Abstract
The study of alternative model organisms has yielded tremendous insights into the regulation of behavioral and physiological traits not displayed by more widely used animal models, such as laboratory rats and mice. In particular, comparative approaches often exploit species ideally suited for investigating specific phenomenon. For instance, comparative studies of socially monogamous prairie voles and polygamous meadow voles have been instrumental toward gaining an understanding of the genetic and neurobiological basis of social bonding. However, laboratory studies of less commonly used organisms, such as prairie voles, have been limited by a lack of genetic tools, including the ability to manipulate the genome. Here, we show that lentiviral vector-mediated transgenesis is a rapid and efficient approach for creating germline transgenics in alternative laboratory rodents. Injection of a green fluorescent protein (GFP)-expressing lentiviral vector into the perivitelline space of 23 single-cell embryos yielded three live offspring (13 %), one of which (33%) contained germline integration of a GFP transgene driven by the human ubiquitin-C promoter. In comparison, transfer of 23 uninjected embryos yielded six live offspring (26%). Green fluorescent protein is present in all tissues examined and is expressed widely in the brain. The GFP transgene is heritable and stably expressed until at least the F(2) generation. This technology has the potential to allow investigation of specific gene candidates in prairie voles and provides a general protocol to pursue germline transgenic manipulation in many different rodent species.
Keywords: behavior, central nervous system, lentiviral vector, lentivirus, prairie vole, trangenesis, transgenic
Lentiviral vector-mediated gene transfer into single-cell embryos results in germline transgenesis in an alternative laboratory rodent species.
INTRODUCTION
The widespread use of the mouse as a model organism has been greatly facilitated by the ease with which its genome can be manipulated. Insertion of foreign transgenes into the mouse genome has become a routine experimental technique [1] and has resulted in notable advances in myriad fields. Yet, transgenic mice also have numerous limitations, especially in relation to behavioral research. Multiple mouse strains are visually impaired [2] and others, including the commonly used C57BL/6 strain, show age-related progressive hearing loss and cochlear degeneration [3, 4]. Another commonly used strain, BALB/c, exhibits reduced corpus collosum volume, which has been linked with decreased sociability [5, 6]. Likewise, a majority of embryonic stem (ES) cell lines used to generate targeted transgenics are derived from the 129 mouse strain, yet 129/Sv mice are impaired in many learning tasks [2]. Even in cases where the aforementioned abnormalities do not hinder behavioral assessment, there are numerous physiological and behavioral traits that are simply not displayed by mice but remain relevant to human health and disease.
In comparison, less commonly used laboratory rodents, such as hamsters, wild mouse species, and voles, are often outbred, free of physiological abnormalities such as blindness or deafness, and exhibit traits not displayed by inbred laboratory mouse strains. For instance, Syrian hamsters (Mesocricetus auratus) [7, 8] have a stereotyped and robust form of territorial aggression, and the comparative approach has been used in various different wild mouse species to study traits ranging from resistance to neurotoxins (Onychomys spp.) [9] to “singing” phenotypes (Scotinomys spp.) [10, 11]. Socially monogamous prairie voles (Microtus ochragaster) remain a premier model for understanding the genetics and neurobiology regulating social bonding and other behaviors associated with monogamy that are not exhibited by polygamous laboratory mouse and rat species [12, 13]. However, study of these organisms has been limited, in part, by a lack of transgenic methodology with which to directly address the role of candidate genes in modulating their various phenotypes of interest.
Traditionally, insertion of foreign DNA into a genome required large numbers of harvested embryos and invasive injection of DNA constructs into the pronucleus. However, recent advances in viral vector technologies have created opportunities to generate transgenic organisms with relatively few embryos and avoid injection into the embryo itself. This approach has successfully been employed in both commonly used laboratory organisms, including mice and rats, as well as a variety of less common organisms spanning agricultural and health research [14]. For instance, lentiviral-mediated transgenesis has now been achieved in monkeys as well as an array of agricultural animals, including pigs, sheep, goats, and cattle [15–17]. Broadly, such technological advances have the potential to provide mechanisms to directly assess gene function via genomic manipulation in a wide array of species, including alternative laboratory rodents. However, to date, there have been no successful attempts to generate germline transgenics in these types of laboratory rodent model species.
