Abstract
Anticancer drugs are often nonselective antiproliferative agents (cytotoxins) that preferentially kill dividing cells by attacking their DNA at some level. The lack of selectivity results in significant toxicity to noncancerous proliferating cells. These toxicities along with drug resistance exhibited by the solid tumors are major therapy limiting factors that results into poor prognosis for patients. Prodrug and conjugate design involves the synthesis of inactive drug derivatives that are converted to an active form inside the body and preferably at the site of action. Classical prodrug and conjugate design has focused on the development of prodrugs that can overcome physicochemical (e.g., solubility, chemical instability) or biopharmaceutical problems (e.g., bioavailability, toxicity) associated with common anticancer drugs. The recent targeted prodrug and conjugate design, on the other hand, hinges on the selective delivery of anticancer agents to tumor tissues thereby avoiding their cytotoxic effects on noncancerous cells. Targeting strategies have attempted to take advantage of low extracellular pH, elevated enzymes in tumor tissues, the hypoxic environment inside the tumor core, and tumor-specific antigens expressed on tumor cell surfaces. The present review highlights recent trends in prodrug and conjugate rationale and design for cancer treatment. The various approaches that are currently being explored are critically analyzed and a comparative account of the advantages and disadvantages associated with each approach is presented.
Keywords: Prodrugs, conjugates, targeted design, nanotechnology, anticancer
1. INTRODUCTION
Cancer is characterized by uncontrolled growth of abnormal cells [1,2]. These cancerous cells may invade the nearby tissues and spread to other parts of body through the blood and lymph systems (metastases) [3]. There are over 550,000 cancer-related deaths in the United States every year and cancer accounts for one of every four deaths in the country [4]. Current cancer treatment strategies employ a combination of surgery, radiation, and chemotherapy. The localized therapies, such as surgery and radiotherapy, succeed only when malignant cells are confined to the treated area. The use of chemotherapy is therefore essential for the systemic treatment of metastases accompanying the local and regional growth of tumors [5]. Metastases are the primary cause of death from cancer. The anticancer drugs used in chemotherapy are systemic anti-proliferative agents that preferentially kill the dividing cells. These cytotoxic agents are antimetabolites, alkylating agents, DNA-complexing agents, mitosis inhibitors or hormones, and they exert their activity by interfering with some aspect of DNA replication, repair, translation or cell division. They mainly rely on enhanced proliferative rate of cancer cells and therefore, are not truly selective for cancer cells. The prolonged use of chemotherapy results in lethal damage to proliferating non-cancerous cells and this is particularly true in the treatment of solid tumors, where majority of the tumor cells do not divide rapidly [5]. It has been observed that the use of cytotoxins in patients with appreciable tumor burdens leads to remissions of limited duration and varying degree, followed by re-growth and spread of more malignant and multi-drug resistant forms of cancer [6]. Despite several decades of intensive research, the long-term outlook for patients with aggressive cancer remains discouraging, and there is a need for innovative approaches to design anticancer drugs with reduced toxicity and improved therapeutic indices [7,8].
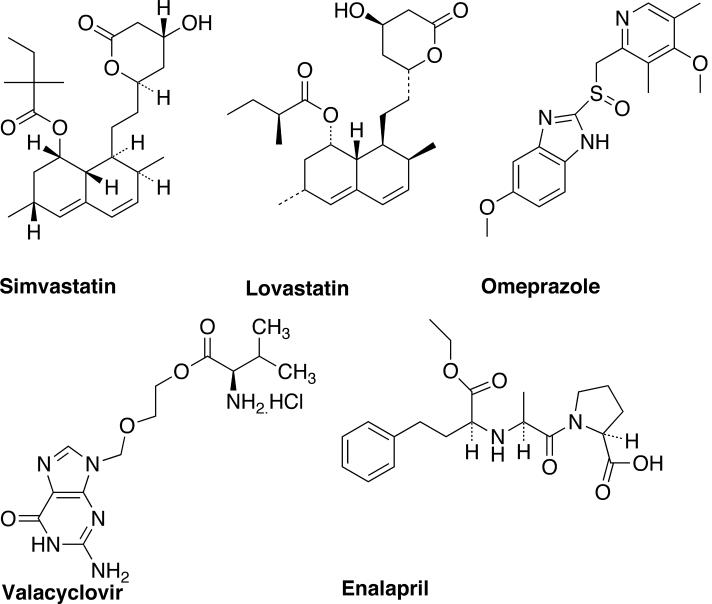
Prodrug therapy provides an alternative approach to design less reactive and less cytotoxic form of anticancer drugs [9]. Albert [10] and Harper [11] introduced the prodrug concept and used it to characterize pharmacologically inert derivatives that can be converted to active drug molecules in vivo, enzymaticaly or non-enzymaticaly, to exert a therapeutic effect [12,13]. The term prodrug therefore implies a covalent linkage between the drug and the chemical moiety causing the inertness. Conventional prodrug design aims to overcome [9,14,15]: (i) pharmaceutical problems such as poor solubility, insufficient chemical stability, unacceptable taste or odor, and irritation or pain; (ii) pharmacokinetic problems such as insufficient oral absorption, inadequate blood-brain barrier permeability, marked presystemic metabolism, and toxicity; and (iii) pharmacodynamic problems such as low therapeutic index and lack of selectivity at the site of action. Thus, the major objective of prodrug design is to temporarily alter the physicochemical properties of drugs to accomplish modification of drug pharmacokinetics, prolongation of action, reduce toxicities and side effects, increased selectivity, and resolve formulation challenges. The classical approach has been successfully used to design and develop prodrugs with improved pharmacokinetic and pharmacodynamic properties. Some of the current blockbuster prodrugs in the market are shown in Fig. (1). Simvastatin and Lovastatin are lipid-lowering agents supplied in the inactive lactone form [9]. They are hydrolyzed to active β-hydroxyacid form in vivo. Simvastatin inhibits the 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA), which is involved in cholesterol biosynthesis. Omeprazole is used to lower stomach acidity [15]. It is supplied as a racemate and converted to an active achiral form in the acidic conditions of stomach, which then reacts with cysteine group in H+/K+ ATPase and destroys the ability of parietal cells to produce gastric acid. Valacyclovir is an amino acid ester of acyclovir [9]. Acyclovir (also a prodrug) is a synthetic purine nucleoside analog with in vitro and in vivo activity against herpes simplex virus (HSV-1 and 2) and varicella-zoster virus (VZV). Valacyclovir is first converted to acyclovir via esterase cleavage and then viral enzymes converts it into acyclovir monophosphate, which is further converted to diphsophate by cellular guanylate kinase and finally into triphosphate by a number of cellular enzymes in vivo. Enalapril is a prodrug used for lowering the blood pressure [9]. It is supplied as ethyl ester derivative of enalaprilat (drug) and converted to active form by hydrolysis of ester in vivo, which inhibits angiotensin-converting enzyme (ACE).
Fig. (1).
Major prodrugs available in market.
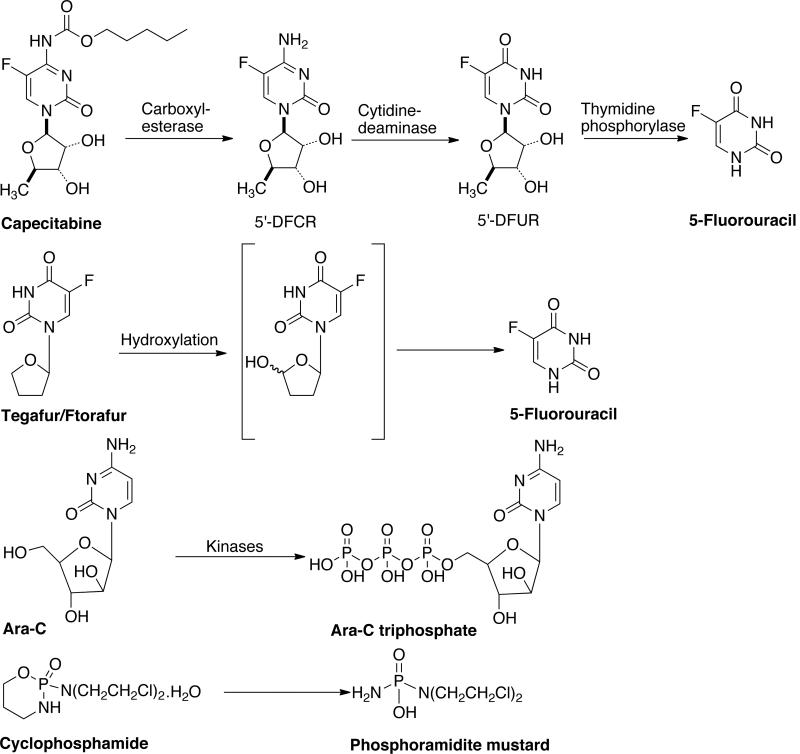
Conventional prodrug design has also been used to develop several anticancer prodrugs [16]. Capecitabine (Xeloda, N4-pentyloxycarbonyl-5'deoxy-5-fluorocytidine) is one prominent example shown in Fig. (2). It is a prodrug of 5'-deoxy-5-fluorouridine (5'-DFUR), a fluoropyrimidine nucleoside agent that can be bioactivated into antitumor drug 5-FU by thymidine phosphorylase and uridine phosphorylase [17]. 5'-DFUR has been clinically used for the treatment of breast, colorectal, and gastric cancer, but known to cause severe gastrointestinal toxicity. The conversion of capecitabine to active drug is achieved in three-steps. In the first step, a hepatic carboxylesterase converts it into 5'-deoxy-5-fluorocytidine. This is subsequently converted to 5'-deoxy-5-fluorouridine by cytidine deaminase enzyme in the liver/tumor. Finally, tumor-associated enzyme thymidine phosphorylase converts 5'-deoxy-5-fluorouridine to 5-fluoruracil, which in turn is converted to 5'-fluorouridine or 5'-fluoro-2-deoxyuridine. The 5'-fluoro-2-deoxyuridine is incorporated into RNA and DNA respectively. This prodrug demonstrates satisfactory gastrointestinal absorption, low toxicity and high conversion to 5'-DFUR.
Fig. (2).
Enzyme activated anticancer prodrugs.
Ftorafur/Tegafur (UFT, 5-fluoro-1-tetrahydro-1-furyl uracil) is another such example [Fig (2)]. Tegafur is a slow-releasing prodrug of 5-FU, which avoids the rapid breakdown of drug in the gastrointestinal tract [18]. The prodrug is activated to 5-FU by cytosolic thymidine phosphorylase and microsomal P450 mainly in liver. 5-FU has been used as anticancer drug for over 20 years and it inhibits cancer growth by thymidylate inhibition and by incorporation into DNA and RNA. Ara-C (Cytarabine) or 4-amino-1-β-D-arabinofuranosyl-2-(1H)-pyrimidinone is antineoplastic agent used for a wide variety of proliferating mammalian cells [19]. It exhibits cell-phase specificity, primarily killing cells undergoing DNA synthesis (S-phase) and under certain conditions blocking the progression of cells from G1 phase to the S-phase. It is metabolized by deoxycytidine kinase and other nucleotide kinases to nucleotide triphosphate, an effective inhibitor of DNA polymerase. Cyclophosphamide or 2H-1,3,4-oxazaphosphorin-2-amine-N-N-bis(2-chloroethyl)-tetrahydro-2-oxide monohydrate is a synthetic antineoplatsic drug related to the nitrogen mustards [20]. Cyclophosphamide is biotransformed primarily in the liver by a mixed function microsomal oxidase system to an active alkylating metabolite. These metabolites interfere with the growth of rapidly proliferating malignant cells and the mechanism of action involves cross-linking of tumor cell DNA. There are several other taxol, etoposide, rapamycin, bisantrene, butyric acid, DTIC and doxorubicin prodrugs, that have been developed to overcome pharmaceutical, pharmacokinetic and pharmacodynamic problems associated with active drug molecules [16]. Such prodrugs are not the focus of this review and the readers are referred to recently published review articles on this subject [9,15,16].
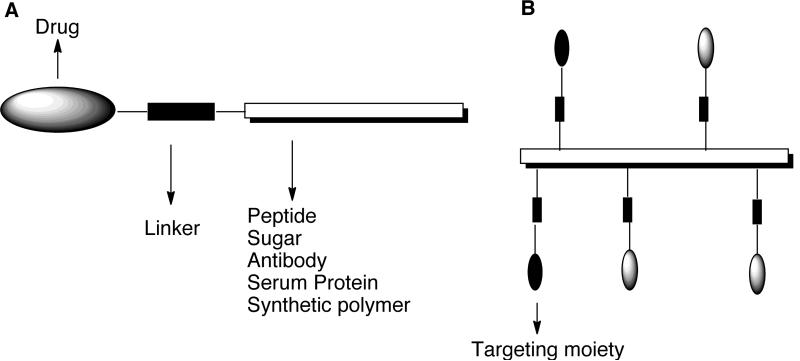
The majority of anticancer prodrugs developed using conventional prodrug designs are known to undergo non-specific activation. Current prodrug designs are therefore focused towards the development of targeted prodrugs [21–24]. Such prodrugs are designed by conjugating the prodrug molecules to low to high molecular weight molecules (often called carriers) like sugars, growth factors, vitamins, antibodies, peptides and synthetic polymers that can transport the drugs to tumors and subsequently release it outside or inside the tumor cells. The drug release in most of the prodrugs is accomplished by conjugating the drug to the carrier through a spacer that incorporates a pre-determined breaking point, which allows the drug to be released at the cellular target site. The general design of carrier-linked prodrug is shown in Fig. (3). The design and development of truly tumor-specific carriers is a challenging task [24]. The first challenge is optimizing the physicochemical properties such as molecular weight, hydrophilicity / hydrophobicity, three-dimensional structure, and immunogenicity of the carrier. Also, tumor heterogeneity, vascularization, interstitial pressure, and expression of multidrug resistant proteins are known to influence the biodistribution of drug carrier and the amount of drug reaching the targeted site. The second challenge is ensuring that the targeting properties of the carrier are preserved on modification with the drug molecules.
Fig. (3).
General design of carrier-linked anticancer prodrugs.
This review provides an overview of recent developments in targeted prodrug and conjugate design. Relevant prodrug and conjugate examples illustrating the salient features of different targeting strategies are described. We have focused on prodrug and conjugate examples in clinical trials or advanced preclinical studies. The advantages and disadvantages associated with each strategy are also discussed.
2. TARGETING STRATEGIES
The prodrugs can be targeted selectively to tumors by employing either active or passive targeting strategies as described below.
2.1. Active targeting
2.1.1. Tumor specific antigens or receptors
This strategy aims to develop prodrugs by conjugating drug molecules to monoclonal antibodies (mAbs) [25,26] or ligands [27,28] for specific interaction with antigens or receptors expressed on tumor cell surface. These cell surface targets are distinguished into two categories: non-internalizing and internalizing. In non-internalizing systems, the drug conjugate is cleaved extracellularly, whereas in internalizing systems drug is cleaved intracellularly after endocytosis. Several antigens and cell surface receptors that have been used for targeting are listed below.
Antigens: Cluster of differentiation (CD20, CD33), Carcinoembryonic antigen, Blood group carbohydrates, Mucin-type glycoproteins (MUC1, CanAg), Lewis Y, Lewis X, Cancer testis antigens (CT7, MAGE-A3), Protease-specific membrane antigen.
Vascular receptors: Integrins (αvβ3, αvβ5), Nucleolin, Aminopeptidase N, Endoglin, Vascular endothelial growth factor (VEGF1-4).
Plasma protein receptors: Low-density lipoprotein, Transferrin
Peptide receptors: Somatostatin receptors, Bombesin receptors, Neuropeptide Y receptors, Leuteinizing-hormone releasing receptors.
Receptors for growth factors and vitamins: Folate receptors (FR-α, FR-β, FR-γ), Epidermal growth factor receptors (EGF1, EGF2, Her2), Transforming growth factor receptor and fibroblast growth factor receptors.
Carbohydrate receptors: Asialoglycoprotein receptor, Galectins (galectin 1, galectin 2), Selectins (E-selectin, P-selectin), Hyaluronic acid receptors (CD44, RHAMM, HARLEC).
Monoclonal antibodies (mAbs) were among the first carrier systems that were investigated for active targeting because of their high binding affinity for respective antigens [29]. mAbs are used as single agents, as drug-antibody conjugates or as antibody enzyme conjugates. Most mAbs belong to the immunoglobulins of the IgG class, which is smallest in size but most abundant antibody found in all biological fluids [30]. The IgG molecule is a symmetric Y-shaped glycoprotein with two light and two heavy chains bound together by disulfide bridges. The heavy chains are glycosylated, and both chains include a constant and variable region with high affinity binding sites for antigens. Proteases enzymes cleave the antibodies into defined fragments: pepsin cleaves the antibody to F(ab)2 fragments, whereas cleavage by papain produces two monovalent Fab fragments. mAbs exert their antitumor effect through various pathways: by simple blockage of antigens and subsequent inhibition of signal transduction, by complement dependent cytolysis (CDC), or by antibody-dependant cell-mediated cytotoxicity [31]. Once the antibody is bound to an antigen, the complement cascade is activated and /or effector cell bind to the Fc regions of the antibody activating natural killer cells and leading to the destruction of the antigenic cells.
Several standard chemotherapeutic agents including antifolates [32], vinca alkaloids [33] or anthracyclines [34] were conjugated to monoclonal antibodies. Earlier work verified that such antibody conjugates indeed display selectivity towards the cells that expresses the respective antigens. It was also established that the antibody conjugates are internalized via receptor-mediated endocytosis after binding to antigens and the drug is released from the lysosome into the cell. As the drug is released into the intracellular compartment, drug efflux by the P-glycoprotein transmembrane pump (associated with multidrug resistance) is reduced as compared to systemic application of the respective drugs alone. Several linkers were used to prepare antibody conjugates but peptide linkers that are stable in serum and can be enzymatically degraded in the intracellular compartment are considered superior [35]. There are elevated level of certain proteolytic enzymes (e.g., cathepsin B) present in tumors, which cleave specific peptide sequences (section 3.1.). They are used for the selective activation of prodrug containing specific peptide spacers (e.g., gly-phe-leu-gly). The disulfide linkers are also considered advantageous because they are cleaved by disulfide exchange with intracellular thiol such as glutathione, which are present at much higher concentrations in tumors than normal cells [36]. mAb conjugates failed in clinical trials because the mAbs used were of murine origin and provoked immune responses. These antibody-drug conjugates led to the development of human anti-mouse antibody responses in patients that caused rapid clearance of the immunoconjugates from the body [28]. It was also observed that the amount of cytotoxic drug that can be conjugated to the antibody (i.e., drug loading) without reducing its capability to bind to antigens, and the expression of antigen on tumors is limited.
