosterone 1.18 ± 0.04 g), RVR was calculated as MAP divided by RBF corrected to kidney weight.
2.4. mRNA and protein expression assessment
A separate cohort of NZGH rats (16–17 weeks of age) was euthanized and the kidneys were harvested. One kidney was preserved with RNAlater (Ambion) for real-time RT–PCR, whereas the other was collected for western blot of renal cortex punches.
mRNA expression of PKC isoforms was assessed by real-time RT–PCR as described previously.34 Pre-validated primers and probes directed toward the PKCα (Rn01496152_m1), PKCδ (Rn00593117_g1), and PKCε (Rn01769089_m1) were obtained via Assays-on-Demand (Applied Biosystems, Foster City, CA, USA). The threshold cycle (Ct) was determined for the target genes and for cyclophilin. These raw values were analysed with a modified ΔCt method using a data analysis program, qBase (version 1.2.2) (http://medgen.ugent.be/qbase/) to obtain normalized relative quantification values.
2.5. Western blot
The tissue samples were homogenized in RIPA buffer and subjected to SDS–PAGE electrophoresis and western blotting as described previously.34 Membranes were probed for proteins of interest using primary antibodies to PKCδ (1:500, Abcam, MA, USA), PKCε (1:500, Abcam), and CPI-17 (1:1000, Abcam). CPI-17 and phospho-CPI-17 were detected using rabbit anti-phospho-CPI-17 (Thr38) antibody (1:1000, Abcam). The protein bands were visualized using fluorescent secondary antibodies (Rockland) and were quantified by densitometric analysis software on the Odyssey Imager (LiCor Biosciences). β-Actin served as a loading control.
2.6. Statistical analysis
All values are the mean ± SEM. One-way analysis of variance (ANOVA) was used to analyse real-time RT–PCR and western blot data. Two-factor ANOVA for repeated measures was used to analyse baseline values and dose–responses to Ang II. The grouping factors were surgery (levels: Sham, castrated, castrated + testosterone) and treatment (levels: baseline values: prior to chelerythrine, recovery, chelerythrine; dose–response curves: Doses of Ang II). Post hoc comparisons were performed with Student Newman Keuls test (Graph Pad Software). Statistical significance was accepted at P < 0.05.
3. Results
3.1. Baseline values in conscious rats
Manipulation of androgens significantly affected baseline blood pressure (ANOVA P = 0.037). Baseline MAP was higher in the sham-operated (183 ± 6 mmHg) and castrated + testosterone treated NZGH (179 ± 4 mmHg) compared with castrated NZGH (163 ± 5 mmHg). However, there were no significant differences in baseline heart rate values amongst the groups (Sham: 352 ± 12 b.p.m.; castrated: 363 ± 5 b.p.m.; castrated + testosterone: 380 ± 11 b.p.m.) (ANOVA P = 0.153).
3.2. Baseline values in anaesthetized rats
The baseline MAP, RVR, and HR values after anaesthesia and inhibition of endogenous Ang II production by enalapril rats are shown in Table 1. Two-way ANOVA failed to reveal an effect of surgery or treatment on MAP (surgery P = 0.292; treatment P = 0.786; interaction P = 0.995) or HR (surgery P = 0.896; treatment P = 0.092; interaction P = 0.563). Similarly, ANOVA revealed no main effect of surgery (P = 0.171) on RVR. Thus, the Sham, castration, or castration + testosterone interventions caused no significant differences in MAP, HR, or RVR. However, there was a significant main effect of treatment (P = 0.001; interaction P = 0.221) on RVR. Post hoc analysis revealed that RVR was increased slightly but significantly during the recovery and chelerythrine periods compared to baseline in the castrated + testosterone group. However, there was no difference in RVR between the recovery and chelerythrine periods in any group.
Table 1.
