Abstract
Study Objectives:
To assess whether dysfunctional autonomic regulation during REM sleep as indexed by heart rate variability (HRV) is a pathophysiological factor in frequent nightmares (NMs).
Design:
Monitoring with polysomnography (PSG) and electrocardiography (ECG) for 3 consecutive nights: Night 1 (N1), adaptation night; N2, administration of partial REM sleep deprivation; N3, recovery night. Differences between NM and control (CTL) groups assessed for ECG measures drawn from wakefulness, REM sleep, and Stage 2 sleep on both N1 and N3.
Setting:
Hospital-based sleep laboratory
Participants:
Sixteen subjects with frequent NMs ( ≥ 1 NM/week; mean age = 26.1 ± 8.7 years) but no other medical or psychiatric disorders and 11 healthy comparison subjects ( < 1 NM/month; mean age = 27.1±5.6 years).
Results:
NM and CTL groups differed on 2 REM sleep measures only on N1; the NM group had longer REM latencies and REM/NREM cycle durations than did the CTL group. No differences were found on time domain and absolute frequency domain ECG measures for either N1 or N3. However, altered HRV for the NM group was suggested by significantly higher LFnu, lower HFnu, and higher LF/HF ratio than for the CTL group.
Conclusions:
Results are consistent with a higher than normal sympathetic drive among NM subjects which is unmasked by high REM sleep propensity. Results also support a growing literature linking anxiety disorders of several types (panic disorder, posttraumatic stress disorder (PTSD), generalized anxiety disorder) to altered HR variability.
Citation:
Nielsen T; Paquette T; Solomonova E; Lara-Carrasco J; Colombo R; Lanfranchi P. Changes in cardiac variability after rem sleep deprivation in recurrent nightmares. SLEEP 2010;33(1):113-122.
Keywords: Parasomnias, nightmares, REM sleep deprivation, heart rate variability, sympathetic arousal
IDIOPATHIC NIGHTMARES (NMS) ARE EPISODES OF INTENSE DYSPHORIC DREAMING—USUALLY INVOLVING FEELINGS OF THREAT, ANXIETY, FEAR OR terror—that arise primarily during REM sleep. Their recurrent form, known as nightmare disorder,1,2 has no known etiology but is nonetheless considered to be distinct from anxiety disorders,2 despite the fact that NMs are highly comorbid with many anxiety disorders and a frequent source of daytime distress. The occurrence of NMs in REM sleep is consistent with an underlying autonomic dysfunction that is periodically exacerbated by the intense autonomic fluctuations that characterize normal REM sleep.3,4 However, that NMs have also been observed occasionally in Stage 2 NREM sleep leaves the exclusivity of this autonomic dysfunction to REM sleep in doubt. The relationship of NM pathology to that of different anxiety disorders also remains unclear.
Several findings suggest that abnormally high sympathetic activity is a factor in NM pathology. First, and most obviously, REM sleep related tachycardia5,6 and accelerated respiration appear in the PSG records of patients just before they awaken from many NM episodes.5 Second, NMs occur primarily late in the sleep period, when REM sleep is most concentrated and its autonomic surges most extreme.3 Third, elevated sympathetic activity has been documented for several conditions that are characterized by severe recurrent NMs, such as posttraumatic stress disorder (PTSD).7,8 Finally, the habitual REM sleep of NM subjects (with or without PTSD) is punctuated by frequent periodic leg movements (PLM) and PLM with microarousals,9 both of which are correlates of patterned rises and falls in heart rate (HR),10,11 increased HR variability (HRV)12 and increased blood pressure (BP).13
The hypothesized increase in sympathetic activity in NM etiology is likely an anomaly of normal REM sleep autonomic activity, which is itself quite volatile in nature. Rapid eye movement bursts are normally accompanied by transient HR surges3,14,15 and an elevation in the low-frequency (LF) spectrum of the ECG, which indexes relative sympathetic activation.4,16 REM sleep is also accompanied by phasic surges in BP17 which may contribute to the early morning BP surges that are thought to increase risk for acute cardiac events.18 Such findings support the suggestion that intense emotionality during dreaming may precipitate life-threatening arrhythmias,3 a possibility consistent with both folklore and the observation of “killer nightmares” preceding coronary events.19 Thus, changes in REM sleep related autonomic activity may be a factor in nightmare formation, although comparisons with Stage 2 sleep and wakefulness are needed to determine whether it is in fact exclusive to REM sleep. Further, the experimental manipulation of REM sleep propensity may be a useful method for unmasking the pathophysiology of this autonomic activity.
HRV measures are an appropriate method for assessing autonomic fluctuations in NM patients. Among other types of anxiety disorders, such as panic and the specific phobias, HR and HRV measures have been used to demonstrate the likely pathological implication of elevated sympathetic activation.20,21 A growing literature also documents the validity of sleep related HRV measures among normal subjects3,4 and sleep disordered patients.7,22,23 For example, insomniac patients have higher HR and higher LF spectral power in all sleep stages than do healthy subjects;23 both measures reflect elevated sympathetic nervous system activity. Similar findings were reported for PTSD patients.7 Despite such advances, however, no studies assessing HRV have been conducted for patients suffering from idiopathic NMs.
Assessment of HRV typically includes time and frequency domain measures. Both types of measures are derived from quantification of R-waves of successive QRST complexes. Time domain measures quantify the mean heart rate (HR) and standard deviation of normal to normal R-R intervals (SDNN) as well as the percentage of N-N intervals that differ markedly (± 50msec) from preceding intervals (pNN50). Frequency domain measures are derived from spectral analysis of R-R intervals and may be expressed in either absolute or normalized terms. Very low frequency (VLF) power reflects the influence of slow regulatory mechanisms of still unknown origin; LF power is believed to reflect sympathetic influences on the heart as well as cardiac baroreflex responsiveness to BP variation.24 HF power primarily reflects respiration driven vagal modulation of the heart.25 Finally, the LF/HF ratio is considered to reflect sympathovagal balance.20,26–28 Normalized unit spectral power measures (HFnu, LFnu) are derived from their absolute power equivalents (HF, LF) over a normalizing denominator such as total power (or total power minus VLF). They, together with the LF/HF ratio, are largely equivalent carriers of information about sympathovagal balance.29 LF in particular is characteristic of anxiety disorders such as PTSD30 and panic31 (see review21).
Typically, during REM sleep, HR, LF, and LFnu increase; while HF and HFnu decrease relative to NREM sleep, suggesting that normal REM sleep is characterized by relative sympathetic activation.4,32–34 In light of findings reviewed above, we selected LFnu, a measure of relative sympathetic activation, as the primary endpoint for the present study.