With the general goal of establishing a protocol to introduce foreign genes into rodent species other than laboratory rats and mice, we report here the first germline transgenic prairie vole. For this initial experiment, we chose a widely used visual reporter transgene, the jellyfish-derived green fluorescent protein (GFP) under the control of the human ubiquitin-C promoter [18]. Prairie and other vole species have long been used as models for studying questions spanning ecology, disease, and, most recently, complex social behavior and monogamy. Within prairie voles, the ability to generate transgenic animals will allow us to directly investigate hypotheses regarding the molecular physiology of complex sociobehavioral traits. Furthermore, because our laboratory maintains an outbred prairie vole colony and routinely adds wild animals to our stock, this is the first demonstration of transgenesis in a virtually wild rodent species. The ability to rapidly and efficiently create transgenic animals will likely greatly enhance the power of the comparative approach and the scientific use of alternative laboratory species.
MATERIALS AND METHODS
Production of Lentivirus
We used a lentiviral vector containing the GFP coding sequence driven by the human ubiquitin-C promoter, referred to as pLVU-GFP. Detailed information on this construct and production of this vector is available from Lois et al. [19]. The same vector has been used previously to generate transgenic mice and monkeys [16, 18]. Briefly, viral vector was cotransfected with plasmid p(Δ)8.9 and pVSVG into Invitrogen HEK293FT packaging cells. Supernatant was collected and concentrated by ultracentrifugation. The resulting concentration of infectious viral particles (titer) was determined by expression of GFP in HEK293FT cells plated at a density of 2.5 × 105 per well in a six-well plate. Titer was determined by multiplying the number of GFP-positive cell colonies by the dilution factor and presented by colony-forming units (cfu)/ml.
We aliquoted high-titer virus (5 × 109 cfu/ml; 3 μl per tube) and stored it at −80°C until use. The titer should not have decreased for at least 1 yr at −80°C, and the titer after thawing is believed to be higher than 1 × 109 cfu/ml based on previous tests in cell culture and transgenic mouse studies.
Generation of Transgenic Prairie Voles
Prairie vole colony maintenance.
All animals were bred in our in house colony and maintained on a 14L:10D cycle with food and water supplied ad libitum. Animals were between 2 mo and 1 yr old at the time of use. Sexually naïve females were housed two or three per cage, whereas sexually experienced individuals were singly housed to avoid aggressive encounters. All procedures were reviewed and approved by the Emory Institutional Animal Care and Use Committee and were conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the National Research Council.
Production of sterile stud males.
A cohort of adult male prairie voles were vasectomized and used to induce pseudopregnancy. An incision was made at the caudal end of the abdominal cavity, and the vas deferens were located, tied off, and then severed. Males were allowed to recover for 2 wk and then cohabitated with a female for 4 wk to ensure sterility. Only confirmed sterile males were used to induce pseudopregnancy. Vasectomized males were used in multiple experiments and retired once they reached 1 yr of age.
Harvesting single-cell embryos.
Prairie voles exhibit induced estrus, and exposure to male olfactory cues (e.g., urine) is necessary to induce behavioral receptivity and follicle development. Ovulation then occurs only if mating takes place [20–22]. To induce receptivity while controlling for initiation of mating and ovulation, pairs consisting of a female and an experienced stud male were placed in cages containing a perforated divider. After 44 h of separated cohabitation, the divider was removed, and time of initial mating was recorded. Any pairs that failed to mate within 2 h of removal of the divider were eliminated from the study.
Females were killed using CO2, and their oviducts were removed into M2 media (Millipore, Billerica, MA) 22–23 h after initiation of mating. Under a stereoscope, a 32-gauge needle was placed into the infundibulum, and oviducts were flushed with ∼0.3 ml of M2 media. Harvested embryos were stored in M16 media (Millipore) microdrops under mineral oil at 37°C and 5% CO2.
Production of psuedopregnant surrogates.
Surrogate females consisted of experienced mothers who had successfully raised at least one litter. These females were placed into divided cages with a vasectomized male at the same time that pairs were caged for embryo harvest. The divider was removed after females in the embryo harvest group had mated, typically after 46–48 h of separated cohabitation. Mating was confirmed visually, and only females who mated received transferred embryos.
Perivitelline injection of lentiviral vector and embryo transfer.
High-titer lentiviral vector (∼1 × 109 infectious units/ml) was mixed with polybrene for a final concentration of 8 μg/ml, and approximately 100–200 pl of the vector mixture was injected into the perivitelline space using a 1- to 2-μm micropipette (inner diameter, see Fig. 1). Injected embryos were transferred to both oviducts of psuedopregnant females via oviduct puncture (three to four embryos per side). After embryo transfer, surrogate females were placed back in the cage with their vasectomized male partner. Experienced prairie vole mothers within our colony routinely give birth to three to seven offspring, with a typical gestation period of 21–23 days. This is similar to previously reported litter sizes and gestation periods for this species [23–25]. We checked surrogate females for pups starting 18 days after embryo transfer. All pups were born 22–23 days after transfer. Resulting offspring were investigated visually using a handheld Sky-blue II epifluorescent light (Youlum Inc., Taiwan) for preliminary detection of GFP expression (Fig. 2).
