Abstract
Background
We explored the relationship of genetic variants of the serotonin transporter gene SLC6A4, a key regulator of the serotonergic neurotransmission, with both depressive symptoms and plasma Interleukin-6 (IL-6) levels.
Methods and Results
We genotyped 20 polymorphisms in 360 male twins (mean age: 54) from the Vietnam Era Twin Registry. Current depressive symptoms were measured with the Beck Depression Inventory-II (BDI-II). IL-6 was assessed using a commercially available ELISA kit. Genotype associations were analyzed using generalized estimating equations. To study how SLC6A4 genetic vulnerability influences the relationship between depressive symptoms and IL-6, bivariate models were constructed using structural equation modeling. Of the 20 polymorphisms examined, the effective number of independent tests was 6 and the threshold of significance after Bonferroni correction was 0.008. There were 6 SNPs significantly associated with BDI (P≤0.008), including rs8071667, rs2020936, rs25528, rs6354, rs11080122 and rs8076005, and 1 SNP borderline associated (rs12150214, P=0.017). Of these 7 SNPs, 3 were also significantly associated with IL-6 (P<0.008), including rs25528, rs6354 and rs8076005, and the other 4 were borderline associated (P=0.009~0.025). The subjects with one copy of the minor allele of these 7 SNPs had higher BDI scores and IL-6 levels. Further bivariate modeling revealed that approximately 10% of the correlation between BDI and IL-6 could be explained by the SLC6A4 gene.
Conclusions
Genetic vulnerability involving the SLC6A4 gene is significantly associated with both increased depressive symptoms and elevated IL-6 plasma levels. Common pathophysiological processes may link depression and inflammation, and implicate the serotonin pathway in neural-immune interactions.
Keywords: atherosclerosis, epidemiology, genetics, inflammation, depression
The relationship between depression and the incidence of coronary artery disease (CAD) is well established. 1 Adverse lifestyle behaviors, lower heart rate variability, and enhanced platelet activation have long been considered potential explanations for this association. 2 Of more recent interest is the role of inflammation in the development and progression of atherosclerosis 3 and its potential association with depression. 4 Major depression and depressive symptoms are associated with higher levels of inflammatory biomarkers such as interleukin-6 (IL-6). 5 However, the causal direction of this association remains unclear and may be bidirectional, such that neurobiological correlates of depression, or physical illness, may result in enhanced inflammation, and the latter, in turn, may increase the risk of depression. 6
It is also plausible that a common genetic vulnerability accounts for the observed association between depression and inflammation, since both phenotypes are heritable. 7, 8 We have recently reported evidence for a common genetic pathway linking depressive symptoms and IL-6 using twins; specifically, we found that over two-thirds of the covariance between these two phenotypes could be explained by genes they have in common. 8 Potential candidates for this shared genetic substrate are genetic variants involved in neurobiological pathways of the stress response, which are associated with both depression and inflammation. Among these, the serotoninergic pathway is of particular interest.
Serotonin (5-HT) is released by human mast cells and platelets following antigen challenge. 9 This neurotransmitter is well known for its role in depression, 10 but there is also evidence that serotonin modulates immune responses 11 and proinflammatory cytokine synthesis. 12 The serotonin transporter (5HTT) is a key protein of the serotonergic system which regulates serotonin concentration in the synaptic cleft and extrasynaptic sites. 13 The human serotonin transporter gene (SLC6A4) is mapped to chromosome 17q11.1–17q12, and several polymorphisms within the SLC6A4 gene have been described. 14 One of the most frequently studied functional polymorphisms of the SLC6A4 gene is the serotonergic transporter-linked polymorphic region (5-HTTLPR) located in the promoter region, which is a 44-bp insertion or deletion polymorphism producing two common alleles, long (L) and short (S) alleles. 15 The homozygous or heterozygous carriage of the S allele is associated with lower transcription of 5-HTT, and therefore with increased serotonin binding and reuptake. 16 Some studies have found an association between 5HTTLPR and depression, 17, 18 while others have not. 19, 20 Only a few studies investigated the relationship of other polymorphisms within the SLC6A4 gene and depression. 21 Furthermore, no study has investigated the association of variants of the SLC6A4 gene with plasma IL-6 levels.
In the present study, we investigated the association between polymorphisms of the SLC6A4 gene on depressive symptoms and IL-6 levels in a well-characterized sample of middle-aged male twins. The study of twins allowed us to quantify the role of the specific genotype of interest in explaining shared genetic effects using bivariate structural equation models (SEM). 22
METHODS
Study Populations
The Twins Heart Study (THS) is an investigation of psychological, behavioral and biological risk factors for subclinical cardiovascular disease using twins. Twins were selected from the Vietnam Era Twin (VET) Registry, 23 which includes 7369 middle-aged male-male twin pairs both of whom served in the United States military during the time of the Vietnam War.