Baseline values in anaesthetized rats
| Group | Prior to chelerythrine |
Recovery |
Presence of chelerythrine |
||||||
|---|---|---|---|---|---|---|---|---|---|
| MAP | RVR | HR | MAP | RVR | HR | MAP | RVR | HR | |
| Sham | 141 ± 6 | 20 ± 1 | 286 ± 18 | 147 ± 9 | 20 ± 3 | 276 ± 18 | 145 ± 8 | 22 ± 4 | 271 ± 21 |
| Cas | 135 ± 5 | 19 ± 2 | 298 ± 23 | 137 ± 8 | 25 ± 4 | 283 ± 24 | 134 ± 8 | 25 ± 4 | 258 ± 26 |
| Cas + T | 141 ± 5 | 24 ± 3 | 276 ± 16 | 144 ± 6 | 31 ± 4*# | 270 ± 10 | 145 ± 7 | 32 ± 5*# | 266 ± 11 |
This table shows values for baseline mean arterial blood pressure (MAP; mmHg), renal vascular resistance (RVR; mmHg/mL/min/g kidney), and heart rate (HR; b.p.m) in anaesthetized NZGH rats prior to chelerythrine treatment, in the recovery period after Ang II infusion, and during chelerythrine treatment (n = 5/group). Values are mean ± SEM. Cas, castrated; Cas + T, castrated + testosterone replacement.
*P < 0.05 compared with the values in the absence of chelerythrine.
#P < 0.05 compared with the values obtained in the same period.
3.3. Pressor and RVR responses to Ang II in anaesthetized rats
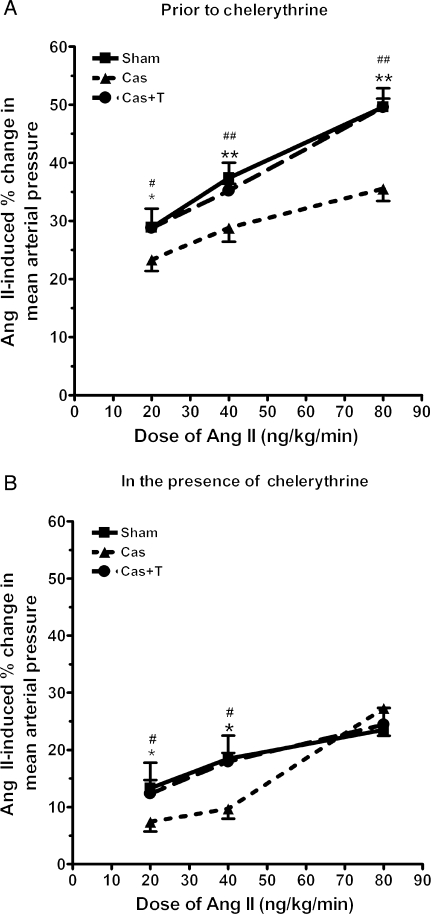
As shown in Figure 1A, cumulative infusions of Ang II increased MAP in each group. ANOVA revealed a significant effect of dose (P < 0.0001), surgery (P = 0.003), and interaction (P = 0.02). Castration significantly attenuated MAP responses to Ang II. Testosterone replacement in castrated NZGH significantly increased Ang II-induced MAP responses such that these responses were similar to those in sham-operated rats. Thus, testosterone treatment of castrated NZGH restored the pressor responses to Ang II.
Figure 1.
Mean arterial blood pressure (MAP) responses to Ang II (20, 40, and 80 ng/kg/min) in anaesthetized sham-operated (Sham n = 5), castrated (Cas n = 5), and castrated + testosterone replacement (Cas + T n = 5) NZGH at 16–17 weeks of age. (A) MAP responses in the absence of chelerythrine. (B) MAP responses in the presence of chelerythrine. Values are mean ± SEM. *P < 0.05 and **P < 0.01 Sham vs. Cas. #P < 0.05 and ##P < 0.01 Cas + T vs. Cas.
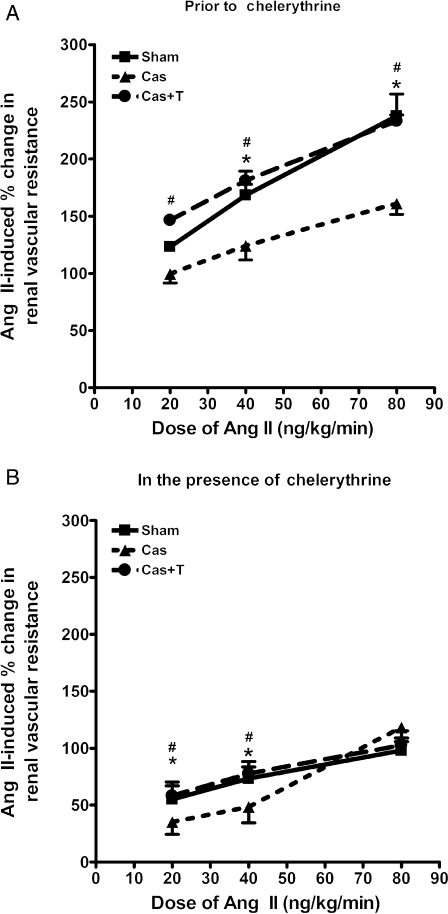
Ang II infusion caused a graded increase in RVR in each group (Figure 2A). ANOVA revealed a significant effect of dose (P < 0.0001), surgery (P = 0.0003), and interaction (P = 0.0001). The RVR responses to Ang II in the sham group ranged between 123 ± 3% and 237 ± 19% over baseline. RVR responses to Ang II were significantly attenuated in male NZGH subjected to castration (100 ± 8% to 161 ± 9% over baseline). Testosterone replacement restored RVR responses to Ang II in castrated NZGH resulting in responses similar to sham-operated animals.