Studies of various psychiatric problems have employed challenge procedures during wakefulness to stimulate autonomic activity and elicit HRV anomalies, challenges such as injections of isoproterenol that induce HR amplitude variability in panic disorder35 and presentations of trauma-related stimuli that inhibit LF power in PTSD.36 In the case of some sleep disorders, total and partial sleep deprivation have been used as challenge procedures. For example, 38 hours of total sleep deprivation have been shown to induce somnambulistic behaviors during recovery sleep among subjects whose symptoms would otherwise go undetected on PSG recordings.37 In line with the notion that NMs are primarily a REM sleep anomaly, we explored the use of partial REM sleep deprivation as a challenge procedure for eliciting the autonomic symptoms of NMs in the laboratory. The success of such a procedure would facilitate laboratory studies of NMs which, with few exceptions, have been hampered by the unexplained absence of reported NM episodes during PSG recordings.38,39
When healthy human subjects are deprived of REM sleep, REM propensity is disproportionately increased during subsequent sleep.40 This increase may take the form of atypically high REM percentage or REM density41 and an increase in the dreamlike quality of REM sleep and hypnagogic dreaming,42 among other changes. In rats, REM sleep deprivation heightens emotional drive, i.e., aggressiveness43 and impairs recall of fear extinction.44 Thus, we expected that higher than normal REM propensity would be brought about by a partial REM sleep deprivation procedure and would provoke measurable autonomic symptoms during the recovery sleep of NM subjects; this was expected to include HRV anomalies and perhaps NM episodes as well. From the literature reviewed earlier, we anticipated that NM sufferers would show evidence of elevated sympathetic activity, especially elevated LFnu, relative to control subjects on pre-REM deprivation measures, and that REM deprivation would further exacerbate these differences on recovery night—particularly during REM sleep. We also expected that this sympathetic activity indicator would be associated in a dose-response fashion with measures of REM propensity.
METHODS
Subjects
Sixteen individuals who reported during a telephone screening recalling at least 1 NM/week for ≥ 6 months (Mage = 26.1 ± 8.7 y) and 11 healthy comparison subjects who reported recalling < 1 NM/month (Mage = 27.1 ± 5.6 y) were recruited by media advertisements and through contacts with laboratory staff. These criteria were taken from the International Classification of Sleep Disorders45 diagnostic criteria for nightmare disorder and indicate at least moderately severe NMs. The groups did not differ in age (F1,25 = 0.106, P = 0.747) or in male to female ratio (NM: 6:10; CTL: 4:7; χ2 = 0.004, P = 0.95). The groups also did not differ in their self-reported use of alcohol (NM: 5/16 none; CTL: 7/11; χ2 = 2.769, P = 0.096), caffeine (NM: 10/16 ≥ 1/day; CTL: 5/11; χ2 = 0.767, P = 0.381), recreational drugs (NM: 14/16 none; CTL: 10/11; χ2 = 0.077, P = 0.782), or tobacco (NM: 13/16 none; CTL: 10/11; χ2 = 0.482, P = 0.488), but did differ marginally in their use of prescription medications (NM: 12/16 none; CTL: 11/11; χ2 = 3.228, P = 0.072). The latter difference was due to 3 NM subjects taking oral contraceptives. Subjects were not seen in a clinical context, were not currently following psychotherapy, were not seeking treatment, and were not given extensive psychiatric evaluations. During intake, none reported having neurological, psychiatric, or other sleep disorders, and none reported having prior traumatic experiences in response to the question “Have you had any traumatic experience in the past such as a physical attack, car accident, etc?” Nonetheless, the NM group scored higher than the CTL group on the following screening questionnaires: Beck Depression Inventory (BDI),46 State Trait Anxiety Inventory-State subscale (STAI-S),47 Symptom Checklist 90-Revised (SCL90-R) global severity index.48 Subjects kept a 1-week daily home log for rating sleep and dream attributes; only the items assessing dream anxiety (1 = not at all; 9 = very) and the per-day frequencies of NMs are reported here.
The protocol was approved by the hospital ethics review board. Subjects were aware they would be paid $25 per laboratory recording night as well as parking and breakfast expenses. Written informed consent was obtained.
Laboratory Procedures
Subjects slept for 3 consecutive nights: baseline (N1), REM deprivation (N2) and REM recovery (N3). On N1, they were fitted with a standard montage of PSG and ECG electrodes and allowed to sleep undisturbed in a comfortable, sound-shielded room until the scheduled morning awakening. Audio-visual surveillance was maintained throughout the night. On N2, subjects were deprived of REM sleep by enforced awakenings (80 dB, 500 Hz, 0.5-sec tone) from every REM sleep episode after the 2nd, beginning 5 min after appearance of the first rapid eye movement of each episode. They were asked to report and rate sleep mentation and then allowed to return to sleep. On N3, mentation was sampled at sleep onset,42 then subjects were allowed to sleep undisturbed until the morning.
Sleep Recordings
Subjects were fitted with a 14-channel recording montage that included 4 referential EEG channels from the international 10-20 electrode placement system (C3, C4, O1, O2); 4 channels for left/right and vertical/horizontal eye movements; 4 EMG channels for chin and right side forearm extensor, leg tibialis and forehead corrugator muscle activities; 1 cardiac channel for bipolar ECG; and 1 respiration channel for nasal thermistry. Tracings were scored and artifacts removed by trained polysomnographers applying standard criteria and using Harmonie v6.0b49 software. An in-house program was used to output standard sleep stage variables.
ECG Analyses
Three-minute R-R and respiration segments were selected from REM sleep, Stage 2 NREM sleep (samples both preceding and following REM sleep, subsequently averaged), and pre-sleep wakefulness. Segments were visually selected to contain only stationary signals, i.e., to contain no microarousals, periodic leg movements, complex REM sleep movements, apneas, or sleep state changes. A trained technologist screened the ECG signal to detect R-waves and to identify and remove arrhythmias and artifacts. R-R variability was then analyzed in both time and frequency domains using Cardiolab software (Fondazione S. Maugeri, Italy). Time-domain variables included mean heart rate (HR), standard deviation of the normal-to-normal RR intervals (SDNN), and percentage of 50-ms or greater differences between adjacent R-R intervals (pNN50). Spectral components were quantified by an autoregressive decomposition algorithm that computed peak powers and central frequencies and classified them into HF (0.15–0.40 Hz), LF (0.04–0.15 Hz), and VLF (< 0.04 Hz) bands. HF and LF R-R variability components were considered in both absolute values and normalized units (HFnu and LFnu ); the latter were obtained by dividing the power of each component by total variance minus the VLF and DC (0 Hz) components x100. The LF/HF ratio was calculated as an estimate of sympathovagal balance. To ascertain whether HRV changes are unique only to REM sleep, cardiac variables were separately averaged within subjects for all REM, NREM, and Awake segments sampled.
Statistical Analyses
To ascertain that groups did not differ on sleep architecture, group comparisons on all measures were made using independent, 2-tailed t-tests. ECG differences were evaluated with a standard battery of 9 measures arranged in a multivariate design to control for intercorrelations among ECG measures. One of these measures (LFnu) was treated as primary endpoint, all others as secondary. ECG measures were entered in 3 separate 2 × 2 MANOVAs (REM, Stage 2, Awake), with Group (NM, CTL) as an independent factor, Night (N1, N3) as a repeated measure, and a multivariate grouping of 9 variables (RRmean, SDNN, pNN50, HF, LF, VLF, HFnu, LFnu, LF/HF) as dependent measures. An error rate of P = 0.05 was applied to the primary endpoint; an error rate correction for all other measures was established for each state at P = 0.05/#measures, or 0.05/8 = 0.006. Pearson correlations were used to assess relationships between psychopathology scores and HRV measures only for the NM group because an insufficient number of data points were available for the CTL group. Correlations were also calculated between a REM sleep propensity measure (REM% on N2) and HRV measures for the entire sample.
