Abstract
Embryo implantation involves direct interaction of the blastocyst with the luminal epithelium of the receptive uterus. MUC1, a transmembrane mucin expressed at the apical surface of uterine epithelia, acts as a barrier to microbial infection and enzymatic attack. Loss of MUC1 is believed to be a prerequisite for a functionally receptive uterus across many species. Human and murine MUC1 regulation by steroid hormones displays important differences. Estrogen (E2) stimulates MUC1 expression in mice, and progesterone (P4) antagonizes E2 action in this regard. MUC1 expression is severely reduced during the receptive uterine state in mice. In contrast, human MUC1 expression is maximal at the receptive or midluteal phase, when P4 levels are high. No information is available regarding regulation of human MUC1 in vivo at the site of embryo attachment. Our aim was to better understand regulation of human MUC1 during early pregnancy in vivo. For this purpose, we used a transgenic mouse carrying full-length human MUC1 gene (Tg(MUC1)79.24Gend) as well as endogenous MUC1 as a model system. Human MUC1 was detected by real-time RT-PCR, Western blotting, and immunohistochemistry during early pregnancy. Our data indicate that human MUC1 persists at reduced (20% relative to Day 1 postcoitum) levels in receptive-phase uteri, including the site of embryo attachment. In contrast, mouse MUC1 was much more severely (>98% relative to Day 1 postcoitum) reduced in the same context. These observations are consistent with distinct regulation between the human and mouse genes. Because these genes are expressed in the same transcriptional context (i.e., mouse uterine epithelia), structural differences between human and murine genes must account for these differences in MUC1 regulation.
Keywords: embryo, female reproductive tract, implantation, MUC1, pregnancy
Human MUC1 is expressed during embryo implantation in a transgenic mouse model, while expression of mouse Muc1 is extinguished, indicating distinct regulatory patterns conferred by each species.
INTRODUCTION
Attachment of mammalian embryos to the uterine epithelium is a highly coordinated and critical biological process representing the initial step of implantation, and it occurs in three distinct stages: apposition, attachment, and penetration [1, 2]. Blastocyst attachment to the luminal epithelium of the receptive endometrium is temporally restricted within a period often referred to as the “window of receptivity” [3]. In mice, implantation takes place during a period of 12–24 h, between Day 4 and Day 5 postcoitum [4]. Under the influence of steroid hormones, “prereceptive” uterine epithelia undergo changes in cell surface composition to transition to a receptive uterine state. The apical surfaces of uterine epithelia are enveloped by a thick, protective glycocalyx that retracts as the uterus becomes accessible to a prospective embryo. Mucin glycoproteins, particularly MUC1, are major components of the apical glycocalyx. MUC1 lubricates and hydrates cell surfaces and functions as a protective barrier against microbial and proteolytic attack. The extensively glycosylated extracellular domain of MUC1 prevents the attachment of mammalian embryos, possibly by inhibiting access to cell surface adhesion-promoting receptors [5, 6]. Interestingly, the cytoplasmic and transmembrane domains of mouse MUC1 retain 87% homology to these regions of the human protein but only a 37% homology in the tandem repeat region of the ectodomain [7]. In addition, the promoter regions of these species show 74% homology but contain no conserved steroid hormone response elements [8].
MUC1 is abundantly expressed at the apical surface of both luminal and glandular epithelia of the uterus in various species [9–13]. Downregulation or a loss of MUC1 at implantation sites is a prerequisite for a functionally receptive uterus in most species studied to date [11–15]. Interestingly, in humans, persistent expression of MUC1 is observed during the receptive state [10, 16, 17], distinct from many other species. However, changes in glycosylation patterns during the receptive phase suggest a more complex role for MUC1 during implantation in humans [16]. MUC1 provides a scaffold for selectin ligands that potentially could support blastocyst interactions via selectins at the maternal-fetal interface [18]. One in vitro study indicates that factors secreted by the blastocyst may contribute to downregulation of MUC1 at attachment sites [19].
In rodents, MUC1 regulation contrasts with certain findings in humans. MUC1 expression in the cycling mouse uterus is highest in proestrus and estrus, correlating with high estrogen (E2) levels [12, 20]. In mice and rats, MUC1 is lost on Day 4 of pregnancy, before blastocyst attachment, an event that is believed to be necessary for a receptive uterine state [12, 14]. Although MUC1 represents only 10% of the uterine epithelial mucin population [21], in vitro assays reveal an increase in blastocyst attachment when MUC1 is genetically ablated [22]. Furthermore, in mice, E2 stimulates MUC1 expression [12]. On the other hand, the expression of MUC1 in humans [10, 23] is stimulated by progesterone (P4). This differential P4 responsiveness appears to be due to differences in the balance of P4 receptor A and B isoforms between humans and mice [23].
No information is available on the regulation of MUC1 at human implantation sites. Understanding the controls of MUC1 expression during implantation could provide new therapeutic avenues for treatment of infertility, preterm abortions, and improving pregnancy success. An ideal model to study expression of the human MUC1 gene in an implantation context is mice harboring the intact MUC1 gene (Tg(MUC1)79.24Gend; previously described as MUC1.Tg), including sequences necessary for tissue-specific expression [24, 25]. Tg(MUC1)79.24Gend mice express the human MUC1 transgene with appropriate tissue specificity as observed in humans [24, 25].