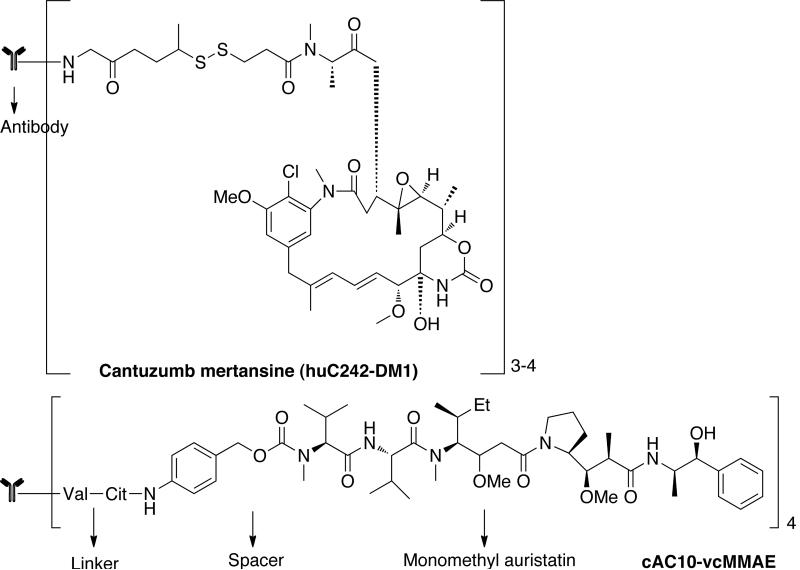
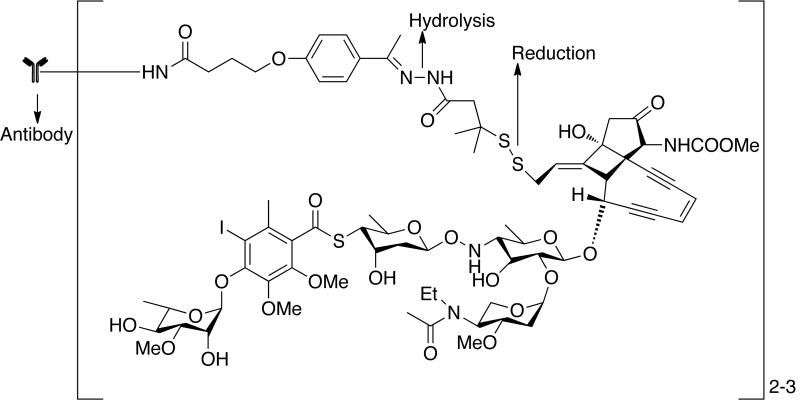
The problem of immunogenicity was resolved by the development of chimeric and humanized antibodies that do not carry murine sequences. From 1998–2004, the FDA approved five chimeric or humanized antibodies such as rituximab (Rituxan), trastuzumab (Herceptin), alemtuzumab (campath), bevacizumab (Avastin) and cetuximab (Erbitux) for the treatment of hematological and solid cancers [37–39]. Several cytotoxic drugs have been investigated for use in drug antibody conjugates. This includes doxorubicin, CC-1065 (from Streptomyces zelensis), second-generation taxanes, monomethyl auristatin E, and geldanamycin. A prominent example in this category is the cantuzumab mertansine conjugate of DM1 [Fig. (4)] [40]. DM1 is maytansinoid derivative containing 19-member macrocyclic lactam of antibiotic ansamycin. These are microtubule inhibitors active in the subnanomolar range [41]. They bind reversibly to assemble tubulin causing disassembly of microtubules thereby inhibiting mitosis. Cantuzumab mertansine is the most advanced disulfide drug conjugate consisting of DM1 coupled to ε-amino group of lysine residues on humanized antibody C242 through a disulfide bond. The likely mechanism of drug release is reductive cleavage of the disulfide bond in endosomes/lysosomes that ensures the intracellular release of DM1. Another example is antibody conjugates of auristatins, cAC10-vcMMAE, obtained by conjugating chimeric mAb cAC10 [42]. This conjugate induces the growth arrest of CD30+ cells in vitro and in vivo to monomethyl auristatin E (MMAE) through a valine-citrulline peptide linker that are selectively cleaved by cathepsin B after internalization. Auristatins are 50 to 200-fold more potent than vinca alkaloid. They are structural analogue of marine pentapeptide dolastatin, which interact with the vinca alkaloid-binding site on α-tubulin preventing the formation of mitotic apparatus.
Fig. (4).
Antibody conjugates of maytansinoid (huC242-DM1) and auristatins (cAC10-vcMMAE).
Mylotarg (gemtuzumab, Wyeth) [43] is the only immunoconjugate that has been approved by the FDA for the treatment of cancer [Fig. (5)]. This immunoconjugate consists of humanized anti-CD33 mAb linked to the cytotoxic antibiotic ozogamicin (N-acetyl-γ calicheamicin). The linker consists of two cleavable bonds. Mylotarg has been approved for the treatment of CD33+ acute myeloid leukemia (AML) in elderly patients who are not eligible for other chemotherapies and who are suffering from their first relapse. Mylotarg demonstrated clinical efficiency in pediatric patients with advanced CD33+ AML. Further studies for this patient group using Mylotarg in combination with standard chemotherapy are in progress. Several other immunoconjugates are currently in clinical trials (Table 1).
Fig. (5).
Structure of Mylotarg. Mylotarg is only approved immunoconjugate for cancer therapy.
Table 1.
Immunoconjugates and folate-targeted prodrugs in clinical trials.
| Prodrugs | Description | Target | Status |
|---|---|---|---|
| (A) Immunoconjugates | |||
| IMGN901 (huN901-DM1) [57] | Humanized anti-CD56 conjugated to DM1 | CD56+ cells | Phase I |
| Solid tumors | Phase I | ||
| Small-cell lung cancer | Phase II | ||
| IMGN242 (huC242-DM4) [57] | Anti-CanAg mAb conjugated to DM4 | Gastric Cancer | Phase I/II |
| MLN-2704 [58] | Anti-PSMA mAb conjugated to DM1 | PSMA expressing prostate cancer | Phase I/II |
| Inotuzumab-ozogamicin [59] | Anti-CD22 mAb conjugated to calicheamicin | CD22 expressing cells in B-cell Leukemia | Phase I |
| AVE9633 [60] | Anti-CD33 mAb conjugated to DM4 | CD33 expressing cells in acute myeloid leukemia | Phase I |
| Trastuzumab-DM1 (T-DM1) [57] | Anti-HER2 mAb conjugated to DM1 | HER2 expressing cells in metastatic breast cancer | Phase I/II |
| SAR3419 [60] | Anti-CD 19 mAb conjugated to DM4 | CD19 expressing cells in B-cell derived cancers | Phase I |
| SGN-35 [61] | Anti-CD30 mAb conjugated to Auristatins | CD 30 expressing cells in hematologic malignancies including Hodgkin Lymphoma | Phase I |
| (B) Immunotoxins | |||
| Transferrin-CRM107 (Trans-MID) [62] | Transferrin linked to diphtheria derived toxins | Transferrin receptors | Phase II |
| Phase I (Children) | |||
| IL13-PE38QQR [62] | IL13 linked to Pseudomonas toxins | IL13 binds to tumors expressing receptors when infused into the brain | Phase III |
| SS1(dsFV)-PE38 [60] | Anti-mesothelin mAb linked to Pseudomonas exotoxins | Mesothelin-expressing malignancies | Phase I |
| (C) Folate-targeted prodrugs | |||
| EC145 [62] | Folate-vinblastine hydrazide | Cancer cells expressing folate | Phase I/II |
| EC17 [62] | Folate-hapten | Cancer cells expressing folate | Phase II |
| EC0225 [62] | Folate conjugated to vinca alkaloid & mitomycin C | Cancer cells expressing folate | Phase I |
There are several cellular receptors besides antigens that provide targets for prodrug design as discussed earlier. Active targeting is achieved by binding drugs to ligands that display high affinity for a particular receptor. The prodrug is taken up by receptor-mediated endocytosis after binding. The drug is then released in endosomes or lysosomes depending on the route of cellular trafficking of the particular receptor. The ligands can be low or high molecular weight compounds such as vitamins, peptides, sugars, native or modified proteins and antibodies.
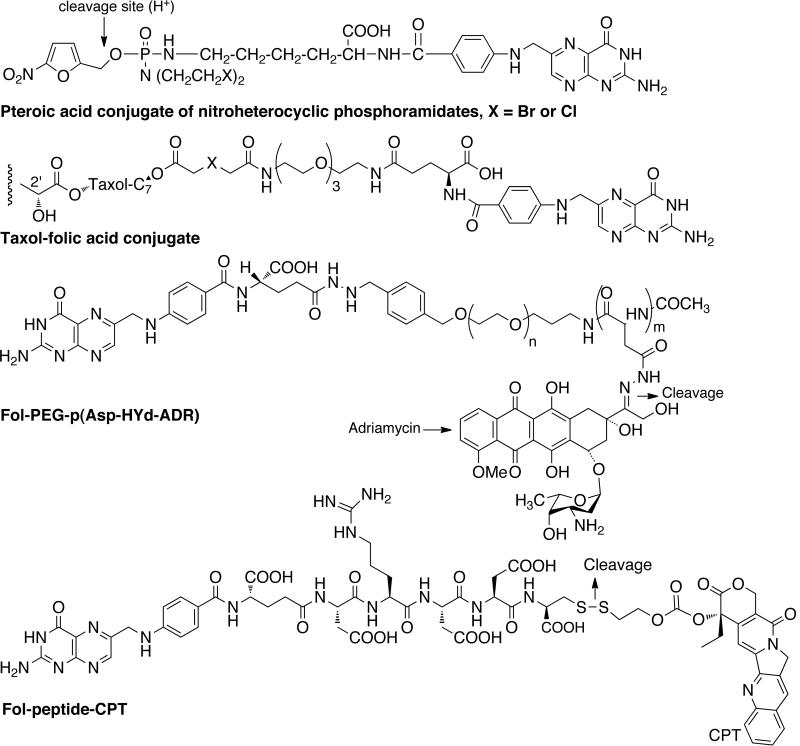
Folic acid is one of the most used ligand as it retains high affinity for its receptor even after modification with drug/carrier molecules. It is overexpressed in many human cancer types and folate conjugates with alkylating agents, platinum complexes, paclitaxel, 5-fluorouracil, camptothecin, doxorubicin and mitomycin have been investigated. For instance, Liu et al. developed a novel synthetic procedure to conjugate folic acid to 5'-OH of 5-fluoro-2'-deoxyuridine-5'-O-monophsophate (FdUMP) by automated synthesis [44]. FA-FDUMP conjugate showed improved cytotoxicity towards human colorectal cells (H630) and 5-FU-resistance colorectal tumor cells (H630–10). Steinberg and Borch synthesized and evaluated pteroic acid conjugates of nitroheterocyclic phosphoramidites (alkylating agent) for folate receptor targeting [Fig. (6)] [45]. Fuchs and coworkers developed a series of taxol derivatives tethered to glutamate and folate through α-alkoxy and α-aminoester linkers [46]. Taxol is a mitotic inhibitor used in cancer chemotherapy. Kataoka and coworkers designed multifunctional polymeric micelles, folate-poly(ethylene glycol)-poly(aspartate-hydrazone-adriamycin) or Fol-PEG-PAsp-Hyd-ADR for folate targeting and pH triggered drug release [47]. Folate was attached at the end of the shell forming PEG chain and adriamycin was attached to the side chain of core forming pAsp segment through an acid sensitive hydrazone bond and the conjugate showed an enhanced cellular uptake. Adriamycin (doxorubicin) is an anthracycline antibiotic known to interact with DNA, and used for the treatment of wide range of cancers. Low and coworkers developed a folate-targeted prodrug by attaching camptothecin (CPT) to folate through a hydrophilic spacer linked to folate via releasable disulfide carbonate linker [48]. The CPT conjugate inhibited the cell proliferation in human KB cells and receptor-mediated uptake was demonstrated. CPT is a cytotoxic quinoline alkaloid which inhibits the DNA enzyme topoisomerase I.
Fig. (6).
Folate-targeted prodrugs.
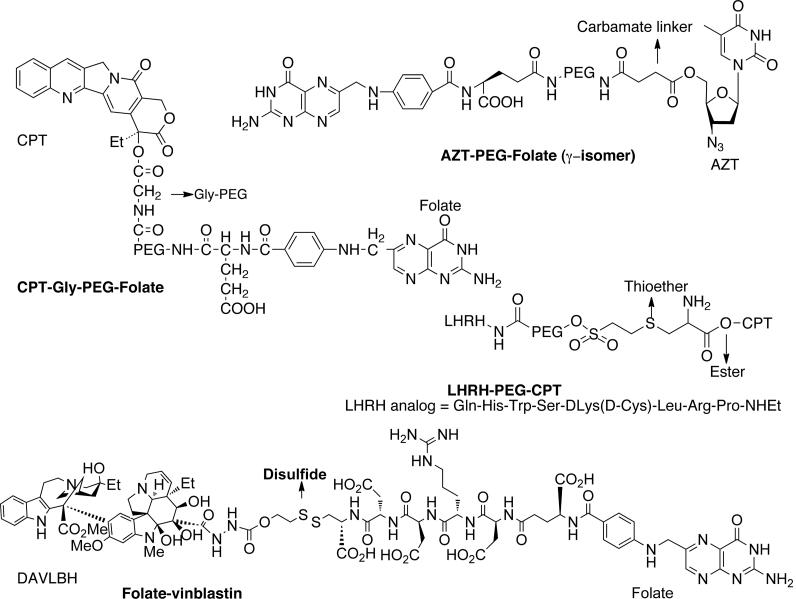
Our group has developed several CPT conjugates for folate targeting. A modular conjugate, CPT-Gly-PEG-Folate was prepared by attaching CPT to one end of bifunctional poly(ethylene glycol)/PEG via a glycine linker and folic acid to the other end as shown in Fig. (7) [49]. The conjugate showed specific interaction with folate receptor on KB cells and demonstrated enhanced cytotoxicity because of receptor-mediated endocytosis. The conjugate CPT-Gly-PEG alone did not show any significant toxicity. Later, a series of CPT conjugates were prepared by incorporating linkers such as glycine (gly), alanine (ala), proline (pro), proline-glycine (pro), proline-alanine (pro-ala), glycine-glycine-glycine (gly-gly-gly) and glycine-glycine-phenylalanine-glycine (gly-gly-phe-gly) [50]. The amino acid and dipeptide linkers were incorporated to control the CPT release by changing the steric hindrance around the linker whereas the tripeptide and tetrapeptide linkers were incorporated to utilize cleavage by cathepsin B enzyme, which are over expressed in cancer cells. The CPT-gly-PEG-Folate was found to be most active in vivo, but from histopathological and systemic toxicity point of view, the conjugate PEG-Gly-Gly-Phe-Gly-folate was found to be most promising. It was also shown that single targeting (folate) was as effective as dual targeting (folate and cathepsin B).
Fig. (7).
Folate and LHRH-targeted prodrugs.
We have been involved in the development of the folic acid conjugate of 3'-azido-3'deoxythymidine (AZT) [51]. Folate-PEG-AZT conjugates were synthesized by incorporating carbamate and succinate linkage. Compared to AZT, conjugates showed ~20-fold greater potency against the ovarian cell line (A2780/AD) that overexpresses the folate receptor. AZT is considered to be responsible for the formation of ultrastructural changes or strand termination in growing DNA strands. Minko and coworkers used PEG as a carrier to attach CPT (apoptosis inducing agent) and luteinizing hormone-releasing hormone (LHRH, targeting moiety) analog [52]. LHRH receptors are overexpressed in the plasma membrane of several types of cancer cells but not in normal visceral organs. Enhanced efficacy of chemotherapy due to amplified apoptosis in tumors was observed. Later, they developed a system that incorporated a synthetic analogue of BCL2 homology 3 domain (BH3) peptide (inhibitor of cellular antiapoptotic defense) to PEG (carrier), apart from CPT (apoptosis inducer) and LHRH peptide (targeting moiety) [53]. This showed higher activity than individual components applied separately. The same group developed another polymeric prodrug system by attaching up to three copies of CPT and LHRH to PEG moiety through citric acid spacer [54]. The multicomponent prodrug system showed about 100-times more toxicity and enhanced antitumor activity. Prodrug EC 145 developed by Leamon and coworkers is probably the most promising folate-targeted prodrug in clinical trials [55,56]. It is composed of vinca alkaloid desacetylvinblastine monohydrazide (DAVLBH) linked to folic acid through reducible disulfide bridge. EC 145 was found to be more active and better tolerated than the free drug in in vivo preclinical studies and showed superior antitumor activity. Several folate-targeted prodrugs in clinical trials are shown in Table 1.
Cyclic peptides that bind to integrins can be used to target vascular receptors. Vascular receptor proteins are crucial for interaction between a cell and the extracellular matrix and they are involved in tumor angiogenesis [63]. Certain integrins (αvβ3, αvβ5) are over expressed on proliferating endothelial cells and some tumor cells. Peptides containing the RGD sequence (Arg-Gly-Asp) that is present in extracellular matrix can be used to target integrins and subsequently inhibit angiogenesis. A number of RGD-drug conjugates with cytostatic and diagnostic agents have been prepared to obtain the proof of concept [64–67].
The asialoglycoprotein receptor (ASGPR) is a membrane bound lectin expressed on hepatocytes and liver cancer [68]. It has been used for prodrug targeting for the treatment of hepatocellular carcinoma. ASGPR has high affinity for terminal β-galactoside or β-N-acetylgalactosamine on glycoproteins and is responsible for the endocytosis of several glycoproteins. The strong interaction of glycoproteins with ASGPR receptor is attributed to the cluster effect (multivalency) in which adjacent saccharide group binds to the receptor with high binding constants [69]. The cluster effect is mainly due to the thermodynamic property of multivalent ligands rather than the presence of multiple receptor binding sites.