Figure 2.
Renal vascular resistance (RVR) responses to Ang II (20, 40, and 80 ng/kg/min) in anaesthetized sham-operated (Sham n = 5), castrated (Cas n = 5), and castrated + testosterone replacement (Cas + T n = 5) NZGH at 16–17 weeks of age. (A) RVR responses in the absence of chelerythrine. (B) RVR responses in the presence of chelerythrine. Values are mean ± SEM. *P < 0.05 and **P < 0.01 Sham vs. Cas. #P < 0.05 and ##P < 0.01 Cas + T vs. Cas.
Chelerythrine infusion significantly attenuated pressor dose–response curves to Ang II in each group as shown in Figure 1B. However, the inhibitory effect of chelerythrine was significantly greater in both sham-operated NZGH and castrated + testosterone NZGH compared with the castrated rats such that the differences in dose–response curves amongst the androgen replete and castrated NZGH were reduced (Figure 1B).
A similar pattern was detected for the RVR responses to Ang II. Chelerythrine treatment significantly reduced renal vasoconstrictor dose-responses to Ang II (Figure 2B) and attenuated the differences in RVR dose–responses amongst the groups. Nevertheless, there remained a significant difference in the RVR response to Ang II at the lowest and middle doses. At the highest dose of Ang II, chelerythrine treatment largely abolished the differences between androgen-replete and androgen-deficient groups (Figure 2B). Accordingly, chelerythrine treatment effectively decreased the differential Ang II-induced pressor and RVR dose-responses observed amongst androgen-replete and androgen-depleted NZGH.
3.4. mRNA expression of PKC isoforms
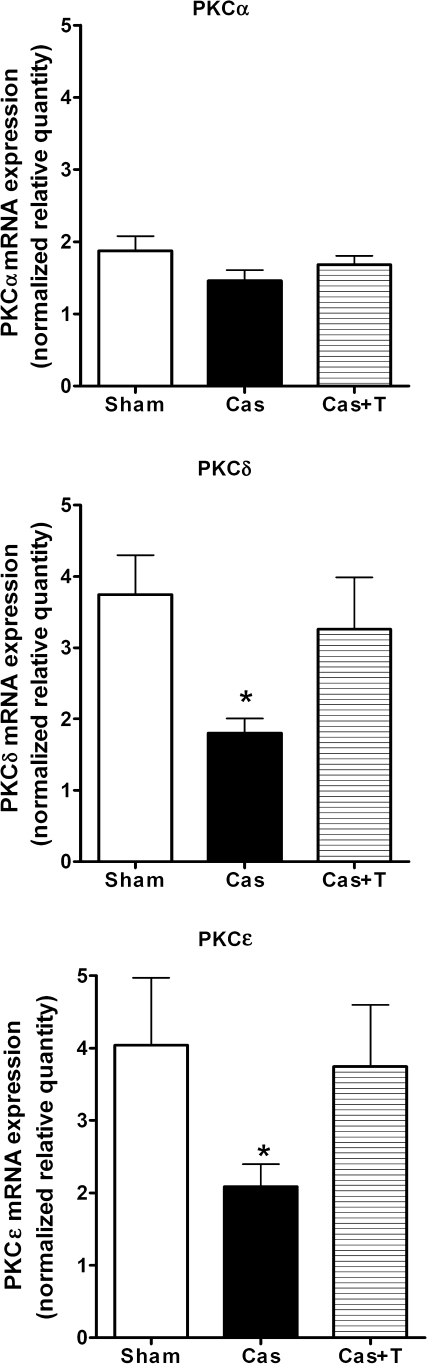
There were no significant differences in PKCα mRNA expression amongst the three groups (Figure 3). However, mRNA levels of PKCδ and PKCε were significantly decreased in castrated NZGH compared with sham-operated rats. The values of PKCδ and PKCε mRNA expression obtained in the castration with testosterone replacement group were not different from those obtained in the sham-operated group.