FIG. 1.
Single-cell embryos were harvested from pregnant female prairie voles, and lentiviral vector was injected into the perivitelline space (A). GFP present in the viral preparation was detectable within the embryos after injections. Brightfield image of embryos is shown in B, and GFP fluorescent filter is shown in C. Bar = 10 μm.
FIG. 2.
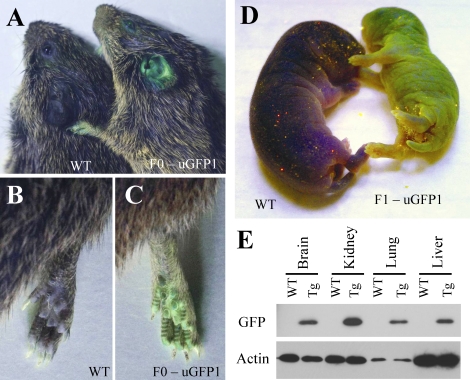
The founder F(0) animal displays GFP expression within the skin (A–C, shown as an adult). GFP is also expressed in the skin of F(1) offspring (D, 1-day-old littermate pups shown). Western blot analysis of internal tissues shows widespread expression of GFP (E).
Genotyping by PCR
Genomic DNA was obtained by incubating ear-punch tissue in lysis buffer (5 mM Tris, pH 8.8; 100 μM ethylenediaminetetraacetic acid [EDTA]; 0.5% Tween-20; and 0.003% proteinase K). Enzyme activity was heat inactivated, and resulting DNA-containing solution was diluted 1:10. Transgene presence was assayed using forward primer 5′-caagcagggagctagaacgattc and reverse primer 5′-caagaacccaaggaacaaagctcc with the following conditions: 95°C for 10 min, 30 times (95°C for 30 sec, 55°C for 40 sec, 72°C for 50 sec), 72°C for 10 min, and 4°C hold. The resulting product was separated on a 1.8% gel, and the presence of a 422-bp fragment indicated amplification of the lentiviral backbone.
Southern Blot Confirmation and Determination of Integration Number
Genomic DNA was purified from ∼5 mm of tail using the Gentra Puregene kit (Qiagen, Valencia, CA). Briefly, tails were incubated overnight in lysis buffer with proteinase K at 55°C, treated with RNase, and purified via ethanol precipitation. Genomic DNA (8 μg) was digested overnight with BamHI, and resulting fragments were separated on a 1% agarose gel. BamHI cuts once within the integrated provirus between the ubiquitin promoter and the GFP coding region (Fig. 3). DNA was then transferred to a Zeta-probe GT membrane (Bio-Rad, Hercules, CA) using standard neutral transfer conditions. DNA was fixed to the membrane via ultraviolet cross-link and rinsed in 2× saline-sodium citrate (SSC; 0.3 M NaCl and 0.03 M sodium citrate, pH 7.0). Transferred blots were dried and stored at room temperature until probed.
FIG. 3.
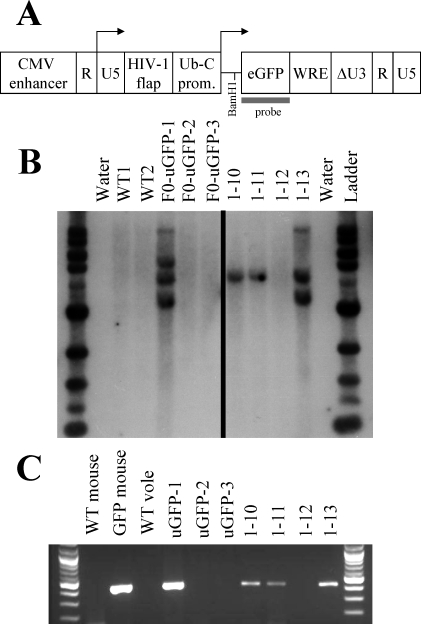
The GFP-expressing lentiviral construct, pLVU-GFP, is shown in A as adapted from Lois et al. [19], with permission from the American Association for the Advancement of Science. Location of the probe and appropriate restriction site for Southern blot analysis are shown. B) Southern blot analysis of all offspring born in the F(0) cohort reveals three integration sites of the GFP transgene within a single founder male (F(0)-uGFP-1), as indicated by the presence of three different-sized bands (left side). F(1) littermate offspring 1–10, 1–11, and 1–13, sired by this founder, inherited copies of the GFP gene, whereas littermate 1–12 did not (right side). C) Polymerase chain reaction analysis also indicates the presence of the GFP transgene in a single founder animal and in F(1) animals 1–10, 1–11, and 1–13 but not 1–12.
