Abstract
Aims
Resveratrol activates Sirtuin 1 (SIRT1), a nicotinamide adenine dinucleotide-dependent deacetylase which modulates metabolic homeostasis and improves several pathophysiological features present in diseases of ageing. In particular, it has been shown that SIRT1 activation improves endothelial dysfunction and suppresses vascular inflammation, two central pathophysiological processes involved in the initiation and progression of cardiovascular disease. The downstream targets of SIRT1 activation in this context, however, remain poorly defined. Therefore, in this study, we aimed to characterize mechanistically how SIRT1 activation regulates the endothelial vasoprotective phenotype.
Methods and results
We demonstrate that SIRT1 activation by resveratrol increases the expression of the transcription factor Krüppel-like factor 2 (KLF2) in human vascular endothelial cells, resulting in the orchestrated regulation of transcriptional programs critical for conferring an endothelial vasoprotective phenotype. Moreover, we show that KLF2 upregulation by resveratrol occurs via a mitogen-activated protein kinase 5/myocyte enhancing factor 2-dependent signalling pathway.
Conclusion
Collectively, these observations provide a new mechanistic framework to understand the vascular protective effects mediated by SIRT1 activators and define KLF2 as a critical mediator of these effects.
Keywords: Endothelial, Vascular, SIRT1, Resveratrol, Krüppel-like factor 2
1. Introduction
In the last decade, an increasing number of studies have demonstrated that resveratrol (3,5,4′-trihydroxystilbene) exerts a wide range of protective effects on the cardiovascular system.1 These effects, which confer anti-inflammatory, vasodilatory, and anti-thrombotic properties to the vasculature, may be explained by the activation of the NAD+-dependent deacetylase Sirtuin 1 (SIRT1).2,3 Previous studies have demonstrated that endothelial-specific overexpression of SIRT1 improves endothelial function and decreases atherosclerosis in ApoE null mice.4 SIRT1 has also been shown to regulate vascular tone via the activation of endothelial nitric oxide synthase (eNOS).5 Given the critical role for SIRT1 in maintaining vascular endothelial homeostasis, SIRT1 activators have received significant attention as potential pharmacological agents for the treatment of cardiovascular disease. Indeed, resveratrol administration has been shown to improve endothelial dysfunction4,6,7 and reduce vascular inflammation in mice.8 Despite these reported benefits, the underlying mechanisms through which resveratrol and SIRT1 improve endothelial dysfunction remain largely unknown.
Our laboratory and others have demonstrated that the transcription factor Krüppel-like factor 2 (KLF2) functions as an as inhibitor of angiogenesis,9 and a critical regulator of the flow-mediated vasoprotective endothelial phenotype,10–12 conferring anti-thrombotic, anti-inflammatory, and vasodilatory properties to the vascular endothelium.12 Moreover, recently published studies have demonstrated that ApoE null mice bearing a hemizygous deficiency in the KLF2 gene exhibit an increase in atherosclerotic lesion area in the aorta when compared with wild-type mice,13 thus validating the vasoprotective function of KLF2 in vivo.
Recently, we performed a small molecule screen to identify novel regulators of KLF2 expression in endothelial cells and uncovered resveratrol as a potent inducer of KLF2 (Mack P.J., Parmar K.M., García-Cardeña G., unpublished results). In this study, we examined whether resveratrol induces a vasoprotective endothelial cell phenotype via KLF2 and have begun to define the molecular mechanisms involved in this process.
2. Methods
2.1. Endothelial cell culture and adenovirus-mediated infection
Human umbilical vein endothelial cells (HUVEC) were isolated from several normal term umbilical cords, pooled, and cultured as described previously14 by the core cell culture laboratory of the Centre for Excellence in Vascular Biology at the Brigham and Women's Hospital. These human tissues are routinely obtained as ‘Discarded Pathology Tissues’ for research purposes, without attributes to specific donor, under existing institutionally approved protocols (BWH Human Research Committee IRB). For Ad-MEK5DN (MOI = 20) and Ad-GFP (MOI = 20) experiments, cells at 85–90% confluency were infected for 12 h, washed with media, and then incubated for an additional 12 h. This was followed by 8 h of incubation with either 100 µM resveratrol (Sigma) or ethanol vehicle. For Ad-MEF2ASA (MOI = 50) and Ad-GFP (MOI = 50) experiments, cells at 85–90% confluency were infected for 24 h, after which the cells were washed and incubated for 16 h. Cells were then treated with 100 µM resveratrol or ethanol vehicle for an additional 8 h.