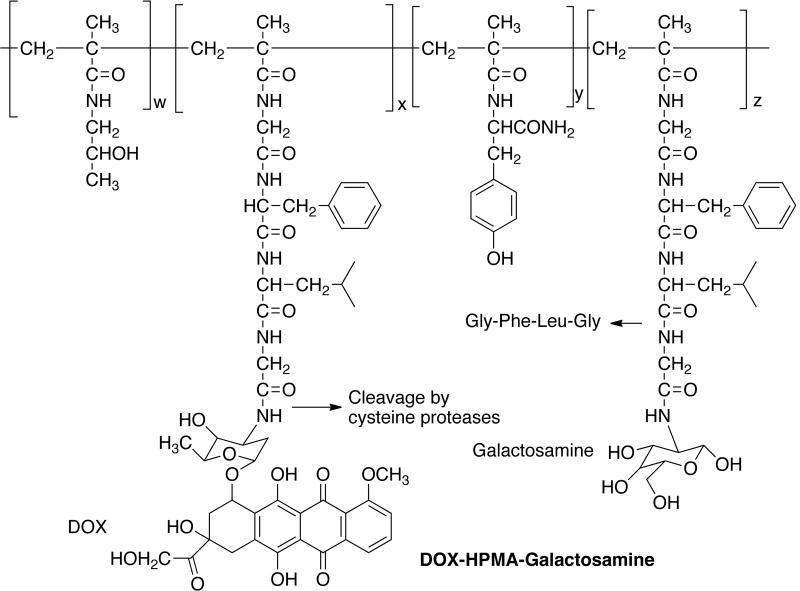
Stephano and coworkers synthesized (6-maleimidocaproyl) hydrazone derivative of DOX and coupled it to lactosaminated human albumin (LHSA) [70]. A hydrazone linker was used because it is stable at neutral plasma pH, but allows the drug release in the endosomal or lysosomal compartments. The animals injected with drug conjugate showed 8 to 14-times higher drug concentration in liver than heart, intestine, spleen and kidney compared to the same dose of free drug. They also showed substantial resistance of rat regenerating hepatocytes to high intracellular concentrations of DOX [71]. This suggests that the drug conjugate can be used as adjuvant chemotherapy after the surgical removal of HCC. It was later shown that the effect of HSA lactosamination was due to the hindered uptake by spleen, and bone marrow [72]. HPMA copolymer-Gly-Phe-Leu-Gly containing galactosamine (PK2, FCE28069) is the only polymer-drug conjugate bearing a targeting ligand to be tested clinically [73,74]. PK2 has Mw ~25000, DOX content (~7.5 wt%), and galactosamine content of 1.5–2.5 mol% [Fig. (8)]. The prodrug showed 30% delivery to the hepatic region in preclinical studies. The prodrug was found to accumulate in tumors also due to the enhanced permeability and retention (EPR) effect and ratio of tumor tissue to normal liver uptake was 1:3 in 24 h. Phase I/III clinical trials were conducted on patients with hepatocellular carcinoma and the prodrug was given by intravenous infusion every three weeks. The maximum tolerated dose was 160 mg m−2 and a dose-limiting toxicity typical to anthracycline was observed. Two patients gave measurable response and there were 11 others with stable disease out of total 23 patients. The galactose-mediated liver targeting was about 15–20% dose at 24 h.
Fig. (8).
ASGPR-targeted prodrug.
The greatest drawback associated with antibody-drug conjugates and drug modified with ligands having affinity for particular receptors is that they are not exclusively tumor specific and cross-reactivity of drug conjugate with normal tissue is observed [24]. The inner regions of solid tumors are often poorly vascularized and exhibit low blood flow. Both factors decrease the amount of macromolecular prodrug reaching these parts of tumors. The antigen and receptors are secreted by the tumor and this limits the amount of drug reaching the tumor, because soluble antigens and receptors neutralize the prodrugs in circulation. Heterogeneity of antigen and receptor expression by tumor cells restricts the number of cells that can be targeted effectively. The preclinical evaluation of the prodrug relies on a mouse model with human transplanted tumors. The biodistribution of prodrugs in such models might not be indicative of biodistribution behavior in humans.
2.1.2. Antibody-directed enzyme prodrug therapy (ADEPT)
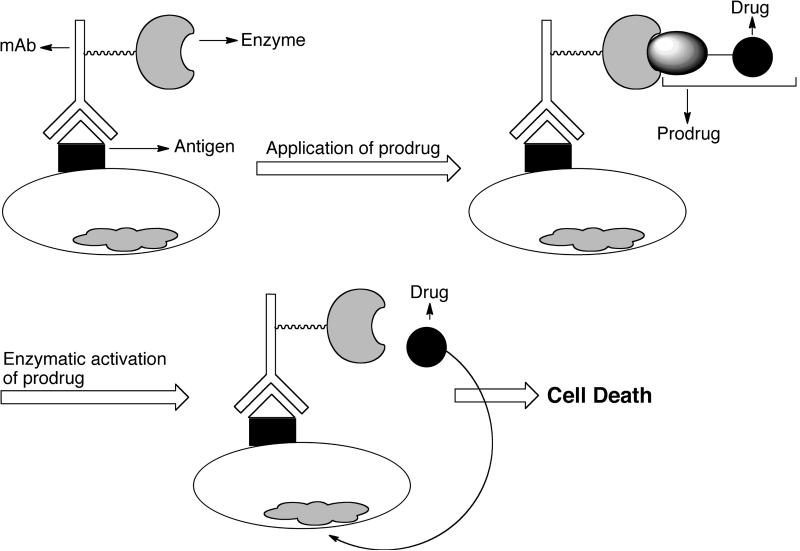
Antibody-directed enzyme prodrug therapy or ADEPT [25,26,75] is a two-step mechanism developed independently by Bagshawee [76] and Senter [77] as shown in Fig. (9). A tumor-associated mAb linked to drug-activating enzyme (usually antibody-enzyme fusion protein) is administered intravenously in the first step, which binds to specific antigen expressed on the tumor cell surface. A non-toxic prodrug is administered systemically in the second step and is converted to the cytotoxic drug by the pre-targeted enzyme.
Fig. (9).
Schematic presentation of antibody-directed enzyme prodrug therapy (ADEPT). mAb-enzyme conjugate is given first, which binds to antigens expressed on tumor surfaces. Prodrug is given next, which is converted to active drug by the pre-targeted enzyme. Redrawn from ChemMedChem 2008, 2, 20.
The ideal drugs for ADEPT are small molecules with ability to diffuse into the tumor tissues to cause a bystander effect. The bystander effect is defined as the capability to kill the surrounding non-dividing/non-expressing tumor cells and is an important requirement for this type of therapy as it amplifies the drug effect. To avoid systemic toxicity in clinical application, the time interval between enzyme and prodrug administration should be optimized so that the conjugate accumulates only in tumors and not the blood and normal tissues. There are several general considerations for ADEPT [75]. The target antigen should either be expressed on tumor cell membrane or secreted into the extracellular matrix of the tumor and use of high-affinity mAb is essential. The enzyme should be able to exert its optimal activity at a pH close to that of tumor extracellular fluid. Moreover, the antibody-enzyme conjugate may be immunogenic and circulating anticonjugate antibodies may interfere with treatment. Therefore, the drug should be dose dependant and cell cycle independent. For effective therapy, the antibody-enzyme conjugate should remain on the cell-surface after binding to the respective antigens and it must also be cleared rapidly from the circulation to prevent toxicity. The enzymes that are used in ADEPT can be divided into following three categories [78]: Class I: enzymes of non-mammalian origin with no mammalian homologues such as alkaline phosphatase, and α-galactosidase; Class II: enzymes of non-mammalian origin with mammalian homologues such as carboxypeptidase A, β-glucuronidase, and nitroreducatse; Class III: enzymes of mammalian origin such as β-lactamase, carboxypeptidase, cytosine deaminase, benzylpenicillin amidase, and phenoxylmethylpenicillin.
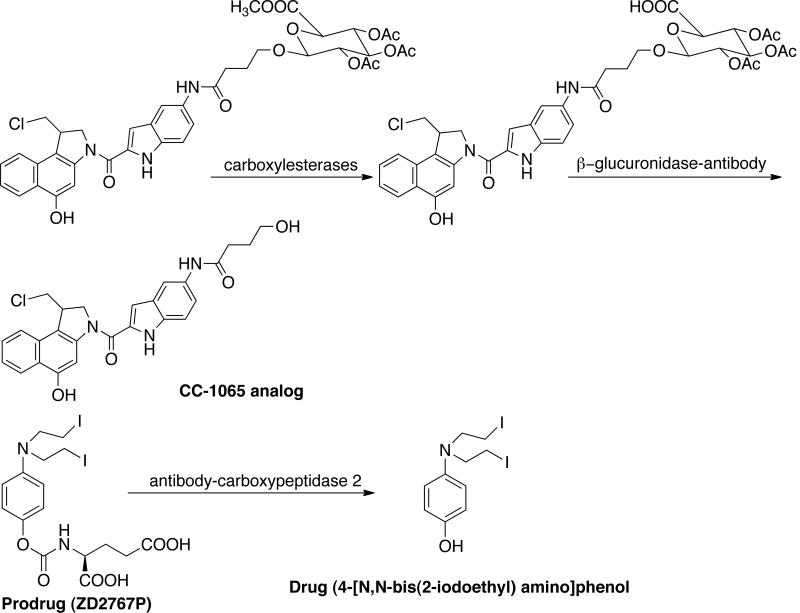
The ADEPT strategy has been used for antitumor drugs such as paclitaxel, anthracyclines and dinitrobenzamides. Wei and Pei developed fluorodeoxyuridine-5'-dipeptidyl derivatives (FdU) for biotransformation by peptidyldeformylase (PDF) enzyme [79]. The enzyme removes the dipeptide formyl N-terminal group thereby releasing the active drug (FU). Wang et al. developed a prodrug constituted by cephalosporin and CC-1065 analog [80]. This undergoes activation by β-lactamase coupled to an antibody. The prodrug showed 10-times less toxicity compared to free drug. Consequently, these authors developed analogs of CC-1065 for use in β-glucuronidase sensitive ADEPT system. [Fig. (10)]
Fig. (10).
Representative examples of prodrugs in ADEPT system.
Currently, there are two ADEPT systems in Phase I/II clinical trials with prodrug ZD2767P or N-{4-[N, N-bisamino]phenoxycarbonyl}-L-glutamic acid [81]. The prodrug is activated by enzyme carboxypeptidase 2 (CPG2) to active drug 4-[N, N-bis(2-iodoethyl)amino]phenol or phenol bisiodide mustard and has been found active against colorectal tumors. The prodrug possesses the best profile in terms of enzyme kinetics, cytotoxicity differential between CPG2 expressing and non-expressing cell lines, and overall in vivo efficacy. Moreover, the prodrug has short half-life and is therefore ideal candidate for ADEPT. The first ADEPT system in a clinical trial used a recombinant fusion of murine anti-carcinoembryonic antigen (CEA) F(ab)2 fused to CPG2 in combination with prodrug ZD2767 for the treatment of advanced colorectal cancer [82]. The second ADEPT study in clinical trials utilized a recombinant fusion of anti-carcinoembryonic antigen (CEA) sFv fused to CPG2, in combination with ZD2767P for the treatment of CEA-expressing tumors [83].
The major advantage of ADEPT over antibody-conjugates is the amplification of the cytotoxic effect due to catalytic activation of prodrug. Another benefit is the ability to kill surrounding tumor cells thereby reducing the risk of tumor evading therapy by antigen loss. The cytotoxic effects of drugs are largely confined to the tumor target and hence the side effects are reduced as compared to systemic administration of chemotherapy. ADEPT has proved highly effective in several tumor xenograft studies but this has not translated into clinical practice. A significant obstacle has been the immunogenicity of enzymes used for prodrug activation and the targeting mAb as both were derived from non-human sources. This problem was resolved by the use of human enzymes in conjunction with humanized or human mAbs. A humanized anticarcinoembryonic antigen (CEA)-antibody human-β-glucuronidase fusion protein plus doxorubicin glucuronide prodrug was developed. This ADEPT system gave superior efficacy and reduced toxicity in tumor xenograft studies in mice as compared to free doxorubicin given at its maximum tolerated dose. The ADEPT system produced 4- to 12- fold higher intra- and up to 5-fold lower extra-tumoral drug concentrations. The use of human enzymes however poses the potential risk of unwanted activation of prodrugs by endogenous enzymes. Although promising, the ADEPT system still needs more evaluation.
2.1.3. Gene-directed enzyme prodrug therapy (GDEPT) / Virus-directed enzyme prodrug therapy (VDEPT)
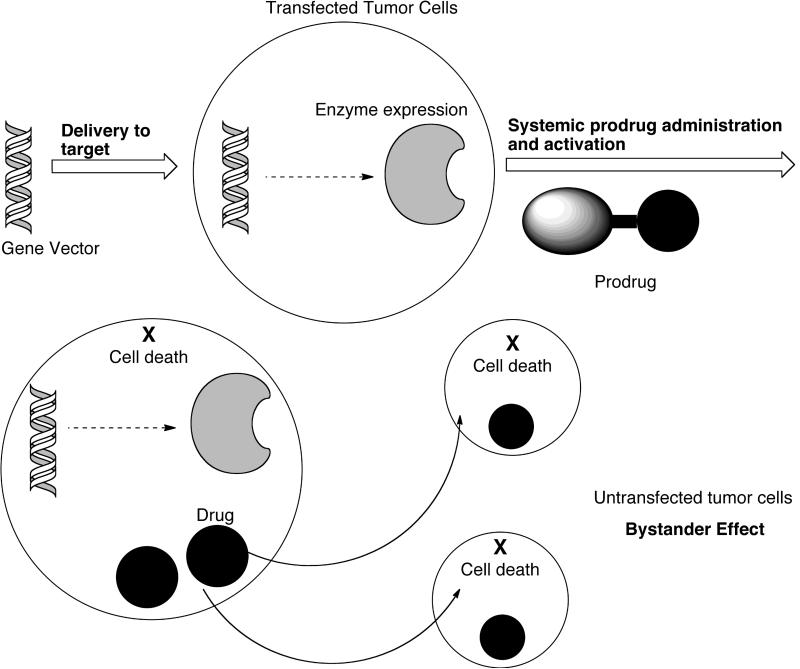
GDEPT is also known as suicide gene therapy [75,84,85]. It involves physical delivery of a gene for a foreign enzyme (not naturally expressed in the host) to tumor cells by a targeting mechanism that leaves the surrounding non-cancerous cells untransformed. The transformed tumor cells express the enzyme, which in turn activates the systemically delivered non-toxic prodrug [Fig. (11)].
Fig. (11).
Schematic presentation of gene-directed enzyme prodrug therapy (GDEPT). Gene for foreign enzyme is transfected to tumor cells, which express the enzyme to activate the systemically administered prodrug. Redrawn from Nature Rev. Cancer 2007, 7, 870.
For GDEPT to be effective, the expressed enzyme or a related protein should not be present in normal human tissues or expressed only at very low concentrations so as to activate the prodrug only in tumor tissues [85]. The enzyme must achieve sufficient expression in the tumors and give high catalytic activity. The prodrug should be lipophilic so that it can diffuse into the tumor cells before it can be converted into cytotoxic drug by the suicide enzyme. Alternatively, if prodrug cleavage takes place extracellularly, the active drug should be capable of diffusing through cell membranes. The drug should be able to kill surrounding non-dividing or non-expressing tumor cells by bystander effect as explained earlier. The half-life of drug should be long enough to induce bystander effect but short enough to avoid leakage into the systemic circulation.
The vector delivery of genes is a major challenge in GDEPT as it is in all areas of gene therapy [86]. A wide variety of vectors are available for gene delivery. Viral vectors are most popular, but they suffer from limited amount and size of plasmid-DNA. There is also the possibility of inducing severe immune responses. Non-viral vectors such as cationic liposomes are less expensive, easier and safer to make, and suitable for long-time storage, but their delivery properties are far from optimal. Gene delivery in general has suffered from an inability to transfect large numbers of cells efficiently and often lacks specificity for the cells of interest. The commonly used vectors for gene-delivery in GDEPT are listed below [85].
Bacteria: The most prevalent organisms used are Salmonella and Clostridium, but others, such as Bifidobacterium and Escherichia coli have also been used.
Virus: The viral vector can be non-replicating or replicating. The common vectors used in this category include Adenovirus (AdV), Adeno-associated virus (AAV), Retrovirus, Herpes Simplex Virus (HSV). The GDEPT approach is recognized as VDEPT (virus-directed enzyme prodrug therapy) when viral vectors are used for gene delivery.
Natural proteins: The most common example is antibody.
Synthetic Vectors: This includes mostly liposomes, which are probably the most utilized non-viral vector for gene-delivery.
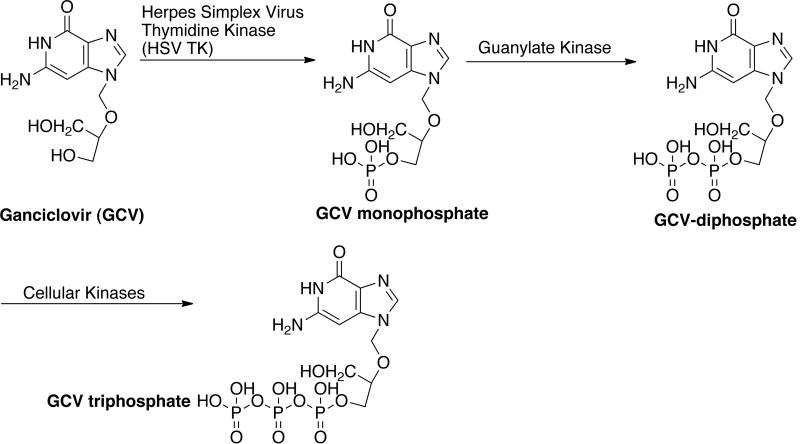
An early example of GDEPT is the combination of Herpes Simplex Virus Thymidine Kinase (HSV-TK) and ganciclovir (GCV). GCV is used to treat HSV infection in humans [84]. The drug is phosphorylated by the HSV-TK and then by cellular kinases to produce GCV-triphosphate, which incorporates into the elongating DNA during the cell division (S-phase) and cause inhibition of DNA polymerase and single strand breaks [Fig. (12)]. These characteristics make HSV TK-GCV useful for eradicating tumor cells invading non-proliferating tissues. The GCV system has a highly charged triphosphate that is insoluble in lipid membranes. This makes the diffusion of drug and cell-to-cell contact for bystander killing difficult. However, several preclinical studies have shown successful tumor regression even with 10% of tumor cells expressing HSV TK. The bystander effect could be due to the transfer of GCV through gap junctions or immune-related. Cytosine deaminase (bacteria and fungi) and 5-fluorocytosine (5-FC) is another GDEPT example [Fig. (2)] [84]. The CD enzyme catalyzes the hydrolytic deamination of cytosine to uracil and converts the prodrug 5-FC into active drug 5-FU (5-fluorouracil). The CD gene was cloned from Escherichia coli and the CD/5-FU GDEPT system was found to be effective against fibrosarcomas, carcinomas, and metastatic formations in animal models. The drug was shown to exert a strong bystander effect that did not require cell-to-cell contact. It was shown that 1–30% cell expression is enough for the drug to exhibit bystander effect. It was realized that the treatment with a single GDEPT strategy might lead to partial response and hence a combination of several gene or treatment modalities was investigated to improve the GDEPT efficiency. CD-HSV TK fusion genes were delivered followed by the prodrug GCV and 5-FC and higher efficacy for combined system was observed. This system gave encouraging results when used in combination with radiotherapy. Improved antitumor activity has been reported for this system by using a catalytically superior Saccharomyces cerevisiae CD.