The GFP coding region was gel purified from XbaI-digested pLVU-GFP plasmid and used as template DNA in a random primer-labeling reaction using the Stratagene Prime It-II kit (Applied Biosystems, Foster City, CA). The resulting probe was purified with a Sephadex G-50 column (GE Healthcare, Piscataway, NJ) and hybridized to the blotted membrane in Rapid-Hyb Buffer (GE Healthcare) at 65°C overnight. The membrane was rinsed repeatedly with washes containing increasing stringency concentrations of SSC containing 0.1% SDS until radioactive signal was detectable by Geiger counter selectively within regions of the blot containing DNA. The resulting hybridized blot was exposed to film overnight. Films were scanned and adjusted for contrast and brightness using Adobe Photoshop.
Western Blot Assessment of GFP Expression
GFP expression was examined using Western blot analysis of proteins extracted from various tissues. Total protein was extracted from liver, brain, lung, and kidney via homogenization in lysis buffer (10 mM HEPES, 50 mM NaCl, 5 mM EDTA, and 1% Triton-X). Protein concentration was determined by bicinchoninic acid assay, and 5 μg of denatured protein extract was loaded into NuPAGE 4%–12% Bis-Tris gel (Invitrogen, Carlsbad, CA). After separation by electrophoresis, proteins were transferred to a nitrocellulose membrane. The blot was blocked and incubated in primary antibody specific for GFP (1:1000; A-11122; Invitrogen). Primary antibody reactivity was detected by incubation in secondary antibody (1:10 000; 9169; Sigma) and detected using Pierce SuperSignal West Pico Chemiluminescence (Pierce, Rockford, IL) followed by film exposure. Blots were stripped with ReStore Plus stripping solution (Pierce) for 15 min and incubated in a horseradish peroxidase-conjugated primary antibody specific to actin (1:5000; Ab20272–100; Abcam) and detected with the SuperSignal West Pico Chemiluminescence prior to film exposure.
Immunohistochemical Investigation of Transgene Expression
Animals were killed using CO2 asphyxiation, and tissue was immediately harvested and placed in 4% paraformaldehyde overnight. After fixation, the tissue was stored in 30% sucrose until sectioning. Tissues were cut into a 1:6 series in 30-μm sections using a freezing microtome and were stored free-floating in cryoprotectant solution at −20°C until immunohistological processing. Sections were removed from cryoprotectant and rinsed in PBS (pH 7.4). The sections were reacted in 0.5% hydrogen peroxide in PBS for 30 min at room temperature to remove residual blood, rinsed, and then incubated in primary antibody directed against GFP (A-11122; Invitrogen) in PBS containing 1% Triton-X overnight at room temperature with a working antibody concentration of 1:100 000. Sections were then rinsed in PBS and incubated in secondary antibody (Vectastain PK6101; Vector Laboratories, Burlingame, CA) for 1 h according to the manufacturer's recommendations. Excess secondary antibody was removed by rinsing in PBS, and the tissue was incubated in avidin-biotin peroxidase complex (ABC Elite Kit PK-6100; Vector Laboratories) at a concentration of 1:200. After rinsing in PBS, GFP-antibody complex was visualized as a brown reaction product using diaminobenzidine containing 0.08% hydrogen peroxide in Tris buffer. The reaction was terminated after 20 min by rinsing in PBS buffer. Sections were mounted out of saline onto gelatin-subbed slides and air dried. Sections were then dehydrated in a series of graded alcohols, cleared in zylene, and coverslipped using Cytoseal XYL (Richard and Allan Scientific).
Assessment of Transgene Transmission Across Multiple Generations
Our transgenic founder, F(0)-uGFP1, was mated to a wild-type (WT) female to produce F(1) pups. After producing three litters with a WT mate, the same founder was paired with one of his female offspring to produce offspring with multiple copies of the GFP transgene. We labeled these offspring F(1.5). Two F(1) males (1–10 and 1–11; see Fig. 3) were also paired with WT females to establish an F(2) generation. Both of these males carry a single copy of the GFP transgene, and thus 50% of their offspring should carry the transgene. F(1), F(1.5), and F(2) offspring were investigated with PCR and confirmed visually for GFP expression to determine rates of transgene heritability. The F(2) generation was also weighed at weaning on Postnatal Day 21 to investigate potential gross developmental differences among the GFP offspring compared with their WT littermates.