2.2. Sirtinol experiments
HUVEC at 90–100% confluency were treated for 1 h with either vehicle (DMSO), 50 µM, or 75 µM sirtinol (Sigma), after which 100 µM resveratrol or ethanol vehicle was added and the cells were incubated for an additional 8 h.
2.3. AMP-activated kinase inhibition
HUVEC at 90–100% confluency were treated for 30 min with either vehicle (DMSO) or 20 µM compound C (Calbiochem),15 then 100 µM resveratrol or ethanol vehicle was added, and the cells incubated for an additional 4 or 8 h.
2.4. RNA isolation and real-time TaqMan PCR
Cells were lysed, RNA isolated, and real-time TaqMan PCR performed as described previously.14
2.5. siRNA experiments
HUVEC were transfected as previously described with minor modifications.14 Briefly, cells were transfected at 30–40% confluency using either Lipofectamine RNAiMAX or Oligofectamine (Invitrogen). At 24 h post-transfection, cells were replated at 90–100% confluency. For siKLF2 experiments, cells at 38 h post-transfection were treated for an additional 24 h with either 100 µM resveratrol or ethanol vehicle. For siSIRT1 and siERK5 experiments, cells at 45 h post-transfection were treated for an additional 8 h with either 100 µM resveratrol or ethanol vehicle. The following Invitrogen Stealth siRNA duplexes were utilized: HSS145585 (KLF2, 30 nM, Lipofectamine RNAiMAX); HSS118729 (SIRT1, 100 nM, Oligofectamine); HSS183373 (ERK5, 100 nM, Oligofectamine). Each Stealth siRNA duplex was matched with the appropriate Stealth siRNA GC negative control from Invitrogen. All results were validated using an additional siRNA targeting a different region of the respective transcript [ERK5 siRNA (s11149; Ambion); SIRT1 siRNA (HSS118730; Invitrogen); KLF2 shRNA (TRCN0000020728; Sigma)]. The efficiency of the siRNA experiments was further validated by analysing SIRT1 and ERK5 protein levels via western blotting as described below.
2.6. Western blotting
HUVEC at 90–100% confluency were incubated with either 100 µM resveratrol, 1 µM simvastatin, or ethanol vehicle for 8 h. After stimulation, cells were washed and lysed at 4°C in RIPA buffer supplemented with protease (Roche Applied Science) and phosphatase (Thermo Scientific) inhibitors. Cells lysates were subjected to SDS–PAGE and immunoblotting as previously described.12 ERK5 polyclonal antibody (1:1000) was from Cell Signaling (3372), monoclonal antibody against alpha-tubulin (1:1000) was from Santa Cruz Biotechnology Inc. (sc-14262), and SIRT1 polyclonal antibody (1:1000) was from Epitomics (1104-1).
2.7. Statistical analysis
Statistical analysis was performed with SPSS Statistics Software. Student's t-test was used for comparing differences between two groups, and one-way ANOVA followed by Tukey's HSD test for comparing differences between multiple groups. Differences were considered significant at P < 0.05.
3. Results
3.1. Resveratrol induces endothelial KLF2 expression
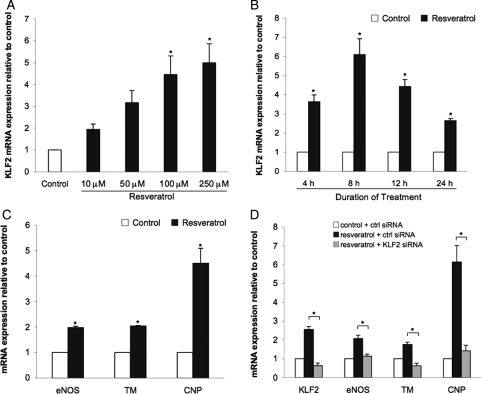
Human endothelial cells treated with increasing concentrations of resveratrol exhibited an induction of KLF2 mRNA expression (Figure 1A). Since 100 µM resveratrol resulted in a similar induction of KLF2 compared with the higher dose, this concentration was used in all subsequent experiments. As shown in Figure 1B, resveratrol induction of KLF2 followed a time-dependent pattern. The resveratrol-mediated increase in KLF2 expression was detected after 4 h of incubation, peaked at 8 h, and gradually decreased at later time points.
Figure 1.
