Abstract
Reduced hypothalamic sensitivity to steroid negative feedback may contribute to the onset of puberty. In high fat-fed rodents, the timing of vaginal opening (VO) is advanced, suggesting that puberty begins earlier. Because obesity can increase androgens, which interfere with normal steroid feedback in adult females, we hypothesized that androgens reduce hypothalamic sensitivity to negative feedback during puberty and that blocking androgen action would prevent advanced VO in high fat-fed mice. Age at VO was examined in mice fed high-fat or low-fat diets from weaning and treated with the androgen receptor antagonist flutamide or vehicle (controls). VO was advanced in high-fat vs. low-fat controls, and flutamide blocked this advancement. VO was also delayed in low fat-fed flutamide-treated females, suggesting involvement of androgens in the timing of normal puberty. We next investigated if high-fat diet-induced insulin resistance contributes to early VO, as elevated insulin can stimulate androgen production. VO was examined in mice on either diet treated with the insulin sensitizer metformin. Metformin blocked high-fat advancement of VO but did not alter the timing of VO in low fat-fed mice. Insulin was elevated in high fat-fed females that had undergone VO compared with age-matched low fat-fed or metformin-treated animals on either diet that had not undergone VO. Together, these data suggest a model in which metabolic changes induced by high-fat diet, including transient increased circulating insulin, act in part by increasing androgen action to influence the timing of puberty in females.
Keywords: androgen receptor, androgens, high-fat diet, hypothalamus, insulin, metabolism, neuroendocrine, puberty
Advancement of vaginal opening in mice by a high-fat diet is blocked by both androgen receptor antagonists and an insulin sensitizer, suggesting that androgens and insulin have a role in the pubertal process.
INTRODUCTION
The pubertal transition involves many endocrine and neuroendocrine changes that lead to activation or reactivation of the hypothalamo-pituitary gonadal (HPG) axis [1, 2]. During normal puberty, reduced hypothalamic sensitivity to steroid negative feedback is one mechanism that is hypothesized to underlie increased activity of this axis [3–5]. The axis loses its extraordinary sensitivity to suppression by estrogen negative feedback, higher concentrations of estradiol are required to suppress gonadotropin-releasing hormone 1 (GnRH) and luteinizing hormone (LH) secretion, and progesterone assumes an increasingly major role in providing ovarian steroid negative feedback to the hypothalamus. Although there are species differences in timing and extent, the reduction of hypothalamic sensitivity to estradiol negative feedback has been reported in numerous species, including rodents and sheep, and in primates during the later stages of sexual maturation [6–8].
The pubertal activation of the GnRH system is influenced by metabolic and hormonal cues [9, 10]. Negative energy balance due to excess exercise or food restriction delays puberty [11–14]; caloric restriction also causes inhibition of GnRH release [15]. Conversely, a high-fat diet has been shown to advance puberty in female rodents [16], and increasing adiposity in girls may be a contributing factor to a trend for earlier puberty [17]. The links between metabolic cues and the pubertal transition are incompletely understood.
Androgens may provide a link between metabolic and endocrine cues for the onset of puberty. Increased androgens are associated with obesity [18–20], and androgens reduce sensitivity to steroid negative feedback in animal models [21, 22] and adults with hyperandrogenemic disorders [23, 24]. Furthermore, insulin can act as a gonadotropin, upregulating the production of androgens at the level of the ovary [25]. We hypothesized that androgens have a role in the reduction of hypothalamic sensitivity during puberty, that high-fat diets advance puberty by increasing insulin-stimulated androgen production, and that blocking androgen action reverses dietary advancement of puberty. To test these hypotheses, we examined the effect of the androgen receptor antagonist flutamide and the effect of the insulin sensitizer metformin on the age at vaginal opening (VO) in female mice fed control and high-fat diets.
MATERIALS AND METHODS
Mice
All procedures were approved by the University of Virginia Animal Care and Use Committee and were conducted in accord with the National Research Council's Guide for the Care and Use of Laboratory Animals. Mice were weaned at age 21 days from timed-pregnant C57Bl6/J mice from Jackson Laboratories (Bar Harbor, ME), were group housed (3–4 animals/cage) on a 14L:10D cycle with lights off at 1830 h (Eastern standard time), and were randomly assigned to either a low-fat chow (10% calories from fat; Research Diets, New Brunswick, NJ, or Harlan 2916 chow; Harlan, Indianapolis, IN [no differences were observed between the groups receiving these two diets]) or a high-fat chow (45% calories from fat; Research Diets) fed ad libitum from the day of weaning until the end of the experiment. All chemicals were obtained from Sigma Chemical Company (St. Louis, MO).
Experiment 1: Flutamide Treatment
Animals on each diet (low fat or high fat) were randomly subdivided into groups that received daily injections of either the androgen receptor blocker flutamide (10 mg/kg per day s.c. in the morning [low fat-fed flutamide n = 11 and high fat-fed flutamide n = 11]) or sesame oil vehicle (low fat-fed control n = 8 and high fat-fed control n = 9). Body mass and pubertal state as indicated by VO were monitored daily.