RESULTS
Generation of Transgenic Prairie Voles
Germline transgenic voles were produced by infecting single-cell embryos with high-titer lentiviral vector (Figs. 1 and 2). A total of 58 embryos were harvested in two experiments from 15 females (3.8 embryos per female). This is consistent with a normal litter size of three to five pups for primiparous prairie voles. We implanted 23 uninjected and 23 virally injected embryos into psuedopregnant surrogate females. Of these, six offspring were born from uninjected embryos (26%) and three from injected embryos (13%). Of the three injected embryos, a single F(0) animal (33%) carried genomically integrated copies of the GFP transgene (Figs. 2 and 3). A subsequent study employing the same methods but using a different transgene yielded one transgenic out of 10 offspring (data not shown). To test for germline integration and heritability of transgene expression, we mated the F(0) transgenic with a WT female. As expected, a portion of the resulting offspring inherited the GFP transgene (see below, and Figs. 2 and 3 and Table 1).
TABLE 1.
Multigenerational transgene heritability.
GFP transgene incorporation into the prairie vole genome and its heritability were further verified via both PCR and Southern blot methods (Fig. 3). The PCR from genomic DNA indicates the presence of lentiviral backbone DNA in our transgenic F(0) and F(1) animals but not within WT animals. Our Southern blot probe for the GFP coding region hybridized to three different bands within the separated, BamHI-digested genomic DNA of the F(0) founder, suggesting three separate transgene integration sites. F(1) littermate offspring sired by this founder inherited copies of the GFP gene. Visual inspection of GFP in these offspring did not reveal appreciably different levels of peripheral green fluorescence in offspring with two copies of the transgene (1–13) compared with those with only one copy (1–10 and 1–11). Although this experiment serves as proof of principle that production of transgenic voles is possible, it will be important to determine potential copy number and integration site effects for future transgenes.
Verification of GFP Expression in F(0) and F(1) Transgenic Prairie Voles
Given that the GFP transgene is under the control of the ubiquitin promoter, we expected widespread expression of GFP. Preliminary visual analysis of GFP fluorescence in skin showed external expression in both pups and adults (Fig. 2). Both Western blot and immunohistological detection of GFP revealed its widespread internal expression in various tissues in F(1) offspring (Figs. 2, 4, and 5). These findings suggest that the ubiquitin promoter is a useful promoter for driving transgene expression in prairie voles and that GFP retains its native properties when expressed in the prairie vole. From a functional standpoint, transgene expression in some of the key brain regions implicated in the social behavior of this species, including the prefrontal cortex, nucleus accumbens, and lateral septum, further suggests that transgenesis will be a useful tool in investigating the genetic basis of social behavior (Fig. 5).
FIG. 4.
Immunohistological analysis shows widespread expression of GFP in adult lung (top row), liver (middle row), and kidney (bottom row) of F(1) GFP transgenics (Tg) compared with WT voles. The different tissues displayed varying levels of background staining; however, the staining was consistently more intense in the transgenic animal compared with the WT. Bar = 100 μm.
FIG. 5.
Immunohistochemical analysis also reveals widespread GFP expression in the brains of an adult transgenic F(1) prairie vole. Higher-magnification images are shown for the prefrontal cortex (top row), nucleus accumbens (middle row), and lateral septum (bottom row). These brain regions are involved in regulating the complex social behavior of this species and represent future anatomical targets for transgenic technology in prairie voles. PFC, prefrontal cortex; NAcc, nucleus accumbens; CP, caudate putamen; ac, anterior commisure; LS, lateral septum. Bar = 100 μm.
Transgene Heritability Across Multiple Generations
To assess the transmission of the GFP transgene across multiple generations, we produced F(1), F(1.5), and F(2) offspring (Table 1). The transgene was heritable in all three sets of offspring as determined by both PCR and visual investigation. Average litter size was similar to that observed in our WT colony at the same time (Table 1). Rates of transgene transmission were significantly lower than expected by chance in the F(1) and F(1.5) generations (F(1) generation, n = 13, χ2 = 5.3, P = 0.021; F(1.5), n = 29, χ2 = 8.6, P = 0.0034; Table 1). Both of these offspring cohorts were sired by the founder male, and this may indicate germline mosaicism (i.e., incomplete lentiviral transduction of the germline progenitor cells during development). However, heritability of GFP in these offspring serves as proof of principle that transgenes can be inserted into the prairie vole genome.