Figure 3.
Normalized relative mRNA expression for the PKC isoforms (n = 8/group). Upper panel: PKCα; middle panel: PKCδ; lower panel: PKCε. Kidney tissue samples were obtained from male NZGH at 16–17 weeks of age. Sham, sham-operated; Cas, castrated; Cas + T, castrated + testosterone replacement. Values are means ± SEM. *P < 0.05 compared with sham.
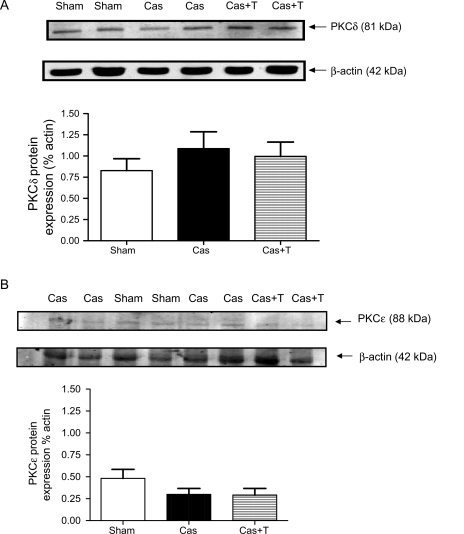
3.5. Western blot
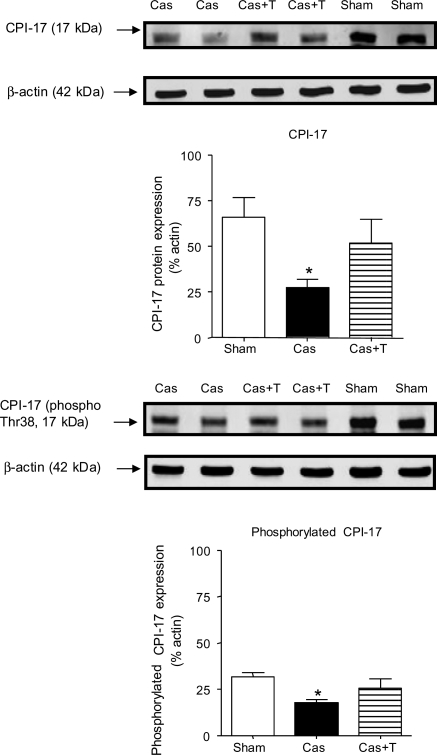
Neither PKCδ nor PKCε protein expression was significantly affected by castration or by testosterone treatment of castrated NZGH (Figure 4). We also assessed the effect of manipulation of androgens on CPI-17, a downstream target of PKC. There was a significant decrease in both CPI-17 and phospho-CPI-17 levels in castrated NZGH compared with sham-operated NZGH (Figure 5), whereas the values observed from the castration with testosterone replacement group were similar to that in the sham-operated group.
Figure 4.
Protein expression for PKCδ and PKCε in punches of renal cortex obtained from NZGH (n = 8/group) at 16–17 weeks of age. The top panel shows an example of PKCδ bands (∼81 kDa), β-actin bands (∼42 kDa) and quantification of the blots expressed as the ratio of PKCδ band and actin band density. The lower panel shows an example of PKCε bands (∼88 kDa), β-actin bands (∼42 kDa) and quantification of the blots expressed as the ratio of PKCδ band and actin band density. Sham, sham-operated; Cas, castrated; Cas + T, castrated + testosterone replacement. Values are means ± SEM.
Figure 5.
CPI-17 and phospho-CPI-17 protein expression as assessed by western blot in the renal cortex of NZGH (n = 8/group) at 16–17 weeks of age. The top part of each panel shows an example of CPI-17 (left panel) and phospho-CPI-17 bands (right panel) (∼17 kDa) and β-actin bands (∼42 kDa). The lower part of each panel shows the quantification of the blots expressed as the phospho-CPI-17/actin ratio. Sham, sham-operated; Cas, castrated; Cas + T, castrated + testosterone replacement. Values are means ± SEM. *P < 0.05 compared with sham.