Experiment 2: Metformin Treatment
Female weanlings were assigned to low-fat or high-fat diet and were given metformin in drinking water containing 0 (control), 1, 2.5, 5, or 10 mg/ml (n = 6–9/group). These doses were based on the observation that mice drink 2–6 ml of water per day and weigh 8–16 g during this pubertal transition. The approximate effective dose delivered to animals was 0, 200, 500, 1000, or 2000 mg/kg per day. The lowest dose, 1 mg/ml, had no effect on age at VO. The 5 and 10 mg/ml doses blocked high-fat advancement of VO but also reduced the growth rate within diet groups, and animals receiving the highest dose did not gain body mass at the same rate as controls. The 2.5 mg/ml dose blocked the advancement of VO without altering the growth rate within diet groups and was thus chosen for metformin treatment.
Female weanlings (low-fat control n = 11, high-fat control n = 11, low-fat metformin-treated n = 13, and high-fat metformin-treated n = 14) were assigned to either a high-fat or low-fat diet and were treated or not treated with 2.5 mg/ml of metformin in drinking water. Body mass, VO, and water consumption were monitored daily (in the morning), and terminal trunk blood samples were taken via decapitation at age 35–38 days after all groups had undergone VO at random estrous cycle stages.
Experiment 3: Metformin Treatment with Earlier Blood Sampling
The experiment was repeated (low-fat control n = 7, high-fat control n = 7, low-fat metformin-treated n = 7, and high-fat metformin-treated n = 8) except that terminal trunk blood samples were taken at Day 27 after all animals in the control high fat-fed group had undergone VO (random estrous cycle stage) and before any other groups underwent VO. Serum insulin and testosterone were assayed.
Hormone Assays
All samples from a single experiment were assayed together. Serum insulin was assayed using an ELISA kit (Crystal Chem, Downers Grove, IL). The intraassay coefficient of variation (CV) was 10% or less, and the sensitivity was 100 pg/ml. Serum testosterone was assayed using a commercial sensitive testosterone kit (TKTT2; Diagnostics Products Inc., Los Angeles, CA). The intraassay CV was 4.3%, and the sensitivity was 15 ng/ml. Serum nonesterified fatty acids (NEFAs) were assayed using a NEFA kit (Wako Diagnostics, Osaka, Japan). The intraassay CV was 0.75%, and the sensitivity was 0.0014 mEq/L.
Statistical Analysis
Data were analyzed with Prism (GraphPad Software, San Diego, CA) using two-way ANOVA, followed by Bonferroni multiple comparisons post hoc test. All values are reported as the mean ± SEM, and P < 0.05 was considered significant.
RESULTS
Androgen Receptor Antagonist Blocks High-Fat Diet-Induced Early VO and Delays Normal VO
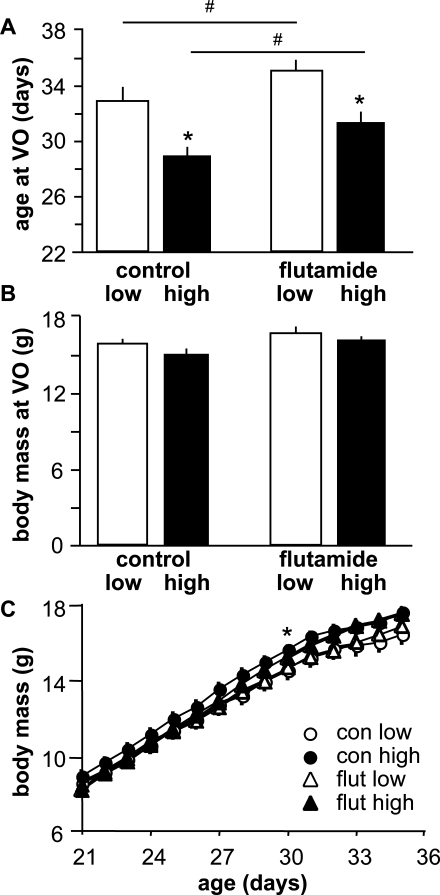
To examine the interaction between dietary fat content and androgen action in the timing of VO, we monitored age at VO in mice fed either a high-fat or low-fat diet in conjunction with flutamide or vehicle treatment from the time of weaning. As reported previously [16], high-fat diet significantly advanced VO in vehicle-treated mice (low-fat n = 8 and high-fat n = 9, P < 0.001) (Fig. 1A). This advancement was blocked by flutamide (n = 11, P > 0.1 vs. vehicle-treated low fat-fed mice). Additionally, flutamide treatment significantly delayed VO in flutamide-treated low fat-fed mice compared with vehicle-treated low fat-fed control mice (n = 11, P < 0.05). There was no difference in body mass at VO (Fig. 1B) or body mass over time (Fig. 1C) among groups, and flutamide treatment had no effect on either growth or appearance of health among the mice. Testosterone levels were not determined because flutamide interferes with the testosterone assay.