The present study was designed to define the expression of MUC1 during the peri-implantation stages of pregnancy in the Tg(MUC1)79.24Gend mouse. This mouse model provides the opportunity to assess whether differences in human and mouse MUC1 expression are due to differences in the transcriptional context or structural differences between these genes. Collectively, our findings demonstrate that unlike murine MUC1 mRNA and protein expression, human MUC1 expression persists at reduced levels during the peri-implantation period in this model. Therefore, it appears that structural differences between the human and mouse gene orthologs account, at least in part, for differences in MUC1 expression between species. We conclude that persistent, low-level human MUC1 expression at implantation sites is insufficient to inhibit embryo implantation.
MATERIALS AND METHODS
Materials
All chemicals used were reagent grade or better. All reagents used for the experiments were purchased from Fisher Scientific (Pittsburgh, PA) or Sigma Aldrich (St. Louis, MO) unless otherwise indicated.
Animals
Human MUC1 transgenic (Tg(MUC1)79.24Gend) mice on a C57BL/6 background were generated as described previously [24, 25] and backcrossed more than 10 times to FvB/N, making them congenic. For our studies, Tg(MUC1)79.24Gend mice on an FvB/N background were maintained as heterozygotes. The transgenics also express the endogenous mouse Muc1 gene. Wild-type FvB/N mice used as controls were purchased from Taconic (Germantown, NY). Mice were bred and maintained under pathogen-free conditions at the University of Delaware Animal Care Facility. All protocols were in accordance with the guidelines for humane treatment of laboratory animals by the National Institutes of Health and the Institutional Animal Care and Use Committee at the University of Delaware. Genotyping was routinely performed by PCR analysis of genomic DNA to confirm presence of the human MUC1 gene in the mice.
Tissue Collection
Adult Tg(MUC1)79.24Gend/FvB or wild-type FvB females were mated with fertile males of the same strain to induce pregnancy. Mice were killed on Days 1, 3, and 5 of pregnancy between 1000 and 1130 h. The morning when the vaginal plug was found was designated Day 1 of pregnancy (or Day 1 postcoitum). Pregnancy was confirmed by flushing eggs from oviducts on Day 3 and embryos from uterine lumina on Day 5 (day of implantation). Endometrial scrapings were collected from the inner wall of the uteri using a scalpel blade for analysis by Western blotting and for extraction of RNA. Uterine horns were frozen in Tissue Tek Optimal Cutting Temperature (Sakura Finetechnical, Torrance, CA) and preserved at −80°C until cryosectioning for immunohistochemistry. Implantation sites were visualized by intravenous injection of 0.3 ml of 1% (w/v) Pontamine Sky Blue 6BX (Alfa Aesar, Ward Hill, MA) in 1× PBS at 1900 h on the evening of Day 5 for 10 min, and mice were later killed to collect uterine horns.
Immunoblotting
Endometrial scrapings were solubilized in sample extraction buffer: 8 M urea; 1% (w/v) SDS; 50 mM Tris, pH 7.0; 1% (v/v) β-mercaptoethanol; and a 1:100 dilution of protease inhibitor cocktail (Sigma), and protein concentration was determined as described by Lowry et al. [26]. Fifty micrograms of total protein extract was incubated for 5 min at 100°C with Laemmli sample buffer [27] and separated by SDS-PAGE using a 10% or 15% (w/v) Porzio and Pearson SDS-PAGE gel [28]. Proteins were transferred from gels to Trans Blot Transfer Medium nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA) at 4°C for 5 h at 40 V. Blots were blocked at room temperature for 1–2 h in Dulbecco PBS plus 0.1% (v/v) Tween-20 (PBS-T) and 3% (w/v) bovine serum albumin (BSA), or with 5% (w/v) nonfat dry milk in PBS-T. The MUC1 primary antibody, 214D4 (kindly provided as hybridoma media by Dr. John Hilkens, The Netherlands Cancer Institute, Amsterdam, The Netherlands) [29, 30], was added to a final dilution of 1:1000. Another MUC1 primary antibody, HMFG1 [29], was added to a final dilution of 1:500. The primary antibody, CT1 [31, 32], was added to a final dilution of 1:1000. Blots were incubated with the primary antibody overnight at 4°C with constant rotary agitation. Blots were rinsed three times for 5 min each at room temperature with PBS-T to remove unbound antibody. Subsequently, blots were incubated for 2 h at 4°C with peroxidase-conjugated sheep anti-mouse (Jackson Immunoresearch, West Grove, PA) or goat anti-rabbit (Sigma) immunoglobulin G (IgG) at a final dilution of 1:200 000 in 5% (w/v) nonfat dry milk or 3% (w/v) BSA/PBS-T, respectively. Finally, the blots were rinsed three times with PBS for 5 min each at room temperature, and antibody binding was detected using the SuperSignal West Dura Extended Duration Substrate ECL system (Pierce, Rockford, IL) as described by the manufacturer. Blots were exposed to x-ray film, and signal intensities were quantified using Scion Image (Scion Corp., Fredrick, MD). Protein extracts from the human uterine epithelial cell line, HES, were used a positive control. Blots were reprobed with the X-260 polyclonal antibody (generously provided by Dr. Warren Schmidt, Vanderbilt Medical School, Nashville, TN) at a 1:1000 dilution to detect cytokeratins (Krt) 18 and 19 as a control for potential changes in uterine epithelial population. Statistical analyses were performed using one-way ANOVA and the Tukey-Kramer multiple comparisons test (GraphPad InStat program; GraphPad Software Inc., San Diego, CA).