4. Discussion
The current work tested the hypothesis that androgens potentiate RVR responses, at least in part, via an effect on the PKC-CPI-17 signalling pathway. The major findings include: (i) castration reduced the MAP and RVR responses to Ang II whereas testosterone treatment of castrated NZGH restored these responses. (ii) Treatment with a PKC inhibitor, chelerythrine, attenuated the differences in MAP and RVR responsiveness to Ang II. (iii) mRNA expression of PKCδ and PKCε were significantly attenuated by castration but were restored by testosterone treatment. However, protein expression as assessed by western blot did not reveal any differences amongst the groups. (iv) Protein expression of CPI-17 and phospho-CPI-17 was decreased in castrated group compared with sham-operated group, whereas testosterone replacement in castrated rats reversed this effect. Taken together, these findings provide new evidence, suggesting that the PKC/CPI-17 pathway may contribute to androgenic potentiation of the pressor and renal vascular responses to Ang II in the NZGH.
Current evidence supports the view that testosterone augments the development of hypertension in genetic models such as the SHR5,7,20,27,37 and NZGH.34 An increase in vascular constrictor activity may contribute to this effect. Chronic treatment with testosterone increased vascular resistance38 and responsiveness to vasoconstrictor agents in animals13,21 and in humans.19 Conversely, castration reduced constrictor responses in rat aorta21 and kidney34–36 and reduced RVR in SHR.6 Our current work is consistent with these data. We observed that early castration (5 weeks of age) markedly attenuated the pressor responses to Ang II in 16 to 17-week-old NZGH rats. Testosterone replacement therapy restored the pressor effects of Ang II to levels approximating those in sham-operated control NZGH. Thus, our current data are compatible with the view that the long-term effects of testosterone increase the pressor effect of Ang II.
In the present work, we focused on the kidney since increased renal vascular reactivity has been linked to the development and maintenance of hypertension.12,42 In particular, enhanced renal vascular reactivity to Ang II has been identified in the SHR12,14,15,42 and pre-hypertensive humans.26 Our previous work also showed that castration of male hypertensive rats reduced renal vascular reactivity in the isolated perfused kidney36 and intact rat.34 The current findings are consistent with these data. Castration reduced Ang II-induced increases in RVR to approximately one-half of that observed in the sham-operated NZGH. Conversely, RVR responses to Ang II in testosterone treated castrated NZGH were not different from those observed in the sham-operated NZGH. Collectively, these data indicate that constrictor mechanisms in the renal vasculature are a target for androgen action in the kidney.
The PKC pathway has been implicated in Ang II-induced renal vasoconstriction.30 Current data concerning the influence of sex steroids on PKC function in vasculature are conflicting. Estrogen increased PKC isoform mRNA expression in rabbit aorta.8 Conversely, estrogen downregulated PKC function in rat aorta whereas androgens had no effect.25 We tested the involvement of PKC in functional studies using a selective PKC inhibitor, chelerythrine. As reported previously by others,22,30 chelerythrine attenuated the renal vasoconstrictor activity of Ang II. Although this effect was observed in all three groups in our study, the inhibitory effect of chelerythrine was much greater in the sham-operated NZGH and castrated NZGH treated with testosterone. These functional data suggest that the PKC signalling pathway contributes importantly to androgen potentiation of the renal vasoconstrictor activity of Ang II.
PKCα, PKCδ, and PKCε are implicated in Ang II stimulation of vascular smooth muscle.23,24,32 Accordingly, we assessed the effect of castration and testosterone supplementation on mRNA expression of the PKCα, PKCδ, and PKCε. Castration was associated with a significant decrease in mRNA expression for PKCδ and PKCε compared with sham-operated NZGH. Testosterone treatment restored mRNA expression of these isoforms to levels observed in sham-operated NZGH. However, in contrast to the mRNA data, western blot failed to detect significant changes in the level of protein expression for PKCδ and PKCε. Others showed that testosterone increased protein expression of PKCδ in coronary vascular smooth muscle.18 The reasons for these discrepancies are not immediately clear but may be related to differences in the source organs (heart vs. kidney) or in methodologies since we used kidney punches and Maddali18 used coronary artery cells in cell culture. Nevertheless, our data are at least consistent with the possibility that androgens may exert offsetting transcriptional and post-translational effects on PKC isoform expression.