Fig. (12).
Herpes simplex virus-thymidine kinase / ganciclovir (HSV TK/GCV) based GDEPT system.
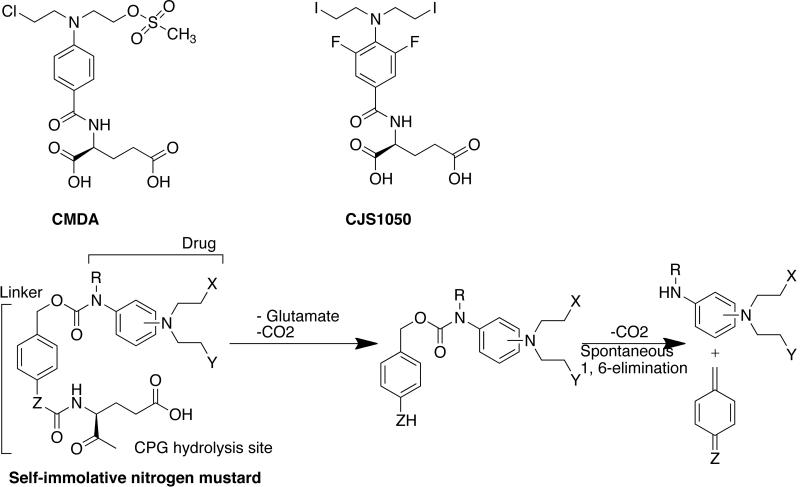
Springer and coworkers have used Pseudomonas carboxypeptidase G2 (CPG2) and nitrogen mustard prodrugs to construct a GDEPT system [85]. CPG2, as explained earlier, catalyzes the hydrolysis of nitrogen mustard prodrugs into glutamic acid and the cognate drug. The commonly used prodrugs are CMDA and ZD2767P [Fig. (10) & (13)]. A self-immolative nitrogen mustard prodrug was also designed by separating the CPG2 hydrolysis site from the effecter end of the molecule through a spacer [87–89]. This prodrug undergoes 1,6 elimination to release the active drug. The advantage of using this prodrug over conventional nitrogen mustard prodrugs is that the structure of linking group and lipophilicity can be changed without affecting the activation kinetics.
Fig. (13).
Common nitrogen mustard prodrugs. The structure of self-immolative prodrug is also shown. Prodrug ZD2767P is shown in Fig. (10).
The CPG2 system has several advantages over other GDEPT systems [85]. For instance, CPG2 has no mammalian equivalent unlike the carboxylesterase, purine nucleoside phosphorylase or CYP450. No additional activation steps are required unlike the CD/5FC system and strong bystander effect is obtained as nitrogen mustard drugs are relatively lipophilic and can pass through the cell membranes. Moreover, CPG2 conjugated to CEA antibody has been investigated in ADEPT system [Fig. (10)]. Marais et al. were first to investigate CPG2-based GDEPT system in vitro using a range of human cancer cell types [90]. The enzyme was not secreted and remained in cytoplasm and was designated as CPG2*. The CPG2* expressing cells showed 11- to 95-fold greater sensitivity to nitrogen mustard prodrug and sensitivity was dependant on the cell type. The system showed a large bystander effect, and about 90% of the surrounding cells were killed when only about 2% of the cells expressed CPG2*.
The Springer group attempted to resolve the problem of enzyme release by expressing CPG2 in cell-surface-tethered form by creating a fusion protein with the transmembrane region of the epidermal growth factor receptor family member ERBB [91]. The surface-tethered CPG2 system gave 10-fold reduction in IC50 of CMDA in MDA MB 361 mammary tumor cells and good bystander effect in vitro and in vivo [92]. The CPG 2 was delivered under the control of TERT promoter (AdV.hTERT-CPG2*) which resulted into infection of AdV and expression of CPG2* with vastly greater titer in tumors than in liver. Addition of prodrug ZD2767P for six weeks led to significant decrease in tumor growth rate and prolonged survival of animals. Similar therapy was tested in SW620 human colorectal adenocarcinoma cells [93]. It was observed that AdV.hTERT-CPG2 with ZD2767P was as effective as cisplatin and free prodrug. Approximately 80% of cells were killed with only 25% of SW620 expressing cells. In xenografts, they observed good tumor regression and increased survival in the GDEPT group of animals compared with prodrug alone group. It was found that tumors express low CARS than lung, gut, liver, or ovaries, but the level of CPG2 in tumor was 20-fold higher than in liver and with little or no expression in other sampled tissues. In animals treated with full GDEPT, presence of prodrug led to increased viral copy and enzyme activity in tumor compared with those treated with virus alone. This suggests that the system does not affect the viral genome, but directly or indirectly increases the adenovirus replication. The prodrug apparently appears to exert positive cooperative effect on the vector and this was further confirmed by an improved bystander effect. The increased survival of animals suggested that either virus has low toxicity towards non-tumor cells or hTERT targeting restricts the distribution to tumors. This system is expected to soon go in Phase I clinical trials.
Several other GDEPT systems have been developed, which are in various stages of preclinical and clinical development [85]. The purinenucleoside phosphorylase (PNP) enzyme in combination with 6-methylpurine (prodrug). The PNP enzyme converts the prodrug into 6-methyl purine (drug), which causes the inhibition of RNA, protein and DNA synthesis. The carboxyesterases enzyme in combination with prodrug Irinotecan (CPT11) [94]. The prodrug is converted into active drug camptothecin (SN38), which binds to nuclear enzyme topoisomerase-I and leads to single strand breaks. The nitroreductase enzyme (Escherichia coli) working in combination with prodrug CB1954, where the prodrug is converted into 5-aziridin-1-yl-4-hydroxylamino-2-nitrobenzamide, which is known to cause DNA interstrand crosslinking [95]. The enzyme cytochrome P450 (CYP450) in combination with prodrug cyclophosphamide is another GDEPT system undergoing evaluations [96]. The prodrug is converted to 4-hydroxycyclophosphamide, which spontaneously disintegrates to acrolein (potential cytotoxin) and a bifunctional alkylating oxazaphosphorine mustard drug (DNA crosslinker). The GDEPT systems in clinical trials are listed in Table 2.
Table 2.
| Enzyme | Prodrug | Target | Status |
|---|---|---|---|
| HSV-TK [97–101] | Ganciclovir | Hepatocellular carcinoma | Phase I |
| Glioma | Phase I | ||
| Ovarian | Phase I | ||
| Prostate | Phase I | ||
| Colorectal | Phase I | ||
| CD with HSV-TK [102] | Ganciclovir & 5-FC | Prostate | Phase I |
| Nitroreductase [103] | CB1954 | Hepatocellular carcinoma | Phase I |
| CYP2B6 [104] | Cyclophosphamide | Breast, melanoma | Phase I |
| Thyroid | Phase I | ||
| HSV-TK [105,106] | Ganciclovir | Melanoma, Breast, Non-small cell lung sarcoma | Phase I |
| Glioma | Phase I/II, III |
CD, cytosine deaminase, CYP2B6, member of cytochrome P450 group of enzymes; 5-FC, 5-fluorocytosine; HSV-TK, Herpes simplex virus - thymidine kinase.
NatureRev. Cancer 2007, 7, 870.
The advantage of the GDEPT approach is the possibility of delivering target-specific cancer therapy with reduced systemic toxicity resulting in a better prognosis for patients. There are certain theoretical risks associated with GDPET such as insertional mutagenesis, anti-DNA antibody, local infection and tumor nodule ulceration, which may restrict its use. The elective delivery and expression of genes may be difficult to achieve. The viral vectors used are mostly replication deficient but there is a slight risk of reversion to wild type virus. The introduction of retrovirus vectors may cause mutagenesis of the host genome. Besides, retroviral vectors target only dividing cells and even in rapidly growing tumor nodules, only 6–20% of cells are in a proliferating stage and in S-phase.
2.1.4. Antibody-targeted, triggered, electrically modified prodrug type strategy (ATTEMPTS)
The prodrug activation in ADEPT strategy largely depends on chemical conjugation and enzyme cleavage respectively. ADEPT is therefore restricted to small-molecule drugs. Macromolecular drugs such as proteins are not suitable drug candidate for ADEPT strategy because chemical conjugation of proteins is more challenging due to the presence of multiple functional groups. Furthermore, owing to their large size, it is difficult to produce proteins specifically for enzymes used for prodrug activation in ADEPT system [107].
Yang and coworkers designed an elegant approach known as antibody targeted, triggered, electrically modified prodrug type strategy (ATTEMPTS) for the delivery of enzyme drugs without their associated toxic side effects [107]. ATTEMPTS delivery system is comprised of large complex made of two components: (i) targeting component consisting of an antibody chemically linked with an anionic heparin molecule; (ii) a drug component consisting of the enzyme drug modified with a cationic moiety. The two components are linked through a tight, but reversible electrostatic attraction. The cationic species conjugated to the enzyme is relatively small (positively charged peptide) and hence the enzyme conjugates retain its catalytic activity. However, this enzyme conjugate is unable to exert its catalytic activity because it is bound to antibody-heparin conjugate via electrostatic bonds. The enzyme inactivity is presumably due to the blocking of enzyme active site by the appended macromolecule. The ATTEPMTS complex is delivered to the targeted site by the attached antibody and the enzyme drug is released at the site by using a triggering agent such as protamine. Protamine is a heparin antidote, which binds to heparin more strongly than most of the cationic species. The released enzyme is then concentrated at the site of action thereby maximizing its catalytic activity towards drug conjugate at the targeted site while minimizing its toxic effects towards the normal cells. The selection of an appropriate cationic moiety is key to success of ATTEMPTS strategy because retention of prodrug after administration and its conversion to active drug relies on the binding strength of modified enzyme towards heparin. An important aspect of this approach is that both chemical and biological methods can be used to insert the cationic moiety.
Yang and colleagues have extensively used this strategy for delivery and selective activation of macromolecular drugs in vitro and in vivo [108,109]. They modified tissue plasminogen activator (t-PA) with a cationic species (Arg7-Cys-) and rendered it inactive by electrostatic binding with negatively charged heparin-antifibrin antibody conjugate. After targeting the complex to the target site, t-PA activity was restored by administration of protamine (heparin antidote).
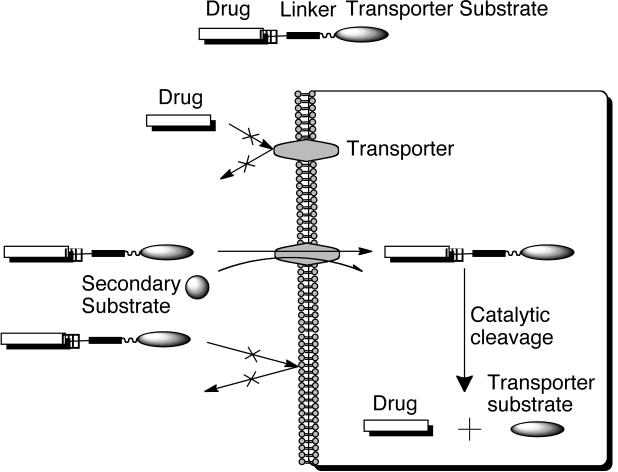
2.1.5. Membrane transporters
Membrane transporters are integral plasma membrane proteins that mediate the uptake of different substrates including, polar nutrients, amino acids and peptides, nucleosides, and sugars (Table 3) [21,110]. Transporters in general fall into two major families: (1) the ATP-binding cassette (ABC) family, which includes those transporters responsible for drug resistance through efflux transporters like Pgp and (2) the solute carrier (SLC) transporters, which are capable of influx into the cell [111]. SLC transporter types include uniports, substrate-ion symports, substrate-ion antiports, substrat-substrate antiports and ion-ion antiports [112]. An example of the SLC transporters are nucleoside transporters, which are responsible for the uptake of nucleosides (uridine, guanosine, adenosine, thymidine, and cytidine) the precursors of nucleotides [113]. Transporters can also be selective for different classes of substrates as is the case for the transporters of purine and pyrimidines. Often transporters require the flux of a secondary substrate, like the peptide transporters, PEPT1 and PEPT2, require the influx of H+ to facilitate their uptake functions [110]. This characteristic distinguishes transporters from their receptor counterparts, which require endocytosis to achieve uptake and can lead to complications of endosomal escape or lysosomal degradation.
Table 3.
Examples of membrane transporters and pharmacologic substrates.
| Transporter | Natural Substrates | Pharmacological Substrates |
|---|---|---|
| SERT | Serotonin | Paroxetine |
| NET | Noradrenaline | Imipramine |
| DAT | Dopamine | Imipramine |
| PEPT1 | Di/tri peptides | Ampicillin |
| RFC1 | Folate/thiamine | Methotrexate |
| CNT1 | Nucleosides | AraC, gemcitabine, FdU |
| ENT1 | Nucleosides | Cladribine, AraC, fludarabine |
| LAT1 | Large neutral L-amino acids | Melphalan |
| Pgp/MDR1 | Multiple classes of compounds | Anthracyclines, taxanes, vinca alkaloids |
| MRP1 | Multiple classes of compounds | Anthracylines, campothecins |
| SVMT | Pantothenate, Biotin, and Lipoate | Biotin-conjugates |
Because many transporters have nutrients as substrates, prodrug design can be manipulated to mask the drug with nutrient moiety as seen in Fig. (14). The use of membrane transporters as prodrug targets has largely stemmed from the discovery of the absorption mechanism of valacyclovir via the PEPT1 transporter. The addition of the L-valyl ester to the parent drug acyclovir vastly improved the bioavailability of acyclovir [114–117]. In 1997, our group first reported that the mechanism of valacyclovir absorption was concentration dependent, saturable, and inhibited by oligopeptides, suggesting the involvement of a transporter [118–120]. Our group and others have been instrumental in elucidating the involvement of PEPT1 in the absorption of valacyclovir [121–126]. Upon absorption, valacyclovir is rapidly converted to its parent drug acyclovir via esterase cleavage. Acyclovir is then free to enter the blood stream, where it can be taken up by cells via nucleoside transporters [113,121,127].
Fig. (14).
Schematic presentation of membrane transporter targeted prodrug uptake. Redrawn from AAPS Pharmsci 2000, 2, 1.
Several properties of PEPT1 make it amenable to drug targeting, in particular for oral absorption. Its level of expression in the small intestine epithelial cells and its low affinity and high capacity profile with Km values ranging from 200 μM to 10 mM depending on the substrate [110,128]. Also PEPT1 has the ability to transport stereoselective peptides (L-enantiomers of amino acids in general have higher affinity than D-enantiomers), as well as peptidomimetic compounds like valacyclovir and β-lactam antibiotics [110]. Valacyclovir was the first example of a novel peptide ester prodrug (a nonpeptidic substrate) that actively targeted the human transporters, PEPT1, for increased oral absorption.
Besides the obvious benefit of increased oral absorption, this method of drug delivery can also be tailored to cancer cells over expressing certain membrane transporters. The malignant ductal pancreatic cancer cell lines AsPc-1 and Capan-2 [129] and the human fibrosarcoma cell line HT-1080 over express the PEPT1 membrane transporter [130]. It has been suggested that if tumor cell uptake of hydrophilic polymer drug conjugates via a specific mechanism of internalization can be achieved; these drugs may avoid the development of or elimination due to multidrug resistance [131,132].
Floxuridin (FUDR) like many of its nucleoside counterparts (acyclovir included) is plagued by low and variable bioavailability, as well as adverse reactions, despite its well-characterized clinical effectiveness [127,133]. Various classical prodrug design methods have been employed, including linking FUDR to aliphatic carboxylic acid chains to improve drug permeability [134]. In an attempt to take advantage of the PEPT1 transporter system, Amidon and coworkers created amino acid ester prodrugs of FUDR in a similar fashion to valacyclovir. The myriad of amino acid esters that were generated led to several conclusions for this targeted drug delivery system in vitro: (i) the structure, the stereochemistry, and site of esterfication of the promoiety affected the rate of prodrug activation, (ii) the amino acid moiety affords both transport and stability characteristics to the prodrug and can be tuned to desired effects, (iii) the amino acid moiety can improve the drugs resistance to metabolism due to thymidine phosphorylase, possibly improving both oral absorption and therapeutic effect [135–137].
β-Carboline is an anticancer drug from a group of intercalating agents that has been conjugated by Zhao et al. via amino acid ester linkages to improve its physicochemical properties and cytotoxicity [138–143]. β-carboline's primary mechanism of action is to intercalate into the DNA helix, which interferes with DNA topoisomerases I and II, causing irrevocable damage to the cellular DNA resulting in cytotoxicity. Functional side chains of amino acids have also been shown to make specific base interactions with DNA [144–146]. Therefore, linking β-carboline to amino acids improved its cytotoxicity in HeLa, MCF-7, and HepG2 cell lines as measured by MTT (3-[4,5-dimehtylthiazol-2yl]-2,5-diphenyltetrazolium bromide) assay [147]. The cytotoxicity was greatest in the prodrugs with lysine and arginine residues giving IC50 values in the 1–7 μM ranges. It was also observed that the Lys and Arg amino acids markedly improved the permeability of β-carboline in Caco-2 cells with apparent permeability coefficients (Papp) of ~12×10−6 cm/s from the apical side to the basolateral side. The increase in permeability was most likely due to the PEPT1 transporter interaction, another example of the potential of transporter targeted prodrug design for increasing absorption.
The sodium-dependent multivitamin transporter (SMVT) is another membrane transporter that has been exploited for targeted prodrug design. This transporter is responsible for the uptake of several essential nutrients including pantothenate, biotin, and lipoate into cells [148,149]. Previously, our group has utilized SMVT to increase the absorption of peptides [150] and shown that PEG conjugates of anti-HIV drugs and anti-cancer drugs containing biotin may target SMVTs for cellular uptake [132,151]. We have also shown that human ovarian carcinoma cell line A2780 and its multidrug resistant form A2780/AD express high levels of SMVT, which can potentially be exploited for the uptake of anti-cancer prodrug conjugates [132]. CPT conjugates (CPT-PEG, CPT-PEG-biotin) were synthesized and shown to increase the cytotoxicity of CPT by 12 and 60-fold respectively in A2780 cells and by 12 and 30- fold respectively in multidrug resistant A2780/AD cells. The cytotoxicity of the conjugates was attributed to the enhanced apoptotic effects by activation of the caspase pathway.