The THS included 180 twin pairs (102 monozygotic [MZ] and 78 dizygotic [DZ]), who were born between 1946 and 1956. The methods of construction of this sample were described in the Supplemental Material and were also described previously. 8 Twin pairs were examined together at the Emory University General Clinical Research Center between March 2002 and March 2006, and maintained an identical schedule during examination. All twins had a comprehensive physical exam and were queried about previous diagnoses of cardiovascular diseases. We also administered the Structured Clinical Interview for DSM-IV (SCID) 24 to classify subjects based on a lifetime history of major depressive disorder (MDD). The final data included 105 twin pairs where neither twin had a history of MDD, 68 twin pairs discordant for history of MDD and 7 twin pairs where both had a history of MDD. Compared to the normal twin pairs, the discordant twin pairs and the concordant-depressed twin pairs were younger, had higher prevalence of diabetes, more depressive symptoms and more likely to use antidepressants. For other study variables, there were no significant differences among these twin pairs. Because few twins (N=8) had a current major depressive episode, we focused on current level of depressive symptoms measured with the continuous Beck Depression Inventory-II score. Therefore, we used all the twins in the analysis rather than study the twins based on discordant and concordant for history of MDD or current major depressive episode. This protocol was approved by the Institutional Review Board at Emory University and informed consent was obtained from all subjects.
Assessment of Depression
Lifetime history of MDD was assessed by means of the SCID, which yields a clinical diagnosis of major depression based on a lifetime history of major depressive episodes. 24 We also collected information on current level of depressive symptoms using the Beck Depression Inventory-II (BDI), a standardized scale providing a continuous measure of depressive symptoms. 25 It has been used extensively in community samples and has satisfactory test-retest and internal consistency reliability. 26
Assessment of Interleukin-6
Plasma level of interleukin-6 was assessed using commercially available high-sensitivity ELISA kits obtained from R and D Systems. All samples were run in duplicate. Inter- and intra-assay variability were reliably <10%. The values of IL-6 were log-transformed to improve distribution. We were also able to evaluate the multiplicative changes due to genetic variants, i.e., percent change of IL-6 for twins with the risk allele compared with those without the risk allele.
Other Measurements
We measured weight, height, blood pressure, high- and low-density lipoprotein (HDL and LDL) cholesterol, and fasting glucose, as reported previously. 8 Physical activity was assessed with a modified version of the Baecke Questionnaire of Habitual Physical Activity; this is a 16-question instrument documenting level of physical activity at work, during sports and non-sports activities. The global physical activity score was used in the analysis. Cigarette smoking was classified into current versus never or past smoker. A history of coronary heart disease (CHD) occurring since 1990 was defined as a previous diagnosis of myocardial infarction or angina pectoris, or previous coronary revascularization procedures. Diabetes mellitus was defined as having a fasting glucose level > 126 mg/dl or being treated with anti-diabetic medications. Framingham risk score, a commonly employed summary index of CAD risk, was also calculated to incorporate information on presence and severity of the following risk factors: age, LDL cholesterol, HDL cholesterol, blood pressure, diabetes mellitus, and current smoking. 27 Information on current use of anti-depressants was also collected.
Genotyping the SLC6A4 Polymorphisms and Multiple Testing
5-HTTLPR Polymorphism Genotyping
Genotyping of the 5-HTTLPR length polymorphism was carried out using polymerase chain reaction (PCR). PCR was performed in 384 well plates in a 10 μl volume with 10 ng of genomic DNA. The PCR products were mixed with a ROX 500 sizing ladder (Applied Biosystems) then separated using an Applied Biosystems 3100 genetic analyzer and analyzed with Applied Biosystems GeneMapper 4.0 software. Fragment lengths for the L-allele were 291 bp and 247 bp for the S-allele. The primers and PCR reaction conditions can be obtained by request.
Single Nucleotide Polymorphisms Genotyping
We used the HapMap Caucasian panel (CEPH, release 24, build 36) to select SLC6A4 SNPs. Twenty-three SNPs with minor allele frequency (MAF) > 0.05 were selected. An additional SNP (rs6355) with MAF of 0.03 was also selected since it was located in the codon region. A total of 24 SNPs were genotyped in our sample using the Beckman GenomeLab SNPstream Genotyping System (Beckman Coulter, Inc., Fullerton, CA), while 5 SNPs failed to perform well in genotyping assays, and were therefore discarded from further analysis. However, these 5 SNPs were in strong linkage disequilibrium (LD) with other 19 SNPs (pair-wise r2 range from 0.85 to 1). Genotyping accuracy for all 19 SNPs was 99% as assessed by inclusion of duplicates (pairs of MZ twins) in the arrays, and negative controls (water blanks) were included on each plate.
Multiple Testing
We used the method developed by Gao et al. for multiple testing correction since there were 20 polymorphisms being studied. 28 Briefly, this method uses composite LD among SNPs to capture the correlation and derives the effective number of independent tests using a principal component approach. Then the Bonforroni correction can be implemented based on the number of independent tests. Gao et al.'s method is accurate and efficient, and is comparable to the permutation test. 28 By using this method, we performed 6 independent tests from 20 correlated polymorphisms. To achieve a nominal significance of 0.05, the threshold after Bonferroni correction was 0.05/6=0.008.