Vascular contraction is dependent on the extent of MLC phosphorylation, which is determined by the balance of MLC kinase and MLC phosphatase activities.33 CPI-17 exerts an inhibitory effect on MLC phosphatase with consequent increases in MLC phosphorylation and vascular constriction.33 The inhibitory effect of CPI-17 is increased more than 1000-fold upon the phosphorylation at Thr-38 by PKC.43 Western blot analysis showed that CPI-17 and phospho-CPI-17 values were significantly lower in the castrated NZGH compared with the sham-operated group. On the other hand, testosterone treatment of castrated NZGH increased CPI-17 expression and, correspondingly, the phospho-CPI-17 values to levels comparable to the sham-operated NZGH. These findings suggest that androgens may modulate renal vascular reactivity to Ang II via an effect on CPI-17 expression and the amount of activated (phosphorylated) CPI-17. These effects may contribute to androgen modulation of renal vascular reactivity and hypertension.
There were some limitations inherent to the design of this study. Castrations and testosterone treatments were done at 5 weeks of age, while testing was performed at 16–17 weeks of age. Accordingly, the design precluded differentiation between the direct effects of testosterone and indirect effects mediated through androgenic effects on growth and development of, for example, blood vessels.4 Thus, the effects we have observed may represent secondary or tertiary effects of testosterone. In addition, the direct acute actions of testosterone on Ang II responsiveness and PKC-CPI-17 signalling were not assessed in the present study. Since acute administration of testosterone elicited vasodilation,4 the acute actions of testosterone on renal vascular responsiveness and PKC signalling are likely quite different from those reported herein. Future work dissecting the critical time points, intermediary steps, and length of exposure for androgen action on angiotensin responsiveness and PKC signalling are necessary.
In conclusion, the findings of this study support the view that androgens amplify the development of genetic hypertension. One mechanism contributing to this effect may be enhancement of signalling through the PKC-CPI-17 pathway. Greater understanding of these mechanisms may lead to the development of new drug targets for the treatment of hypertension.
Supplementary material
Supplementary Material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by the National Institutes of Health (grants #2R01HL063053-05A2 to D.S.M.) and from the American Heart Association (grants #0515443Z predoctoral fellowship to J.S. and #0515443Z grant in aid to D.S.M.).
Supplementary Material
References
- 1.Bazan E, Campbell AK, Rapoport RM. Protein kinase C activity in blood vessels from normotensive and spontaneously hypertensive rats. Eur J Pharmacol. 1992;227:343–348. doi: 10.1016/0922-4106(92)90014-m. [DOI] [PubMed] [Google Scholar]
- 2.Crofton JT, Share L. Gonadal hormones modulate deoxycorticosterone-salt hypertension in male and female rats. Hypertension. 1997;29:494–499. doi: 10.1161/01.hyp.29.1.494. [DOI] [PubMed] [Google Scholar]
- 3.Dahl LK, Knudsen KD, Ohanian EV, Muirhead M, Tuthill R. Role of the gonads in hypertension-prone rats. J Exp Med. 1975;142:748–759. doi: 10.1084/jem.142.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Card Res. 2002;53:688–708. doi: 10.1016/s0008-6363(01)00527-2. [DOI] [PubMed] [Google Scholar]
- 5.Fortepiani LA, Reckelhoff JF. Treatment with tetrahydrobiopterin reduces blood pressure in male SHR by reducing testosterone synthesis. Am J Physiol. 2005;288:R733–R736. doi: 10.1152/ajpregu.00500.2004. [DOI] [PubMed] [Google Scholar]
- 6.Fortepiani A, Yanes L, Zhang H, Racusen LC, Reckelhoff JF. Role of androgens in mediating renal injury in aging SHR. Hypertension. 2003;42:952–955. doi: 10.1161/01.HYP.0000099241.53121.7F. [DOI] [PubMed] [Google Scholar]