Immunohistochemistry
Uterine sections of 10 μm thickness were prepared using a Leica CM3050 S Cryostat (Leica Microsystems Inc., Wetzlar, Germany) mounted on Superfrost blue slides (Fisher Scientific). Sections were fixed in methanol for 10 min at room temperature, rehydrated in PBS (Ca2+ and Mg2+ free) for 30 min at room temperature, and blocked in 5% (w/v) BSA/PBS for 30 min. Sections were incubated with primary antibody for 1 h at 37°C in a humidified chamber. After rinsing in PBS twice for 5 min, sections were incubated with secondary antibody for 1 h at 37°C in a humidified chamber. Double staining involved staining of sections first with the primary antibody, CT-1 (1:10 dilution), and Alexa Fluor-568 (1:50 dilution) anti-rabbit IgG (Invitrogen, Carlsbad, CA) was used as a secondary antibody. Sections then were incubated at room temperature for 1 h with mouse monoclonal anti-human MUC1 antibody, 214D4 (Upstate Biotechnology Inc., Lake Placid, NY), and Alexa Fluor-488 Zenon mouse IgG labeling reagent (Invitrogen) at 1:40 dilution in a humidified chamber at room temperature. For immunohistochemistry, the 214D4 commercial antibody was used per the requirements of the protocol for Zenon labeling. One microgram of the 214D4 antibody was used to prepare the Zenon labeling complex according to the manufacturer's instructions. Sections were rinsed three times with PBS for 5 min each at room temperature, followed by a postfixation step for 10 min at room temperature in 4% (w/v) paraformaldehyde in PBS. Draq5 (Biostatus Limited, Shepshed, U.K.) at a 1:3000 dilution was used to counterstain nuclei. Specificity of commercial 214D4 antibody was determined by incubating sections with mouse IgG1 (data not shown). Finally, sections were mounted in Gel/Mount (BioMeda Corp., Foster City, CA) antifading reagent. Sections were examined and imaged with a Zeiss LSM 510 confocal microscope.
RNA Isolation and Real-Time RT-PCR
Uterine tissue from Tg(MUC1)79.24Gend mice was collected. Total RNA was extracted from endometrial scrapings using TRIzol reagent (Invitrogen), DNAse treated (Ambion, Austin, TX) as per the manufacturer's instructions and quantified by ultraviolet spectrophotometry. One microgram of total RNA was reverse transcribed using Omniscript RT (Qiagen, Valencia, CA) for 1 h at 37°C. Real-time RT-PCR was performed using the SYBR green PCR master mix (Applied Biosystems, Foster City, CA) for 15 sec at 95°C and 60 sec at 63.8°C (MUC1) or 58°C (KRT18 and MUC1) for 45 cycles, on an ABI Prism 7000 sequence detection system (Applied Biosystems). Primer sequences were designed using the SDSC Biology Workbench (http://workbench.sdsc.edu/) and are as follows: human MUC1, reverse 5′-TGACATCCTGTCCCTGAGTG and forward 5′-AGAGAAGTTCAGTGCCCAGC; mouse Muc1, reverse 5′-TACCACTCCAGTCCACAGCA and forward 5′-GTCTTCAGGAGCTCTGGTGG; and mouse cytokeratin 18 (Krt18), reverse 5′-GGGCTTCATTTGCTGTCTGT and forward 5′-TAGTCCCAGCATTGGGTAGC. The relative amounts of MUC1/Muc1 to Krt18 mRNA were determined using the comparative threshold cycle method (User Bulletin No.2; ABI Prism 7700 Sequence Detection system).
RESULTS
Expression of Human MUC1 mRNA During the Peri-Implantation Stage in Human MUC1 Transgenic Mouse Endometrium
To contrast behavior of human versus mouse MUC1 gene expression in the same cellular context, we exploited the use of a transgenic mouse harboring the entire human MUC1 gene. Previous studies demonstrate this mouse expresses human MUC1 in an appropriate tissue- and cell type-specific fashion. Initially, we examined the relative levels of expression of human MUC1 mRNA in comparison to murine Muc1 mRNA during early pregnancy. Real-time RT-PCR revealed that Muc1 mRNA expression fell to barely detectable levels between Day 1 and Day 4.5 postcoitum, consistent with previous reports from our lab. Interestingly, although human MUC1 mRNA expression decreased similarly between Days 1 and 3, approximately 20% remained at Day 4.5 (Fig. 1). These data demonstrated that although human MUC1 mRNA expression decreases during early pregnancy, it contrasts from the behavior of the mouse gene in that a significant amount is maintained at the time of embryo attachment; i.e., Day 4.5 postcoitum.
FIG. 1.