The heritability of the transgene in the F(2) generation, where germline mosaicism is highly unlikely, was not statistically different from the expected 50% transmission rate (n = 33, χ2 = 1.13, P = 0.29; Table 1). Furthermore, weanling weight assessed on Postnatal Day 21 did not differ significantly according to genotype in the F(2) generation (n = 10 transgene and 17 WT; transgene weight was 14.7 g, WT weight was 16.6 g; t test, P = 0.20). These offspring all carried one copy of the transgene. The potential effects of carrying multiple copies of the transgene were not assessed.
DISCUSSION
Here, we demonstrate for the first time that lentiviral-mediated gene transfer is a viable and effective technique for generating germline transgenic animals when working with alternative rodent species. The potential uses and implications for this technology are widespread and will allow researchers to address a variety of questions unanswerable with laboratory rat and mouse strains. For instance, within prairie voles, we anticipate that this technology will provide a powerful tool for directly testing the behavioral functions of various genes and provide valuable resources for understanding the neurogenetic mechanisms governing complex social behaviors.
As a species, prairie voles exhibit complicated reproductive physiology and behavior that would make transgenic production using pronuclear injection a potentially daunting task. Like many other rodents, prairie voles exhibit an alternative reproductive cycle, and females must be induced into behavioral estrus, requiring complicated experimental manipulations to produce single-cell embryos from multiple females at once. Despite administering multiple superovulation hormone protocols that work in other rodents, we did not find a hormone regimen that increased our embryo harvest (data not shown). Even in other vole species where superovulation has been reported, the results are highly variable and generally require unusual hormone doses [26–28]. Additionally, the success rate of births from transplanted embryos in this study was relatively low: 26% and 13% for uninjected and injected embryos, respectively. For prairie voles, birth rates increase if the male remains present during pregnancy [23]. Therefore, we left our sterile stud males with the surrogate females for at least the first 2 wk of pregnancy. As a result, sterile stud males could not be used more than once every 3 wk. These factors combine to make it difficult to obtain more than 50 embryos in a single experiment. However, because of the advantages of using lentiviral-mediated gene transfer, we were able to produce a transgenic line despite these challenges. We have now created an additional transgenic prairie vole line carrying a different transgene using this same approach. In sum, this suggests that lentiviral transgene delivery is a viable technology for a wide variety of rodent species despite reproductive and physiological variation.
Applications of Lentiviral Transgenics
Lentiviruses remain a promising technology for performing a wide variety of transgenic manipulations in alternative model species. Although the primary limitation of working with viral vectors is a restricted insert size (10 kb), there remain a wide variety of potential experiments that can be performed even within this limit [14]. Using tissue- or cell type-restricted promoters, cDNAs of interest can be expressed in a highly selective fashion. Likewise, use of recombinase systems or drug-sensitive promoters can be employed to regulate the temporal and localized expression of a transgene [29]. Alternatively, interfering RNAs, known as siRNAs, targeting the degradation of a specific mRNA can be inserted into the genome to create knockdown animals with decreased expression of a single protein [30, 31]. This may prove to be an especially powerful technique. Within mice, transgenic siRNAs can result in nearly total reduction of protein levels, and in many cases this technique may circumvent the need to generate targeted gene knockouts [32–34]. It can also be combined with the previously mentioned approaches to target mRNA knockdown with temporal and/or regional specificity [31, 35, 36].
Lentiviral transgenesis often results in founder animals that carry multiple, randomly integrated copies of the transgene [18]. As a result, transgene integration can potentially cause insertional mutations. Although this represents a challenge in many experiments, it also creates an opportunity for insertion mutagenesis screening. Such random integrations also mean that this technique can be optimized to generate subtly different lines using the same transgene. By breeding for individual insertions, we may be able to create lines with varying but reproducible levels of transgene expression according to integration site effects. This may prove to be most useful with siRNA technologies where different insertion sites may yield different levels of knockdown. In sum, through creative and insightful use of genetic tools previously developed in mice and other commonly used genetic model organisms, lentiviral transgenesis can be used to manipulate genes in a wide variety of ways in many organisms.
Within prairie voles, we anticipate that some of the most useful applications of this technology will be to alter gene expression in the brain via a combination of the methods mentioned above. Pharmacological and other manipulations have implicated a variety of brain regions and molecules in the modulation of social behavior, and we have been able to establish that it is possible to express a genomically integrated transgene within these regions (Fig. 5). Transgenic manipulation of specific genes and brain regions has the potential to directly identify brain mechanisms mediating social behavior and elucidate the mechanisms responsible for generating variation in social traits.
