Abstract
Study Objectives:
Research has shown variability in the correlations observed between subjective sleepiness and objective polysomnographic sleep latency. The present study evaluated whether eliciting subjective judgments of sleepiness after a 1-minute period of quiet with eyes closed would strengthen the relationship between subjective and objective measures.
Design:
Subjective judgments of sleepiness were collected following three 1-minute conditions (eyes-closed, eyes-open fixed gaze, and visual reaction time task) using the Stanford Sleepiness Scale and a visual analogue scale, prior to a measure of polysomnographic sleep latency. For each participant, subjective and objective measures were obtained a total of 12 times half-hourly from 20:00 to 01:30.
Setting:
Sleep laboratory of the Flinders University.
Participants:
Participants were 12 young adult good sleepers.
Results:
Within-subjects correlations between subjective and objective sleepiness across an evening period of increasing sleepiness were generally high (means approximately −0.63) and significant (P < 0.05). Contrary to expectation, there were no differences in correlations among the 3 conditions. Unexpectedly, when the correlations were calculated across subjects, correlations were noticeably weaker (means around −0.42), whereas the correlation means calculated across subjects, controlling for clock time, were close to 0.
Conclusions:
In controlled laboratory conditions, high correlations between subjective and objective measures of sleepiness within subjects were found across a large range of sleepiness (20:00 to 01:30) for all conditions. Calculating correlations within subjects and across a range of the circadian variation in sleepiness contributed substantially to the strength of the relationship found. These results suggest that the variability of prior research findings may be due, at least partly, to the way in which correlations were derived.
Citation:
Short M; Lack L; Wright H. Does subjective sleepiness predict objective sleep propensity? SLEEP 2010;33(1):123-129.
Keywords: Sleepiness, sleep onset latency, subjective sleepiness, MSLT, Stanford sleepiness scale
IN VARIOUS SITUATIONS, PEOPLE MAKE SUBJECTIVE ASSESSMENTS ABOUT HOW SLEEPY THEY ARE AND WHETHER THEIR SLEEPINESS POSES A RISK TO themselves or others. It is on these subjective assessments that decisions are made about whether to take a break, have a nap, or to continue with their previous activities. Unfortunately, people's subjective assessment of sleepiness does not always accurately reflect their propensity to fall asleep as assessed by objective sleepiness measures such as the Multiple Sleep Latency Test (MSLT).1–4
Studies that have examined the relationship between subjective and objective sleepiness have had varied results, with some reporting moderate to high correlations5–9 and others reporting correlations that were low or nonsignificant.1–4,10–13 These large variations in the strength of the relationship observed between subjective and objective sleepiness raise the question of whether a reliable or significant relationship exists at all. If subjective sleepiness ratings are not reliably related to objective sleepiness, then the conclusions drawn in the research setting may be questionable, and reliance upon subjectively experienced sleepiness as a safeguard against potential sleep-related accidents would be foolhardy.
Previous research examining this relationship varies in a number of important ways. The type of measures used is one important difference. Objective measures used include MSLT, electroencephalography, or various performance measures. The type of subjective sleepiness measure used is also important, in particular whether or not the measures are sensitive to diurnal variations in subjective sleepiness. Other varying factors include the study population (patient vs nonpatient), whether or not participants are subject to sleep deprivation prior to assessment, time of day of assessments, and use of within- or between-subjects analyses. These methodologic differences may help to explain the inconsistent correlational findings. Another factor that may help to explain this inconsistency is the context in which the subjective ratings are made. Two studies finding low or nonsignificant correlations between subjective and objective sleepiness collected subjective ratings after 90 minutes of free time between trials.4,14 During this time, participants engaged in light physical activity and social interaction, both of which have been shown to lower subjective sleepiness ratings. Studies examining factors affecting subjective ratings of sleepiness have supported the notion that more stimulating activities can alter subjective sleepiness.14–17 Making subjective ratings in situations in which there are no distractions and minimal or no physical activity (which is the context in which MSLTs are conducted) may strengthen the relationship between subjective and objective sleepiness.