Statistical Analyses
The main purpose of our analyses was to test the associations between each single polymorphism and haplotype variations in the SLC6A4 gene with depressive symptoms and IL-6 plasma levels. We further investigated to what extent the covariance between depressive symptoms and IL-6 could be explained by variations in the SLC6A4 gene.
We used Haploview 4.0 software for computing Hardy-Weinberg Equilibrium (HWE), MAF and pair-wise LD between the studied polymorphisms, where one twin was randomly selected from each pair for the calculation. 29 Association analyses were performed using Generalized Estimating Equations (GEE), which corrects for the relatedness between twins. The exchangeable correlation structure was used for the GEE. Analyses were first done separately for each of the studied polymorphisms and followed up by haplotype analysis. For individual polymorphism analysis, we tested the additive model of polymorphisms on depressive symptoms and IL-6 levels respectively, where the polymorphisms were coded as 0, 1 or 2 depending on the carrier status of the minor allele. In the presence of a significant association, we further adjusted for a priori specified covariates including body mass index (BMI), physical activity, history of CHD, use of antidepressants and Framingham risk score. Using GEE we also examined the association between SLC6A4 polymorphisms and MDD as a binary outcome variable.
The haplotype trend regression (HTR) approach was adapted to test for associations of statistically inferred haplotypes with depressive symptoms and IL-6 respectively as outlined by Zaykin et al, 30 where linear regression was replaced with the GEE procedure to take into account the relatedness between twins. Briefly, HTR is based on the regression of a trait on a design matrix that includes the expected proportions of haplotypes. The contributions of haplotypes are weighted with the design matrix, such that unambiguous pairs of haplotypes are coded 1 for the haplotypes of homozygotes, 0.5 for each of the haplotypes of a heterozygote, and 0 for all other haplotypes. However, the contributions of ambiguous pairs of haplotypes in the design matrix are based on the probabilities of haplotype pairs (divided by 2) as estimated by PHASE 2.0. 31 The most frequent haplotype was used as the reference with which the other haplotypes were contrasted. Haplotypes with estimated frequencies <5% were pooled together. The analyses were repeated after adjusting for the CAD risk factors and use of antidepressants.
We further constructed bivariate structural equation models (SEM), which models and takes account of residual twin covariance, to study the interplay of depressive symptoms and IL-6 with the SLC5A4 genetic vulnerability. Details of the approach for incorporating the measured genotypes in SEM have been described previously. 22 Compared with univariate analysis, bivariate modeling has the advantage of allowing genetic effects on the means, variances and relations between depressive symptoms and IL-6. The models were fitted with Mx software. 32 Most of twins from the THS are Caucasians (94%). Further adjustment for race did not change the results. To avoid the potential influences of race/ethnicity, we repeated all the analyses after exclusion of Africa-Americans. The results were virtually identical and are not reported.
RESULTS
Of the 360 THS twins, 82 twins had a lifetime history of MDD. The mean duration of major depressive episodes was 8.3 months. However, the majority of twins with MDD were in remission, with only 8 subjects meeting DMS-IV criteria for a current major depressive episode and 77% having had the last depressive episode > 1 year before examination. Of the 82 MDD twins, 27 (33%) were on antidepressants. There was no association between SCL6A4 polymorphisms and lifetime history of MDD. Further adjustment for the covariates did not change the results (data not shown). Table 1 shows the demographic characteristics and CAD risk factors of the THS twins. The mean age (±SD) was 54 years (±2.89), with a range of 47 to 60 years. Twenty percent of the participants were current smokers and about 10% had a history of CHD. Fifty-two subjects (14.4%) were using antidepressants.
Table 1.
Characteristics in the Twins Heart Study subjects
| Characteristic | Mean or Percent |
|---|---|
| Number | 360 |
| Age, years, mean ± SD | 54.4 ± 2.89 |
| Systolic blood pressure, mm Hg, mean ± SD | 129.4 ± 15.8 |
| Diastolic blood pressure, mm Hg, mean ± SD | 80.7 ± 10.7 |
| LDL-cholesterol, mg/dL, mean ± SD | 122.6 ± 33.8 |
| HDL-cholesterol, mg/dL, mean ± SD | 38.6 ± 9.69 |
| Diabetes, n (%) | 33 (9.19) |
| Body mass index, mean ± SD | 29.2 ± 4.86 |
| Current smoker, n (%) | 70 (20.0) |
| Framingham Risk Score, mean ± SD | 5.26 ± 2.07 |
| Physical activity (Baecke) score, mean ± SD | 7.43 ± 1.59 |
| Prior coronary heart disease, n (%) | 35 (9.72) |
| Posttraumatic stress disorder, n (%) | 23 (6.4) |
| Current antidepressant use, n (%) | 52 (14.4) |
| Interleukin-6, mg/dL, mean ± SD | 2.86 ± 6.33 |
| Depressive symptoms (BDI) score, mean ± SD | 4.94 ± 6.62 |
SD, standard deviation; BDI, Beck depression inventory
A total of 20 polymorphisms in the SLC6A4 gene were genotyped, including an ins/del polymorphism located in the promoter region (5HTTLPR), a missense mutation in the codon region (rs6355), two SNPs in un-translated regions (UTR, rs6354 and rs1042173) and 16 SNPs in introns. The location of these polymorphisms is shown in Supplemental Figure 1. Mutation types on all polymorphisms and MAF are presented in Table 2, along with the HWE tests. Sixteen polymorphisms were common with MAF>10%, 2 SNPs had MAF<10% but >5% and 2 SNPs had MAF<5%. No significant deviation from HWE was found for any polymorphism after the multiple testing corrections.