The most paramount advantage to the use of transporters like PEPT1 and SMVT is the potential for increased oral absorption. Therefore, prodrugs utilizing membrane transporters may be afforded the luxury of an oral formulation, which is the gold standard of administration. Another advantage in the case of amino acid ester prodrugs is the variability of the amino acid side chains, which can be used to alter the prodrug's physicochemical properties [21,147]. Likewise, the broad specificity of many of these transporters for multiple substrates increases the potential flexibility in prodrug design. Also because transporter-targeting substrates are generally sugars, vitamins, or peptides, the byproducts of the prodrug's conversion yields nontoxic nutrients. Even though the expression profile of these transporters can be seen as an advantage for increasing oral absorption, it can also be disadvantageous. For example PEPT1 was mentioned earlier to be overexpressed by some cancer cells, but PEPT1 is also found in cells of the small intestine, kidney, bile duct, and pancreas. Therefore, if PEPT1 substrates are being used as targeting moieties, toxicity in these cell types may occur. Another disadvantage could arise if the prodrug upon absorption by the transporter is quickly converted to parent drug, as is the case for valacyclovir. Once this occurs the parent drug is now passively distributed throughout the systemic circulation, which could in turn cause systemic toxicity.
2.2. Passive targeting
Prodrugs can also be targeted to tumors by passive processes. This is achieved by attaching the drug to larger molecules (synthetic or biopolymers) or nanoparticles (liposomes, nanospheres) that act as inert carriers [152,153]. These macromolecules or nanoparticles do not necessarily interact with tumor cells but strongly influence drug biodistribution. The underlying concept behind this is to take advantage of the enhanced permeability and retention (EPR) effect elucidated by Maeda and coworkers [154].
Angiogenesis is induced in tumors to accommodate their ever-increasing demand for nutrition and oxygen as the tumor cells multiply and cluster together to reach the size of 2–3 nm [155,156]. The neovasculature in tumors differs greatly from the microscopic anatomical architecture of normal tissue. For instance, the blood vessels in tumors are irregular in shape, dilated, leaky or defective, and the endothelial cells are poorly aligned or disorganized with large fenestrations. The perivascular cells and the smooth muscle layers are frequently absent or abnormal in the vascular wall. Tumor vessels have wide lumens, whereas tumor tissues have poor lymphatic drainage. This results into extensive leakage of blood plasma components such as macromolecules, nanoparticles, and lipidic particles in tumor tissues. These macromolecules and nanoparticles are retained in tumors due to the slow venous return in tumor tissues and poor lymphatic drainage. This phenomenon is known as the EPR effect.
The pore size of tumor microvessels may vary from 100 to 1200 nm in diameter whereas the tight junctions between endothelial cells of microvessels are mostly under 2 nm (exceptions: ~6 nm in kidney, liver and spleen) [156]. Macromolecular carriers, on the other hand, have hydrodynamic radii in the range of 2–10 nm, which allows their extravasation into tumor tissues and not in normal tissue. It must be mentioned that enhanced permeability is true for smaller molecules also. However, large molecules with molecular weight of ≥ 40 kDa show decreased clearance from tumors (i.e., they also demonstrate enhanced retention). As a result, small molecules are rapidly cleared from the tumor interstitium, whereas the large molecules are retained. Thus, it is a combination of enhanced permeability and impaired clearance that account for the accumulation of macromolecular prodrugs in tumors due to the EPR effect. It should be cautioned that molecular weight is absolutely important, but not the sole criterion for predicting the molecule's biodistribution. The chemical nature of polymer, as well as shape and conformation in water, may also influence its molecular size.
The EPR effect is influenced by a number of other factors [156]. Longer plasma residence time is found to improve the targeting by EPR. Large tumors with extensive vascular regions are less EPR active than smaller tumors. The neovasculature is a prerequisite for EPR and this explains why macromolecular prodrugs are unable to target small metastases at pre-angiogenic stage. It has been observed that transplanted tumors are better vascularized than spontaneously grown tumors. High blood pressure and vascular mediators like bradykinin, nitric oxide, prostaglandins, metalloproteinases, and peroxy nitrite also impact the tumor vascular permeability. EPR effect has been used to achieve 10 to 50-fold (1–5% and in some cases 20% of injected dose per gram of tumor) high local concentration of drugs in the tumor tissues than in normal tissues within 1–2 days of injection.
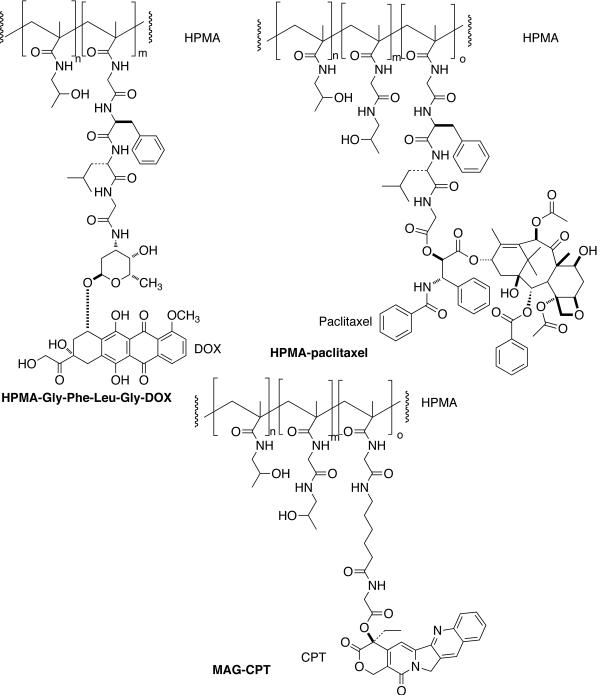
Duncan and Kopecek developed an N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer-Gly-Phe-Leu-Gly-doxorubicin conjugate (PK1, FCE28068) currently in clinical trials [Fig. (15)] [157].
Fig. (15).
HPMA-copolymer conjugates of doxorubicin (DOX), paclitaxel (CT), and camptothecin (CPT) for passive targeting of prodrugs.
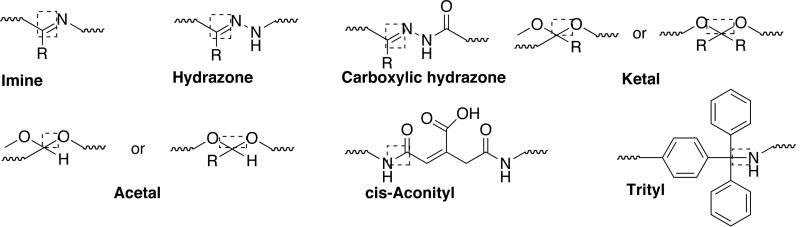
It was shown that the tetrapeptidyl linker is stable in circulation, but cleaved by thiol-dependant protease cathepsin B after endocytic uptake of conjugate from the tumor interstitium. The prodrug has Mw ~30000 Da and a doxorubicin content of ~8.5 wt%. It was administered as a short infusion every three weeks (maximum tolerated dose: 320 mg m−2). Antitumor activity was seen in patients considered to be chemotherapy resistant/refractory and at lower doses (80–180 mg m−2). This suggests targeting through EPR. The conjugate showed prolonged half-life, no liver accumulation, and rapid elimination. Polymer-bound DOX was always detected in plasma at higher levels than free DOX and cumulative doses of >20 g m−2 could be administered without any sign of immunogenicity or polymer-related toxicity. Later, HPMA-DOX conjugates were developed using pH sensitive cis-aconityl, hydrazone and acetal linkers to trigger drug-release following the conjugate internalization. pH sensitivity takes advantage of the fact that proton pump present in the endosomal and lysosomal membranes creates an acidic intervesicular environment (pH = 6.5–4.0). One such HPMA copolymer-DOX conjugate containing hydrazone linkage showed improved antitumor activity against lymphoma in vivo compared with tetrapeptide conjugate [158]. HPMA-paclitaxel (PNU166945) [159] and HPMA-camptothecin (MAG-CPT) [160] were developed and evaluated in clinical trials with disappointing results [Fig. (15)]. MAG-CPT displayed dose-limiting cumulative bladder toxicity whereas HPMA-paclitaxel displayed neurotoxicity.
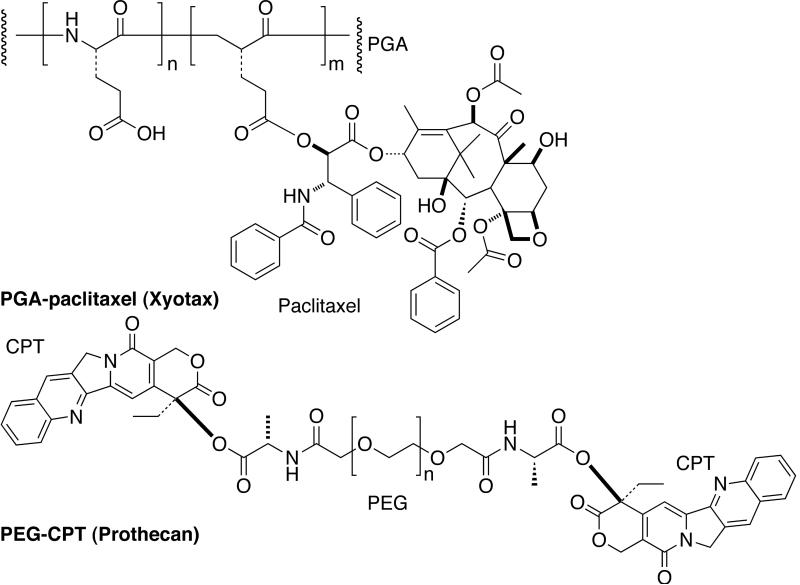
Several other polymers such as polyglutamate (PGA) and poly(ethylene glycol) (PEG) have also been used for conjugation with drugs [Fig. (16)]. PGA-paclitaxel (CT-2103, Xyotax) was developed and evaluated in clinical trials [161]. It contains 37 wt% of paclitaxel linked through 2'-position via an ester bond to the γ-carboxylic of PGA (Mw ~40000). PGA is a biodegradable polymer and its backbone is cleaved by cathepsin B to liberate diglutamyl-paclitaxel. The PGA-paclitaxel has proven to be the most promising candidate in this category [162]. It was administered intravenously as a single agent for 30 min every three weeks (maximum tolerated dose: 266 mg m−2). A significant number of patients showed partial responses or stable disease (mesothelioma, renal cell carcinoma, non small cell lung cancer and paclitaxel resistant ovarian cancer patient). Xyotax is currently being evaluated in combination with cisplatin and carboplatin.
Fig. (16).
Passively targeted anticancer prodrugs: Xyotax and Prothecan.
PEG-camptothecin (PROTHECAN) uses PEG of Mw 40,000 Da and the drug is bound via the C-20-OH position thus favoring the desired lactone ring formation [Fig. (16)] [152,162]. Alanine spacer was used instead of glycine to improve the stability in human blood plasma. Drug loading was rather low (10.7 wt%) illustrating a major limitation associated with PEG. In a Phase I study, PEG-CPT was administered every three weeks at doses of up to 4800 mg m−2 (maximum tolerated dose: 200 mg m−2). A Phase II program with PEG-CPT is ongoing. PEG is one of the most versatile polymers for medical applications characterized by chemical inertness of the polyether backbone and excellent solubility in aqueous media. PEG's are nontoxic, non-immunogenic, and non-biodegradable making them suitable for modification of various biologically active compounds. Other polymeric prodrugs in Phase I/II clinical trials are HPMA-platinates [163], and PGA-camptothecin [152].
Improved water solubility, prolonged stay in blood circulation, and reduced toxicities can be achieved besides passive targeting by conjugating the drug molecule to polymers. The polymer to be used for such applications should be biocompatible (nontoxic, nonimmunogenic, preferably biodegradable), able to carry the required drug payload, able to protect the drug against premature metabolism, display active/passive targeting, able to liberate the drug at a rate appropriate to its mechanism of action, and able to enter tumor cells by endocytosis (if designed for lysosomotropic release). Recent efforts have focused on combining the advantage of linear polymers with dendridic structures, which have resulted in the development of very interesting polymeric materials such as graft copolymers, star-like block copolymers, multivalent copolymers, dendronized linear polymers, start-like polymer with terminal dendrons, and bow-tie hybrids. These polymeric architectures can be used as carrier for passive targeting of anticancer drugs. Several conjugates have been prepared using these modified materials and evaluated in preclinical studies.
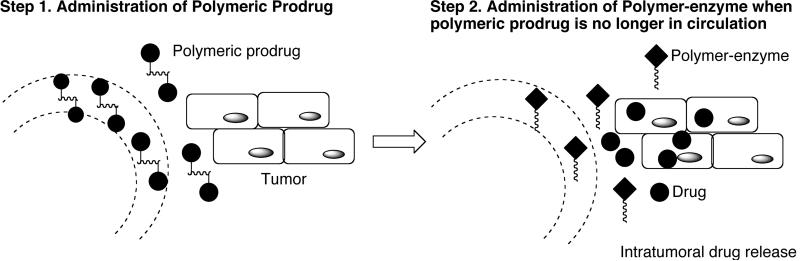
2.2.1. Polymer-directed enzyme prodrug therapy (PDEPT) and Polymer-directed enzyme liposome therapy (PELT)
Polymer-directed enzyme prodrug therapy (PDEPT) is a two-step antitumor approach like ADEPT that combines a polymeric prodrug and a polymer-enzyme to generate cytotoxic drug at the tumor site [Fig. (17)] [152,162].
Fig. (17).
Schematic presentation of PDEPT strategy. Polymeric prodrug is administered first to promote the tumor targeting followed by polymer-enzyme conjugate for prodrug activation. Redrawn from Nature Rev. Drug Disc. 2003, 2, 347.
PDEPT involves the initial administration of the polymeric prodrug to promote tumor targeting before administration of the activating polymer-enzyme conjugate. The process utilizes the EPR effect to target the polymeric prodrug as well as polymer enzyme conjugate. Polymer-enzyme conjugates are well known and PEG-L-asparginase (Oncaspar) is commercially available. PDEPT has certain advantages over ADEPT/GDEPT. For instance PEGylated / polymeric enzymes show little or no toxicity unlike antibody enzyme conjugates. Synthesis and purification of polymer-enzyme conjugates is easier compared to antibody-enzyme conjugate. The prodrug is administered first, which circumvents the problems associated with premature prodrug activation in the circulation.
Duncan and coworkers were first to evaluate the feasibility of PDEPT concept using a PK1 prodrug and HPMA copolymer-cathepsin B [164]. PK1 has HPMA-copolymer conjugated to doxorubicin through a cysteine proteases sensitive linker Gly-Phe-Leu-Gly. The prodrug was administered to B16F10 melanoma-bearing mice. The level of free DOX was found to remain constant with time. After 5 hours, HPMA-cathepsin B conjugate was administered and a marked increased in tumoral free DOX level was noticed. Overall, 3–4 fold increase in free DOX AUC was obtained compared to PK1 alone. Little free DOX was detected in blood at any time point (<10 ng) suggesting complete clearance of PK1. Animals bearing B16F10 showed increased survival with no signs of weight loss or toxic deaths. A significant decrease in the tumor growth rate was also observed. Cathepsin B alone was not active suggesting targeting by EPR.
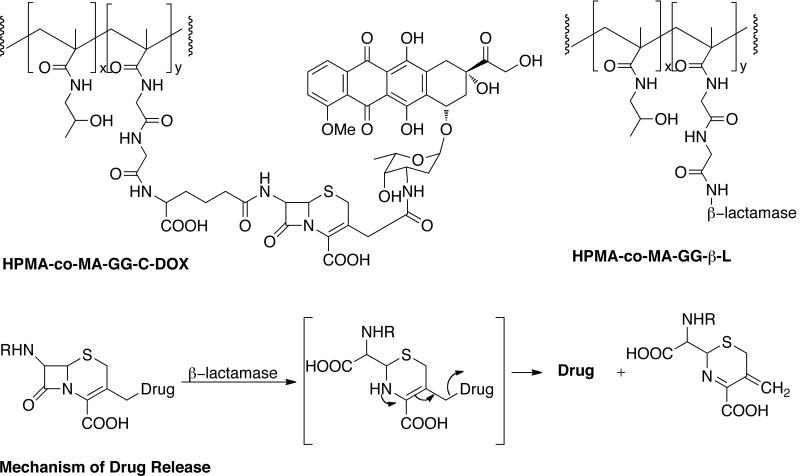
They developed another PDEPT system consisting of HPMA copolymer-methacryloyl-Gly-Gly-cephalosporin-doxorubicin (HPMA-co-MA-GG-C-DOX) as polymeric prodrug and HPMA copolymer-methacryloyl-Gly-Gly-β-lactamase (HPMA-co-MA-GG-β-L) as polymer-enzyme conjugate to activate the prodrug [Figure (18)] [165]. The β-lactamases is small (35–40 kDa), soluble monomeric enzymes that are capable of hydrolyzing β-lactams to substituted β-amino acids. They act on penicillins or cephalosporins and the mechanism of hydrolysis involves expulsion of 3'-leaving group. β-Lactamases are particularly tolerant to wide variety of substituent at this position. The polymeric prodrug has a molecular weight of ~31600 Da and C-DOX content of 5.85 wt%. The polymer-enzyme conjugate has a molecular weight of 75–150000 Da and was shown to retain 80% of the enzyme activity against cephalosporin C after conjugation. The prodrug was injected intravenously to mice bearing subcutaneous B16F10 melanoma. After 5 hours, polymer enzyme conjugate was administered resulting into the release of free DOX. The PDEPT combination caused a significant decrease in tumor growth (T/C = 132%) whereas neither free DOX nor polymeric prodrug alone displayed this activity. The PDEPT system did not show any toxicity at the doses used. This system is currently undergoing further preclinical studies. Shabat and coworkers developed a slightly modified approach were they used HPMA conjugated to catalytic antibody for prodrug activation [166].
Fig. (18).
PDEPT system consisting HPMA copolymer-C-Dox and HPMA copolymer-β-lactamase. The mechanism of drug release is also shown.
Duncan's group has proposed the liberation of active drug from liposome by the action of HPMA-copolymer-phospholipase conjugates. This alternative strategy for prodrug activation is known as Polymer-directed enzyme liposome therapy (PELT) [152]. Liposomes are used to deliver prodrug through EPR followed by polymer enzyme, which activates the prodrug. Robinson and coworkers have developed an approach called “Lectin-directed Enzyme-Activated Prodrug Therapy (LEAPT)” for prodrug targeting [167]. This involves administration of glycosylated-enzyme conjugates, which binds to cells surfaces expressing specific lectin receptors. The enzyme then activates the systemically administered prodrug at the site of action. Polymeric anticancer prodrugs currently in various stages of clinical trials are collected in Table 4.