FIG. 1.
Blocking androgen receptors delays puberty in both low fat-fed and high fat-fed female mice. A) Mean ± SEM age at VO. B) Mean ± SEM body mass at VO. C) Mean ± SEM body mass over time. Open bars, low-fat diet; black bars, high-fat diet; con, control; flut, flutamide. *P < 0.05 vs. low fat-fed mice in same treatment and #P < 0.05 vs. vehicle-treated controls on the same diet.
Insulin-Sensitizing Treatment Blocks Dietary-Induced Advancement of VO
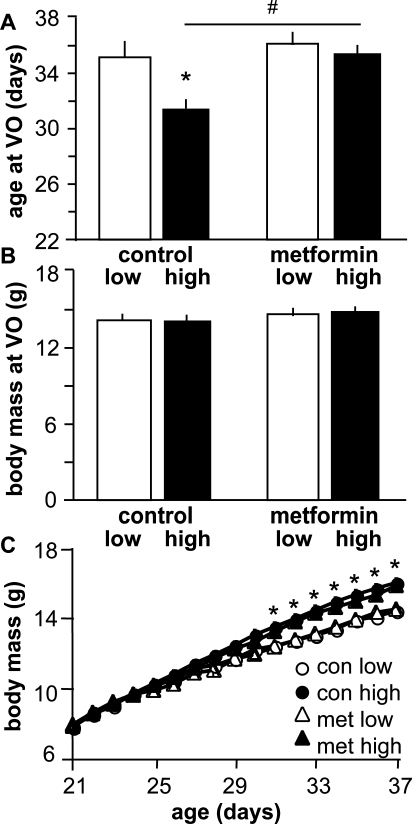
These data indirectly implicate androgen action as a component of the pubertal process in females. We surmised that one possible mechanism by which high-fat diet might increase androgens is by inducing insulin resistance and thus increasing circulating insulin levels via compensatory insulin release [26–28]. To assess the effects of insulin and its possible increase by a high-fat diet, we administered the insulin-sensitizing drug metformin at a dose determined in a pilot study (2.5 mg/ml in drinking water [∼500 mg/kg per day per mouse]) to female weanlings. There were no differences in water consumption over a 24-h period between cages with metformin-treated water vs. untreated water, indicating that treatment did not alter water intake (data not shown). Mice in all treatment groups underwent VO later than in the previous experiment; however, VO was still advanced in high fat-fed controls (n = 11) vs. low fat-fed controls (n = 11) (P < 0.001) (Fig. 2A). This high-fat diet advancement of VO was blocked by metformin (n = 14, P > 0.05 vs. low fat-fed controls). Metformin did not delay VO in low fat-fed mice (n = 13, P > 0.05 vs. low fat-fed control mice). There was no difference in body mass at VO (Fig. 2B), but high fat-fed animals were larger (P < 0.05 [age, 31–37 days]) than low fat-fed animals regardless of treatment. These data suggest that sensitization to insulin blocks the ability of a high-fat diet to advance VO but does not interfere with the timing of VO in the setting of a normal-fat diet.
FIG. 2.
An insulin sensitizer, metformin, delays puberty in high fat-fed female mice. A) Mean ± SEM age at VO. *P < 0.05 vs. low fat-fed mice in same treatment and #P < 0.05 vs. high fat-fed vehicle-treated controls. B) Mean ± SEM body mass at VO. C) Mean ± SEM body mass over time. *P < 0.05 body mass of high fat-fed mice vs. low fat-fed mice. Open bars, low-fat diet; black bars, high-fat diet; con, control; met, metformin.
Endocrine Measures
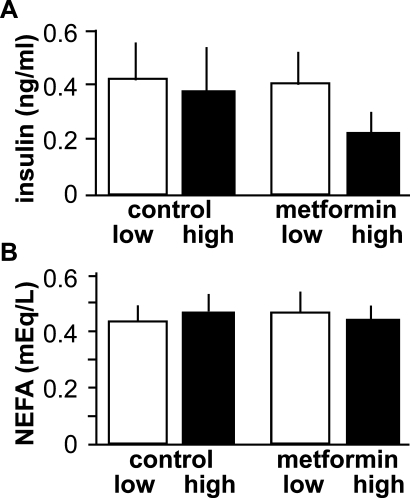
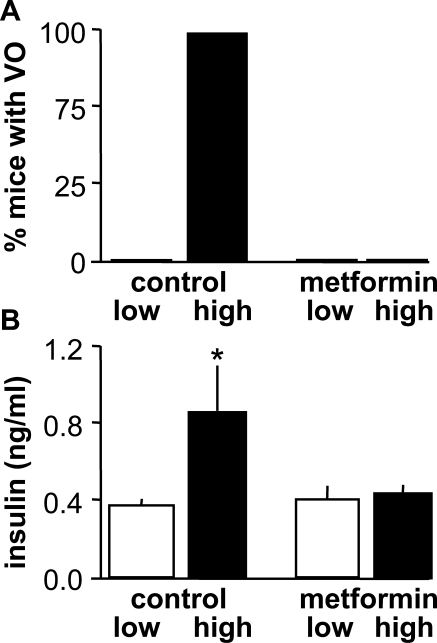
Because of the small size of prepubertal mice and the possible negative impact of stress upon the pubertal process, we first attempted to sample trunk blood at the conclusion of the study after all mice had undergone VO. There were no observed differences in serum insulin or NEFAs among groups (P > 0.1) (Fig. 3). In contrast, when serum was sampled on Day 27, after animals in the vehicle-treated high-fat group had undergone VO but before VO in the other groups, insulin levels were elevated in the high fat-fed animals compared with all other treatments (P < 0.05) (Fig. 4). Testosterone was below the limit of detection for all samples in both groups of animals (data not shown).