Resveratrol induces the expression of KLF2 and KLF2-dependent endothelial atheroprotective genes. (A) KLF2 mRNA levels in HUVEC cultured for 8 h with 10, 50, 100, and 250 µM of resveratrol and compared with ethanol vehicle treatment (*P < 0.05 vs. ethanol control; n = 3; ANOVA, Tukey's HSD test). (B) KLF2 expression in HUVEC incubated with 100 µM resveratrol for the indicated periods of time (*P < 0.05 vs. its time-corresponding ethanol vehicle control; n = 4; Student's t-test). (C) Expression of known KLF2 targets in HUVEC after 24 h of resveratrol (100 µM) stimulation (*P < 0.05 vs. ethanol vehicle control; n = 3; Student's t-test). (D) Effect of KLF2 silencing on expression of resveratrol-induced vasoprotective targets (*P < 0.05; n = 3; ANOVA, Tukey's HSD test). Data expressed as mean ± SEM.
3.2. Resveratrol-induced expression of endothelial vasoprotective genes is dependent on KLF2 expression
We and others have previously shown that atheroprotective flow and statins increase the expression of several KLF2 downstream transcriptional targets whose expression confers a vasoprotective endothelial phenotype.12,16 To test whether resveratrol exerts the same effects on gene expression, endothelial cells were incubated for 24 h with resveratrol and the expression of eNOS, thrombomodulin (TM), and c-type natriuretic peptide (CNP) was analysed by quantitative PCR. As shown in Figure 1C, stimulation of endothelial cells with resveratrol resulted in a significant induction of the vasoprotective genes studied. To document whether the expression of these vasoprotective genes was KLF2-dependent, the resveratrol-mediated effect on eNOS, TM, and CNP expression was assessed in endothelial cells where the expression of KLF2 was silenced. These experiments clearly showed that when KLF2 expression was repressed, resveratrol failed to induce the expression of the vasoprotective genes analysed (Figure 1D).
3.3. Resveratrol induces KLF2 expression through SIRT1
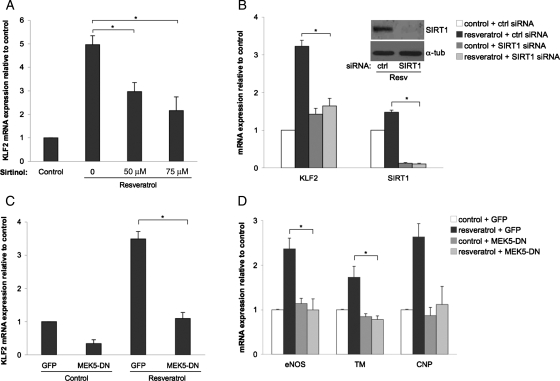
To investigate whether resveratrol induction of KLF2 expression in endothelial cells is mediated by sirtuins in general, and SIRT1 in particular, we performed the following experiments. First, endothelial cells were treated with two different concentrations of the non-specific sirtuin inhibitor sirtinol, and the effect of these treatments on resveratrol-mediated KLF2 induction was assessed. As demonstrated in Figure 2A, sirtinol inhibited resveratrol-mediated KLF2 induction in a concentration-dependent manner, suggesting that sirtuin activity is necessary for KLF2 induction by resveratrol. Second, endothelial cells were transfected using a specific siRNA targeting SIRT1, and the induction of KLF2 was determined after treatment with resveratrol. These experiments clearly documented that SIRT1 mediates the resveratrol-dependent induction of KLF2 (Figure 2B).
Figure 2.
Resveratrol induces KLF2 expression via SIRT1 and MEK5. (A) KLF2 mRNA expression in HUVEC stimulated with resveratrol (100 µM) for 8 h in the absence or presence of increasing concentrations of the non-specific sirtuin inhibitor sirtinol (*P < 0.05; n = 4; ANOVA, Tukey's HSD test). (B) Effect of sirtuin-1 (SIRT1) silencing on induction of KLF2 expression by resveratrol (*P < 0.05; n = 3; ANOVA, Tukey's HSD test). Insert shows representative western blot demonstrating SIRT1 silencing efficiency. (C) KLF2 mRNA expression in HUVEC infected with MEK5-DN or GFP-control virus followed by incubation with resveratrol or its vehicle (#P < 0.01; n = 3; ANOVA, Tukey's HSD test). (D) Effect of MEK5-DN on the resveratrol-dependent expression of KLF2 transcriptional targets (*P < 0.05; n = 3; ANOVA, Tukey's HSD test). Data expressed as mean ± SEM.