Table 4.
Polymer-conjugates in clinical trials.
| Prodrug | Description | Target | Status |
|---|---|---|---|
| EZN-2208 (PEG-SN38) [60] | PEG linked to 7-ethyl-10-hydroxycamptothecin | Passive targeting to solid tumors | Phase I |
| CT-21006 [168] | Polyglutamate linked to camptothecin | Passive targeting | Phase II |
| Xyotax (CT-2103) [60] | Polyglutamate linked to paclitaxel | Passive targeting | Phase I |
| Genoxol-PM [60] | Polymeric micelles of PEG-poly(D, L-lactide) | Biodegradable micelles for passive targeting | Phase II |
| Inno-206 (DOX-EMCH) [60] | Doxorubicin-EMCH | EMCH is acid-sensitive, binds to albumins, passive targeting | Phase II |
| ProLindac [169] | HPMA copolymer-diaminocyclohexane platinum (II) | Passive targeting & drug-release in acid milieu | Phase II |
3. DRUG RELEASE AT THE TUMOR SITE
Prodrugs, once inside the tumor, must be activated (cleaved) to exert their antitumor effect. The activation of the free drug can occur intracellularly or extracellularly. This section gives a brief overview of major approaches that are used for prodrug cleavage at the tumor site.
3.1. Enzymatic cleavage
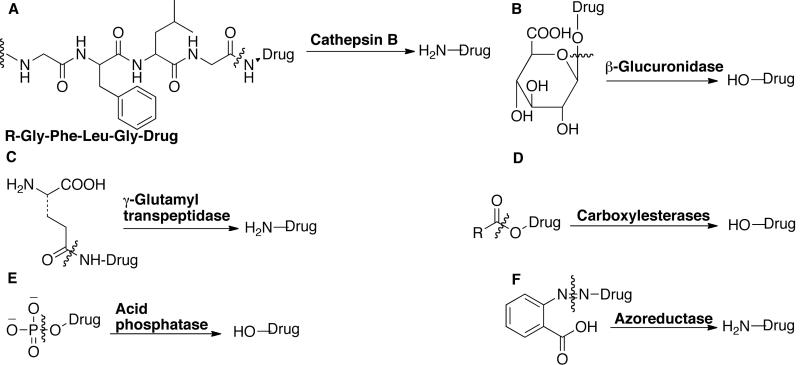
Prodrug activation can be achieved by tumor-associated enzymes, which are expressed either intracellularly or extracellularly, by malignant cells. The drug release by enzymatic cleavage is achieved by following mechanisms [24]: (i) a peptide linker is attached directly to the drug, which is cleaved by the enzyme to release the drug; (ii) the peptide sequence itself is enzymatically cleaved to release the drug-peptide derivative, which is further cleaved or hydrolyzed to the active drug molecule. Another possibility is to attach self-immolative spacer to the peptide linker (Section 3.5). Cleavage by an enzyme produces a labile self-immolative spacer-drug derivative that undergoes spontaneous elimination to liberate the anticancer drug. Some common tumor-associated enzymes are listed below and representative examples are shown in Fig. (19) [27,170].
Fig. (19).
Bond cleavage by tumor-associated enzymes. The cleavage sites are marked.
Cathepsin B, D, H and L
Several cancer cells, particularly those in fast-growing or aggressive neoplastic disease stage, express proteolytic enzymes such as cathepsin B, D, H and L. These enzymes are either membrane-bound or secreted extracellularly. They are responsible for the digestion of the basement membrane, as well as activation of enzymes, growth factors, and other proteases, as part of the metastatic cascade. Clinical detection of tumor and serum levels of cathepsin D and its proform in breast cancer is considered a prognostic indicator of metastatic disease progression, which correlates inversely to survival. Different approaches are being developed to inhibit metastases that rely on protease inhibitors. Cathepsin B, H and L are responsible for lysosomal degradation of proteins. Prodrugs with the general structure Arg-Arg-X, Ala-Leu-X, Gly-Leu-Phe-Gly-X, Gly-Phe-Leu-Gly-X and Ala-Leu-Ala-Leu-X are substrate for this enzyme. Cathepsin D is responsible for degradation of extracellular matrix. Prodrugs with general structure Phe-Ala-Ala-Phe(NO2)-Phe-Val-Leu-OM4P-X and Bz-Arg-Gly-Phe-Phe-Pro-4mβNA are substrate for this enzyme.
Plasmin
Serum plasminogen activator is produced in many tumor cells. Plasminogen is converted to plasmin by protease thus producing high-level of plasmin in the tumor cells. These plasmins are degraded rapidly in the plasma and hence tissues remote to tumor are not exposed to plasmin. Plasmin is responsible for the fibrinolysis and degradation of blood plasma proteins. Prodrugs of general structure D-Val-Le-Lys-X or D-Ala-Phe-Lys-X or D-Ala-Trp-Lys-X are some of the substrate examples for plasmin.
uPA/tPA
These are responsible for activation of plasmin formation and prodrugs of the general structure Gly-Gly-Gly-Arg-Arg-Arg-Val-X are good substrates for it.
Prostate-specific antigen
Responsible for liquefaction of semen and prodrugs of general structure morpholinocarbonyl-His-Ser-Ser-Lys-Leu-Gln-Leu-X are substrate for it.
Matrix metalloproteaes (MMP-2 and MM-9)
These are responsible for degradation of extracellular matrix and collagens and cleave prodrugs with general structure Ac-Pro-Leu-Gln-Leu-X, and Gly-Pro-Leu-Gly-Ile-Ala-Gly-Gln-X.
β-Glucuronidase
Glycosidase enzymes are responsible for the hydrolysis of glucuronide moieties. Of all the enzymes, β-Glucuronidase has been most widely explored. The advantage of using this enzyme is that it is normally found in lysosomes or microsomes with very low serum level.
γ-Glutamyl transpeptidase
High levels of this enzyme are found in some liver cancers. γ-Glutamyl derivative of p-phenylenediamine mustard, cytosine arabinoside, adriamycin, activicin and galactosamine can be substrates for this enzyme.
Carboxylesterases
This enzyme is responsible for the hydrolysis or transesterification of drugs, and xenobiotics. Produgs incorporating esters or carbamate functional groups are good substrate for this enzyme.
Acid phosphatase
Human prostatic tumors contain high levels of acid phosphatase. This enzyme can cleave prodrugs incorporating acid phosphatase.
Azoreductase
Hepatocellular carcinomas in humans express high levels of azoreductases. Several azo derivatives of mustards have been prepared, which are inert and nontoxic, but could be converted to cytotoxic compound with azoreducatase.
3.2. Acid sensitive linkers
Acid sensitive linkages are incorporated into the prodrug to cleave it under the acidic conditions present in tumors, endosomes, and lysosomes [24,27]. The environment in tumor tissues is often more acidic (0.5–1.0 pH units lower) than the normal tissues. A more prominent pH shift takes place during the prodrug uptake from blood or extracellular spaces (pH = 7.2–7.4) to intracellular compartments (pH = 4.0–6.5). These changes in pH can be utilized to cleave acid-sensitive prodrugs extracellularly, particularly when the prodrug stays in tumor interstitium for longer durations. Examples of acid sensitive linkage used in prodrug and conjugate design are imine, hydrazone, carboxylic hydrazone, ketal, acetal, cis-aconityl, and trityl bonds [Fig. (20)]. Polymeric materials incorporating acid sensitive linkages such as ketal, acetal and cis-aconityl bonds in their backbone have also been developed to degrade the polymer under acidic conditions after the cellular uptake [24].
Fig. (20).
Acid sensitive linkages used in prodrug design. The cleavage sites are enclosed in box.
3.3. Hypoxia
The presence of hypoxia or regions of low levels of oxygen in human tumors was postulated by Thomlinson and Gray [171]. Some regions in tumor tissues are poorly vascularized and hypoxic cells are formed, which show low oxygen tension, low pH, low nutrient levels, and overexpression of angiogenic factors [172]. These hypoxic cells are resistant to radiation and chemotherapy as they are distant from blood vessels, and not adequately exposed to anticancer drugs. Furthermore, cellular proliferation decreases as a function of the distance from blood vessels. Hypoxia selects cells that have lost sensitivity to p53-mediated apoptosis and this decreases the sensitivity to some anticancer agents. Additionally, hypoxia upregulates genes involved in drug resistance, including the P-glycoproteins.
A common mechanism for converting non-toxic prodrug to a toxic drug in a hypoxic environment involves the reduction by one or two electrons of the prodrug to form a radical that becomes a substrate for back-oxidation by oxygen to the original compound [Fig. (21)] [172]. The non-toxic prodrug must be a substrate for reducatses such as cytochrome P450 reductase or DT-diaphorase. With the exception of AQ4N, most of the first-generation hypoxic anticancer prodrugs were derivatives of nitro compounds, quinones, or N-oxides, which could be metabolized to reactive electrophiles (chemical species with high affinity for electron rich centers) or free radicals (chemical species with unpaired electron) that are unable to escape from the hypoxic cells, in which they are generated.
Fig. (21).
Prodrug activation in hypoxic environment. Prodrug is reduced to free radicals, which is converted back to prodrug by oxygen present in the oxic cells. The process is irreversible in hypoxic cells and prodrug free radical is converted to active drug. Also shown is activation mechanism of Tirapazamine (TPZ) and Anthraquinone (AQ4N). Redrawn from Nature Rev. Cancer 2004, 4, 437.
Prodrugs activated in hypoxic environment comprise three modular domains: a trigger unit that is reduced under hypoxia; an effector (the drug molecule); and a linker, which transmits the triggering event to the drug. The examples of hypoxic prodrugs in clinical trials include Tirapazamine (TPZ) [173] and Anthraquinone (AQ4N) [174] shown in Fig. (21). TPZ or tirapazamine is a di-N-oxide and it is activated to benzotrizinyl (BTZ) radical or hydroxide radical, which gives rise to double strand breaks through topoisomerase II-dependant process. AQ4N or anthraquinone is also a di-N-oxide. It is a prodrug of AQ4, which is a potent DNA intercalator/topoisomerase poison. The active drug is formed by reduction of two tertiary amine N-oxide groups by two-electron reduction. Other examples include SN 23862 and CB 1954. SB 23862 is dinetrobenzamide mustard [175] and reduction of either of nitro group acts as electron switch redistributing the electron density in the aromatic ring to activate the nitrogen mustard. CB 1954 is reduced to corresponding hydroxylamine with in the tumors by rat-diaphorase. Following reaction of hydroxylamine with acetyl CoA, the later becomes a very potent crosslinking agent.
One of the limitations of restricting prodrug activation to hypoxic tissue is that a large proportion of such tissues are necrotic, lacking the enzymes and cofactors are needed to reduce the prodrugs. An alternate approach for prodrug activation under hypoxia involves reduction by ionizing radiations, which are used for the treatment of tumors. Radiolysis of water generates aquated electron, which is much more powerful reducing agent than an enzyme, and is readily scavenged by O2 in oxic cells to form superoxide [176]. Three prodrugs have been described that are efficiently activated by ionizing radiations under hypoxia: nitrobenzyl quaternary ammonium salts [177], cobalt (III) complexes [172], and oxypropyl-substituted 5-fluoruracil derivatives [178].
3.4. Immunotoxins
Immunotoxins are antibody conjugates of highly potent drugs (DOX is frequently used) and immunotoxins. Immunotoxins contain a toxin made by plants; insects or microorganisms and examples include Pseudomonas exotoxin A (PE), diphtheria toxin (DT), and ricin. Several immunotoxins were constructed by conjugating mAbs to whole toxins via a disulfide linkage [16,24]. The disulfide bonds are cleaved in the reducing environment present in endosomes/lysosomes and the process usually involves thiol-exchange reaction. Modified immunotoxins with mutated or no binding domains were developed to reduce the side effects to normal cells. Thioether and acid sensitive linkages were used to improve the stability in plasma. Immunotoxins where the cytotoxic drug is conjugated by a peptide linker are also known. A widely investigated example is the BR96-DOX conjugate, where an average of 8 molecules of DOX are linked to chimeric mAB BR 96 through an acid-sensitive hydrazone linkage [179]. Promising immunotoxins currently in clinical trials include TransMID 107 (transferrin-CRM107), PRECISE (IL13-PEI-301-R03) [24].
3.5. Self-immolative spacers
The self-immolative spacers have three components: drug, linker, and trigger [24]. The tumor specific cleavage reaction takes place between trigger and the linker to form a drug-linker derivative, which then degrades spontaneously by elimination or cyclization to release the free drug [180–184]. The general mechanism of action for self-immolative (self-eliminating) spacer is shown in Fig. (22). Dubowchik and coworkers prepared a series of doxorubicin derivatives with cathepsin B-cleavable dipeptides [185]. They observed that in the presence of the target enzyme, only those derivatives that incorporated the self-immolative moiety liberated the drug. An example of self-immolative spacer has been mentioned earlier in section 2.1.3 [Fig. (13)]. Self-immolative or cascade-release dendrimers have also been reported by others [186,187].
Fig. (22).
Self-immolative spacers in prodrug design.
4. CONCLUSION AND PERSPECTIVES
The aim of this review has been to present an overview of recent trends in the design of anticancer prodrugs. Prodrug targeting through tumor-specific antigens has shown success in clinical trials and Mylotarg has already been approved for use. Development of humanized and/or chimeric mAb has to an extent address the safety concern associated with the use of mAb and has provided a fresh impetus to their use in targeted prodrug design. The ADEPT approach has certain advantage over immunoconjugates. However, it should be noted that most of the enzymes need cofactors that are present only inside the cells. Enzymes delivered by ADEPT may therefore need to gain access inside the cell before they can optimally activate prodrugs. This requirement is limited by the fact that large-sized antibody-enzyme conjugates often show poor penetration. GDEPT/VDEPT approach can deliver gene-encoding enzymes selectively to target tissue, but the gene delivery issues are yet to be fully resolved. Enhanced permeability and retention of polymers and nanoparticles provide an attractive approach to target anticancer prodrugs selectively to tumors by passive targeting. Several polymeric prodrugs have already been developed that are in advanced stage of pre-clinical and clinical development. The development of PDEPT strategy makes passive targeting more attractive. However, the drug loading and amount of drug reaching the target tissue needs further optimization.
It must be accepted that overall success achieved so far with targeted prodrug design is far from satisfactory. Only a limited number of prodrugs have been approved so far despite the fact that fairly large numbers of prodrugs undergo clinical trial every year. The limited clinical success of targeted prodrug design could be attributed to several factors. Prodrug design is often an afterthought, which is implemented when a particular drug shows undesirable pharmacokinetic and pharmacodynamic properties. We believe that more successful prodrugs are likely to reach the market if prodrug design is incorporated upon inception of a new therapeutic.
The preclinical evaluation of prodrugs relies mostly on mouse models with human transplanted tumors. Prodrugs found to be effective in such models are moved into the clinical trials. These models though useful cannot account for species specificity. The development of new and dynamic animal models more reflective of human disease states is a major challenge in targeted prodrug design, as it is any other drug design.
Design of targeted prodrug involves conjugation of active drug to carrier molecules such as sugars, growth factors, vitamins, antibodies, peptides and synthetic polymers through a linker. Optimization of synthetic procedure is a major challenge as the chemistries associated with drug molecule and the carrier may not always be compatible. There is a possibility that the carrier may induce immunogenic responses and/or increase the lipophilicity upon conjugation. An unbalanced increase in lipophilicity may reduce the aqueous solubility and therefore the drug bioavailability. Attachment to the carrier also influences the drug stability and activity and any undesirable change may compromise the clinical efficiency of the prodrug. The amount of drug that can be attached to the carrier (drug loading) is often limited due to the number of attachment sites available. The drug loading remains a major impediment to clinical success of targeted prodrug design as it determines the amount of drug reaching the target site. The drug loading can be augmented by using multiple copies drugs. Attachment of multiple drug copies can improve the in vitro drug activity but this may not necessarily translate into increased therapeutic benefit. There is a need to correlate drug loading with corresponding clinical efficacy.
A major concern in targeted prodrug design is the fact that the targeting strategies are based on the assumption that a particular enzyme or receptor is elevated in tumor tissues. The prodrugs that are converted to active drug by tumor-associated enzymes or internalized by binding to receptors should improve the therapeutic index. However, normal tissues or organs can express the same enzymes endogeneously, thus there is a possibility of off target effects. This problem is further compounded by the fact that tumors are heterogeneous, therefore their enzyme expression profiles are variable. Consequently, prodrug designed for particular tumor-associated enzyme may undergo differential activation in tumors or may not be activated at all thereby influencing the therapeutic efficacy. The choice of appropriate target antigen and/receptor is key to efficient targeted prodrug design.
The strategies we have described in this review range from simple modifications to complex systems involving several pharmaceutical and biological manipulations. However, the greatest benefit of prodrug design is the flexibility in it techniques and components. The development so far are significant and they are expected to provide framework for future research efforts in targeted prodrug design, which in turn will lead to the development of some useful anticancer prodrugs and conjugates. As the field grows, and new polymers, antibodies, targets, are identified, prodrug design is expected to become the norm in therapeutics instead of the afterthought.
ACKNOWLEDGEMENTS
The National Institutes of Health (R37 AI/DK-51214) and the US Army Medical Research and Materiel Command (W81XWH-05-1-0342) supported the work. We acknowledge our graduate students, postdoctoral fellows and research faculty who contributed to this work over the years. Their names appear in the references.