ECG samples were less numerous for Night 3 because 2 NM and 2 CTL subjects dropped out after Night 1. In addition, there were stage differences in the number of valid ECG epochs used for analysis because Stage 2 sleep was sampled for both ascending and descending subtypes (producing twice as many epochs as for REM sleep), and because fewer valid epochs of Awake than of sleep ECG were available.
RESULTS
Subject Characteristics
The NM group scored higher (M = 11.13 ± 8.4) than the CTL group (M = 4.73 ± 6.0) on Beck depression (t25 = 2.17, P = 0.04) and marginally higher on Spielberger state anxiety (36.3 ± 8.9 vs. 30.3 ± 5.2; t24 = 1.93, P = 0.065) and SCL-90-R global severity (59.0 ± 10.6 vs. 50.1 ± 10.8; t24 = 1.93, P = 0.061). The NM group rated their pre-laboratory home dreams as being more anxious (M = 5.98 ± 1.4) than did the CTL group (M = 2.59 ± 1.5; t24 = 3.13, P = 0.007), and more of them were NMs (M = 0.20 ± .26 per day) than for the CTL group (M = 0.00 ± 0.00; Mann Whitney U = 21.0, Z = −2.284, P = 0.056, 2-tailed).
For NM subjects, trait anxiety scores correlated with time domain measures on both nights, especially with Stage 2 SDNN and pNN50 and to a lesser extent Awake SDNN and pNN50 (Table 1). State anxiety scores correlated more marginally only with Stage 2 frequency domain measures LFnu, HFnu, and LF/HF. However, none of the preceding correlations survived application of a conservative (Bonferroni) error correction for multiple correlations (0.05/72 = 0.0007).
Table 1.
Pearson correlations between State and Trait Anxiety measures and HRV time and frequency domain measures on Night 1 (N1; N = 16) and Night 3 (N3; N = 14) for subjects with frequent NMs
| Time Domain | Statea |
Traitb |
|||
|---|---|---|---|---|---|
| r | P | r | P | ||
| REM | HR-N1 | −0.071 | ns | 0.141 | ns |
| HR-N3 | 0.010 | ns | 0.156 | ns | |
| SDNN-N1 | −0.113 | ns | −0.410 | ns | |
| SDNN-N3 | −0.154 | ns | −0.524 | 0.054 | |
| pNN50-N1 | 0.010 | ns | −0.351 | ns | |
| pNN50-N3 | −0.016 | ns | −0.420 | ns | |
| St2 | HR-N1 | 0.111 | ns | 0.245 | ns |
| HR-N3 | 0.072 | ns | 0.236 | ns | |
| SDNN-N1 | −0.307 | ns | −0.637 | 0.008 | |
| SDNN-N3 | −0.418 | ns | −0.665 | 0.009 | |
| pNN50-N1 | −0.186 | ns | −0.580 | 0.019 | |
| pNN50-N3 | −0.406 | ns | −0.615 | 0.019 | |
| Awake | HR-N1 | 0.130 | ns | 0.314 | ns |
| HR-N3 | −0.199 | ns | 0.228 | ns | |
| SDNN-N1 | −0.439 | ns | −0.553 | 0.026 | |
| SDNN-N3 | −0.445 | ns | −0.623 | 0.017 | |
| pNN50-N1 | −0.195 | ns | −0.452 | 0.079 | |
| pNN50-N3 | −0.225 | ns | −0.556 | 0.039 | |
| Frequency Domain | |||||
| REM | LFnu-N1 | −0.397 | ns | 0.058 | ns |
| LFnu-N3 | −0.142 | ns | 0.199 | ns | |
| HFnu-N1 | 0.382 | ns | −0.085 | ns | |
| HFnu-N3 | 0.104 | ns | −0.234 | ns | |
| LF/HF-N1 | −0.251 | ns | 0.310 | ns | |
| LF/HF-N3 | −0.117 | ns | 0.302 | ns | |
| St2 | LFnu-N1 | −0.462 | 0.072 | 0.007 | ns |
| LFnu-N3 | 0.029 | ns | 0.017 | ns | |
| HFnu-N1 | 0.503 | 0.047 | −0.045 | ns | |
| HFnu-N3 | −0.032 | ns | −0.107 | ns | |
| LF/HF-N1 | −0.542 | 0.030 | 0.089 | ns | |
| LF/HF-N3 | 0.057 | ns | 0.145 | ns | |
| Awake | LFnu-N1 | −0.145 | ns | 0.322 | ns |
| LFnu-N3 | −0.189 | ns | 0.029 | ns | |
| HFnu-N1 | 0.081 | ns | −0.364 | ns | |
| HFnu-N3 | 0.215 | ns | −0.046 | ns | |
| LF/HF-N1 | −0.300 | ns | 0.053 | ns | |
| LF/HF-N3 | −0.383 | ns | −0.071 | ns | |
Spielberger State Anxiety subscale;
SCL-90-R Anxiety subscale (T-scores); only P < 0.08 are shown (Bonferroni corrected P = 0.0007). N1, night 1; N3, night 3; HR, mean heart rate; SDNN, standard deviation of normal to normal (N-N) intervals; pNN50, percentage of N-N intervals that differ ± 50msec from preceding intervals; LFnu, low frequency power in normalized units; HFnu, high frequency power in normalized units; LF/HF, ratio of low frequency power to high frequency power.
General Sleep Characteristics
As shown in Table 2, the NM and CTL groups differed very little on standard sleep measures for the 3 nights of the study. The only measures differentiating the groups were N1 REM latency (P = 0.021; NM > CTL), N1 NREM/REM cycle duration (P = 0.004; NM > CTL) and, marginally, N1 #REM periods (NM < CTL). A Group × Night interaction effect for average REM density was marginal (F1,14 = 3.762, P = 0.073), and NM and CTL groups did not differ on this measure for either N1 (NM: 0.123 ± 0.06; CTL: 0.163 ± 0.04; t14 = 1.32, P = 0.209) or N3 (NM: 0.102 ± 0.94; CTL: 0.095 ± 0.05; t14 = −0.34, P = 0.740).
Table 2.