3.4. KLF2 induction by resveratrol is MEK5 and MEF2 dependent but ERK5 independent
To further investigate the signalling pathway leading to KLF2 upregulation by resveratrol, we assessed the role of the three known components of the flow-mediated KLF2 signalling pathway, namely, mitogen-activated protein kinase 5 (MEK5), ERK5, and myocyte enhancing factor 2 (MEF2).12,17
The role of MEK5 was determined by quantifying the induction of KLF2 and its downstream transcriptional targets (eNOS, TM, and CNP) in resveratrol-treated endothelial cells infected with control adenovirus or virus expressing a dominant-negative MEK5 mutant (MEK5-DN). As shown in Figure 2C and D, MEK5-DN abrogated the resveratrol-mediated upregulation of KLF2 and that of its downstream transcriptional targets eNOS and TM, demonstrating that MEK5 involvement is conserved in both biomechanical and resveratrol-mediated vasoprotective gene expression.
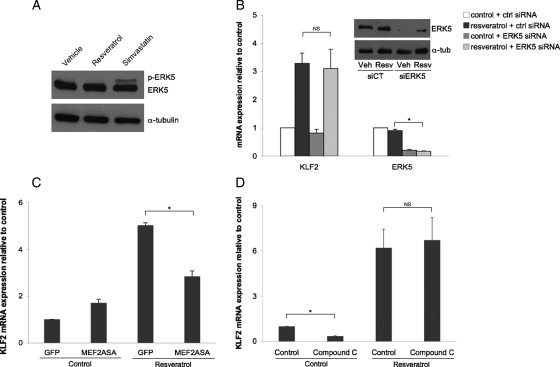
Since ERK5, a known target of MEK5, is activated by biomechanical stimuli and statins12,18 (Garcia-Cardena, unpublished results), we next explored whether ERK5 was necessary for the resveratrol-mediated induction of KLF2. ERK5 activation was assessed by determining its phosphorylation state in cells incubated with resveratrol or simvastatin, which served as a positive control. As seen in Figure 3A, resveratrol did not induce ERK5 phosphorylation, demonstrating that phosphorylation of this kinase is not required for the resveratrol-mediated KLF2 induction. This finding was further confirmed by documenting the effect of ERK5 silencing on KLF2 induction by resveratrol. ERK5 silencing did not modify KLF2 induction by resveratrol (Figure 3B). These findings demonstrate that ERK5 is not necessary for the resveratrol-mediated KLF2 induction.
Figure 3.
KLF2 induction by resveratrol is ERK5 and AMPK independent, but partly dependent on MEF2. (A) Western blot of phospho-ERK5, ERK5, and house keeping protein alpha-tubulin from HUVEC stimulated for 8 h with ethanol vehicle, resveratrol (100 µM) or simvastatin (1 µM). (B) Effect of ERK5 silencing on resveratrol-induced KLF2 expression in HUVEC (*P < 0.05; n = 3; ANOVA, Tukey's HSD test). Insert shows representative western blot demonstrating ERK5 silencing efficiency. (C) KLF2 mRNA expression in HUVEC infected with MEF2ASA or GFP-control virus and stimulated with resveratrol or its vehicle (*P < 0.05; n = 3; ANOVA, Tukey's HSD test). (D) Effect of AMPK inhibition on resveratrol-mediated KLF2 expression in HUVEC (*P < 0.05; n = 3; ANOVA, Tukey's HSD test). Data expressed as mean ± SEM.
Previous studies have shown that the transcription factor MEF2 is necessary for the induction of KLF2 by biomechanical and other types of stimuli,12,14,19,20 therefore we next investigated whether MEF2 was required for the resveratrol-mediated increase in KLF2. As seen in Figure 3C, cells expressing a dominant-negative MEF2 mutant (MEF2ASA) exhibited a significant reduction in KLF2 induction by resveratrol when compared with cells expressing GFP control. These observations demonstrate that MEF2 is an important component of the resveratrol-KLF2 signalling pathway.
3.5. Resveratrol-mediated KLF2 induction is AMP-activated kinase independent
A recent report from our group has shown that the activation of the AMP-activated kinase (AMPK) by flow leads to the expression of KLF2 in cultured endothelial cells.15 Since SIRT1 has also been shown to activate AMPK,21 we next examined whether this kinase is involved in the resveratrol-mediated KLF2 induction. As shown in Figure 3D, AMPK inhibition by compound C caused a significant reduction in the basal expression of KLF2, however, it did not affect the resveratrol-mediated upregulation of KLF2 expression after 8 h of treatment. Similar results were obtained evaluating a shorter (4 h) stimulation period (data not shown).