- 7.Ganten U, Schroder Witt G, Zimmermann F, Ganten D, Stock G. Sexual dimorphism of blood pressure in spontaneously hypertensive rats: effect of antiandrogen treatment. J Hypertens. 1989;7:721–726. [PubMed] [Google Scholar]
- 8.Goel A, Thor D, Anderson L, Rahimian R. Sexual dimorphism in rabbit aortic endothelial function under acute hyperglycemic conditions and gender-specific responses to acute 17beta-estradiol. Am J Physiol. 2008;294:H2411–H2420. doi: 10.1152/ajpheart.01217.2007. [DOI] [PubMed] [Google Scholar]
- 9.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of dahl hypertension. Hypertension. 2000;35:484–489. doi: 10.1161/01.hyp.35.1.484. [DOI] [PubMed] [Google Scholar]
- 10.Iams SG, Wexler BC. Inhibition of the development of spontaneous hypertension in SH rats by gonadectomy or estradiol. J Lab Clin Med. 1979;94:608–616. [PubMed] [Google Scholar]
- 11.Ishikura F, Asanuma T, Beppu S. Low testosterone levels in patients with mild hypertension recovered after antidepressant therapy in a male climacterium clinic. Hypertens Res. 2008;31:243–248. doi: 10.1291/hypres.31.243. [DOI] [PubMed] [Google Scholar]
- 12.Jackson EK, Herzer WA, Vyas SJ, Kost CK., Jr Angiotensin II-induced renal vasoconstriction in genetic hypertension. J Pharmacol Exp Ther. 1999;291:329–334. [PubMed] [Google Scholar]
- 13.Karanian JW, Ramwell PW. Effect of gender and sex steroids on the contractile response of canine coronary and renal blood vessels. J Cardiovasc Pharmacol. 1996;27:312–319. doi: 10.1097/00005344-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Kost CK, Jr, Herzer WA, Li P, Jackson EK. Vascular reactivity to angiotensin II is selectively enhanced in the kidneys of spontaneously hypertensive rats. J Pharmacol Exptl Ther. 1994;269:82–88. [PubMed] [Google Scholar]
- 15.Kost CK, Jr, Jackson EK. Enhanced renal angiotensin II subtype 1 receptor responses in the spontaneously hypertensive rat. Hypertension. 1993;21:420–431. doi: 10.1161/01.hyp.21.4.420. [DOI] [PubMed] [Google Scholar]
- 16.Lange DL, Haywood JR, Hinojosa-Laborde C. Role of the adrenal medullae in male and female DOCA-salt hypertensive rats. Hypertension. 1998;31:403–408. doi: 10.1161/01.hyp.31.1.403. [DOI] [PubMed] [Google Scholar]
- 17.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 18.Maddali KK, Korzick DH, Tharp DL, Bowles DK. PKCdelta mediates testosterone-induced increases in coronary smooth muscle Cav1.2. J Biol Chem. 2005;280:43024–43029. doi: 10.1074/jbc.M509147200. [DOI] [PubMed] [Google Scholar]
- 19.Malkin CJ, Jones RD, Jones TH, Channer KS. Effect of testosterone on ex vivo vascular reactivity in man. Clin Sci (Lond) 2006;111:265–274. doi: 10.1042/CS20050354. [DOI] [PubMed] [Google Scholar]
- 20.Martin D, Biltoft S, Redetzke R, Vogel E. Castration reduces blood pressure and autonomic venous tone in spontaneously hypertensive rats. J Hypertens. 2005;23:2229–2236. doi: 10.1097/01.hjh.0000191903.19230.79. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda K, Ruff A, Morinelli TA, Mathur RS, Halushka PV. Testosterone increases thromboxane A2 receptor density and responsiveness in rat aortas and platelets. Am J Physiol. 1994;267:H887–H893. doi: 10.1152/ajpheart.1994.267.3.H887. [DOI] [PubMed] [Google Scholar]
- 22.Nagahama T, Hayashi K, Ozawa Y, Takenaka T, Saruta T. Role of protein kinase C in angiotensin II-induced constriction of renal microvessels. Kidney Int. 2000;57:215–223. doi: 10.1046/j.1523-1755.2000.00822.x. [DOI] [PubMed] [Google Scholar]
- 23.Nelson CP, Willets JM, Davies NW, Challiss RA, Standen NB. Visualizing the temporal effects of vasoconstrictors on PKC translocation and Ca2+ signaling in single resistance arterial smooth muscle cells. Am J Physiol. 2008;295:C1590–C1601. doi: 10.1152/ajpcell.00365.2008. [DOI] [PubMed] [Google Scholar]
- 24.Ohanian V, Ohanian J, Scarth LS, Parker P, Heagerty A. Identification of protein kinase c isoforms in rat mesenteric small arteries and their possible role in agonist induced contraction. Circ Res. 1996;78:806–812. doi: 10.1161/01.res.78.5.806. [DOI] [PubMed] [Google Scholar]