Sleep architecture measures for subjects with (NM) and without (CTL) frequent nightmares
| Night 1 |
Night 2 |
Night 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| General sleep architecture measures | NM (n = 16) Mean (SD) | CTL (n = 11) Mean (SD) | P† | NM (n = 14) Mean (SD) | CTL (n = 11) Mean (SD) | P† | NMs (n = 14) Mean (SD) | CTLs (n = 8) Mean (SD) | P† |
| Total sleep time (min) | 426.4 (56.4) | 410.8 (44.1) | ns | 386.8 (56.6) | 353.1 (57.8) | ns | 398.3 (64.6) | 395.8 (54.8) | ns |
| Sleep efficiency (%) | 95.5 (5.0) | 96.1 (2.1) | ns | 83.1 (8.5) | 81.0 (8.2) | ns | 97.3 (2.8) | 98.7 (1.0) | ns |
| Awakenings (#)a | 14.6 (6.6) | 18.2 (9.9) | nsb | 18.5 (9.5) | 22.8 (13.8) | ns | 12.4 (8.6) | 11.1 (9.5) | ns |
| Wake after sleep onset (min)a | 19.2 (22.8) | 15.9 (8.3) | ns | 76.9 (39.3) | 79.0 (33.4) | ns | 11.0 (12.9) | 5.5 (4.6) | ns |
| Sleep latency (min)a | 11.8 (9.4) | 12.4 (13.5) | ns | 9.4 (7.3) | 10.2 (15.7) | ns | 6.9 (4.4) | 5.8 (3.3) | ns |
| Latency to persistent sleep (min)a | 16.0 (10.9) | 14.0 (14.0) | ns | 10.5 (7.3) | 12.5 (18.3) | ns | 9.6 (7.3) | 6.4 (4.8) | ns |
| Latency to Stage 2 (min)a | 17.1 (11.3) | 14.6 (14.1) | ns | 13.5 (8.6) | 13.7 (18.6) | ns | 10.1 (5.4) | 8.6 (4.8) | ns |
| Latency to Stage 3-4 (min)a | 14.3 (8.9) | 11.5 (6.2) | ns | 13.5 (8.3) | 13.5 (14.0) | ns | 11.6 (7.2) | 9.8 (4.8) | ns |
| Awake (%)a | 4.3 (4.9) | 3.8 (2.1) | ns | 16.0 (8.3) | 18.1 (7.9) | ns | 2.5 (2.8) | 1.3 (1.0) | ns |
| Stage 1 (%)a | 5.6 (3.3) | 4.6 (2.1) | ns | 8.3 (4.5) | 7.3 (4.2) | ns | 5.5 (3.3) | 3.4 (1.4) | ns |
| Stage 2 (%) | 52.9 (8.5) | 49.3 (8.2) | ns | 52.5 (6.5) | 51.0 (8.2) | ns | 46.5 (10.6) | 44.6 (7.7) | ns |
| Stage 3–4 (%) | 21.9 (7.2) | 25.6 (8.5) | ns | 24.2 (7.2) | 27.7 (8.5) | ns | 22.8 (7.5) | 23.5 (6.2) | ns |
| REM sleep measures | |||||||||
| Latency to REM (min) | 115.4 (64.5) | 73.5 (11.1) | 0.021b | 108.2 (66.1) | 83.5 (28.3) | nsb | 66.7 (31.7) | 51.0 (22.8) | ns |
| REM/NREM cycle duration (min) | 112.3 (29.8) | 86.2 (6.9) | 0.004b | 90.7 (28.0) | 78.5 (16.7) | ns | 86.6 (15.0) | 80.5 (6.7) | ns |
| REM periods (#) | 3.8 (0.9) | 4.5 (1.0) | 0.065 | 5.4 (1.7) | 5.5 (1.8) | ns | 4.0 (1.0) | 4.4 (1.1) | ns |
| REM (%) | 19.7 (6.5) | 20.5 (4.8) | ns | 15.0 (5.3) | 14.0 (5.4) | ns | 25.2 (7.6) | 28.5 (3.4) | ns |
| REM efficiency (%) | 85.4 (11.2) | 88.2 (6.0) | ns | 85.1 (12.3) | 83.9 (13.2) | ns | 85.0 (13.1) | 88.7 (5.8) | ns |
| REM fragmentation (#stage shifts within REM period) | 13.9 (7.1) | 14.0 (4.5) | ns | 12.0 (5.3) | 11.7 (4.5) | ns | 15.9 (7.3) | 18.0 (7.2) | ns |
2-tailed, independent t-tests, P-values > 0.15 not displayed;
Variable log(X+1) transformed for statistical comparisons;
Comparison used unequal variance assumption (Levene P < 0.05)
REM Sleep Deprivation and Rebound Effects
Selective REM sleep deprivation successfully reduced REM% for the NM group from 19.7% ± 6.5% on N1 to 15.0% ± 5.3% on N2 (t14 = 4.103, P = 0.0004). REM% was similarly reduced for CTL subjects from 20.5% ± 4.8% on N1 to 14.0% ± 5.4% on N2 (t10 = 4.316, P = 0.0003). A differential REM rebound for the 2 groups was apparent only when REM% was examined by thirds of the night. A marginal Group (NM, CTL) × Thirds (1st, 2nd, 3rd) × Night (N1, N3) interaction (F2,42 = 2.843, P = 0.069) indicated that, relative to N1, NM subjects rebounded in the 1st third (4.7% vs. 2.3%; t13 = −3.182, P = 0.007) and 2nd third (9.2% vs. 6.5%; t13 = −2.708, P = 0.018), but not the 3rd third (11.3% vs. 10.7%; t13 = −0.438, P = 0.669) of N3, whereas CTL subjects rebounded in the 1st third (5.5% vs. 2.6%; t8 = −5.529, P = 0.001) and 3rd third (13.5% vs. 9.1%; t8 = −4.733, P = 0.001) but not the 2nd third (8.2% vs. 7.9%; t8 = −0.243, P = 0.814) of N3.
HRV Measures
There were no significant differences between NM and CTL groups in the number of ECG epochs analyzed for any stage.
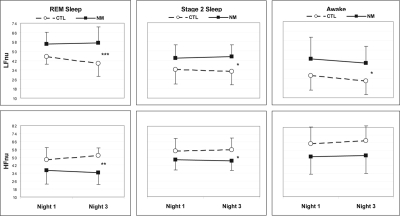
The overall multivariate analysis for ECG measures taken in REM sleep revealed a significant Group × Night interaction (Hotelling T = 2.257, F9,12 = 3.01, P = 0.039) which was also present for LFnu considered alone (T = 0.260, F1,20 = 5.19, P = 0.034; Figure 1). This interaction indicated that LFnu was higher for NM than for CTL subjects on N3 (F1,20 = 16.969, P = 0.0005), but only marginally so on N1 (F1,20 = 3.682, P = 0.069). The only other REM sleep ECG measure to reflect this interaction was a trend for HFnu (T = 0.199, F1,20 = 3.990, P = 0.060; all other interactions: P > 0.18); HFnu was lower for NM than for CTL subjects on N3 (F1,20 = 13.613, P = 0.001), but not on N1 (F1,20 = 3.431, P = 0.083). LF/HF did not produce a similar interaction effect (P = 0.663), even though simple effects followed the same pattern as for LFnu, i.e., NM subjects higher than CTL subjects on N3 (F1,20 = 9.627, P = 0.006), but only marginally so on N1 (F1,20 = 3.704, P = 0.069).
Figure 1.
Normalized low frequency (LFnu) and high frequency (HFnu) power in REM sleep, Stage 2 sleep, and Awake for Nights 1 and 3. From Night 1 to Night 3, REM LFnu decreases and REM HFnu increases for the CTL but not the NM group. The pattern reflects a continuing relative sympathetic activation and parasympathetic withdrawal following REM deprivation for the NM group. Plots are for 2 × 2, Groups (NM, CTL) × Nights (N1, N3), MANOVA interaction effects (see text). Group simple effects: ***P < 0.0005; **P < 0.01; *P < 0.05.
The multivariate analysis for Stage 2 sleep ECG measures showed no overall Group × Night interaction (T = 0.433, F9,12 = 0.577, P = 0.793) and no significant interaction for LFnu (P = 0.533) or any other dependent measures (all P > 0.32). The multivariate analysis for Awake also showed no overall Group × Night interaction (T = 1.130, F9,12 = 1.506, P = 0.250) and no interaction for LFnu (T = 0.001, F9,12 = 0.017, P = 0.898) or any other dependent measures (all P > 0.32), with the possible exception of a trend for VLF (T = 0.189, F9,12 = 3.789, P = 0.066).