REFERENCES
- [1].Hanahan D, Weinberg RA. Cell. 2000;100:57. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [2].Finkel T, Serrano M, Blasco MA. Nature. 2007;448:767. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- [3].Chambers AF, Groom AC, MacDonald IC. Nature Rev. Cancer. 2002;2:563. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- [4].American Cancer Society Cancer Facts and Figures. 2008 [Google Scholar]
- [5].Skeel RT, editor. Handbook of Cancer Chemotherapy. Lippincott Williams and Wilkins; USA: 2007. [Google Scholar]
- [6].Gottesman MM, Fojo T, Bates SE. Nature Rev. Cancer. 2002;2:48. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- [7].Chabner BA, Roberts TG., Jr. Nature Rev. Cancer. 2005;5:65. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- [8].Kamb A, Wee S, Lengauer C. Nature Rev. Drug Disc. 2007;6:115. doi: 10.1038/nrd2155. [DOI] [PubMed] [Google Scholar]
- [9].Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen J. Nature Rev. Drug Disc. 2008;7:255. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- [10].Albert A. Nature. 1958;182:421. doi: 10.1038/182421a0. [DOI] [PubMed] [Google Scholar]
- [11].Harper NJ. Prog. Drug Res. 1962;4:221. [Google Scholar]
- [12].Bundgaard HE. Prodrug design. Elsevier; Amsterdam: 1985. [Google Scholar]
- [13].Friis GJ, Bundgaard H. A textbook of drug design and development. Harwood Academic Publishers; Amsterdam: 1996. [Google Scholar]
- [14].Testa B. Biochem. Pharmacol. 2004;68:2097. doi: 10.1016/j.bcp.2004.07.005. [DOI] [PubMed] [Google Scholar]
- [15].Ettmayer P, Amidon GL, Clement B, Testa B. J. Med. Chem. 2004;47:2393. doi: 10.1021/jm0303812. [DOI] [PubMed] [Google Scholar]
- [16].Sinhababu AK, Thakker DR. Adv. Drug Del. Rev. 1996;19:241. [Google Scholar]
- [17].Hwang JJ, Marshall JL. Expert Opin. Pharmacother. 2002;3:733. doi: 10.1517/14656566.3.6.733. [DOI] [PubMed] [Google Scholar]
- [18].Takiuchi H, Ajani JA. J. Clin. Oncol. 1998;16:2877. doi: 10.1200/JCO.1998.16.8.2877. [DOI] [PubMed] [Google Scholar]
- [19].Breistol K, Balzarini J, Sandvold ML, Myheren F, Matrtinsen M, Clercq ED, Fodstad O. Cancer Res. 1999;59:2944. [PubMed] [Google Scholar]
- [20].Boddy AV, Yule SM. Clin. Pharmacokinet. 2000;38:291. doi: 10.2165/00003088-200038040-00001. [DOI] [PubMed] [Google Scholar]
- [21].Han H-K, Amidon GL. AAPS Pharmsci. 2000;2:1. doi: 10.1208/ps020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Denny A. Eur. J. Med. Chem. 2001;36:577. doi: 10.1016/s0223-5234(01)01253-3. [DOI] [PubMed] [Google Scholar]
- [23].Silva ATA, Chung MC, Castro LF, Guido RVC, Ferreira EI. Mini Rev. Med. Chem. 2005;5:893. doi: 10.2174/138955705774329528. [DOI] [PubMed] [Google Scholar]
- [24].Kratz F, Muller IA, Ryppa C, Warnecke A. ChemMedChem. 2008;3:20. doi: 10.1002/cmdc.200700159. [DOI] [PubMed] [Google Scholar]
- [25].Carter P. Nature Rev. Cancer. 2001;1:118. doi: 10.1038/35101072. [DOI] [PubMed] [Google Scholar]
- [26].Schrama D, Reisfeld RA, Becker JC. Nature Rev. Drug Disc. 2006;5:147. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- [27].Dubowchik GM, Walker MA. Pharmacol. Ther. 1999;83:67. doi: 10.1016/s0163-7258(99)00018-2. [DOI] [PubMed] [Google Scholar]
- [28].Allen TM. Nature Rev. Cancer. 2002;2:750. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- [29].Weiner LM. Nature Rev. Cancer. 2007;7:701. doi: 10.1038/nrc2209. [DOI] [PubMed] [Google Scholar]
- [30].Harris LJ, Skalestky E, McPherson A. J. Mol. Biol. 1998;275:861. doi: 10.1006/jmbi.1997.1508. [DOI] [PubMed] [Google Scholar]
- [31].Hale G, Clarke M, Waldman H. J. Immunol. 1985;134:3056. [PubMed] [Google Scholar]
- [32].Shen WC, Ballou B, Ryser HJ, Hakala TR. Cancer Res. 1986;46:3912. [PubMed] [Google Scholar]
- [33].Johnson DA, Laguzza BC. Cancer Res. 1987;47:3118. [PubMed] [Google Scholar]
- [34].Dillman RO, Johnson DE, Shawler DL, Koziol JA. Cancer Res. 1988;48:6097. [PubMed] [Google Scholar]
- [35].Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, DeBlanc RL, Gearing RP, Bovee TD, Siegall CB, Francisco JA, Wahl AF, Meyer DL, Senter PD. Nature Biotechnol. 2003;21:778. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- [36].Jaracz S, Chen J, Kuznetsova LV, Ojima I. Bioorg. Med. Chem. 2005;13:5043. doi: 10.1016/j.bmc.2005.04.084. [DOI] [PubMed] [Google Scholar]
- [37].Adams GP, Weiner LM. Nature Biotechnol. 2005;23:1147. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- [38].Fanale MA, Younes A. Drugs. 2007;67:333. doi: 10.2165/00003495-200767030-00002. [DOI] [PubMed] [Google Scholar]
- [39].Zhang Q, Chen G, Liu X, Qian Q. Cell Res. 2007;17:89. doi: 10.1038/sj.cr.7310143. [DOI] [PubMed] [Google Scholar]
- [40].Tolcher AW, Ochaa L, Hammond LA, Patnaik A, Edwards T, Takimoto C, Smith L, Bano JD, Scwartz G, Mays T, Janak ZL, Johnson R, Dewhite M, Martino H, Audette C, Maes K, Chari RVJ, Lambert JM, Rowinsky EK. J. Clin. Oncol. 2003;21:211. doi: 10.1200/JCO.2003.05.137. [DOI] [PubMed] [Google Scholar]
- [41].Jordan MA, Wilson L. Nature Rev. Cancer. 2004;4:253. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- [42].Francisco JA, Cerveny CC, Meyer DL, Mixan BJ, Klussman K, Chace DF, Rejniak SX, Gordon KA, deBlanc R, Toki BE, Law C-L, Doronina SO, Siegall CB, Senter PD, Wahl AF. Blood. 2003;102:1458. doi: 10.1182/blood-2003-01-0039. [DOI] [PubMed] [Google Scholar]
- [43].Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, Roy S, Sridhara R, Rahman A, Williams G, Padzur R. Clin. Cancer Res. 2001;7:1490. [PubMed] [Google Scholar]
- [44].Liu J, Kolar C, Lawson TA, Gmeiner WH. J. Org. Chem. 2001;66:5655. doi: 10.1021/jo005757n. [DOI] [PubMed] [Google Scholar]
- [45].Steinberg G, Borch G. J. Med. Chem. 2001;44:69. doi: 10.1021/jm000306g. [DOI] [PubMed] [Google Scholar]
- [46].Lee JW, Lu JY, Low PS, Fuchs PL. Bioorg. Med. Chem. 2002;10:2397. doi: 10.1016/s0968-0896(02)00019-6. [DOI] [PubMed] [Google Scholar]
- [47].Bae Y, Jang W-D, Nishiyama N, Fukushima S, Kataoka K. Mol. Biosyst. 2005;1:242. doi: 10.1039/b500266d. [DOI] [PubMed] [Google Scholar]
- [48].Henne WA, Doornweered DD, Hilgenbrink AR, Kularatne SA, Low PS. Bioorg. Med. Chem. Lett. 2006;16:5350. doi: 10.1016/j.bmcl.2006.07.076. [DOI] [PubMed] [Google Scholar]
- [49].Paranjpe PV, Chen Y, Kholodovych V, Welsh W, Stein S, Sinko PJ. J. Controlled Release. 2004;100:275. doi: 10.1016/j.jconrel.2004.08.030. [DOI] [PubMed] [Google Scholar]
- [50].Paranjpe PV, Stein S, Sinko PJ. Anti-Cancer Drugs. 2005;16:763. doi: 10.1097/01.cad.0000172834.78068.7c. [DOI] [PubMed] [Google Scholar]
- [51].Vortherms AR, Doyle RP, Gao D, Debrah O, Sinko PJ. Nucleosides Nucleotides Nucleic Acids. 2008;27:173. doi: 10.1080/15257770701795946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dharap SS, Wang Y, Chandna P, Khandare JJ, Qiu B, Gunaseelan S, Sinko PJ, Stein S, Farmanfarmaian A, Minko T. Proc. Natl. Acad. Sci. USA. 2005;102:12962. doi: 10.1073/pnas.0504274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chandna P, Saad M, Wang Y, Ber E, Khandare JJ, Vetcher AA, Soldatenkov VA, Minko T. Mol. Pharm. 2007;4:668. doi: 10.1021/mp070053o. [DOI] [PubMed] [Google Scholar]
- [54].Khandare JJ, Chandna P, Wang Y, Pozharov VP, Minko T. J. Pharmacol. Exp. Ther. 2006;317:929. doi: 10.1124/jpet.105.098855. [DOI] [PubMed] [Google Scholar]
- [55].Vlahov IR, Santhapuram HKR, Kleindl PJ, Howard SJ, Stanford KM, Leamon CP. Bioorg. Med. Chem. Lett. 2006;16:5093. doi: 10.1016/j.bmcl.2006.07.030. [DOI] [PubMed] [Google Scholar]
- [56].Reddy JA, Dorton R, Westrick E, Dawson A, Smith T, Xu L-C, Vetzel M, Kleindl P, Vlahov IR, Leamon CP. Cancer Res. 2007;67:4434. doi: 10.1158/0008-5472.CAN-07-0033. [DOI] [PubMed] [Google Scholar]
- [57].Immunogen 2008 http://www.immunogen.com.
- [58].Galsky MD, Eisenberger M, Moore-Cooper S, Kelly WK, Slovin SF, DeLaCruz A, Lee Y, Webb IJ, Scher I. J. Clin. Oncol. 2008;26:2147. doi: 10.1200/JCO.2007.15.0532. [DOI] [PubMed] [Google Scholar]
- [59].Wyeth 2008 http://www.clinicalbreastcancer.com/publication/sabcs_gne/sabcs_gne.pdf#page=13.
- [60].National Cancer Institute 2008 http://www.cancer.gov.
- [61].Seattle Genetics 2008 http://www.seattlegenetics.com.
- [62].Clinicaltrials.gov 2008 http://www.clinicaltrials.gov.
- [63].Dechantsreiter MA, Planker E, Matha B, Lohof E, Holzemann G, Jonczyk A, Goodman SL, Kessler H. J. Med. Chem. 1999;42:3033. doi: 10.1021/jm970832g. [DOI] [PubMed] [Google Scholar]
- [64].Su Z-F, Liu G, Gupta S, Zhu Z, Rusckowski M, Hnatowich DJ. Bioconjugate Chem. 2002;13:561. doi: 10.1021/bc0155566. [DOI] [PubMed] [Google Scholar]
- [65].Janssen ML, Oyen WJ, Dijkgraaf I, Massuger LF, Frielink F, Edwards DS, Rajopadhye M, Boonstra H, Corstens FH, Boerman OC. Cancer Res. 2002;62:6146. [PubMed] [Google Scholar]
- [66].Boturyn D, Coll J-L, Garanger E, Favrot M-C, Dumy P. J. Am. Chem. Soc. 2004;126:5730. doi: 10.1021/ja049926n. [DOI] [PubMed] [Google Scholar]
- [67].Jin Z-H, Josserand V, Foillard S, Boturyn D, Dumy P, Favrot M-C, Coll J-L. Mol. Cancer. 2007;6:41. doi: 10.1186/1476-4598-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lis H, Sharon N. Chem. Rev. 1998;98:637. doi: 10.1021/cr940413g. [DOI] [PubMed] [Google Scholar]
- [69].Lundquist JL, Toone EJ. Chem. Rev. 2002;102:555. doi: 10.1021/cr000418f. [DOI] [PubMed] [Google Scholar]
- [70].Di Stefano G, Kratz F, Lanza M, Fiume L. Digest. Liver Dis. 2003;35:428. doi: 10.1016/s1590-8658(03)00212-3. [DOI] [PubMed] [Google Scholar]
- [71].Di Stefano G, Derenzini M, Kratz F, Lanza M, Fiume L. Liver Int. 2004;24:246. doi: 10.1111/j.1478-3231.2004.0916.x. [DOI] [PubMed] [Google Scholar]
- [72].Di Stefano G, Luigi F, Baglioni M, Busi C, Chieco P, Kratz F, Mattioli A. Eur. J. Pharm. Sci. 2007;30:136. doi: 10.1016/j.ejps.2006.10.012. [DOI] [PubMed] [Google Scholar]
- [73].Julyan PJ, Seymour LW, Ferry DR, Daryani S, Bovin CM, Doran J, David M, Anderson D, Christodoulou C, Young AM, Hesslewood S, Kerr DJ. J. Controlled Release. 1999;57:281. doi: 10.1016/s0168-3659(98)00124-2. [DOI] [PubMed] [Google Scholar]
- [74].Seymour LW, Ferry DR, Anderson D, Hesslewood S, Julyan PJ, Payner R, Doran J, Young AM, Burtles S, Kerr DJ. J. Clin. Oncol. 2002;20:1668. doi: 10.1200/JCO.2002.20.6.1668. [DOI] [PubMed] [Google Scholar]
- [75].Xu G, McLeod HL. Clin. Cancer Res. 2001;7:3314. [PubMed] [Google Scholar]
- [76].Bagshawe KD. Brit. J. Cancer. 1987;56:531. doi: 10.1038/bjc.1987.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Senter PD, Saulnier MG, Schreiber GJ, Hirschberg DL, Brown JP, Hellstrom I, Hellstrom KE. Proc. Natl. Acad. Sci. USA. 1988;85:4842. doi: 10.1073/pnas.85.13.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Senter PD, Springer CJ. Adv. Drug Del. Rev. 2001;53:247. doi: 10.1016/s0169-409x(01)00206-x. [DOI] [PubMed] [Google Scholar]
- [79].Wei Y, Pei D. Bioorg. Med. Chem. Lett. 2000;10:1073. doi: 10.1016/s0960-894x(00)00167-0. [DOI] [PubMed] [Google Scholar]
- [80].Wang Y, Yuan H, Wright SC, Wang H, Larrick JW. BMC Chem. Biol. 2001;1:4. doi: 10.1186/1472-6769-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Niculescu-Duvaz D, Scanlon I, Niculescu-Duvaz I, Springer CJ. Tetrahedron Lett. 2005;46:6919. [Google Scholar]
- [82].Francis RJ, Sharma SK, Springer CJ, Green AJ, Hope-Stone LD, Sena L, Martin J, Adamson KL, Robbins A, Gumbrell L, O'Malley D, Tsiompanou E, Shahnakhti H, Webley S, Hochhauser D, Hilson AJ, Blakey D, Begent RHJ. Brit. J. Cancer. 2002;87:600. doi: 10.1038/sj.bjc.6600517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chester K, Pedley B, Tolner B, Violet J, Mayer A, Sharma S, Boxer G, Green A, Nagl S, Begent R. Tumour Biol. 2004;25:91. doi: 10.1159/000077727. [DOI] [PubMed] [Google Scholar]
- [84].Greco O, Dachs GU. J. Cell. Physiol. 2001;187:22. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1060>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- [85].Hedley D, Oglivie L, Springer C. Nature Rev. Cancer. 2007;7:870. doi: 10.1038/nrc2247. [DOI] [PubMed] [Google Scholar]
- [86].Ring CJA. J. Gen. Virol. 2002;83:491. doi: 10.1099/0022-1317-83-3-491. [DOI] [PubMed] [Google Scholar]
- [87].Niculescu-Duvaz D, Niculescu-Duvaz I, Friedlos F, Martin J, Spooner R, Davies L, Marais R, Springer CJ. J. Med. Chem. 1998;41:5297. doi: 10.1021/jm980425k. [DOI] [PubMed] [Google Scholar]
- [88].Niculescu-Duvaz I, Niculescu-Duvaz D, Friedlos F, Spooner R, Martin J, Marais R, Springer CJ. J. Med. Chem. 1999;42:2485. doi: 10.1021/jm980696v. [DOI] [PubMed] [Google Scholar]
- [89].Niculescu-Duvaz D, Niculescu-Duvaz I, Friedlos F, Martin J, Lehouritis P, Marais R, Springer CJ. J. Med. Chem. 2003;46:1690. doi: 10.1021/jm020462i. [DOI] [PubMed] [Google Scholar]
- [90].Marais R, Spooner R, Light Y, Martin J, Springer CJ. Cancer Res. 1996;56:4735. [PubMed] [Google Scholar]
- [91].Marais R, Spooner R, Stribbling SM, Light Y, Martin J, Springer CJ. Nature Biotechnol. 1997;15:1373. doi: 10.1038/nbt1297-1373. [DOI] [PubMed] [Google Scholar]
- [92].Stribbling SM, Friedlos F, Martin J, Davies L, Spooner R, Marais R, Springer CJ. Human Gen. Ther. 2000;11:285. doi: 10.1089/10430340050016021. [DOI] [PubMed] [Google Scholar]
- [93].Schepelmann S, Oglivie LM, Hedley D, Friedlos F, Martin J, Scanlon I, Chen P, Marais R, Springer CJ. Cancer Res. 2007;67:4949. doi: 10.1158/0008-5472.CAN-07-0297. [DOI] [PubMed] [Google Scholar]
- [94].Springer CJ, Antoniw P, Bagshawe KD, Searle F, Bisset GMF, Jarman M. J. Med. Chem. 1990;33:677. doi: 10.1021/jm00164a034. [DOI] [PubMed] [Google Scholar]
- [95].Race PR, Lovering AL, White SA, Grove JI, Searle PF, Wrighton W, Hyde EI. J. Mol. Biol. 2007;368:481. doi: 10.1016/j.jmb.2007.02.012. [DOI] [PubMed] [Google Scholar]
- [96].Knox RJ, Connors TA. Pathol. Oncol. Res. 1997;3:309. doi: 10.1007/BF02904292. [DOI] [PubMed] [Google Scholar]