FIG. 3.
Neither diet nor treatment with the insulin sensitizer metformin affected serum parameters examined after puberty as assessed by VO. Mean ± SEM serum insulin (A) and NEFAs (B). Open bars, low-fat diet; black bars, high-fat diet.
FIG. 4.
Serum insulin levels are elevated by high-fat diet during the pubertal process. A) Percentage of animals in each group that had undergone VO at the time of blood sampling. B) Mean ± SEM serum insulin levels. Open bars, low-fat diet; black bars, high-fat diet. *P < 0.05.
DISCUSSION
Puberty in many species involves reduced sensitivity of the hypothalamo-pituitary axis to steroid negative feedback [3–5, 29]. We hypothesized that androgens have a role in the reduction of hypothalamic sensitivity to negative feedback during puberty and that high-fat diets advance puberty by elevating the insulin drive to androgen production. We repeated prior observations that high-fat diet advances VO in rodents and extended these findings by demonstrating that treatment with either an androgen receptor antagonist or an insulin sensitizer blocked this diet-induced advancement of VO. Notably, androgen receptor blockade also delayed VO in low fat-fed mice, suggesting that androgen action has a role in the normal pubertal process. Insulin levels were transiently elevated by high-fat diet, potentially providing a source of increased drive for androgen synthesis by this metabolic gonadotropin.
Based on these observations, we propose the following model: androgens are a component of a mechanism causing desensitization of the HPG axis to negative feedback during puberty, leading to eventual acquisition of adult GnRH pulse patterns. High-fat diet causes insulin resistance [30, 31], increasing circulating insulin, which subsequently stimulates premature ovarian androgen production, leading to premature activation of the HPG axis and thus advanced VO. An alternative possibility is that increased androgens serve as precursors for the estrogens that have been shown to initiate or advance VO in rats [32] independent of increased hypothalamo-pituitary drive to steroidogenesis. Although a role for estrogens cannot be excluded, the blockade of the advancement of VO by flutamide indicates an androgen receptor-mediated action.
One argument against this model is that no group differences in testosterone levels were observed after all groups had gone through puberty. Attempts to measure this hormone both when pubertal state (as measured by VO) differed among groups and just after all animals had undergone VO failed because of assay sensitivity limitations. It is possible, however, that biologically meaningful changes in androgen levels occurred below the sensitivity of the assay. In this regard, rat serum testosterone increases progressively preceding VO and approaching first cycle [33, 34]. Rat theca cells develop the capacity for steroidogenesis as early as age 5 days coincident with the ability to respond to LH or human chorionic gonadotropin stimulation [35], suggesting that androgen synthesis is possible well before the onset of the pubertal process.
Androgens, particularly mild elevations, can have stimulatory effects at the hypothalamo-pituitary level. Dihydrotestosterone increases GnRH neuron activity and excitatory neurotransmission to GnRH neurons [22, 36]. In cultured gonadotrophs from adult ovariectomized rats, androgens can exert both negative and positive effects on GnRH-stimulated response, with positive effects on LH-beta (Lhb) mRNA synthesis or resultant LH release when repeated GnRH pulses are given [37, 38]. Another mechanism for stimulatory effects of androgens may be their ability to interfere with negative feedback by other steroids. Androgen-mediated hypothalamic insensitivity to steroid negative feedback occurs in women with polycystic ovary syndrome (PCOS), the most common female fertility disorder. Women with PCOS have elevated LH, hyperandrogenemia, insulin resistance, and high prevalence of metabolic syndrome [39, 40]. In these women, greater concentrations of progesterone are needed to inhibit LH pulse frequency [23]; the sensitivity to progesterone can be restored by treatment with flutamide to block androgen action [41]. Similarly in mice, dihydrotestosterone reduces the ability of progesterone to inhibit GnRH neuron activity [22, 36].