To further explore the specificity of the LFnu effect to REM sleep on N3, the univariate simple effects for Group observed for N3 LFnu were also examined for Stage 2 and Awake on both N1 and N3. These comparisons are detailed in Table 3 (for N1) and Table 4 (for N3). For N1, no Group differences were noted for ECG measures in any stage. However, for N3, parallel, albeit diminished, effects were found for both Stage 2 and Awake. Stage 2 LFnu was higher for NM subjects than for CTL subjects on N3 (F1,20 = 6.902, P = 0.016) but only marginally so on N1 (F1,20 = 3.706, P = 0.069); Awake LFnu was marginally higher for NM than for CTL subjects on both N3 (F1,20 = 4.282, P = 0.052) and N1 (F1,20 = 3.300, P = 0.084). None of these differences exceeded the error-corrected P = 0.006 threshold.
Table 3.
Night 1 (baseline sleep) heart rate variability (HRV) measures for subjects with and without frequent nightmares
| Nightmare (N = 16) |
Control (N = 11) |
Group comparisons (P)† |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time domain | REM | St 2 | Awake | REM | St 2 | Awake | REM | St 2 | Awake | |
| HR | M | 66.8 | 62.2 | 64.5 | 63.6 | 60.9 | 63.0 | ns | ns | ns |
| SD | 12.5 | 11.7 | 10.9 | 6.7 | 5.8 | 7.0 | ||||
| SDNNa | M | 71.3 | 60.6 | 55.7 | 77.8 | 67.9 | 58.9 | ns | ns | ns |
| SD | 38.8 | 35.5 | 28.5 | 38.0 | 29.5 | 29.7 | ||||
| pNN50b | M | 11.0 | 13.0 | 12.6 | 14.7 | 19.1 | 16.4 | ns | ns | ns |
| SD | 8.1 | 8.6 | 9.6 | 9.4 | 8.7 | 10.8 | ||||
|
Frequency domain: Absolute | ||||||||||
| VLF | M | 1416.4 | 693.1 | 552.4 | 1160.4 | 629.6 | 172.9 | ns | ns | ns |
| SD | 1834.3 | 483.2 | 693.0 | 856.2 | 383.0 | 233.2 | ||||
| LF | M | 2095.6 | 1527.0 | 1304.6 | 1875.7 | 1547.2 | 809.4 | ns | ns | ns |
| SD | 2736.7 | 1544.3 | 2116.1 | 1599.8 | 1536.8 | 578.3 | ||||
| HF | M | 2070.3 | 2024.8 | 1542.1 | 2622.0 | 2790.9 | 2299.8 | ns | ns | ns |
| SD | 3612.1 | 3333.3 | 2307.8 | 2977.7 | 3375.7 | 3081.5 | ||||
|
Frequency domain: Normalized | ||||||||||
| LFnuc | M | 56.0 | 44.0 | 42.8 | 45.3 | 34.2 | 28.6 | 0.069 | 0.069 | ns |
| SD | 13.6 | 11.1 | 17.2 | 10.3 | 12.4 | 18.2 | ||||
| HFnuc | M | 36.8 | 47.9 | 51.4 | 47.6 | 56.7 | 65.2 | ns | ns | ns |
| SD | 13.9 | 10.9 | 18.1 | 12.1 | 12.9 | 17.4 | ||||
| LF/HF | M | 2.9 | 1.6 | 1.5 | 1.4 | 1.0 | 0.7 | 0.069 | ns | ns |
| SD | 2.0 | 0.9 | 1.3 | 1.0 | 0.9 | 0.7 | ||||
2-tailed, univariate F-tests, P-values > 0.08 not displayed;
SDNN = standard deviation of R-R intervals;
pNN50 = proportion of R-R intervals differing from preceding interval by ± 50msec;
Normalized values calculated as a proportion of total power minus VLF
Table 4.
Night 3 (post-REM deprivation recovery) heart rate variability (HRV) measures for subjects with and without frequent nightmares
| Nightmare (N = 14) |
Control (N = 8) |
Group comparisons (P)† |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time domain | REM | St 2 | Awake | REM | St 2 | Awake | REM | St 2 | Awake | |
| HR | M | 65.6 | 62.6 | 67.1 | 62.8 | 59.9 | 65.2 | ns | ns | ns |
| SD | 11.5 | 11.4 | 11.0 | 8.3 | 6.4 | 7.5 | ||||
| SDNNa | M | 68.6 | 60.4 | 46.8 | 69.6 | 64.2 | 57.8 | ns | ns | ns |
| SD | 28.7 | 32.1 | 23.5 | 23.9 | 27.4 | 21.9 | ||||
| pNN50b | M | 10.6 | 12.5 | 10.1 | 16.6 | 18.5 | 18.9 | ns | ns | 0.053 |
| SD | 7.7 | 9.2 | 9.4 | 8.5 | 9.1 | 10.2 | ||||
|
Frequency domain: Absolute | ||||||||||
| VLF | M | 1104.9 | 874.0 | 387.5 | 1052.6 | 611.7 | 639.0 | ns | ns | ns |
| SD | 1181.8 | 750.6 | 387.5 | 632.7 | 303.2 | 417.0 | ||||
| LF | M | 1885.0 | 1563.8 | 590.3 | 1377.5 | 1405.2 | 497.7 | ns | ns | ns |
| SD | 1438.3 | 1477.0 | 601.6 | 902.9 | 1252.3 | 217.1 | ||||
| HF | M | 1410.6 | 1690.6 | 1189.8 | 2030.4 | 2446.7 | 2294.3 | ns | ns | ns |
| SD | 1822.2 | 2286.1 | 1552.9 | 2084.1 | 2900.1 | 2806.5 | ||||
|
Frequency domain: Normalized | ||||||||||
| LFnuc | M | 57.2 | 45.1 | 39.2 | 39.5 | 32.7 | 24.1 | 0.0005 | 0.016 | 0.052 |
| SD | 11.1 | 10.2 | 17.7 | 6.3 | 11.5 | 14.1 | ||||
| HFnuc | M | 34.5 | 46.5 | 52.8 | 51.9 | 58.0 | 68.1 | 0.001 | 0.023 | 0.063 |
| SD | 11.9 | 9.7 | 18.6 | 7.6 | 11.9 | 15.3 | ||||
| LF/HF | M | 2.8 | 1.5 | 1.6 | 1.0 | 0.9 | 0.5 | 0.006 | ns | ns |
| SD | 1.5 | 0.8 | 2.4 | 0.4 | 0.9 | 0.4 | ||||
2-tailed univariate F-tests, P-values >.08 not displayed;
SDNN = standard deviation of R-R intervals;
pNN50 = proportion of R-R intervals differing from preceding interval by ± 50msec;
Normalized values calculated as a proportion of total power minus VLF
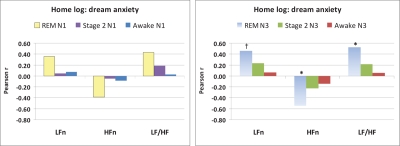
Whole-sample Pearson correlations between the 3 measures that most clearly distinguished NM and CTL groups (LFnu, HFnu, LF/HF) and a measure of REM sleep propensity (REM% on N2) were uniformly nonsignificant (all P > 0.16). Pearson correlations between these 3 measures for REM, Stage 2, and Awake states (N1 and N3) and mean dream content anxiety ratings from the home logs revealed weak relationships for N1 (Figure 2, left panel). For N3, however, correlations were observed only for REM sleep (Figure 2, right panel), i.e., REM LFnu (r15 = 0.456, P = 0.088), REM HFnu (r15 = −0.542, P = 0.037), REM LF/HF (r15 = 0.524, P = 0.045); however, with a conservative correction for multiple correlations (0.05/9 = 0.006), none of the correlations with dream anxiety remain significant.