Previously, region-specific infusion of viral vectors in alternative model species has been an informative tool for dissecting the neurobiology of behavior. For instance, in prairie voles, increased expression of virally delivered vasopressin V1a receptor within the ventral pallidum increases affiliative behaviors in nonmonogamous meadow voles (Microtus pennsylvanicus) [37]. Although this approach has been very informative, it has its limitations. Localized viral injection results in heterogenous infection of cell populations, which introduces experimental variability. Germline transgenesis, in comparison, provides uniform integration of foreign DNA across cells and across generations. This reproducibility facilitates investigation of the physiological mechanisms underlying phenotypic changes due to transgene presence. For instance, through reproducible genomic manipulation of V1a receptor distribution in voles, we will be able to identify more easily the molecular and electrophysiological mechanisms underlying resulting changes in affiliative social behaviors.
In sum, we feel that the extension of transgenic technologies to less commonly used rodent species has the potential to overcome a previous limitation of working with these species. As a result, we will be able to directly explore the genetic components of traits not displayed by mice. We hope that through the adoption of this and other techniques, scientific communities will be able to reshape the way we define a model species, ultimately choosing the right organism to answer a question rather than fitting a question to an existing model.
Acknowledgments
We would like to thank Jeff Roffman for whole-animal GFP photography. The pLVU-GFP vector was originally a gift from Carlos Lois to Anthony Chan. We thank Heather Ross, Catherine Barrett, and Meera Modi for technical assistance with immunohistochemistry, genotyping, and Western blot analysis.
Footnotes
Supported by National Institutes of Health (NIH) grant nos. MH064692 to L.J.Y. and RR00165 to Yerkes National Primate Research Center, and National Science Foundation Science and Technology Centers IBN-9876754. Z.R.D. is supported by a Howard Hughes Predoctoral Fellowship, and A.W.S.C. is supported by the National Center for Research Resources at the NIH (RR018827-04).
REFERENCES
- Palmiter RD, Chen HY, Brinster RL.Differential regulation of metallothionein-thymidine kinase fusion genes in transgenic mice and their offspring. Cell 1982; 29: 701–710. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997; 132: 107–124. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Noben-Trauth K.Strain background effects and genetic modifiers of hearing in mice. Brain Res 2006; 1091: 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JG, Parrish JL, Hughes LF, Toth LA, Caspary DM.Hearing in laboratory animals: strain differences and nonauditory effects of noise. Comp Med 2005; 55: 12–23. [PMC free article] [PubMed] [Google Scholar]
- Brodkin ES.BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res 2007; 176: 53–65. [DOI] [PubMed] [Google Scholar]
- Wahlsten D.Heritable aspects of anomalous myelinated fibre tracts in the forebrain of the laboratory mouse. Brain Res 1974; 68: 1–18. [DOI] [PubMed] [Google Scholar]
- Albers HE, Bamshad M.Role of vasopressin and oxytocin in the control of social behavior in Syrian hamsters (Mesocricetus auratus). Prog Brain Res 1998; 119: 395–408. [DOI] [PubMed] [Google Scholar]
- Wommack JC, Delville Y.Stress, aggression, and puberty: neuroendocrine correlates of the development of agonistic behavior in golden hamsters. Brain Behav Evol 2007; 70: 267–273. [DOI] [PubMed] [Google Scholar]
- Rowe AH, Rowe MP.Physiological resistance of grasshopper mice (Onychomys spp.) to Arizona bark scorpion (Centruroides exilicauda) venom. Toxicon 2008; 52: 597–605. [DOI] [PubMed] [Google Scholar]
- White SA, Fisher SE, Geschwind DH, Scharff C, Holy TE.Singing mice, songbirds, and more: models for FOXP2 function and dysfunction in human speech and language. J Neurosci 2006; 26: 10376–10379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps S. The integrative biology of social behavior: rodents as extended model systems. National Science Foundation Workshop Report 2006.