- [97].Immonen A, Vapalahti M, Tyynela K, Hurskainen H, Sandmair A, Vanninen R, Langford G, Murray N, Yla-Herttuala S. Mol. Ther. 2004;10:967. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- [98].Alvarez RD, Gomez-Navarro J, Wang M, Barnes MN, Strong TV, Arani RB, Arafat W, Hughes JV, Siegal GP, Curiel DT. Mol. Ther. 2000;2:524. doi: 10.1006/mthe.2000.0194. [DOI] [PubMed] [Google Scholar]
- [99].Kubo H, Gardner TA, Wada Y, Koeneman KS, Gotoh A, Yang L, Kao C, Lim SD, Amin MB, Yang H, Black ME, Matsubara S, Nakagawa M, Gillenwater JY, Zhau HE, Chung WK. Human Gen. Ther. 2003;14:227. doi: 10.1089/10430340360535788. [DOI] [PubMed] [Google Scholar]
- [100].Nasu Y, Saika T, Ebara S, Kusaka N, Kaku H, Abarzu F, Manabe D, Thompson TC, Kumon H. Mol. Ther. 2007;15:834. doi: 10.1038/sj.mt.6300096. [DOI] [PubMed] [Google Scholar]
- [101].Freytag SO, Khil M, Stricker H, Peabody J, Menon M, DeParalata-Venturia M, Nafziger D, Pegg J, Paielli D, Barton K, Lu M, Aguilar-Cordova E, Kim JH. Cancer Res. 2002;62:4968. [PubMed] [Google Scholar]
- [102].Freytag SO, Stricker H, Peabody J, Pegg J, Paielli D, Movsas B, Barton K, Brown S, Lu M, Kim JH. Mol. Ther. 2007;15:636. doi: 10.1038/sj.mt.6300068. [DOI] [PubMed] [Google Scholar]
- [103].Braybrooke JP, Slade A, Deplanque G, Harrop R, Madhusudan S, Forster MD, Gibson R, Makris A, Talbot DC, Steiner J, White L, Kan O, Naylor S, Carroll MW, Kingsman SM, Harris AL. Clin. Cancer Res. 2005;11:1512. doi: 10.1158/1078-0432.CCR-04-0155. [DOI] [PubMed] [Google Scholar]
- [104].Singh S, Cummingham C, Buchanan A, Jolly DJ, Nemunaitis J. Mol. Ther. 2001;4:157. doi: 10.1006/mthe.2001.0430. [DOI] [PubMed] [Google Scholar]
- [105].Valery CA, Seihean D, Boyer O, Maroo B, Hauw J-J, Kemeny J-L, Marsault C, Philippon J, Klatzmann D. Neurology. 2002;58:1109. doi: 10.1212/wnl.58.7.1109. [DOI] [PubMed] [Google Scholar]
- [106].Ryan PC, Jakubczak JL, Stewart DA, Hawkins LK, Cheng C, Clarke LM, Ganesh S, Hay C, Huang Y, Kaloss M, Marinov A, Phipps SS, Reddy PS, Shirley PS, Skripchenko Y, Xu L, Yang J, Forry-Schaudies S, Hallenback PL. Cancer Gene Ther. 2004;11:555. doi: 10.1038/sj.cgt.7700735. [DOI] [PubMed] [Google Scholar]
- [107].Park Y-J, Liang J-F, Song H, Li YT, Naik S, Yang VC. Adv. Drug Del. Rev. 2003;55:251. doi: 10.1016/s0169-409x(02)00181-3. [DOI] [PubMed] [Google Scholar]
- [108].Naik SS, Liang JF, Park YJ, Lee WK, Yang VC. J. Controlled Release. 2005;101:35. doi: 10.1016/j.jconrel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- [109].Yang VC, Naik SS, Song H, Dombokowski AA, Crippen G, Liang JF. J. Controlled Release. 2005;110:164. doi: 10.1016/j.jconrel.2005.09.027. [DOI] [PubMed] [Google Scholar]
- [110].Rubio-Aliaga I, Daniel H. Trends Pharmacol.Sci. 2002;23:434. doi: 10.1016/s0165-6147(02)02072-2. [DOI] [PubMed] [Google Scholar]
- [111].Huang Y. Cancer Metastasis Rev. 2007;26:183. doi: 10.1007/s10555-007-9050-6. [DOI] [PubMed] [Google Scholar]
- [112].Landry Y, Gies J-P. Fundamentals and Clinical Pharmacol. 2008;22:1. doi: 10.1111/j.1472-8206.2007.00548.x. [DOI] [PubMed] [Google Scholar]
- [113].Balimane PV, Sinko PJ. Adv. Drug. Deliv. Rev. 1999;39:183. doi: 10.1016/s0169-409x(99)00026-5. [DOI] [PubMed] [Google Scholar]
- [114].Jacobson MA. J. Med. Virol. 1993;(Suppl 1):150. doi: 10.1002/jmv.1890410529. [DOI] [PubMed] [Google Scholar]
- [115].Beauchamp LM, Krenitsky T. Drugs Future. 1993;18:619. [Google Scholar]
- [116].Beauchamp LM, Orr G, de Miranda P, Doucette M, Burnette T, Krenitsky T. Antiviral Chem. Chemother. 1992;3:157. [Google Scholar]
- [117].Smith C, Klein A, Zimmerman T. 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy.1993. [Google Scholar]
- [118].Balimane P, Sinko PJ. Pharm. Res. 1997;14(suppl):S–649. [Google Scholar]
- [119].Cook T, Jiansheng Y, Sinko P. Pharm. Res. 1997;14(suppl):S–20. doi: 10.1023/a:1012150405949. [DOI] [PubMed] [Google Scholar]
- [120].Hu P, Sinko P. Pharm. Res. 1997;14(suppl):S–29. [Google Scholar]
- [121].Balimane PV, Tamai I, Guo A, Nakanishi T, Kitada H, Leibach FH, Tsuji A, Sinko PJ. Biochem. Biophys. Res. Commun. 1998;250:246. doi: 10.1006/bbrc.1998.9298. [DOI] [PubMed] [Google Scholar]
- [122].Sinko PJ, Balimane PV. Biopharm Drug Dispos. 1998;19:209. doi: 10.1002/(sici)1099-081x(199805)19:4<209::aid-bdd93>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- [123].Ganapathy ME, Huang W, Wang H, Ganapathy V, Leibach FH. Biochem. Biophys. Res. Commun. 1998;246:470. doi: 10.1006/bbrc.1998.8628. [DOI] [PubMed] [Google Scholar]
- [124].Han H, de Vrueh RL, Rhie JK, Covitz KM, Smith PL, Lee CP, Oh DM, Sadee W, Amidon GL. Pharm. Res. 1998;15:1154. doi: 10.1023/a:1011919319810. [DOI] [PubMed] [Google Scholar]
- [125].Guo A, Hu P, Balimane PV, Leibach FH, Sinko PJ. J. Pharmacol. Exp. Ther. 1999;289:448. [PubMed] [Google Scholar]
- [126].Balimane P, Sinko P. Biopharm. Drug. Dispos. 2000;21:165. doi: 10.1002/1099-081x(200007)21:5<165::aid-bdd225>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- [127].Shin HC, Kim JS, Vig BS, Song X, Drach JC, Amidon GL. Biol. Pharm. Bull. 2006;29:247. doi: 10.1248/bpb.29.247. [DOI] [PubMed] [Google Scholar]
- [128].Daniel H, Herget M. Am. J. Physiol. 1997;273:F1. doi: 10.1152/ajprenal.1997.273.1.F1. [DOI] [PubMed] [Google Scholar]
- [129].Gonzalez DE, Covitz KM, Sadee W, Mrsny RJ. Cancer Res. 1998;58:519. [PubMed] [Google Scholar]
- [130].Nakanishi T, Tamai I, Sai Y, Sasaki T, Tsuji A. Cancer Res. 1997;57:4118. [PubMed] [Google Scholar]
- [131].Minko T, Kopeckova P, Pozharov V, Kopecek J. J Controlled Release. 1998;54:223. doi: 10.1016/s0168-3659(98)00009-1. [DOI] [PubMed] [Google Scholar]
- [132].Minko T, Paranjpe PV, Qiu B, Lalloo A, Won R, Stein S, Sinko PJ. Cancer Chemother. Pharmacol. 2002;50:143. doi: 10.1007/s00280-002-0463-1. [DOI] [PubMed] [Google Scholar]
- [133].Grem JL. Invest. New Drugs. 2000;18:299. doi: 10.1023/a:1006416410198. [DOI] [PubMed] [Google Scholar]
- [134].Nishizawa Y, Casida JE. Biochem. Pharmacol. 1965;14:1605. doi: 10.1016/0006-2952(65)90015-8. [DOI] [PubMed] [Google Scholar]
- [135].Vig BS, Lorenzi PJ, Mittal S, Landowski CP, Shin HC, Mosberg HI, Hilfinger JM, Amidon GL. Pharm. Res. 2003;20:1381. doi: 10.1023/a:1025745824632. [DOI] [PubMed] [Google Scholar]
- [136].Landowski CP, Song X, Lorenzi PL, Hilfinger JM, Amidon GL. Pharm. Res. 2005;22:1510. doi: 10.1007/s11095-005-6156-9. [DOI] [PubMed] [Google Scholar]
- [137].Landowski CP, Vig BS, Song X, Amidon GL. Mol. Cancer Ther. 2005;4:659. doi: 10.1158/1535-7163.MCT-04-0290. [DOI] [PubMed] [Google Scholar]
- [138].Beljanski M, Beljanski MS. Exp. Cell Biol. 1982;50:79. doi: 10.1159/000163131. [DOI] [PubMed] [Google Scholar]
- [139].Funayama Y, Nishio K, Wakabayashi K, Nagao M, Shimoi K, Ohira T, Hasegawa S, Saijo N. Mutat. Res. 1996;349:183. doi: 10.1016/0027-5107(95)00176-x. [DOI] [PubMed] [Google Scholar]
- [140].Laronze M, Boisbrun M, Leonce S, Pfeiffer B, Renard P, Lozach O, Meijer L, Lansiaux A, Bailly C, Sapi J, Laronze JY. Bioorg. Med. Chem. 2005;13:2263. doi: 10.1016/j.bmc.2004.12.045. [DOI] [PubMed] [Google Scholar]
- [141].Peczynska-Czoch W, Pognan F, Kaczmarek L, Boratynski J. J. Med. Chem. 1994;37:3503. doi: 10.1021/jm00047a008. [DOI] [PubMed] [Google Scholar]
- [142].Pognan F, Saucier JM, Paoletti C, Kaczmarek L, Nantka-Namirski P, Mordarski M, Peczynska-Czoch W. Biochem. Pharmacol. 1992;44:2149. doi: 10.1016/0006-2952(92)90341-f. [DOI] [PubMed] [Google Scholar]
- [143].Sobhani AM, Ebrahimi SA, Mahmoudian M. J. Pharm. Pharm. Sci. 2002;5:19. [PubMed] [Google Scholar]
- [144].Dias N, Goossens JF, Baldeyrou B, Lansiaux A, Colson P, Di Salvo A, Bernal J, Turnbull A, Mincher DJ, Bailly C. Bioconjugate Chem. 2005;16:949. doi: 10.1021/bc050065x. [DOI] [PubMed] [Google Scholar]
- [145].Hastings CA, Barton JK. Biochemistry. 1999;38:10042. doi: 10.1021/bi982039a. [DOI] [PubMed] [Google Scholar]
- [146].Pabo CO, Sauer RT. Annu. Rev. Biochem. 1992;61:1053. doi: 10.1146/annurev.bi.61.070192.005201. [DOI] [PubMed] [Google Scholar]
- [147].Zhao M, Bi L, Wang W, Wang C, Baudy-Floc'h M, Ju J, Peng S. Bioorg. Med. Chem. 2006;14:6998. doi: 10.1016/j.bmc.2006.06.021. [DOI] [PubMed] [Google Scholar]
- [148].Chatterjee NS, Kumar CK, Ortiz A, Rubin SA, Said HM. Am. J. Physiol. 1999;277:C605. doi: 10.1152/ajpcell.1999.277.4.C605. [DOI] [PubMed] [Google Scholar]
- [149].Prasad PD, Wang H, Huang W, Fei YJ, Leibach FH, Devoe LD, Ganapathy V. Arch. Biochem. Biophys. 1999;366:95. doi: 10.1006/abbi.1999.1213. [DOI] [PubMed] [Google Scholar]
- [150].Ramanathan S, Pooyan S, Stein S, Prasad PD, Wang J, Leibowitz MJ, Ganapathy V, Sinko PJ. Pharm. Res. 2001;18:950. doi: 10.1023/a:1010932126662. [DOI] [PubMed] [Google Scholar]
- [151].Ramanathan S, Qiu B, Pooyan S, Zhang G, Stein S, Leibowitz MJ, Sinko PJ. J. Controlled Release. 2001;77:199. doi: 10.1016/s0168-3659(01)00474-6. [DOI] [PubMed] [Google Scholar]
- [152].Duncan R. Nature Rev. Drug Disc. 2003;2:347. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- [153].Duncan R. Nature Rev. Cancer. 2006;6:688. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- [154].Maeda H. Adv. Enzyme Regul. 2001;41:189. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- [155].Maeda H, Wu J, Sawa T, Matsummura Y, Hori K. J. Controlled Release. 2000;65:271. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- [156].Iyer AK, Khaled G, Fang J, Maeda H. Drug Disc. Today. 2006;11:812. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- [157].Vasey PA, Kaye SB, Morrison R, Twelves C, Wilson P, Duncan R, Thomson AH, Murray LS, Hilditch TE, Murray T, Burtles S, Fraier D, Friegerio E, Cassidy J. Clin. Cancer Res. 1999;5:83. [PubMed] [Google Scholar]
- [158].Etrych T, Jelinkova M, Rihova B, Ulbrich K. J. Controlled Release. 2001;73:89. doi: 10.1016/s0168-3659(01)00281-4. [DOI] [PubMed] [Google Scholar]
- [159].Meerum Terwogt JM, Huinink WWB, Schellens JHM, Schot M, Mandjes IAM, Zurio MG, Rocchetti M, Rosing H, Koopman FJ, Beijnen JH. Anti-Cancer Drugs. 2001;12:315. doi: 10.1097/00001813-200104000-00003. [DOI] [PubMed] [Google Scholar]
- [160].Schoemaker NE, Kesteren CV, Rosing H, Jansen S, Swart M, Liverst J, Fraier D, Breda M, Pellizzoni C, Spinelli R, Porro MG, Beinjnen JH, Schellens JHM, Huinink WWB. Brit. J. Cancer. 2002;87:608. doi: 10.1038/sj.bjc.6600516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Li C, Yu D-F, Newman RA, Cabral F, Stephens CS, Hunter N, Milas L, Wallace S. Cancer Res. 1998;58:2404. [PubMed] [Google Scholar]
- [162].Duncan R, Gac-Breton S, Keane R, Musila R, Sat YN, Satchi R, Searle F. J. Controlled Release. 2001;74:135. doi: 10.1016/s0168-3659(01)00328-5. [DOI] [PubMed] [Google Scholar]
- [163].Gianasi E, Wasil M, Evagoru EG, Keddle A, Wilson G, Duncan R. Eur. J. Cancer. 1999;35:994. doi: 10.1016/s0959-8049(99)00030-1. [DOI] [PubMed] [Google Scholar]
- [164].Satchi R, Connors TA, Duncan R. Brit. J. Cancer. 2001;85:1070. doi: 10.1054/bjoc.2001.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Satchi-Fainaro R, Hailu H, Davies JW, Summerford C, Duncan R. Bioconjugate Chem. 2003;14:797. doi: 10.1021/bc020091k. [DOI] [PubMed] [Google Scholar]
- [166].Satchi-Fainaro R, Wasidlo W, Lode HN, Shabat D. Bioorg. Med. Chem. 2002;10:3023. doi: 10.1016/s0968-0896(02)00156-6. [DOI] [PubMed] [Google Scholar]
- [167].Robinson MA, Chariton ST, Garnier P, Wang X-T, Davis SS, Perkins AC, Frier M, Duncan R, Savage TJ, Wyatt DA, Watson SA, Davis BG. Proc. Natl. Acad. Sci. USA. 2004;101:14527. doi: 10.1073/pnas.0303574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Enzon 2008 http://www.enzon.com.
- [169].Acess Pharma 2008 htttp://www.accesspharma.com.
- [170].Rooseboom M, Commandeur JM, Vermeulen NPE. Pharmacol. Rev. 2004;56:53. doi: 10.1124/pr.56.1.3. [DOI] [PubMed] [Google Scholar]
- [171].Thomlinson RH, Gray LH. Brit. J. Cancer. 1955;9:539. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Brown JM, Wilson WR. Nature Rev. Cancer. 2004;4:437. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- [173].Daniels JS, Gates KS. J. Am. Chem. Soc. 1996;118:3380. [Google Scholar]
- [174].Patterson LH. Drug Metab. Rev. 2002;34:581. doi: 10.1081/dmr-120005659. [DOI] [PubMed] [Google Scholar]
- [175].Siim BG, Denny WA, Wilson WR. Oncol. Res. 1997;9:357. [PubMed] [Google Scholar]
- [176].Wilson WR, Tercel M, Anderson RF, Denny WA. Anti-Cancer Drug Design. 1998;13:663. [PubMed] [Google Scholar]
- [177].Kriste AG, Tercel M, Anderson RF, Ferry DM, Wilson WR. Radiation Res. 2002;158:753. doi: 10.1667/0033-7587(2002)158[0753:porfoh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [178].Shibamoto Y, Zhou L, Hatta H, Mori M, Nishimoto S. Jpn. J. Cancer Res. 2000;91:433. doi: 10.1111/j.1349-7006.2000.tb00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Barbour NP, Paborji M, Alexander TC, Coppola WP, Bogardus JB. Pharm. Res. 1995;12:215. doi: 10.1023/a:1016274825322. [DOI] [PubMed] [Google Scholar]
- [180].Damen EWP, Nevalainen TJ, van den Bergh TJM, de Groot FMH, Scheeren H. Bioorg. Med. Chem. 2002;10:71. doi: 10.1016/s0968-0896(01)00235-8. [DOI] [PubMed] [Google Scholar]
- [181].Wang B, Zhang H, Zheng A, Wang W. Bioorg. Med. Chem. 1998;6:417. doi: 10.1016/s0968-0896(98)00014-5. [DOI] [PubMed] [Google Scholar]
- [182].de Groot FMH, van Berkom LWA, Scheeren H. J. Med. Chem. 2000;43:3093. doi: 10.1021/jm0009078. [DOI] [PubMed] [Google Scholar]
- [183].Greenwald RB, Choe YH, Conover CD, Shum K, Wu D, Royzen M. J. Med. Chem. 2000;43:475. doi: 10.1021/jm990498j. [DOI] [PubMed] [Google Scholar]
- [184].Rivault F, Tranoy-Opalinski I, Gesson J-P. Bioorg. Med. Chem. 2004;12:675. doi: 10.1016/j.bmc.2003.11.026. [DOI] [PubMed] [Google Scholar]
- [185].Dubowchik GM, Firestone RA, Padilla L, Wilner D, Hofstead SJ, Mosure K, Knipe JO, Lasch SJ, Trail PA. Bioconjugate Chem. 2002;13:855. doi: 10.1021/bc025536j. [DOI] [PubMed] [Google Scholar]
- [186].McGrath DV. Mol. Pharm. 2005;2:253. doi: 10.1021/mp050047x. [DOI] [PubMed] [Google Scholar]
- [187].Gopin A, Ebner S, Attali B, Shabat D. Bioconjugate Chem. 2006;17:1432. doi: 10.1021/bc060180n. [DOI] [PubMed] [Google Scholar]