Figure 2.
Pearson correlations between average home log dream anxiety ratings (1 to 9 scale) and normalized REM sleep, Stage 2 sleep and Awake state cardiac variability measures for laboratory Night 1 (N1, left panel) and Night 3 (N3, right panel). *P < 0.05; †P = 0.088.
DISCUSSION
REM sleep deprivation proved to be a moderately effective challenge procedure for uncovering HRV anomalies among subjects with frequent NMs. Whereas standard HRV measures assessed during wakefulness or on the baseline recording night revealed minimal differences between NM subjects and controls, some of the same measures produced differences when assessed during post-deprivation recovery sleep. The differences on N3 were apparent even though the two groups did not differ on standard sleep stage measures at this time. The HRV differences were detected almost exclusively after normalization of ECG power values, a procedure strongly recommended by the Task Force of the European Society of Cardiology,28 but one that is implemented only rarely in sleep studies. Among the normalized measures assessed, those derived from REM sleep produced by far the clearest group differences. Specifically, in REM sleep NM subjects had higher than normal LFnu power and LF/HF ratios and lower than normal HFnu power. Moreover, these recovery night REM sleep measures were especially likely to correlate with subject-rated anxiety in home dreams, in that high dream anxiety was associated with relative sympathetic arousal (high LFnu power, high LF/HF ratio, low HFnu power). These findings support to some extent our expectation that sympathetic activity during the REM sleep of NM subjects would be abnormally elevated and related to nightmare pathogenesis. Contrary to our expectations, however, there was no evidence that any HRV measure differentiated groups on the baseline recording night.
Rather, the appearance of group differences and correlations with dream anxiety exclusively during recovery sleep supports to some extent the related notions that (a) REM sleep specific autonomic processes are more easily disrupted by REM sleep deprivation among NM subjects than they are among control subjects, and (b) moderate REM sleep deprivation may be an aggravating pathophysiological factor in the perpetuation of NMs. If so, situational and dispositional factors that are known to influence REM sleep may be examined for their potential to disrupt the autonomic activity of susceptible individuals and for consequent heightening or reduction of NM episodes. For example, REM% is a function of dispositional factors such as neuroticism,40 in that high neuroticism subjects have a much lower post-deprivation REM% and report more NMs than do low neuroticism subjects.50 Indeed, our NM subjects displayed low post-deprivation REM rebound and elevated indicators of anxiety and general psychopathology that are consistent with a high neuroticism profile. Also, REM amount and intensity is affected by recent learning51 and may be particularly crucial for emotional learning such as cued fear conditioning52 and retention of negative stimuli.53,54 A high transient demand on such emotion-related processes, or high “affect load,”55 may contribute to NMs by disturbing normal REM sleep functions.
The pattern of results also raises the possibility that the deprivation-induced increase in REM propensity provoked or unmasked a generalized disruption of autonomic activity that affected all sleep/wake states. The effects of increased REM propensity on REM sleep are well documented and include REM sleep rebound,56 reduced 8.25–11 Hz power,57 and more dreamlike sleep mentation42 on recovery nights. However, increased REM propensity has also been found to influence NREM sleep, e.g., more prominent muscle atonia during some NREM episodes.41 Thus, our finding of marginal group differences during Stage 2 sleep and (to a lesser extent) during wakefulness support the notion that elevated REM propensity produced a generalized sympathetic activation for NM subjects, i.e., one that affected all sleep/wake states but that was brought into starker relief during REM sleep. It may be useful to evaluate whether the marginal group differences seen for Stage 2 sleep reflect the fact that the NREM-to-REM shift toward relative sympathetic activation takes place during Stage 2 sleep that precedes REM sleep by up to several minutes.32,58
The marked REM sleep difference we observed may have been facilitated by background autonomic activity that is much less stable during REM sleep than it is in other states. As mentioned in the Introduction, phasic REM bursts are accompanied by HR and BP surges3,14,15 and elevated LF power.4,16 Our findings suggest that, in normal subjects, these autonomic events change as a function of REM sleep deprivation and reflect a shift to relative parasympathetic activity during recovery. This shift did not occur for the NM subjects, however; their elevated sympathetic activity was not ameliorated. It is even possible—although we did not assess this possibility in order to preserve the integrity of subjects' recovery sleep—that the dreams occurring during the REM sleep from which our ECG samples were drawn had been rendered more dysphoric by the REM propensity manipulation, causing an increase in sympathetic arousal.
The precise relationship between REM propensity and autonomic disruption in NM subjects remains to be elucidated. Correlational analyses did not reveal dose-response relationships between one REM propensity measure (REM% on N2) and HRV measures. However, the high cross-night reliability of correlations between trait anxiety and time domain HRV measures (SDNN, pNN50) and between state anxiety and frequency domain measures suggests that trait and state anxiety may mediate relationships between HRV and autonomic activity in a more complex manner.
It might be argued that normalized frequency domain measures discriminated between groups because they were weighted by the VLF power measure, which is much more predominant in REM sleep than in any other sleep/wake state.32 However, this possibility is doubtful both because we found no difference in absolute VLF power between NM and CTL groups and because the LF/HF ratio, a measure not weighted by VLF power, revealed a group effect that paralleled effects for the LFnu and HFnu measures. As mentioned earlier, LFnu, HFnu, and LF/HF measures provide largely equivalent information about relative sympathetic activation.29
To a limited extent, the autonomic profile of our NM sample resembles that of anxious normal subjects59 in that, during wakefulness, they were marginally lower on some HRV measures (pNN50, VLF, HF) and higher than normal on the LF/HF ratio. Unlike anxious normals, however, our NM subjects did not have lower than normal awake LF or LFnu values. Our NM subjects' lower HF and HFnu scores also parallel those for insomniac patients assessed either during wakefulness60 or sleep.23 On the whole, our findings are consistent with a growing body of work demonstrating abnormal HRV findings for individuals with anxiety disorders20,21 and raise the question of whether nightmares share a common pathophysiology with one or more such anxiety disorders.
An important limitation to the present study is the fact that no habituation night was employed to stabilize sleep before recording of the baseline PSG. It is therefore possible that the baseline differences observed for NM subjects, though minor, are due to the NM subjects' higher sensitivity to the laboratory situation. Such a “first-night effect” is well documented to involve primarily REM sleep measures, such as skipped early REM periods,61,62 prolonged REM latencies,62 and longer REM/NREM cycles.63 One possibility is that NM subjects manifest a more extreme first night effect than do CTL subjects.64 However, the fact that REM% did not differentiate NM and CTL groups on the first night, even though REM% is the most sensitive65 and most consistently reported indicator of the first-night effect,62,63,65 tends to discount this explanation.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest
ACKNOWLEDGMENTS
The research was supported by grants to Tore Nielsen from the Canadian Institutes of Health Research (FRN – 57836) and the Natural Sciences and Engineering Research Council of Canada (RGPIN – 312277-05). The authors acknowledge Dominique Petit and Philippe Stenstrom for help with data collection and proof-reading.