- Lim MM, Young LJ.Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav 2006; 50: 506–517. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL.Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev 1995; 19: 303–314. [DOI] [PubMed] [Google Scholar]
- Park F.Lentiviral vectors: are they the future of animal transgenesis? Physiol Genomics 2007; 31: 159–173. [DOI] [PubMed] [Google Scholar]
- Whitelaw CB, Lillico SG, King T.Production of transgenic farm animals by viral vector-mediated gene transfer. Reprod Domest Anim 2008; 43(suppl 2):355–358. [DOI] [PubMed] [Google Scholar]
- Yang SH, Cheng PH, Banta H, Piotrowska-Nitsche K, Yang JJ, Cheng EC, Snyder B, Larkin K, Liu J, Orkin J, Fang ZH, Smith Y, et al. Towards a transgenic model of Huntington's disease in a non-human primate. Nature 2008; 453: 921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AW, Chong KY, Martinovich C, Simerly C, Schatten G.Transgenic monkeys produced by retroviral gene transfer into mature oocytes. Science 2001; 291: 309–312. [DOI] [PubMed] [Google Scholar]
- Yang SH, Agca Y, Cheng PH, Yang JJ, Agca C, Chan AW.Enhanced transgenesis by intracytoplasmic injection of envelope-free lentivirus. Genesis 2007; 45: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D.Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 2002; 295: 868–872. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Wolf KN, Sprangel ME, Rall WF, Wildt DE.Prolonged mating in prairie voles (Microtus ochrogaster) increases likelihood of ovulation and embryo number. Biol Reprod 1999; 60: 756–762. [DOI] [PubMed] [Google Scholar]
- Richmond M, Conaway CH.Management, breeding, and reproductive performance of the vole, Microtus ochrogaster, in a laboratory colony. Lab Anim Care 1969; 19: 80–87. [PubMed] [Google Scholar]
- Gray GD, Dewsbury DA.A quantitative description of copulatory behavior in prairie voles (Microtus ochrogaster). Brain Behav Evol 1973; 8: 426–452. [PubMed] [Google Scholar]
- McGuire B, Russell KD, Mahoney T, Novak M.The effects of mate removal on pregnancy success in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus). Biol Reprod 1992; 47: 37–42. [DOI] [PubMed] [Google Scholar]
- Solomon NG.Age of pairing affects reproduction in prairie voles. Lab Anim 1991; 25: 232–235. [DOI] [PubMed] [Google Scholar]
- Wolff JO, Dunlap AS.Multi-male mating, probability of conception, and litter size in the prairie vole (Microtus ochrogaster). Behav Processes 2002; 58: 105–110. [DOI] [PubMed] [Google Scholar]
- Oksanen TA, Koskela E, Mappes T.Hormonal manipulation of offspring number: maternal effort and reproductive costs. Evolution 2002; 56: 1530–1537. [DOI] [PubMed] [Google Scholar]
- Mystkowska ET.Preimplantation development in vivo and in vitro in bank voles, Clethrionomys glareolus, treated with PMSG and HCG. J Reprod Fertil 1975; 42: 287–292. [DOI] [PubMed] [Google Scholar]
- Adachi M, Tanaka R, Yoshida T, Tsushima N.Studies on induced superovulation in mature nulliparous Japanese voles (Microtus montebelli) following gonadotropin administration. J Reprod Dev 1999; 39: 7–10. [Google Scholar]
- Lundberg C, Bjorklund T, Carlsson T, Jakobsson J, Hantraye P, Deglon N, Kirik D.Applications of lentiviral vectors for biology and gene therapy of neurological disorders. Curr Gene Ther 2008; 8: 461–473. [DOI] [PubMed] [Google Scholar]
- Singer O, Tiscornia G, Ikawa M, Verma IM.Rapid generation of knockdown transgenic mice by silencing lentiviral vectors. Nat Protoc 2006; 1: 286–292. [DOI] [PubMed] [Google Scholar]
- Chang HS, Lin CH, Chen YC, Yu WC.Using siRNA technique to generate transgenic animals with spatiotemporal and conditional gene knockdown. Am J Pathol 2004; 165: 1535–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Zhang P.Transgenic RNA interference in mice. Physiology 2007; 22: 161–166. [DOI] [PubMed] [Google Scholar]
- Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus MT, Gertler FB, Scott ML, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet 2003; 33: 401–406. [DOI] [PubMed] [Google Scholar]
- Tiscornia G, Singer O, Ikawa M, Verma IM.A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc Natl Acad Sci U S A 2003; 100: 1844–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasim V, Miyagishi M, Taira K.Control of siRNA expression using the Cre-loxP recombination system. Nucleic Acids Res 2004; 32: e66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiscornia G, Tergaonkar V, Galimi F, Verma IM.CRE recombinase-inducible RNA interference mediated by lentiviral vectors. Proc Natl Acad Sci U S A 2004; 101: 7347–7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ.Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 2004; 429: 754–757. [DOI] [PubMed] [Google Scholar]