Table 2.
Summary data on 20 polymorphisms in the serotonin transporter gene and their associations with depressive symptoms and interleukin-6 levels
| BDI Scores | Ln IL-6 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Polymorphisms | Allelesa | MAFb | HWTc | βd | SE | Pe | βd | SE | Pe |
| 5-HTTLPR | L:S | 0.431 | 0.08 | −0.14 | 0.50 | 0.78 | −0.02 | 0.06 | 0.80 |
| rs16965628 | G:C | 0.065 | 0.28 | 0.76 | 0.99 | 0.44 | 0.18 | 0.12 | 0.14 |
| rs8071667* | G:A | 0.135 | 1.00 | 2.36 | 0.75 | 0.002 | 0.22 | 0.09 | 0.016 |
| rs4251417 | G:A | 0.124 | 0.84 | −0.10 | 0.79 | 0.90 | 0.03 | 0.09 | 0.79 |
| rs2066713 | C:T | 0.350 | 0.89 | −0.58 | 0.55 | 0.29 | −0.10 | 0.07 | 0.13 |
| rs12150214† | G:C | 0.172 | 0.95 | 1.59 | 0.66 | 0.017 | 0.18 | 0.08 | 0.025 |
| rs2020936* | T:C | 0.153 | 0.38 | 2.00 | 0.69 | 0.004 | 0.22 | 0.08 | 0.009 |
| rs2020939 | C:T | 0.484 | 0.16 | −0.63 | 0.50 | 0.20 | −0.04 | 0.06 | 0.46 |
| rs25528* | A:C | 0.146 | 0.24 | 2.06 | 0.69 | 0.003 | 0.23 | 0.08 | 0.005 |
| rs6354* | A:C | 0.143 | 0.97 | 2.07 | 0.73 | 0.005 | 0.24 | 0.09 | 0.006 |
| rs6355 | G:C | 0.026 | 1.00 | −0.92 | 1.61 | 0.57 | −0.15 | 0.19 | 0.45 |
| rs11080122* | C:T | 0.145 | 1.00 | 2.09 | 0.73 | 0.004 | 0.20 | 0.09 | 0.019 |
| rs8076005* | A:G | 0.170 | 0.40 | 1.75 | 0.66 | 0.008 | 0.23 | 0.08 | 0.003 |
| rs2020942 | G:A | 0.337 | 0.90 | −0.30 | 0.55 | 0.58 | −0.07 | 0.06 | 0.27 |
| rs140700 | A:G | 0.062 | 0.57 | −0.08 | 1.10 | 0.94 | 0.20 | 0.14 | 0.15 |
| rs140701 | C:T | 0.481 | 0.02 | −0.41 | 0.49 | 0.41 | −0.004 | 0.06 | 0.95 |
| rs2054847 | C:T | 0.487 | 0.03 | −0.39 | 0.49 | 0.43 | −0.003 | 0.06 | 0.96 |
| rs11657536 | G:A | 0.019 | 1.00 | −0.75 | 1.94 | 0.70 | 0.17 | 0.23 | 0.46 |
| rs4325622 | C:T | 0.471 | 0.14 | 0.65 | 0.49 | 0.19 | 0.04 | 0.06 | 0.49 |
| rs1042173 | C:A | 0.466 | 0.26 | 0.55 | 0.50 | 0.27 | 0.05 | 0.06 | 0.39 |
BDI, Beck depression inventory; Ln, logarithm transformation; IL-6, interleukin-6
Major allele vs. minor allele
MAF, minor allele frequency
P values of chi-square testing for Hardy-Weinberg equilibrium
Regression coefficient and standard error (SE) using generalized estimating equations
P values derived from generalized estimating equations
P values reached the nominal significance after multiple testing correction (P=0.008) for both depressive symptoms and IL-6, or at least one of them
P values <0.05 for both depressive symptoms and IL-6, but not reached the significance after multiple testing correction (P=0.008)
Pairwise LD coefficients of the 20 polymorphisms are displayed in Supplemental Figure 1. There was strong LD among rs8071667, rs12150214, rs2020936, rs25528, rs6354, rs11080122 and rs8076005 (pair-wise r2 of 0.72–0.95). Two pairs of SNPs, rs2066713 and rs2020942, showed almost perfect LD (r2=0.96), as well as rs4325622 and rs1042173 (r2=0.98). Strong LD was also found among rs2020939, rs140701 and rs2054847 (r2=0.85–0.9).