4. Discussion
SIRT1 activators are being actively investigated as potential therapeutic agents for diseases of ageing such as type II diabetes and atherosclerosis.22,23 Indeed, SIRT1 activation has been shown to improve vascular function via an increase in eNOS expression, as well as suppress atherosclerotic lesion formation.4 Despite these protective effects, the underlying molecular mechanisms involved remain largely unknown.
Here, we reveal the transcription factor KLF2 as a critical mediator of the endothelial vasoprotective phenotype conferred by the SIRT1 activator resveratrol. Our data demonstrate that resveratrol induces the expression of KLF2 in cultured human endothelial cells, and importantly, that this upregulation is necessary for the resveratrol-mediated increase in the endothelial vasoprotective genes studied. Additionally, we show that the induction of KLF2 by resveratrol is dependent on SIRT1, suggesting that KLF2 may also be required for the vascular protection evoked by caloric restriction, a physiological activator of SIRT1.24
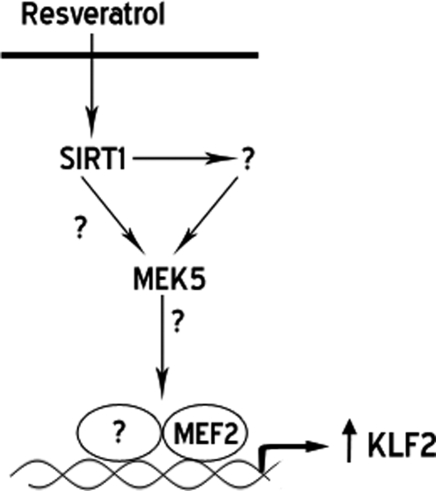
SIRT1 is capable of regulating gene expression via multiple pathways.25 Since the screen that led us to the identification of resveratrol as a KLF2 inducer was based on the transcriptional activity of the proximal KLF2 promoter, we focused our investigation on the MEK5/ERK5/MEF2 signalling pathway. This pathway has been previously described to be necessary and sufficient for the induction of KLF2 by atheroprotective flow.12,17 Our data demonstrate that the induction of KLF2 by resveratrol is MEK5 and MEF2 dependent, but ERK5 independent, highlighting a central role of the MEK5/MEF2 pathway in the expression of KLF2 in the vascular endothelium. Consistent with these observations, a recent report demonstrated that Angiopoietin-1 induction of KLF2 requires MEF2 but not ERK5.20 A working model for the induction of KLF2 by resveratrol, summarizing the herein presented data, is depicted in Figure 4.
Figure 4.
Working model for the resveratrol induction of KLF2. SIRT1 (Sirtuin 1), MEK5 (mitogen-activated protein kinase 5), MEF2 (myocyte enhancing factor 2), KLF2 (Krüppel-like factor 2).
Previous studies have shown that resveratrol regulates the AMPK signalling pathway via the SIRT1-mediated deacetylation of LKB1.21 Additionally, we have recently shown that the flow-mediated activation of AMPK leads to the expression of KLF2 in endothelial cells.15 On the basis of these observations, in this study, we explored the potential involvement of AMPK in the upregulation of KLF2 by resveratrol, and document that the pharmacological inhibition of AMPK does not affect the resveratrol-mediated increase of KLF2 expression. Due to the limited knowledge on additional signalling pathways linking SIRT1 and MEK5 activation, high-throughput approaches are currently being employed to mechanistically define how resveratrol leads to the activation of the MEK5/MEF2 signalling pathway.
Collectively, these observations provide a new mechanistic framework to better understand the effects of SIRT1 activators on the cardiovascular system, and will allow us to examine the possibility that dysregulation of the SIRT1/KLF2 axis is involved in the transcriptional changes driving endothelial dysfunction in the diseases of ageing.
Funding
This work was supported by the National Institutes of Health [Grants HL-076686 and HL-090856 to G.G.-C.]; the Department of Innovation, Universities and Enterprise, Government of Catalonia, Spain [2007 BP-A 00137 to J.G.-S.]; the Howard Hughes Medical Institute Research Training Fellowship for Medical Students (G.V.); and the American Federation for Aging Research/National Institute on Aging [T35 AG026781 to G.V.].
Acknowledgements
We thank Kay Case and Vanessa Davis for isolation and culture of human endothelial cells, and Leonidha Pulluqi for assistance in graphic design.