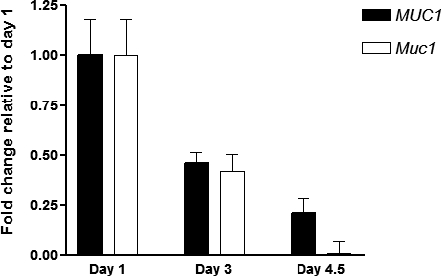
Human MUC1 and mouse Muc1 mRNA expression in early pregnancy. Endometrial extracts from Days 1, 3, and 4.5 postcoitum of Tg(MUC1)79.24Gend were obtained and analyzed for MUC1, Muc1, and Krt18 by real-time RT-PCR as described in Materials and Methods. Human MUC1 and mouse Muc1 mRNA expression were determined relative to that of mouse Krt18. The bars indicate the mean ± SD values from triplicate determinations of triplicate samples in each case and are expressed relative to Day 1. Note that although Muc1 expression declines almost to zero, MUC1 persists at approximately 20% of the level observed on Day 1.
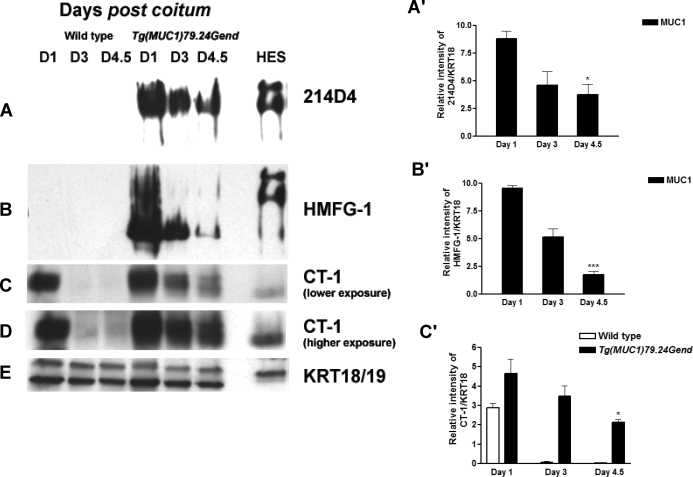
Human MUC1 Protein Expression During the Peri-Implantation Stage by Western Blotting
We next sought to determine whether human MUC1 protein expression also persisted during early pregnancy in this model. Endometrial extracts from wild-type and human MUC1 transgenics were analyzed by immunoblotting with the human MUC1-specific antibodies 214D4 and HMFG1, as well as the pan-species-reactive CT-1 antibody (Fig. 2). Stage-matched endometrial extracts from wild-type mice served as controls for mouse MUC1 expression. Because the uterine epithelial component can change in response to hormone levels and early pregnancy, we used Western blotting for the epithelial marker proteins, cytokeratins 18/19 (KRT 18/19), to control for these variations (Fig. 2E). The antibodies specific for human MUC1 showed no reactivity with wild-type endometrial extracts (Fig. 2, A and B). In contrast, both antibodies strongly reacted with high-molecular weight products in Tg(MUC1)79.24Gend mice. Although human MUC1 protein expression declined during early pregnancy, it nonetheless persisted on Day 4.5 compared with Day 1 (Fig. 2, A and B). The CT-1 antibody, which recognizes the highly conserved cytoplasmic domain, demonstrated almost complete loss of mouse MUC1 between Days 1 and 4.5; however, CT1 reactivity persisted in Tg(MUC1)79.24Gend, reflecting the presence of the human MUC1 (Fig. 2, C and D). Quantitation of these data was generally in good agreement with real-time RT-PCR: Mouse MUC1 was almost completely lost between Days 1 and 4.5, whereas human MUC1 persisted at Day 4.5 at approximately 20% of the level observed at Day 1 (Fig. 2, A–C). Collectively, these results indicate that unlike its murine counterpart, human MUC1 protein expression persists on Day 4.5 postcoitum, albeit at reduced levels.
FIG. 2.
Human MUC1 expression in early pregnancy series in human MUC1 transgenics. Endometrial extracts from Days 1, 3, and 4.5 postcoitum (D1, D3, and D4.5, respectively) from both wild-type and human MUC1 transgenics were analyzed by Western blotting as described in Materials and Methods. Human MUC1 detection was with either 214D4 (A) or HMFG-1 (B) monoclonal antibodies specific for human MUC1. No signal was observed for either antibody in extracts from wild-type mice. Expression of human MUC1 is reduced, but it persists during early pregnancy. C) Detection of human and mouse MUC1 by CT-1 antibody. Almost complete loss of MUC1 expression is detected in the wild type, whereas MUC1 expression persists at Day 4.5 in the transgenics, reflecting the presence of the human MUC1. D) A longer exposure of samples shown in C. E) Blots were reprobed with antibody specific for mouse cytokeratin 18/19 (KRT 18/19), epithelial cell markers serving both as a load control and to control for changes in total epithelial cell populations during pregnancy. Total protein extract from HES cells was used a positive control. Bar graphs in A′, B′, and C′ represent densitometric analyses to quantify MUC1 expression normalized to KRT18 of the above data provided as mean ± SD values in each case. *P < 0.05 relative to human MUC1 Day 1; ***P < 0.001 relative to human MUC1 Day 1.