A similar desensitization may occur during the pubertal process. In normal-weight prepubertal and peripubertal adolescent girls, an overnight increase in LH, testosterone, estradiol, and progesterone is observed [42]. The overnight increase in progesterone and estradiol may suppress GnRH pulse frequency during the next day. There is a gradual loss of hypothalamic sensitivity to estrogen (and perhaps progesterone) negative feedback, and LH pulses increase in frequency and begin to occur during the day [1]. Increases in testosterone across puberty may mediate the gradual decrease in hypothalamic sensitivity during the day. In contrast to this situation in normal-weight girls, ovarian steroid levels in obese girls with elevated androgen levels remain tonically high throughout the day-night cycle, and obese girls exhibit no daytime suppression of LH frequency in Tanner stages 3 and 4 [42–44]. Unlike healthy girls, hyperandrogenemic peripubertal girls also exhibit rapid LH pulses during the day, indicating elevated GnRH pulsatility [1, 45, 46]. Together, these data suggest that obese hyperandrogenemic girls are less responsive to steroid negative feedback at the hypothalamus because of a premature, albeit mild, elevation of androgens.
The increase in circulating insulin levels observed in high fat-fed control mice when that group, but no other, had undergone VO provides evidence for endocrine changes that could drive androgen production at that time, as insulin resistance can lead to compensatory increases in insulin release and insulin can increase testosterone biosynthesis [26–28, 47]. Normal puberty is associated with relative insulin insensitivity and an increase in glucose-stimulated insulin release independent of adiposity [48, 49]. High-fat diet may advance the onset of this insulin resistance and downstream effects of insulin resistance. Taken in conjunction with the present observation that androgen receptor blockade could delay VO, a role for androgens in the timing of the onset of puberty appears plausible.
Numerous connections exist between metabolic factors and androgens. Long-term treatment of precocious pubarchal girls (who have elevated androgens, insulin resistance, and increased adiposity) and low-birth-weight girls with metformin lengthened the pubertal growth period, delayed menarche, lowered insulin resistance, and decreased adiposity [50, 51]. This reversal of insulin resistance by metformin may parallel results in the present study. Specifically, the blockade of VO advancement in metformin-treated mice may arise from a similar alleviation of insulin abnormalities, evidenced by the lower insulin levels in metformin-treated vs. control mice on a high-fat diet. This lower insulin level would decrease the drive to ovarian androgen production. Metformin is thought to increase insulin sensitivity by activating AMP kinase, leading to both reduced hepatic gluconeogenesis and increased glucose uptake in skeletal muscle and adipocytes [52]. Metformin may also act at the ovary; it reduces androgen production by cultured human theca cells from both healthy women and women with PCOS [53, 54]. This suggests that in the present experiments metformin could reduce the androgen action that appears critical for high-fat advancement of VO via multiple mechanisms. Metformin action is still incompletely understood. Metformin may also decrease fatty acid oxidation [55], effectively decreasing the amount available for utilization, perhaps providing yet another potential explanation for the ability of metformin to block the advancement of VO in high fat-fed mice.
Many questions remain regarding the role of metabolic changes during the pubertal process and how these signals are communicated to the reproductive system. Adequate energy reserves and the adipocyte-derived hormone leptin are established permissive factors for puberty; without adequate reserves or leptin signaling, puberty does not proceed [56, 57]. However, accumulation of a certain percentage of fat does not necessarily cause puberty, and neither circulating leptin levels nor body fat correlates with the onset of puberty [58]. Ghrelin, an orexogenic peptide secreted by the gastrointestinal tract, decreases GnRH-stimulated LH secretion [59], but administration of ghrelin to peripubertal female rats had no effect on age at VO [52]. Kisspeptin, a strongly activating neuromodulator of GnRH release, can induce puberty in an undernutrition paradigm [60] and has been proposed as another possible link between metabolic status and reproductive competency. Gonadal steroids influence kisspeptin levels and the response of GnRH neurons to kisspeptin, suggesting that this system is a possible point of integration between metabolic and steroid cues in controlling fertility [61, 62].
The availability of metabolic fuels is critical to fertility, with both pharmacologically induced glucoprivation and lipoprivation reducing episodic LH release via central mechanisms [63, 64] and possibly having a role in pubertal timing [11, 65]. In this regard, acute treatment with insulin can inhibit pulsatile LH release by producing hypoglycemia and an accompanying stress response [66, 67]. This is a different role for insulin than that proposed by the present work, in which increased insulin in the circulation is produced in likely a more gradual manner by dietary manipulation and is proposed to serve at least in part as a driver of androgen synthesis. The effects of insulin and androgens on the timing of the onset of puberty need not be mutually exclusive and perhaps instead represent complementary processes for modulating the HPG axis. Rising androgen levels likely have a role in reprogramming the sensitivity of the HPG axis, while the amount of oxidizable fuels throughout the pubertal transition may provide a “gate” to nutritional status for reproductive competency. The gonadotropic effects of insulin on the ovary provide additional evidence for an overlap in the roles of insulin and androgens.