REFERENCES
- 1.American Academy of Sleep Medicine, Task Force Chair HP. ICSD-II. International classification of sleep disorders: Diagnostic and coding manual. 2nd ed. Chicago: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edition, text revision (DSM-IV-TR) Washington DC: American Psychiatric Association Press; 2000. [Google Scholar]
- 3.Verrier RL, Muller JE, Hobson JA. Sleep, dreams, and sudden death: the case for sleep as an autonomic stress test for the heart. Cardiovas Res. 1996;31:181–211. [PubMed] [Google Scholar]
- 4.Berlad I, Shlitner A, Ben-haim S, Lavie P. Power spectrum analysis and heart rate variability in stage 4 and REM sleep: Evidence for state-specific changes in autonomic dominance. J Sleep Res. 1993;2:88–90. doi: 10.1111/j.1365-2869.1993.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 5.Fisher C, Byrne J, Edwards A, Kahn E. A psychophysiological study of nightmares. J Am Psychoanal Assoc. 1970;18:747–82. doi: 10.1177/000306517001800401. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen TA, Zadra AL. Nightmares and other common dream disturbances. In: Kryger M, Roth N, Dement WC, editors. Principles and practice of sleep medicine, 4th edition. 4th ed. Philadelphia: Elsevier Saunders, ; 2005. pp. 926–35. [Google Scholar]
- 7.Woodward SH, Murburg MM. Heart rate variability in PTSD: Mid-frequency power is inversely related to trauma severity and nightmares. Psychophysiol. 1996;33:S91. [Google Scholar]
- 8.Woodward SH, Arsenault NJ, Voelker K, et al. Autonomic activation during sleep in Posttraumatic Stress Disorder and Panic: A mattress actigraphic study. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.01.005. 10.1016/j.biopsych.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Germain A, Nielsen TA. Sleep pathophysiology in PTSD and idiopathic nightmare sufferers. Biol Psychiatry. 2003;54:1092–98. doi: 10.1016/s0006-3223(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 10.Sforza E, Nicolas A, Lavigne G, Gosselin A, Petit D, Montplaisir J. EEG and cardiac activation during periodic leg movements in sleep: support for a hierarchy of arousal responses. Neurology. 1999;52:786–91. doi: 10.1212/wnl.52.4.786. [DOI] [PubMed] [Google Scholar]
- 11.Fantini ML, Michaud M, Gosselin N, Lavigne G, Montplaisir J. Periodic leg movements in REM sleep behavior disorder and related autonomic and EEG activation. Neurology. 2002;59:1889–94. doi: 10.1212/01.wnl.0000038348.94399.f6. [DOI] [PubMed] [Google Scholar]
- 12.Sforza E, Pichot V, Barthelemy JC, Haba-Rubio J, Roche F. Cardiovascular variability during periodic leg movements: a spectral analysis approach. Clin Neurophys. 2005;116:1096–104. doi: 10.1016/j.clinph.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Pennestri M-H, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–18. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 14.Dickerson LW, Huang AH, Thurnher MM, Nearing BD, Verrier RL. Relationship between coronary hemodynamic changes and the phasic events of rapid eye movement sleep. Sleep. 1993;16:550–557. doi: 10.1093/sleep/16.6.550. [DOI] [PubMed] [Google Scholar]
- 15.Rowe K, Moreno R, Lau TR, et al. Heart rate surges during REM sleep are associated with theta rhythm and PGO activity in cats. Am J Physiol. 1999;277:R843–R849. doi: 10.1152/ajpregu.1999.277.3.R843. [DOI] [PubMed] [Google Scholar]
- 16.Versace F, Mozzato M, De Min TG, Cavallero C, Stegagno L. Heart rate variability during sleep as a function of the sleep cycle. Biol Psychol. 2003;63:149–62. doi: 10.1016/s0301-0511(03)00052-8. [DOI] [PubMed] [Google Scholar]
- 17.Sei H, Morita Y. Why does arterial blood pressure rise actively during REM sleep? J Med Invest. 1999;46:11–17. [PubMed] [Google Scholar]
- 18.Lavery CE, Mittleman MA, Cohen MC, Muller JE, Verrier RL. Nonuniform nighttime distribution of acute cardiac events: a possible effect of sleep states. Circulation. 1997;96:3321–27. doi: 10.1161/01.cir.96.10.3321. [DOI] [PubMed] [Google Scholar]
- 19.Parmar MS, Luque-Coqui AF. Killer dreams. Can J Cardiol. 1998;14:1389–91. [PubMed] [Google Scholar]
- 20.Cohen H, Benjamin J. Power spectrum analysis and cardiovascular morbidity in anxiety disorders. Auton Neurosci. 2006;128:1–8. doi: 10.1016/j.autneu.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol. 2007;74:185–99. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Khoo MC, Kim TS, Berry RB. Spectral indices of cardiac autonomic function in obstructive sleep apnea. Sleep. 1999;22:443–51. doi: 10.1093/sleep/22.4.443. [DOI] [PubMed] [Google Scholar]
- 23.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60:610–615. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Sleight P, La Rovere MT, Mortara A, et al. Physiology and pathophysiology of heart rate and blood pressure variability in humans: is power spectral analysis largely an index of baroreflex gain? Clin Sci (Lond) 1995;88:103–9. doi: 10.1042/cs0880103. [DOI] [PubMed] [Google Scholar]
- 25.Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ. Transfer function analysis of the circulation: unique insights into cardiovascular regulation. Am J Physiol. 1991;261:H1231–H1245. doi: 10.1152/ajpheart.1991.261.4.H1231. [DOI] [PubMed] [Google Scholar]
- 26.Pagani M, Lombardi F, Guzzetti S, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–93. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 27.Acharya UR, Joseph KP, Kannathal N, Lim CM, Suri JS. Heart rate variability: a review. Medical & Biological Engineering & Computing. 2006;44:1031–51. doi: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- 28.Malik M. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 29.Burr RL. Interpretation of normalized spectral heart rate variability indices in sleep research: a critical review. Sleep. 2007;30:913–19. doi: 10.1093/sleep/30.7.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen H, Kotler M, Matar MA, et al. Analysis of heart rate variability in posttraumatic stress disorder patients in response to a trauma-related reminder. Biol Psychiatry. 1998;44:1054–59. doi: 10.1016/s0006-3223(97)00475-7. [DOI] [PubMed] [Google Scholar]
- 31.Rechlin T, Weis M, Spitzer A, Kaschka WP. Are affective disorders associated with alterations of heart rate variability? J Affect Disord. 1994;32:271–75. doi: 10.1016/0165-0327(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 32.Busek P, Vankova J, Opavsky J, Salinger J, Nevsimalova S. Spectral analysis of the heart rate variability in sleep. Physiological Research. 2005;54:369–76. [PubMed] [Google Scholar]
- 33.Monti A, Medigue C, Nedelcoux H, Escourrou P. Autonomic control of the cardiovascular system during sleep in normal subjects. Eur J App Physiol. 2002;87:174–81. doi: 10.1007/s00421-002-0597-1. [DOI] [PubMed] [Google Scholar]