The association of each SLC6A4 polymorphism with depressive symptoms and IL-6 are shown in Table 2. No significant association was observed between 5-HTTLPR and depressive symptoms. However, six SNPs in other locations were significantly associated with BDI scores (P<0.008), including rs8071667, rs2020936, rs25528, rs6354, rs11080122 and rs8076005, and 1 SNP was borderline associated (rs12150214, P=0.017). Of these 7 SNPs, 3 were also significantly associated with IL-6 (P<0.008), including rs25528, rs6354 and rs8076005, and the other 4 were borderline associated (P=0.009–0.025). For these 7 SNPs, the minor allele was associated with higher BDI scores and IL-6 levels. Adjustment for covariates did not change the results.
The haplotype analysis using these 7 SNPs reflected the single SNP analysis since they were highly correlated. About 94% of the genetic variability of the haplotypes consisting of these 7 SNPs was explained by two common haplotypes, including G-G-T-A-A-C-A (Hap1) and A-C-CC-C-T-G (Hap2) with frequencies of 81% and 13% respectively (Supplemental Table 1). Haplotype-based association tests are shown in Table 3. The beta coefficient represents the effect size for haplotype homozygote. Compared with the subjects homozygous for Hap1, those homozygous for Hap2 had, on average, a 98% higher BDI score (P=0.006), and a 56% higher IL-6 level (P=0.01). Adjustment for covariates did not change the results. In addition, we performed a full haplotype analysis using all 20 polymorphisms. No significant associations were found (data not shown).
Table 3.
Haplotype association tests for depressive symptoms and interleukin-6
| BDI Scores | Ln IL-6 | |||||||
|---|---|---|---|---|---|---|---|---|
| Haplotypes | Freq | βa | SE | Pb | βa | SE | Pb | |
| Hap1c | GGTAACA | 0.81 | ref | - | - | ref | - | - |
| Hap2 | ACCCCTG | 0.13 | 4.29 | 1.56 | 0.006 | 0.45 | 0.18 | 0.01 |
| Other Haplotypesd | - | 0.06 | 0.09 | 2.06 | 0.96 | 0.38 | 0.23 | 0.10 |
BDI, Beck depression inventory; Ln, logarithm transformation; IL-6, interleukin-6
SNPs in the order of rs8071667-rs12150214-rs2020936-rs25528-rs6354-rs11080122-rs8076005
Regression coefficient and standard error (SE) using generalized estimating equations
P values derived from generalized estimating equations
Most frequent haplotype chosen as the reference
Haplotypes with estimated frequencies <5% pooled together
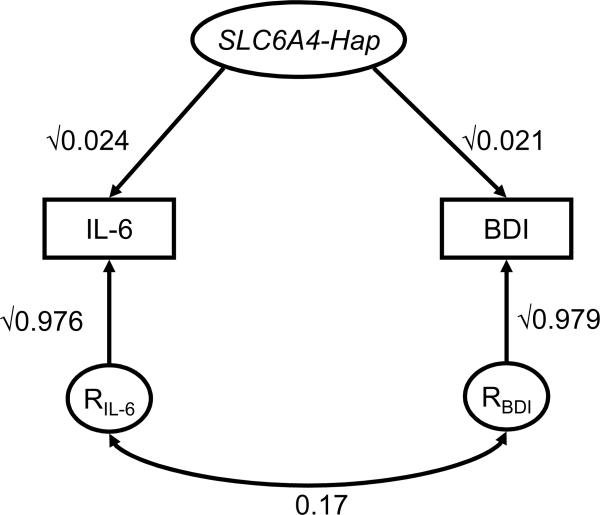
To avoid fitting multiple SNP models, we used the haplotypes consisting of these 7 SNPs for the bivariate SEM construction instead of using each single SNP. The bivariate SEM effects of the SLC6A4 risk haplotype (Hap2) on BDI scores (β =4.299, P=0.02) and IL-6 levels (β=0.469, P=0.01) were comparable to the β values obtained from GEE as shown in Table 3. Moreover, the bivariate SEM provided the estimations of SLC6A4 haplotypes contributing to the correlation between BDI and IL-6. As shown in Figure 1, the explained proportions of the phenotypic variance by SLC6A4 haplotypes were 2.1% and 2.4% for BDI and I-6 respectively. The overall phenotypic correlation between BDI and IL-6 was 0.19 (P<0.001). After taking into account the effect of SLC6A4 haplotypes, the residual correlation was estimated as 0.17 (P<0.001) (Figure 1), indicating that about 10% of this correlation could be explained by the SLC6A4 haplotypes. As we have previously reported, 8 over two-thirds of this correlation is due to the genetic factors. This implies that the SLC6A4 gene contributes approximately 15% of the shared genetic correlation between these two phenotypes.
Figure 1.
Bivariate structural equation model for the variances of depressive symptoms (BDI) and interleukin-6 (IL-6) explained by the haplotypes of serotonin transporter gene (SLC6A4). The variance for each trait is divided into two components: effect of the SLC6A4 haplotypes and residual factors (RIL-6 and RBDI). Paths, which are the standardized regression coefficients, must be squared to equal the proportion of variance accounted for. The symbol “√” indicates the square-root. The residual phenotypic correlation between depressive symptoms and IL-6 is shown as the lower curve.