Conflict of interest: none declared.
References
- 1.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 2.Kaur G, Roberti M, Raul F, Pendurthi UR. Suppression of human monocyte tissue factor induction by red wine phenolics and synthetic derivatives of resveratrol. Thromb Res. 2007;119:247–256. doi: 10.1016/j.thromres.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, et al. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80:191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das S, Alagappan VK, Bagchi D, Sharma HS, Maulik N, Das DK. Coordinated induction of iNOS-VEGF-KDR-eNOS after resveratrol consumption: a potential mechanism for resveratrol preconditioning of the heart. Vascul Pharmacol. 2005;42:281–289. doi: 10.1016/j.vph.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Do GM, Kwon EY, Kim HJ, Jeon SM, Ha TY, Park T, et al. Long-term effects of resveratrol supplementation on suppression of atherogenic lesion formation and cholesterol synthesis in apo E-deficient mice. Biochem Biophys Res Commun. 2008;374:55–59. doi: 10.1016/j.bbrc.2008.06.113. [DOI] [PubMed] [Google Scholar]
- 8.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawanami D, Mahabeleshwar GH, Lin Z, Atkins GB, Hamik A, Haldar SM, et al. Kruppel-like factor 2 inhibits hypoxia-inducible factor 1{alpha} expression and function in the endothelium. J Biol Chem. 2009;284:20522–20530. doi: 10.1074/jbc.M109.025346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, Seppen J, et al. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol. 2005;167:609–618. doi: 10.1016/S0002-9440(10)63002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huddleson JP, Ahmad N, Srinivasan S, Lingrel JB. Induction of KLF2 by fluid shear stress requires a novel promoter element activated by a phosphatidylinositol 3-kinase-dependent chromatin-remodeling pathway. J Biol Chem. 2005;280:23371–23379. doi: 10.1074/jbc.M413839200. [DOI] [PubMed] [Google Scholar]
- 12.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atkins GB, Wang Y, Mahabeleshwar GH, Shi H, Gao H, Kawanami D, et al. Hemizygous deficiency of Kruppel-like factor 2 augments experimental atherosclerosis. Circ Res. 2008;103:690–693. doi: 10.1161/CIRCRESAHA.108.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parmar KM, Nambudiri V, Dai G, Larman HB, Gimbrone MA, Jr, Garcia-Cardena G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J Biol Chem. 2005;280:26714–26719. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- 15.Young A, Wu W, Sun W, Larman HB, Wang N, Li YS, et al. Flow activation of AMP-activated protein kinase in vascular endothelium leads to Kruppel-like factor 2 expression. Arterioscler Thromb Vasc Biol. 2009;29:1902–1908. doi: 10.1161/ATVBAHA.109.193540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, et al. KLF2 is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinderlerer AR, Ali F, Johns M, Lidington EA, Leung V, Boyle JJ, et al. KLF2-dependent, shear stress-induced expression of CD59: a novel cytoprotective mechanism against complement-mediated injury in the vasculature. J Biol Chem. 2008;283:14636–14644. doi: 10.1074/jbc.M800362200. [DOI] [PubMed] [Google Scholar]
- 18.Yan C, Takahashi M, Okuda M, Lee JD, Berk BC. Fluid shear stress stimulates big mitogen-activated protein kinase 1 (BMK1) activity in endothelial cells. Dependence on tyrosine kinases and intracellular calcium. J Biol Chem. 1999;274:143–150. doi: 10.1074/jbc.274.1.143. [DOI] [PubMed] [Google Scholar]
- 19.Sen-Banerjee S, Mir S, Lin Z, Hamik A, Atkins GB, Das H, et al. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720–726. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]
- 20.Sako K, Fukuhara S, Minami T, Hamakubo T, Song H, Kodama T, et al. Angiopoietin-1 induces Kruppel-like factor 2 expression through a phosphoinositide 3-kinase/AKT-dependent activation of myocyte enhancer factor 2. J Biol Chem. 2009;284:5592–5601. doi: 10.1074/jbc.M806928200. [DOI] [PubMed] [Google Scholar]
- 21.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins—novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- 24.Haigis MC, Guarente LP. Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 25.Potente M, Dimmeler S. Emerging roles of SIRT1 in vascular endothelial homeostasis. Cell Cycle. 2008;7:2117–2122. doi: 10.4161/cc.7.14.6267. [DOI] [PubMed] [Google Scholar]