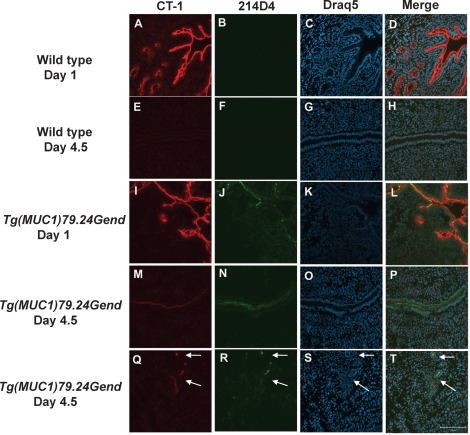
Human MUC1 Protein Expression by Immunohistochemistry During Early Pregnancy
To identify the sites of human MUC1 protein expression in early pregnancy in the Tg(MUC1)79.24Gend model, immunohistochemistry was performed on frozen sections from Days 1 and 4.5 postcoitum (Fig. 3). Stage-matched frozen sections from wild-type mice were used as controls (Fig. 3, A–H). Robust mouse MUC1 expression was observed on Day 1 in luminal and glandular epithelia (Fig. 3, A and D) and was greatly reduced on Day 4.5 (Fig. 3, E and H) in luminal epithelia using the pan-species-reactive CT-1 antibody. Confirming the results from Western blots, no reactivity with the human MUC1-specific 214D4 antibody was observed in sections of wild-type mice (Fig. 3, B and F). In Tg(MUC1)79.24Gend mice, CT-1 staining was observed on Day 1 in both luminal and glandular epithelia (Fig. 3, I and L) and, to a lesser extent, on Day 4.5 in luminal and glandular epithelia (Fig. 3, M and P). Human MUC1 expression was detected on Day 1 (Fig. 3, J and L) and also was detected on Day 4.5 postcoitum (Fig. 3, N and P) in luminal and glandular epithelia (Fig. 3, R and T). Collectively, these data confirm that human MUC1 expression was restricted to endometrial epithelia during the peri-implantation period.
FIG. 3.
Human MUC1 expression at Days 1 and 4.5 of pregnancy in human MUC1 transgenics. Images show double labeling using CT-1, an antibody that recognizes both mouse and human MUC1, and 214D4, a monoclonal antibody that specifically recognizes human MUC1 at Days 1 and 4.5 in wild-type FvB and human MUC1 transgenics. Sections were analyzed by indirect immunofluorescence with anti-CT-1 (red), anti-214D4 (green), and draq5 (arbitrarily assigned the color blue) as described in Materials and Methods. Sections from Days 1 (A–D) and 4.5 postcoitum (E–H) from uteri of wild-type mice were used as controls to compare to human MUC1 transgenic uterine sections from Days 1 (I–L) and 4.5 postcoitum (M–P). Human MUC1 expression in glandular epithelia (indicated by arrows) on Day 4.5 postcoitum is also detected (Q–T). Bar = 100 μm.
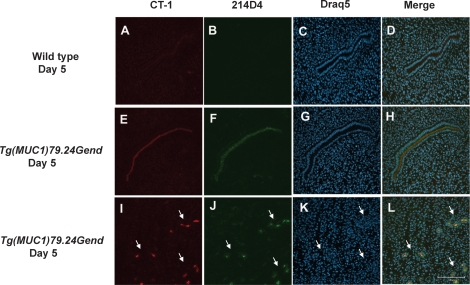
Human MUC1 Protein Expression at Interimplantation and Implantation Sites
To further examine the presence of human MUC1 expression during early pregnancy, we stained interimplantation (Fig. 4) and implantation (Fig. 5) sites from wild-type and human MUC1 transgenic mice with 214D4 and CT-1. A nearly complete loss of expression of mouse MUC1 was observed in luminal and glandular epithelia at interimplantation sites (Fig. 4, A and D). No human MUC1 reactivity was observed in the wild-type controls (Fig. 4B). In contrast, MUC1 expression was observed in the luminal epithelia at interimplantation sites in Tg(MUC1)79.24Gend mice, using both CT-1 and 214D4 (Fig. 4, E, F, and H). Additionally, glandular epithelia stained positively for both CT-1-reactive MUC1 and 214D4-reactive human MUC1 at the interimplantation sites on Day 5 (Fig. 4, I, J, and L).
FIG. 4.
Human MUC1 expression in interimplantation sites on Day 5 of pregnancy. Sections of Day 5 mouse uterine interimplantation sites from wild-type and human MUC1 transgenics were analyzed by indirect immunofluorescence with anti-CT-1 (red), anti-214D4 (green), and draq5 (arbitrarily assigned the color blue) as described in Materials and Methods. Sections of Day 5 wild-type uteri (A–D) were used as controls to compare to human MUC1 transgenic uterine sections (E–L). Human MUC1 expression persists in the luminal (E, F, and H) and glandular (I, J, and L) epithelia (indicated by arrows) at the interimplantation sites compared with mouse MUC1 expression (A, B, and D). Bar = 100 μm.
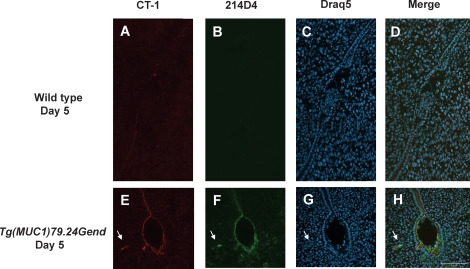
FIG. 5.