The present investigations into the mechanisms by which high-fat diets affect the timing of female puberty corroborate a number of studies implicating metabolic abnormalities in disruption of normal sexual maturation. The ability of both flutamide and metformin to block the high-fat diet-induced advancement of puberty in female mice, as well as the ability of flutamide to delay puberty in low fat-fed animals, points to a role for insulin and androgens in the timing of normal puberty.
Acknowledgments
We thank Debra Fisher for technical assistance and Kimberly Cox, Christopher McCartney, Justyna Pielecka-Fortuna, Alison Roland, and Jianli Sun for editorial comments.
Footnotes
Supported by The Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health U54 HD28934, and a Summer Research Fellowship from the Endocrine Society to D.S.B.
REFERENCES
- Apter D, Butzow TL, Laughlin GA, Yen SS.Gonadotropin-releasing hormone pulse generator activity during pubertal transition in girls: pulsatile and diurnal patterns of circulating gonadotropins. J Clin Endocrinol Metab 1993; 76: 940–949. [DOI] [PubMed] [Google Scholar]
- Watanabe G, Terasawa E.In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology 1989; 125: 92–99. [DOI] [PubMed] [Google Scholar]
- Foster DL, Ryan KD.Endocrine mechanisms governing transition into adulthood: a marked decrease in inhibitory feedback action of estradiol on tonic secretion of luteinizing hormone in the lamb during puberty. Endocrinology 1979; 105: 896–904. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL.The neural basis of puberty and adolescence. Nat Neurosci 2004; 10: 1040–1047. [DOI] [PubMed] [Google Scholar]
- Andrews WW, Advis JP, Ojeda SR.The maturation of estradiol-negative feedback in female rats: evidence that the resetting of the hypothalamic “gonadostat” does not precede the first preovulatory surge of gonadotropins. Endocrinology 1981; 109: 2022–2031. [DOI] [PubMed] [Google Scholar]
- Foster DL, Jackson LM. Puberty in the Sheep. New York:: Elsevier Academic Press;; 2006. [Google Scholar]
- Ojeda SR, Skinner MK. Puberty in the Rat. New York:: Elsevier Academic Press;; 2006. [Google Scholar]
- Plant TM, Witchel SF. Puberty in Nonhuman Primates and Humans. New York:: Elsevier Academic Press;; 2006. [Google Scholar]
- Kennedy GC, Mitra J.Body weight and food intake as initiating factors for puberty in the rat. J Physiol 1963; 166: 408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch RE, McArthur JW.Menstrual cycles: fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science 1974; 185: 949–951. [DOI] [PubMed] [Google Scholar]
- Wade GN, Schneider JE.Metabolic fuels and reproduction in female mammals. Neurosci Biobehav Rev 1992; 16: 235–272. [DOI] [PubMed] [Google Scholar]
- Frisch RE, Gotz-Welbergen AV, McArthur JW, Albright T, Witschi J, Bullen B, Birnholz J, Reed RB, Hermann H.Delayed menarche and amenorrhea of college athletes in relation to age of onset of training. JAMA 1981; 246: 1559–1563. [PubMed] [Google Scholar]
- Manning JM, Bronson FH.Suppression of puberty in rats by exercise: effects on hormone levels and reversal with GnRH infusion. Am J Physiol 1991; 260: R717–R723. [DOI] [PubMed] [Google Scholar]
- Foster DL, Olster DH.Effect of restricted nutrition on puberty in the lamb: patterns of tonic luteinizing hormone (LH) secretion and competency of the LH surge system. Endocrinology 1985; 116: 375–381. [DOI] [PubMed] [Google Scholar]
- I'Anson H, Manning JM, Herbosa CG, Pelt J, Friedman CR, Wood RI, Bucholtz DC, Foster DL.Central inhibition of gonadotropin-releasing hormone secretion in the growth-restricted hypogonadotropic female sheep. Endocrinology 2000; 141: 520–527. [DOI] [PubMed] [Google Scholar]
- Frisch RE, Hegsted DM, Yoshinaga K.Carcass components at first estrus of rats on high-fat and low-fat diets: body water, protein, and fat. Proc Natl Acad Sci U S A 1977; 74: 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfield RL, Lipton RB, Drum ML.Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics 2009; 123: 84–88. [DOI] [PubMed] [Google Scholar]
- Korhonen S, Hippelainen M, Vanhala M, Heinonen S, Niskanen L.The androgenic sex hormone profile is an essential feature of metabolic syndrome in premenopausal women: a controlled community-based study. Fertil Steril 2003; 79: 1327–1334. [DOI] [PubMed] [Google Scholar]
- Coviello AD, Legro RS, Dunaif A.Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab 2006; 91: 492–497. [DOI] [PubMed] [Google Scholar]
- Barber TM.Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf) 2006; 65: 137–145. [DOI] [PubMed] [Google Scholar]