DISCUSSION
We report for the first time that genetic variations in the serotonin transporter gene SLC6A4 are associated with both depression and inflammation in middle-aged males. Participants who carried a specific haplotype consisting of seven SNPs in the SLC6A4 gene had both significantly increased depressive symptoms and elevated IL-6 plasma levels. This risk haplotype explained about 10% of correlation between these two phenotypes. Our results indicate that the SLC6A4 gene underlies a shared pathophysiological pathway linking depression and inflammation. Our data also suggest that other genetic pathways, yet to be discovered, are involved in the link between depression and inflammation.
Growing evidence has implicated the serotonergic neurotransmission pathway, and its related genes, in the pathophysiology of depression. 33 The gene encoding the serotonin transporter is a fundamental regulator of serotonin signaling and therefore has been the focus of many previous studies. 13 Several studies have investigated the relationship of a common functional polymorphism in the promoter region of this gene (5-HTTLPR) and depression, but results are inconsistent. 17–20 In the present study, we found no association between 5-HTTLPR and depressive symptoms. It is possible the previously reported inconsistent results may be due to an interaction between stressful life events and 5-HTTLPR, whereby carriers of the S allele are more likely to suffer from MDD, but only if they had experienced multiple traumatic events. 34
Only a few studies examined the relationship of other SNPs within the SLC6A4 gene and depression. A recent study examined the 5-HTTLPR and additional four SNPs in this gene in relation to depressive symptoms. For these four SNPS, no significant main effects were observed, although an interaction with stressful life events was found. 21 Three of the four SNPs examined in this previous study (rs2020942, rs140700 and rs1042173) were also genotyped in our study. The SNP rs3794808 was not genotyped, but three SNPs (rs2020939, rs140701 and rs2054847) in strong LD with it (based on HapMap data) were studied in the present study. No significant associations of these 6 SNPs with depressive symptoms were observed in our study.
In contrast, we found that 6 additional SNPs in the SLC6A4 gene were significantly associated with depressive symptoms and 1 SNP was borderline, where each minor allele was associated with increased BDI scores. These 7 SNPs were in strong LD with each other. Small to moderate LD was observed between these 7 SNPs and other polymorphisms. Six of the 7 SNPs were located in introns and 1 SNP was in 5' UTR (rs6354). Our study is the first to report an association between these SNPs and depressive symptoms. Further study is necessary to explore if these SNPs affect SLC6A4 gene expression.
We found that these same 7 SNPs were also significantly or borderline associated with IL-6 plasma levels. Substantial previous data suggest that serotonin plays a role in immune function and inflammation. 11 An in vivo study showed that autologous serotonin is required for optimal T-cell activation and that the activation of suboptimally stimulated T-cells can be augmented with low doses of exogenously added serotonin. 35 In addition, serotonin induces NF-kB activation in human vascular smooth muscle cells and enhances IL-6 synthesis via the 5-HT2A receptor. 12 Despite this evidence, no previous study examined the association between genetic variants in the serotoninergic system and IL-6 levels.
In a previous twin study using SEM we reported that depression and inflammation share a common genetic substrate. More than two-thirds of the correlation between depressive symptoms and IL-6 plasma levels was explained by shared genetic effects. 8 The present study extends our previous work and shows that the SLC6A4 gene plays a fundamental role in the shared genetic liability to both depressive symptoms and inflammation. We found that variants in this gene explained about 10% of the shared phenotypic correlation of depressive symptoms and inflammation and 15% of their shared genetic correlation. These results suggest that the serotonin system is pathophysiologically implicated in both depression and inflammation, and that these two phenotypes may be the expression of a common pathophysiological mechanism involving neural-immune dysregulation. Our data also indicate that the remaining portion (85%) of the shared genetic correlation between depressive symptoms and IL-6 is still unexplained. Future studies should evaluate other genetic pathways that are involved in the link between depression and inflammation.
Our study is cross-sectional, thus limited in the ability to discern the temporal order between depression and inflammation. However, based on our results, the covariation of these two phenotypes is due in large part to a common genetic precursor rather than being a cause-effect relationship. Another limitation is that few twins met the criteria for a current major depressive episode, preventing us from examining current clinical depression. Because of this low prevalence, our analysis focused on current depressive symptoms, which are also related to CAD risk, 1 and which showed a graded effect with increasing depressive symptoms in this sample as previously described. 8 Lastly, because our twins were all middle-aged male military veterans, caution should be used in generalizing our results to women or older individuals. Our study provides the foundation for future investigations including diverse sociodemographic groups.
In conclusion, we have discovered a specific genetic vulnerability involving the serotonin transporter gene that is shared between depressive symptoms and elevated IL-6 plasma levels. Our results are consistent with the hypothesis that common pathophysiological processes link depression and inflammation, and provide important insight into the role of serotonin in the pathophysiology of depression and the modulation of neural-immune interactions. Our data suggest that combining genetic and psychiatric profiling may ultimately prove useful in identifying depressed patients at highest risk for cardiovascular consequences and potentially aid in the design of novel drug targets which may benefit clinical treatments.