Human MUC1 expression at the site of implantation. Sections of mouse implantation sites at Day 5 from wild-type and human MUC1 transgenics were analyzed by indirect immunofluorescence with anti-CT-1 (red), anti-214D4 (green), and draq5 (arbitrarily assigned the color blue) as described in Materials and Methods. Sections of an implantation site (A–D) from uteri of wild-type FvB expressing only mouse MUC1 were used as controls to compare to human MUC1 transgenic uteri sections (E–H). Human MUC1 expression persists at the site of implantation (E, F, and H) compared with mouse MUC1 expression (A, B, and D) which is lost at the site of implantation. Arrows indicate glandular epithelia. Bar = 100 μm.
We next sought to determine whether human MUC1 expression persisted at implantation sites (Fig. 5). Mouse MUC1 expression by CT-1 staining was barely detectable at the implantation site in wild-type mice, and staining for human MUC1 was negative (Fig. 5B). Conversely, both CT-1 and 214D4 staining were evident at implantation sites in the Tg(MUC1)79.24Gend mouse uterus, both at the apical aspect of luminal epithelia surrounding the blastocyst, and in nearby glandular epithelia (Fig. 5, E, F, and H). Collectively, these data confirm that unlike mouse MUC1, human MUC1 expression persists in early pregnancy in the Tg(MUC1)79.24Gend model and is maintained at the site of embryo attachment.
DISCUSSION
Our data confirm restricted expression of human MUC1 in uterine epithelial compartments of Tg(MUC1)79.24Gend during early pregnancy. The acquisition of a receptive uterine state is associated with MUC1 loss either locally or throughout the uterus in most species [2]. It is believed that MUC1 loss results in removal of a barrier, which then results in an apical surface that is permissive for embryo implantation [12, 14, 20, 22]. In human uteri, MUC1 regulation drastically differs from that of rodents. MUC1 mRNA and protein expression in human endometrium is hormonally regulated, with a maximal level of expression during the early and midsecretory (receptive) phase, a time when implantation occurs in humans [10, 16, 19]. This regulation corresponds to a time when circulating levels of P4 are high [10]. In contrast, E2 stimulates murine MUC1 expression, whereas P4 antagonizes E2 action in this regard [10, 12, 20, 23]. Estrogen actions on MUC1 expression appear to be indirect, because estrogen receptors (ERs) do not directly regulate the Muc1 gene [33].
Murine Muc1 mRNA expression was barely detectable by Day 4.5 postcoitum, consistent with previous reports [12]. Relative to Day 1 postcoitum levels, about 20% of human MUC1 mRNA and protein expression persists by Day 4.5 postcoitum. Maximal human MUC1 mRNA and protein expression has been reported in human endometrium during the P4-dominated midsecretory phase [10]. Progesterone levels also rise by Day 4.5 postcoitum in mice, although human MUC1 expression declines at this stage in the Tg(MUC1)79.24Gend uterus. Thus, it is likely that differences in regulation of the human MUC1 gene in this mouse model are due to differences in the transcriptional context between human and mouse uterine epithelia. Alternatively, the site of insertion of the human MUC1 transgene may have an impact, although this is difficult to assess.
Several possible explanations exist for the differential regulation of mouse vs. human MUC1 gene expression observed in this model. Although human and mouse Muc1 promoters exhibit a 74% overall homology, the steroid response elements are not conserved [7, 8]. In humans and mice, progesterone receptors and ERs are hormonally regulated throughout the cycle and at the time of embryo implantation [34, 35]. In mice, the predominant isoform is PRA [36], and both E2-induced murine MUC1 expression and P4-induced human MUC1 expression are repressed in the presence of PRA [23]. Therefore, it is possible that PRA is responsible for reduction of human MUC1 in a P4-dominated stage in this mouse model. It is interesting to note that on Day 1 postcoitum, an E2-dominant stage, human MUC1 expression is high. High MUC1 levels at Day 1 postcoitum (a state comparable to estrus) may be due to the combination of the presence of low circulating levels of P4 [37] and higher expression of the positive regulator of human MUC1-PRB isoform in mouse uterine epithelial cells. Additionally, indirect hormonal regulation, possibly through stromal receptors, may also contribute to the regulation of human MUC1 expression during the peri-implantation stage. Western blotting observations confirm that human MUC1 protein expression persists to a degree similar to MUC1 mRNA. Thus, control of MUC1 mRNA levels, rather than potential differences in MUC1 protein stability, account for the differences in MUC1 expression.
The decreased expression of human MUC1 on Day 4.5 postcoitum reveals that the transition to a receptive state is accompanied by reduced MUC1 levels. Nonetheless, persistence of MUC1 expression contrasts with murine MUC1 expression on Day 4.5 postcoitum. Thus, complete removal of MUC1 is not essential to support embryo attachment. We observed that litter sizes produced by the Tg(MUC1)79.24Gend females vs. the wild-type females were not significantly different (data not shown). Thus, mice with this level of persistent MUC1 at embryo attachment sites are fully fertile. Our data demonstrate that 80% reduction is sufficient for this purpose and are similar to results obtained in previous in vitro studies in which partial mucin removal (approximately 60%) resulted in a functionally receptive state [22]. In addition, different glycoforms of human MUC1 were detected by 214D4 and HMFG1 during the peri-implantation stages, in accordance with previous studies profiling MUC1 expression in human endometrium and uterine cells [10, 16, 31]. Previous studies have determined that detection of epitopes in the MUC1 ectodomain by these antibodies is affected by glycosylation [31]. Interestingly, both the precursor HMFG1-reactive and the mature 214D4-reactive species were detected by Western blotting during early pregnancy in the transgenics. These ectodomain-directed antibodies do not recognize murine MUC1 because the protein sequences in the tandem repeat regions are not conserved between humans and mice [7, 24]. The observation that human MUC1 expression persists at sites of embryo attachment also raises the possibility that human MUC1 actually may facilitate embryo attachment. This could be accomplished if MUC1 carried attachment-promoting motifs (e.g., selectin ligands). In this regard, it has been shown previously that MUC1 can carry selectin ligands under certain conditions, including receptive-phase uteri [18, 38].