- 25.Orshal JM, Khalil RA. Gender, sex hormones and vascular tone. Am J Physiol. 2004;286:R233–R249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 26.Palmgren E, Widgren B, Aurell M, Herlitz H. Increased renal vascular sensitivity to angiotensin II in hypertension is due to decreased response to prostaglandins. J Hypertens. 2003;21:969–976. doi: 10.1097/00004872-200305000-00022. [DOI] [PubMed] [Google Scholar]
- 27.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 28.Reckelhoff JF. Sex steroids, cardiovascular disease, and hypertension: unanswered questions and some speculations. Hypertension. 2005;45:170–174. doi: 10.1161/01.HYP.0000151825.36598.36. [DOI] [PubMed] [Google Scholar]
- 29.Reckelhoff JF, Zhang H, Srivastava K, Granger JP. Gender differences in hypertension in spontaneously hypertensive rats. Role of androgens and androgen receptor. Hypertension. 1999;34:920–923. doi: 10.1161/01.hyp.34.4.920. [DOI] [PubMed] [Google Scholar]
- 30.Ruan X, Arendshorst WJ. Role of protein kinase C in angiotensin II-induced renal vasoconstriction in genetically hypertensive rats. Am J Physiol. 1996;270:F945–F952. doi: 10.1152/ajprenal.1996.270.6.F945. [DOI] [PubMed] [Google Scholar]
- 31.Salamanca DA, Khalil RA. Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol. 2005;70:1537–1547. doi: 10.1016/j.bcp.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirisawa Y, Rutland T, Young J, Dean D, Benoit J. Modulation of protein kinase C (PKC)-mediated contraction and the possible role of PKC epsilon in rat mesenteric arteries. Front Biosci. 2003;8:133–138. doi: 10.2741/1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 34.Song J, Kost CK, Jr, Martin DS. Androgens augment renal vascular responses to ANG II in New Zealand genetically hypertensive rats. Am J Physiol. 2006;290:R1608–R1615. doi: 10.1152/ajpregu.00364.2005. [DOI] [PubMed] [Google Scholar]
- 35.Song J, Kost CK, Jr, Martin DS. Androgens potentiate renal vascular responses to angiotensin II via amplification of the Rho kinase signaling pathway. Card Res. 2006;72:456–463. doi: 10.1016/j.cardiores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Song J, Martin DS. Rho kinase contributes to androgen amplification of renal vasoconstrictor responses in the spontaneously hypertensive rat. J Cardiovasc Pharmacol. 2006;48:103–109. doi: 10.1097/01.fjc.0000245403.45406.d8. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan JC, Sasser JM, Pollock JS. Sexual dimorphism in oxidant status in spontaneously hypertensive rats. Am J Physiol. 2007;292:R764–R768. doi: 10.1152/ajpregu.00322.2006. [DOI] [PubMed] [Google Scholar]
- 38.Tatchum-Talom R, Martel C, Marette A. Effects of ethinyl estradiol, estradiol, and testosterone on hindlimb endothelial function in vivo. J Cardiovasc Pharmacol. 2002;39:496–502. doi: 10.1097/00005344-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Toot J, Jenkins C, Dunphy G, Boehme S, Hart M, Milsted A, et al. Testosterone influences renal electrolyte excretion in SHR/y and WKY males. BMC Physiol. 2008;8:5. doi: 10.1186/1472-6793-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turla MB, Webb RC. Enhanced vascular reactivity to protein kinase C activators in genetically hypertensive rats. Hypertension. 1987;9:III150–154. doi: 10.1161/01.hyp.9.6_pt_2.iii150. [DOI] [PubMed] [Google Scholar]
- 41.Turla MB, Webb RC. Vascular responsiveness to protein kinase C activators in mineralocorticoid-hypertensive rats. J Hypertens. 1991;9:209–215. doi: 10.1097/00004872-199103000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Vyas SJ, Jackson EK. Angiotensin II: enhanced renal responsiveness in young genetically hypertensive rats. J Pharmacol Exp Ther. 1995;273:768–777. [PubMed] [Google Scholar]
- 43.Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca(2+) sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol. 2001;535:553–564. doi: 10.1111/j.1469-7793.2001.t01-1-00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.