- 34.Vanoli E, Adamson P, Lin B, Pinna G, Lazzara R, Orr W. Heart rate variability during specific sleep stages: A comparison of healthy subjects with patients after myocardial infarction. Circulation. 1995;91:1918–22. doi: 10.1161/01.cir.91.7.1918. [DOI] [PubMed] [Google Scholar]
- 35.Yeragani VK, Pohl R, Bar KJ, Chokka P, Tancer M. Exaggerated beat-to-beat R amplitude variability in patients with panic disorder after intravenous isoproterenol. Neuropsychobiol. 2007;55:213–18. doi: 10.1159/000108380. [DOI] [PubMed] [Google Scholar]
- 36.Cohen H, Benjamin J, Geva AB, Matar MA, Kaplan Z, Kotler M. Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiat Res. 2000;96:1–13. doi: 10.1016/s0165-1781(00)00195-5. [DOI] [PubMed] [Google Scholar]
- 37.Joncas S, Zadra A, Paquet J, Montplaisir J. The value of sleep deprivation as a diagnostic tool in adult sleepwalkers. Neurology. 2002;58:936–40. doi: 10.1212/wnl.58.6.936. [DOI] [PubMed] [Google Scholar]
- 38.Hartmann E. A note on the nightmare. Int Psychiatr Clin. 1970;7:192–97. [PubMed] [Google Scholar]
- 39.Woodward SH, Arsenault NJ, Michel GE, et al. Polysomnographic characteristics of trauma-related nightmares. Sleep. 2000;23(S2):A356. [Google Scholar]
- 40.Cohen DB. Sleep & dreaming: Origins, nature & functions. New York: Pergamon; 1979. [Google Scholar]
- 41.Werth E, Achermann P, Borbely AA. Selective REM sleep deprivation during daytime. II. Muscle atonia in non-REM sleep. Am J Physiol Regul Integr Comp Physiol. 2002;283:R527–R532. doi: 10.1152/ajpregu.00466.2001. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen TA, Stenstrom PM, Takeuchi T, et al. Partial REM sleep deprivation increases the dream like quality of mentation from REM sleep and sleep onset. Sleep. 2005;28:1083–89. doi: 10.1093/sleep/28.9.1083. [DOI] [PubMed] [Google Scholar]
- 43.Hicks RA, Moore JD, Haynes C, Phillips N, Hawkins J. REM sleep deprivation increases aggressiveness in male rats. Physiol Behav. 1979;22:1097–100. doi: 10.1016/0031-9384(79)90263-4. [DOI] [PubMed] [Google Scholar]
- 44.Fu J, Li P, Ouyang X, et al. Rapid eye movement sleep deprivation selectively impairs recall of fear extinction in hippocampus-independent tasks in rats. Int J Psychoanal. 2007;144:1186–92. doi: 10.1016/j.neuroscience.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 45.Diagnostic Classification Steering Committee TMJC. ICSD - The international classification of sleep disorders: Diagnostic and coding manual. Rochester, Minnesota: American Sleep Disorders Association; 1990. [Google Scholar]
- 46.Beck AT, Epstein N, Brown G, Steer R. An inventory for measuring clinical anxiety. J Consult Clin Psychol. 1988;56:893–97. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 47.Spielberger CD, Gorsuch Rc, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologist's Press; 1970. [Google Scholar]
- 48.Derogatis L. Manual for the SCL-90 Revised Version. Baltimore: L. Derogatis; 1977. [Google Scholar]
- 49.Stellate Systems. 2006. Harmonie v6.0b. Montréal, Québec, Canada.
- 50.Nakazawa Y, Kotorii M, Kotorii T, Tachibana H, Nakano T. Individual differences in compensatory rebound of REM sleep, with particular reference to their relationship to personality and behavioral characteristics. J Nerv Ment Dis. 1975;161:18–25. doi: 10.1097/00005053-197507000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Smith CT, Nixon MR, Nader RS. Posttraining increases in REM sleep intensity implicate REM sleep in memory processing and provide a biological marker of learning potential. Learn Mem. 2004;11:714–19. doi: 10.1101/lm.74904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silvestri AJ, Root DH. Effects of REM deprivation and an NMDA agonist on the extinction of conditioned fear. Physiol Behav. 2007 doi: 10.1016/j.physbeh.2007.08.020. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 53.Wagner U, Fischer S, Born J. Changes in emotional responses to aversive pictures across periods rich in slow-wave sleep versus rapid eye movement sleep. Psychosom Med. 2002;64:627–34. doi: 10.1097/01.psy.0000021940.35402.51. [DOI] [PubMed] [Google Scholar]
- 54.Lara-Carrasco J, Nielsen T, Solomonova E, Levrier K, Popova A. Overnight emotional adaptation to negative stimuli is altered by REM sleep deprivation and is correlated with intervening dream emotions. J Sleep Res. 2009;18:178–87. doi: 10.1111/j.1365-2869.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- 55.Nielsen T, Levin R. Nightmares: A new neurocognitive model. Sleep Med Rev. 2007;11:295–310. doi: 10.1016/j.smrv.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Franken P. Long-term vs. short-term processes regulating REM sleep. J Sleep Res. 2002;11:17–28. doi: 10.1046/j.1365-2869.2002.00275.x. [DOI] [PubMed] [Google Scholar]
- 57.Endo T, Roth C, Landolt HP, et al. Selective REM sleep deprivation in humans: effects on sleep and sleep EEG. Am J Physiol. 1998;274:R1186–R1194. doi: 10.1152/ajpregu.1998.274.4.R1186. [DOI] [PubMed] [Google Scholar]
- 58.Bonnet MH, Arand DL. Heart rate variability: sleep stage, time of night, and arousal influences. Electroencephalogr Clin Neurophysiol. 1997;102:390–396. doi: 10.1016/s0921-884x(96)96070-1. [DOI] [PubMed] [Google Scholar]
- 59.Piccirillo G, Elvira S, Bucca C, Viola E, Cacciafesta M, Marigliano V. Abnormal passive head-up tilt test in subjects with symptoms of anxiety power spectral analysis study of heart rate and blood pressure. Int J Cardiol. 1997;60:121–31. doi: 10.1016/s0167-5273(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 60.Fang SC, Huang CJ, Yang TT, Tsai PS. Heart rate variability and daytime functioning in insomniacs and normal sleepers: preliminary results. J Psychosom Res. 2008;65:23–30. doi: 10.1016/j.jpsychores.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Mendels J, Hawkins DR. Sleep laboratory adaptation in normal subjects and depressed patients (“first night effect”) Electroencephalogr Clin Neurophysiol. 1967;22:556–58. doi: 10.1016/0013-4694(67)90063-6. [DOI] [PubMed] [Google Scholar]
- 62.Agnew HW, Webb WE, Williams RL. The first night effect: An EEG study. Psychophysiol. 1966;2:263–66. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 63.Lorenzo JL, Barbanoj MJ. Variability of sleep parameters across multiple laboratory sessions in healthy young subjects: the “very first night effect”. Psychophysiol. 2002;39:409–13. doi: 10.1017.S0048577202394010. [DOI] [PubMed] [Google Scholar]
- 64.Nielsen TA, Paquette T, Solomonova E, Lara-Carrasco J, Popova A, Levrier K. REM sleep characteristics of nightmare sufferers before and after REM sleep deprivation. Sleep Med. 2008 doi: 10.1016/j.sleep.2008.12.018. in press. [DOI] [PubMed] [Google Scholar]
- 65.Schmidt HS, Kaelbling R. The differential laboratory adaptation of sleep parameters. Biol Psychiatry. 1971;3:33–45. [PubMed] [Google Scholar]