In summary, we show that human MUC1 is expressed during early pregnancy in the Tg(MUC1)79.24Gend mouse model. Similarly to murine MUC1, levels of human MUC1 are reduced during early implantation. The attenuation of human MUC1 in the implantation process is consistent with the general notion that MUC1 is a barrier to embryo attachment. Nonetheless, the persistence of reduced levels of MUC1 at implantation sites raises the possibility that MUC1 may play more complex roles in this process. Tg(MUC1)79.24Gend mice should provide a useful model to study the control of human MUC1 gene expression and MUC1 function during embryo implantation and in other contexts.
Acknowledgments
We greatly appreciate the many helpful discussions with JoAnne Julian and all members of the Carson and Farach-Carson laboratories. We wish to thank the excellent staff at the Office of Laboratory Animal Medicine, University of Delaware, for assistance in the project. We also appreciate Dr. Melissa Brayman for critically reading the manuscript, Sharron Kingston for her expert secretarial assistance, Ben Rohe for help designing primers, and Dr. Kirk Czymmek for assistance with confocal microscopy. The authors wish to thank Dr. John Hilkens for providing the 214D4 antibody.
Footnotes
Supported by NIH grant HD 29963 to D.D.C. and University of Delaware Dissertation Fellowship Award to N.D.
REFERENCES
- Wang H, Dey SK.Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 2006; 7: 185–199. [DOI] [PubMed] [Google Scholar]
- Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K.Embryo implantation. Dev Biol 2000; 223: 217–237. [DOI] [PubMed] [Google Scholar]
- Psychoyos A.Uterine receptivity for nidation. Ann N Y Acad Sci 1986; 476: 36–42. [DOI] [PubMed] [Google Scholar]
- Psychoyos A.Hormonal control of ovoimplantation. Vitam Horm 1973; 31: 201–256. [DOI] [PubMed] [Google Scholar]
- Thathiah A, Carson DD.Mucins and blastocyst attachment. Rev Endocr Metab Disord 2002; 3: 87–96. [DOI] [PubMed] [Google Scholar]
- Jentoft N.Why are proteins O-glycosylated? Trends Biochem Sci 1990; 15: 291–294. [DOI] [PubMed] [Google Scholar]
- Spicer AP, Parry G, Patton S, Gendler SJ.Molecular cloning and analysis of the mouse homologue of the tumor-associated mucin, MUC1, reveals conservation of potential O-glycosylation sites, transmembrane, and cytoplasmic domains and a loss of minisatellite-like polymorphism. J Biol Chem 1991; 266: 15099–15109. [PubMed] [Google Scholar]
- Vos HL, de Vries Y, Hilkens J.The mouse episialin (Muc1) gene and its promoter: rapid evolution of the repetitive domain in the protein. Biochem Biophys Res Commun 1991; 181: 121–130. [DOI] [PubMed] [Google Scholar]
- Hoffman LH, Olson GE, Carson DD, Chilton BS.Progesterone and implanting blastocysts regulate Muc1 expression in rabbit uterine epithelium. Endocrinology 1998; 139: 266–271. [DOI] [PubMed] [Google Scholar]
- Hey NA, Graham RA, Seif MW, Aplin JD.The polymorphic epithelial mucin MUC1 in human endometrium is regulated with maximal expression in the implantation phase. J Clin Endocrinol Metab 1994; 78: 337–342. [DOI] [PubMed] [Google Scholar]
- Hild-Petito S, Fazleabas AT, Julian J, Carson DD.Mucin (Muc-1) expression is differentially regulated in uterine luminal and glandular epithelia of the baboon (Papio anubis). Biol Reprod 1996; 54: 939–947. [DOI] [PubMed] [Google Scholar]
- Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Julian J, Pimental RA, Wegner CC, Dey SK, Carson DD.Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology 1995; 136: 3639–3647. [DOI] [PubMed] [Google Scholar]
- Julian J, Enders AC, Fazleabas AT, Carson DD.Compartmental distinctions in uterine Muc-1 expression during early pregnancy in cynomolgous macaque (Macaca fascicularis) and baboon (Papio anubis). Hum Reprod 2005; 20: 1493–1503. [DOI] [PubMed] [Google Scholar]
- DeSouza MM, Mani SK, Julian J, Carson DD.Reduction of mucin-1 expression during the receptive phase in the rat uterus. Biol Reprod 1998; 58: 1503–1507. [DOI] [PubMed] [Google Scholar]
- Bowen JA, Bazer FW, Burghardt RC.Spatial and temporal analyses of integrin and Muc-1 expression in porcine uterine epithelium and trophectoderm in vivo. Biol Reprod 1996; 55: 1098–1106. [DOI] [PubMed] [Google Scholar]
- DeLoia JA, Krasnow JS, Brekosky J, Babaknia A, Julian J, Carson DD.Regional specialization of the cell membrane-associated, polymorphic mucin (MUC1) in human uterine epithelia. Hum Reprod 1998; 13: 2902–2909. [DOI] [PubMed] [Google Scholar]