- Pielecka J, Moenter SM.Effect of steroid milieu on gonadotropin-releasing hormone-1 neuron firing pattern and luteinizing hormone levels in male mice. Biol Reprod 2006; 74: 931–937. [DOI] [PubMed] [Google Scholar]
- Pielecka J, Quaynor SD, Moenter SM.Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology 2006; 147: 1474–1479. [DOI] [PubMed] [Google Scholar]
- Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC.Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 1998; 83: 582–590. [DOI] [PubMed] [Google Scholar]
- Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC.Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 2000; 85: 4047–4052. [DOI] [PubMed] [Google Scholar]
- Nestler JE, Jakubowicz DJ, Falcon de Vargas A, Brik C, Quintero N, Medina F.Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab 1998; 83: 2001–2005. [DOI] [PubMed] [Google Scholar]
- Barbieri RL, Makris A, Randall RW, Daniels G, Kistner RW, Ryan KJ.Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J Clin Endocrinol Metab 1986; 62: 904–910. [DOI] [PubMed] [Google Scholar]
- Chakrabarty S, Miller BT, Collins TJ, Nagamani M.Ovarian dysfunction in peripubertal hyperinsulinemia. J Soc Gynecol Invest 2006; 13: 122–129. [DOI] [PubMed] [Google Scholar]
- Matveyenko AV, Veldhuis JD, Butler PC.Adaptations in pulsatile insulin secretion, hepatic insulin clearance, and {beta}-cell mass to age-related insulin resistance in rats. Am J Physiol Endocrinol Metab 2008; 295: E832–E841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Fernandez DL.Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev 2001; 22: 111–151. [DOI] [PubMed] [Google Scholar]
- Zhao S, Chu Y, Zhang C, Lin Y, Xu K, Yang P, Fan J, Liu E.Diet-induced central obesity and insulin resistance in rabbits. J Anim Physiol Anim Nutr (Berl) 2008; 92: 105–111. [DOI] [PubMed] [Google Scholar]
- Schoelson SE, Lee J, Goldfine AB.Inflammation and insulin resistance. J Clin Invest 2006; 116: 1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez VD, Sawyer CH.Advancement of puberty in the female rat by estrogen. Endocrinology 1965; 76: 1158–1168. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF, Ahmed CE.The onset of female puberty: studies in the rat. Recent Prog Horm Res 1986; 42: 385–442. [DOI] [PubMed] [Google Scholar]
- Andrews WW, Advis JP, Ojeda SR.The first proestrus in the female rat: circulating steroid levels preceding and accompanying the preovulatory LH surge. Proc Soc Exp Biol Med 1980; 163: 305–309. [DOI] [PubMed] [Google Scholar]
- Gelety TJ, Magoffin DA.Ontogeny of steroidogenic enzyme gene expression in ovarian theca-interstitial cells in the rat: regulation by a paracrine theca-differentiating factor prior to achieving luteinizing hormone responsiveness. Biol Reprod 1997; 56: 938–945. [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM.GABAergic integration of progesterone and androgen feedback to gonadotropin-releasing hormone neurons. Biol Reprod 2005; 72: 33–41. [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Waring DW.Androgen modulation of luteinizing hormone secretion by female rat gonadotropes. Endocrinology 1999; 140: 1767–1774. [DOI] [PubMed] [Google Scholar]
- Yasin M, Dalkin AC, Haisenleder DJ, Marshall JC.Testosterone is required for gonadotropin-releasing hormone stimulation of luteinizing hormone-beta messenger ribonucleic acid expression in female rats. Endocrinology 1996; 137: 1265–1271. [DOI] [PubMed] [Google Scholar]
- Franks S, Robinson S, Willis DS.Nutrition, insulin, and polycystic ovary syndrome. Rev Reprod 1996; 1: 47–53. [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN;PCOS/Troglitazone Study Group. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2006; 91: 48–53. [DOI] [PubMed] [Google Scholar]
- Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC.PCOS: evidence that flutamide restores sensitivity of the GnRH pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 2000; 85: 4047–4052. [DOI] [PubMed] [Google Scholar]
- McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S, Marshall JC.Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab 2007; 92: 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ, Foster CM, Marshall JC.The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab 2006; 91: 1714–1722. [DOI] [PubMed] [Google Scholar]
- McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC.Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab 2009; 94: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra S, McCartney CR, Yoo RY, Eagleson CA, Chang RJ, Marshall JC.Progesterone inhibition of the hypothalamic gonadotropin-releasing hormone pulse generator: evidence for varied effects in hyperandrogenemic adolescent girls. J Clin Endocrinol Metab 2005; 90: 2810–2815. [DOI] [PubMed] [Google Scholar]