There is growing evidence that depression is associated with increased levels of inflammatory markers, but whether this link is casual remains speculative. We sought to determine whether a common genetic vulnerability may be an alternative explanation for the observed association between depression and inflammation. Consistent with this hypothesis, in a study of middle-age male twins we found that the serotonin transporter gene (SLC6A4) is a common precursor of both depressive symptoms and circulating interleukin-6 (IL-6) levels. Approximately 10% of the correlation between these two phenotypes can be explained by the SLC6A4 genetic variants. Our results provide important insight into the role of serotonin in the pathophysiology of both depression and inflammation.
Supplementary Material
ACKNOWLEDGMENTS
The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance in the conduct of this study, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Institutes of Health; National Opinion Research Center; the National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible.
FUNDING SOURCES This study was supported by K24HL077506, R01 HL68630 and R01 AG026255 from the National Institutes of Health; by grants 0245115N, 0725513B, 0730100N and 09SDG2140117 from the American Heart Association; by grant KL2 RR025009 from the Atlanta Clinical and Translational Science Institute and grant NIH NINR P20NR007798, Center for the Study of Symptoms, Symptom Interactions and Health Outcomes; and by the Emory University General Clinical Research Center MO1-RR00039.
Footnotes
DISCLOUSRES None.
REFERENCES
- 1.Wulsin LR, Singal BM. Do Depressive Symptoms Increase the Risk for the Onset of Coronary Disease? A Systematic Quantitative Review. Psychosom Med. 2003;65:201–210. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- 2.Lett HS, Blumenthal JA, Babyak MA, Sherwood A, Strauman T, Robins C, Newman MF. Depression as a Risk Factor for Coronary Artery Disease: Evidence, Mechanisms, and Treatment. Psychosom Med. 2004;66:305–315. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 3.Ross R. Atherosclerosis--an Inflammatory Disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 4.McCaffery JM, Frasure-Smith N, Dube MP, Theroux P, Rouleau GA, Duan Q, Lesperance F. Common Genetic Vulnerability to Depressive Symptoms and Coronary Artery Disease: A Review and Development of Candidate Genes Related to Inflammation and Serotonin. Psychosom Med. 2006;68:187–200. doi: 10.1097/01.psy.0000208630.79271.a0. [DOI] [PubMed] [Google Scholar]
- 5.Empana JP, Sykes DH, Luc G, Juhan-Vague I, Arveiler D, Ferrieres J, Amouyel P, Bingham A, Montaye M, Ruidavets JB, Haas B, Evans A, Jouven X, Ducimetiere P. Contributions of Depressive Mood and Circulating Inflammatory Markers to Coronary Heart Disease in Healthy European Men: The Prospective Epidemiological Study of Myocardial Infarction (Prime) Circulation. 2005;111:2299–2305. doi: 10.1161/01.CIR.0000164203.54111.AE. [DOI] [PubMed] [Google Scholar]
- 6.Wrona D. Neural-Immune Interactions: An Integrative View of the Bidirectional Relationship between the Brain and Immune Systems. J Neuroimmunol. 2006;172:38–58. doi: 10.1016/j.jneuroim.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Su S, Snieder H, Miller AH, Ritchie J, Bremner JD, Goldberg J, Dai J, Jones L, Murrah NV, Zhao J, Vaccarino V. Genetic and Environmental Influences on Systemic Markers of Inflammation in Middle-Aged Male Twins. Atherosclerosis. 2008;200:213–220. doi: 10.1016/j.atherosclerosis.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su S, Miller AH, Snieder H, Bremner JD, Ritchie J, Maisano C, Jones L, Murrah NV, Goldberg J, Vaccarino V. Common Genetic Contributions to Depressive Symptoms and Inflammatory Markers in Middle-Aged Men: The Twins Heart Study. Psychosom Med. 2009;71:152–158. doi: 10.1097/PSY.0b013e31819082ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kushnir-Sukhov NM, Brown JM, Wu Y, Kirshenbaum A, Metcalfe DD. Human Mast Cells Are Capable of Serotonin Synthesis and Release. J Allergy Clin Immunol. 2007;119:498–499. doi: 10.1016/j.jaci.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Deakin JF. The Role of Serotonin in Panic, Anxiety and Depression. Int Clin Psychopharmacol. 1998;13:S1–5. doi: 10.1097/00004850-199804004-00001. [DOI] [PubMed] [Google Scholar]
- 11.Mossner R, Lesch KP. Role of Serotonin in the Immune System and in Neuroimmune Interactions. Brain Behav Immun. 1998;12:249–271. doi: 10.1006/brbi.1998.0532. [DOI] [PubMed] [Google Scholar]
- 12.Ito T, Ikeda U, Shimpo M, Yamamoto K, Shimada K. Serotonin Increases Interleukin-6 Synthesis in Human Vascular Smooth Muscle Cells. Circulation. 2000;102:2522–2527. doi: 10.1161/01.cir.102.20.2522. [DOI] [PubMed] [Google Scholar]