- Hey NA, Li TC, Devine PL, Graham RA, Saravelos H, Aplin JD.MUC1 in secretory phase endometrium: expression in precisely dated biopsies and flushings from normal and recurrent miscarriage patients. Hum Reprod 1995; 10: 2655–2662. [DOI] [PubMed] [Google Scholar]
- Carson DD, Julian J, Lessey BA, Prakobphol A, Fisher SJ.MUC1 is a scaffold for selectin ligands in the human uterus. Front Biosci 2006; 11: 2903–2908. [DOI] [PubMed] [Google Scholar]
- Meseguer M, Aplin JD, Caballero-Campo P, O'Connor JE, Martin JC, Remohi J, Pellicer A, Simon C.Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biol Reprod 2001; 64: 590–601. [DOI] [PubMed] [Google Scholar]
- Braga VM, Gendler SJ.Modulation of Muc-1 mucin expression in the mouse uterus during the estrus cycle, early pregnancy and placentation. J Cell Sci 1993; 105(pt 2):397–405. [DOI] [PubMed] [Google Scholar]
- Pimental RA, Julian J, Gendler SJ, Carson DD.Synthesis and intracellular trafficking of Muc-1 and mucins by polarized mouse uterine epithelial cells. J Biol Chem 1996; 271: 28128–28137. [DOI] [PubMed] [Google Scholar]
- DeSouza MM, Surveyor GA, Price RE, Julian J, Kardon R, Zhou X, Gendler S, Hilkens J, Carson DD.MUC1/episialin: a critical barrier in the female reproductive tract. J Reprod Immunol 1999; 45: 127–158. [DOI] [PubMed] [Google Scholar]
- Brayman MJ, Julian J, Mulac-Jericevic B, Conneely OM, Edwards DP, Carson DD.Progesterone receptor isoforms A and B differentially regulate MUC1 expression in uterine epithelial cells. Mol Endocrinol 2006; 20: 2278–2291. [DOI] [PubMed] [Google Scholar]
- Peat N, Gendler SJ, Lalani N, Duhig T, Taylor-Papadimitriou J.Tissue-specific expression of a human polymorphic epithelial mucin (MUC1) in transgenic mice. Cancer Res 1992; 52: 1954–1960. [PubMed] [Google Scholar]
- Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ.Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res 1998; 58: 315–321. [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ.Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265–275. [PubMed] [Google Scholar]
- Laemmli UK.Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–685. [DOI] [PubMed] [Google Scholar]
- Porzio MA, Pearson AM.Improved resolution of myofibrillar proteins with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim Biophys Acta 1977; 490: 27–34. [DOI] [PubMed] [Google Scholar]
- Burchell J, Gendler S, Taylor-Papadimitriou J, Girling A, Lewis A, Millis R, Lamport D.Development and characterization of breast cancer reactive monoclonal antibodies directed to the core protein of the human milk mucin. Cancer Res 1987; 47: 5476–5482. [PubMed] [Google Scholar]
- Wesseling J, van der Valk SW, Hilkens J.A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol Biol Cell 1996; 7: 565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Julian JA, Carson DD.The MUC1 HMFG1 glycoform is a precursor to the 214D4 glycoform in the human uterine epithelial cell line, HES. Biol Reprod 2008; 78: 290–298. [DOI] [PubMed] [Google Scholar]
- Pemberton L, Taylor-Papadimitriou J, Gendler SJ.Antibodies to the cytoplasmic domain of the MUC1 mucin show conservation throughout mammals. Biochem Biophys Res Commun 1992; 185: 167–175. [DOI] [PubMed] [Google Scholar]
- Zhou X, DeSouza MM, Julian J, Gendler SJ, Carson DD.Estrogen receptor does not directly regulate the murine Muc-1 promoter. Mol Cell Endocrinol 1998; 143: 65–78. [DOI] [PubMed] [Google Scholar]
- Tan J, Paria BC, Dey SK, Das SK.Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology 1999; 140: 5310–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mote PA, Balleine RL, McGowan EM, Clarke CL.Heterogeneity of progesterone receptors A and B expression in human endometrial glands and stroma. Hum Reprod 2000; 15(suppl 3):48–56. [DOI] [PubMed] [Google Scholar]
- Schneider W, Ramachandran C, Satyaswaroop PG, Shyamala G.Murine progesterone receptor exists predominantly as the 83-kilodalton ‘A' form. J Steroid Biochem Mol Biol 1991; 38: 285–291. [DOI] [PubMed] [Google Scholar]
- McCormack JT, Greenwald GS.Progesterone and oestradiol-17beta concentrations in the peripheral plasma during pregnancy in the mouse. J Endocrinol 1974; 62: 101–107. [DOI] [PubMed] [Google Scholar]
- Hey NA, Aplin JD.Sialyl-Lewis x and Sialyl-Lewis a are associated with MUC1 in human endometrium. Glycoconj J 1996; 13: 769–779. [DOI] [PubMed] [Google Scholar]