- Apter D, Butzow T, Laughlin GA, Yen SS.Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. J Clin Endocrinol Metab 1994; 79: 119–125. [DOI] [PubMed] [Google Scholar]
- Bergh C, Carlsson B, Olsson JH, Selleskog U, Hillensjo T.Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil Steril 1993; 59: 323–331. [DOI] [PubMed] [Google Scholar]
- Moran A, Jacobs DR, Steinberger J, Hong CP, Prineas R, Luepker R, Sinaiko AR.Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes 1999; 48: 2039–2044. [DOI] [PubMed] [Google Scholar]
- Caprio S, Plewe G, Diamond MP, Simonson DC, Boulware SD, Sherwin RS, Tamborlane WV.Increased insulin secretion in puberty: a compensatory response to reductions in insulin sensitivity. J Pediatr 1989; 114: 963–967. [DOI] [PubMed] [Google Scholar]
- Ibanez L, Ong K, Valls C, Marcos MV, Dunger DB, de Zegher F.Metformin treatment to prevent early puberty in girls with precocious pubarche. J Clin Endocrinol Metab 2006; 91: 2888–2891. [DOI] [PubMed] [Google Scholar]
- Ibanez L, Valls C, Ong K, Dunger DB, de Zegher F.Metformin therapy during puberty delays menarche, prolongs pubertal growth, and augments adult height: a randomized study in low-birth-weight girls with early-normal onset of puberty. J Clin Endocrinol Metab 2006; 91: 2068–2073. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez R, Martini AC, Navarro VM, Castellano JM, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M.Novel signals for the integration of energy balance and reproduction. Mol Cell Endocrinol 2006; 254–255: 127–132. [DOI] [PubMed] [Google Scholar]
- Mansfield R, Galea R, Brincat M, Hole D, Mason H.Metformin has direct effects on human ovarian steroidogenesis. Fertil Steril 2003; 79: 956–962. [DOI] [PubMed] [Google Scholar]
- Attia GR, Rainey WE, Carr BR.Metformin directly inhibits androgen production in human thecal cells. Fertil Steril 2001; 76: 517–524. [DOI] [PubMed] [Google Scholar]
- Perriello G, Misericordia P, Volpi E, Santucci A, Santucci C, Ferrannini E, Ventura MM, Santeusanio F, Brunetti P, Bolli GB.Acute antihyperglycemic mechanisms of metformin in NIDDM: evidence for suppression of lipid oxidation and hepatic glucose production. Diabetes 1994; 43: 920–928. [DOI] [PubMed] [Google Scholar]
- Zeinoaldini S, Swarts JJ, Van de Heijning BJ.Chronic leptin infusion advances, and immunoneutralization of leptin postpones pubertal onset in normally fed and feed restricted female rats. Peptides 2006; 27: 1652–1658. [DOI] [PubMed] [Google Scholar]
- Mounzih K, Lu R, Chehab FF.Leptin treatment reduces the sterility of genetically obese ob/ob males. Endocrinology 1997; 138: 1190–1193. [DOI] [PubMed] [Google Scholar]
- Bronson FH.Puberty in female mice is not associated with increases in either body fat or leptin. Endocrinology 2001; 142: 4758–4761. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez R, Tena-Sempere M, Aguilar E, Pinilla L.Ghrelin effects on gonadotropin secretion in male and female rats. Neurosci Lett 2004; 362: 103–107. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 2005; 146: 3917–3925. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M.Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 2004; 145: 4565–4574. [DOI] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM.Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 2008; 149: 1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani S, Bucholtz DC, Murahashi K, Estacio MA, Tsukamura H, Foster DL, Maeda KI.Reduction of glucose availability suppresses pulsatile luteinizing hormone release in female and male rats. Endocrinology 1996; 137: 1166–1170. [DOI] [PubMed] [Google Scholar]
- Sajapitak S, Iwata K, Shahab M, Uenoyama Y, Yamada S, Kinoshita M, Bari FY, I'Anson H, Tsukamura H, Maeda KI.Central lipoprivation-induced suppression of luteinizing hormone pulses is mediated by paraventricular catecholaminergic inputs in female rats. Endocrinology 2008; 149: 3016–3024. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Wade GN.Availability of metabolic fuels controls estrous cyclicity of Syrian hamsters. Science 1989; 244: 1326–1328. [DOI] [PubMed] [Google Scholar]
- Caraty A, Grino M, Locatelli A, Guillaume V, Boudouresque F, Conte-Devolx B, Oliver C.Insulin-induced hypoglycemia stimulates corticotropin-releasing factor and arginine vasopressin secretion into hypophysial portal blood of conscious, unrestrained rams. J Clin Invest 1990; 85: 1716–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MD, Ordog T, O'Byrne KT, Goldsmith JR, Connaughton MA, Knobil E.The insulin hypoglycemia-induced inhibition of gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: roles of vasopressin and corticotropin-releasing factor. Endocrinology 1996; 137: 2012–2021. [DOI] [PubMed] [Google Scholar]