- 13.Canli T, Lesch KP. Long Story Short: The Serotonin Transporter in Emotion Regulation and Social Cognition. Nat Neurosci. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- 14.Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic Variation of Human Serotonin Transporter Gene Expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 15.Hariri AR, Holmes A. Genetics of Emotional Regulation: The Role of the Serotonin Transporter in Neural Function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of Anxiety-Related Traits with a Polymorphism in the Serotonin Transporter Gene Regulatory Region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 17.Cervilla JA, Rivera M, Molina E, Torres-Gonzalez F, Bellon JA, Moreno B, de Dios Luna J, Lorente JA, de Diego-Otero Y, King M, Nazareth I, Gutierrez B. The 5-Httlpr S/S Genotype at the Serotonin Transporter Gene (Slc6a4) Increases the Risk for Depression in a Large Cohort of Primary Care Attendees: The Predict-Gene Study. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:912–917. doi: 10.1002/ajmg.b.30455. [DOI] [PubMed] [Google Scholar]
- 18.Gonda X, Juhasz G, Laszik A, Rihmer Z, Bagdy G. Subthreshold Depression Is Linked to the Functional Polymorphism of the 5ht Transporter Gene. J Affect Disord. 2005;87:291–297. doi: 10.1016/j.jad.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Mendlewicz J, Massat I, Souery D, Del-Favero J, Oruc L, Nothen MM, Blackwood D, Muir W, Battersby S, Lerer B, Segman RH, Kaneva R, Serretti A, Lilli R, Lorenzi C, Jakovljevic M, Ivezic S, Rietschel M, Milanova V, Van Broeckhoven C. Serotonin Transporter 5httlpr Polymorphism and Affective Disorders: No Evidence of Association in a Large European Multicenter Study. Eur J Hum Genet. 2004;12:377–382. doi: 10.1038/sj.ejhg.5201149. [DOI] [PubMed] [Google Scholar]
- 20.Lasky-Su JA, Faraone SV, Glatt SJ, Tsuang MT. Meta-Analysis of the Association between Two Polymorphisms in the Serotonin Transporter Gene and Affective Disorders. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:110–115. doi: 10.1002/ajmg.b.30104. [DOI] [PubMed] [Google Scholar]
- 21.Lazary J, Lazary A, Gonda X, Benko A, Molnar E, Juhasz G, Bagdy G. New Evidence for the Association of the Serotonin Transporter Gene (Slc6a4) Haplotypes, Threatening Life Events, and Depressive Phenotype. Biol Psychiatry. 2008;64:498–504. doi: 10.1016/j.biopsych.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 22.van den Oord EJ, Snieder H. Including Measured Genotypes in Statistical Models to Study the Interplay of Multiple Factors Affecting Complex Traits. Behav Genet. 2002;32:1–22. doi: 10.1023/a:1014474711118. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam Era Twin Registry. Twin Res. 2002;5:476–481. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- 24.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for Dsm Iv-Patient Edition (Scid-P) American Psychiatric Press; Washington, D.C: 1995. [Google Scholar]
- 25.Beck AT, Steer RA, Brown GK. Bdi-Ii. Beck Depression Inventory. Second Edition Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- 26.Osman A, Kopper BA, Barrios F, Gutierrez PM, Bagge CL. Reliability and Validity of the Beck Depression Inventory--Ii with Adolescent Psychiatric Inpatients. Psychol Assess. 2004;16:120–132. doi: 10.1037/1040-3590.16.2.120. [DOI] [PubMed] [Google Scholar]
- 27.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of Coronary Heart Disease Using Risk Factor Categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 28.Gao X, Becker LC, Becker DM, Starmer JD, Province MA. Avoiding the High Bonferroni Penalty in Genome-Wide Association Studies. Genet Epidemiol. 2009 doi: 10.1002/gepi.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and Visualization of Ld and Haplotype Maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 30.Zaykin DV, Westfall PH, Young SS, Karnoub MA, Wagner MJ, Ehm MG. Testing Association of Statistically Inferred Haplotypes with Discrete and Continuous Traits in Samples of Unrelated Individuals. Hum Hered. 2002;53:79–91. doi: 10.1159/000057986. [DOI] [PubMed] [Google Scholar]
- 31.Stephens M, Smith NJ, Donnelly P. A New Statistical Method for Haplotype Reconstruction from Population Data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6th ed. Virginia Commonwealth University Medical School; Richmond: 2003. [Google Scholar]
- 33.Mann JJ. Role of the Serotonergic System in the Pathogenesis of Major Depression and Suicidal Behavior. Neuropsychopharmacology. 1999;21:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- 34.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of Life Stress on Depression: Moderation by a Polymorphism in the 5-Htt Gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 35.Young MR, Kut JL, Coogan MP, Wright MA, Young ME, Matthews J. Stimulation of Splenic T-Lymphocyte Function by Endogenous Serotonin and by Low-Dose Exogenous Serotonin. Immunology. 1993;80:395–400. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.