Yang, Lin, and Spielman18 investigated whether participants who sat quietly for 1 minute with their eyes closed prior to making a subjective sleepiness rating would make ratings that more closely reflected objective sleepiness as assessed by sleep latency. They argued that objective measures tap into physiologic sleepiness, whereas subjective measures reflect physiologic sleepiness plus other activating factors such as physical and mental activity, motivation, and context. According to the authors, 1 minute of quiet, seated, eyes-closed time should reduce these activating factors and thus lead to subjective sleepiness ratings that more closely reflect the objective measure. The authors randomly allocated 30 participants into either the eyes-closed or eyes-open group. Each participant made 1 set of subjective sleepiness ratings (using the Stanford Sleepiness Scale [SSS] and a visual analog scale [VAS]) and completed 1 sleep-latency trial at 1 of 3 possible time points across the day: 09:00, 14:00, or 18:00. Their argument was supported by significant, moderately high correlations between subjective measures of sleepiness and sleep-onset latency (SOL) in the eyes-closed group (r = −0.58 and r = −0.62) but nonsignificant in the control group (r = −0.35 and r = −0.19). However, the difference between the correlations for the eyes-closed and control groups did not reach significance.
The present study aimed to replicate the promising findings of Yang and colleagues and build on them in several ways. This study examined correlations between subjective and objective sleepiness in 3 conditions: an eyes-closed condition, an eyes-open condition with the eyes fixated in an unchanging visual environment, and an eyes-open condition in which participants visually fixated on a computer screen and performed a serial reaction-time task. This would distinguish whether closing one's eyes is solely responsible for a stronger relationship between subjective and objective sleepiness. Alternatively, if fixating on a computer screen with eyes open produces a higher correlation than after a serial reaction time task, it would support the argument of Yang, Lin, and Spielman, that it is reduced activation, not closing the eyes per se, that strengthens the correlation between subjective and objective sleepiness.
METHODS
Participants
Participants were 9 females and 3 males, aged between 16 and 37 years (mean = 25.42, SD = 7.14). All participants were good sleepers who scored 5 or less on the Pittsburgh Sleep Quality Index.19 Although medical screening was not conducted, chronotype was assessed using a 7-day sleep-wake diary to determine normal circadian-phase entrainment. The sleep-wake diary captured information on daily sleep-onset time, wake time, SOL, total sleep time, nighttime awakenings, daytime napping, use of caffeine and alcohol, and food intake. Normal phase entrainment was defined as a consistent pattern of sleep onset between 22:00 and 24:00, a sleep latency of less than 30 minutes, wake time after sleep onset of less than 10 minutes, and a minimum of 7 hours sleep per night. Participants drank no more than 2 cups of tea, coffee, or caffeinated soft drinks or 2 standard drinks of alcohol per day. Excluded were cigarette smokers, respondents who were taking any medications that may interfere with sleep, and those who had scores of more than 12 on any of the 3 subscales on the Depression, Stress and Anxiety Scale Short Form.20,21
Approval for the study protocol was given by the Social and Behavioural Ethics Committee of the Flinders University of South Australia, and informed consent was obtained from all study participants. Seven participants completed the study as 1 option for completing part of their undergraduate course requirement. Five participants were recruited externally and were compensated $75 for their involvement.
The Sleep Laboratory
Continuous polysomnographic recordings were made throughout the laboratory session. Sleep onset was determined according to the standard criteria,22 via inspection of continuous raw electroencephalography, electrooculography, and electromyography (PSG, E-Series, Compumedics, Victoria, Australia). Sleep onset was defined as 3 consecutive 30-second epochs of any stage of sleep. The sleep laboratory was sound attenuated and, between sleep-latency trials, the rooms were illuminated to less than 50 lux. The laboratory was free of time cues and was temperature controlled to 22°C.
Procedure
Participants each completed a single 7-hour evening session in the sleep laboratory. On the night prior to the laboratory session, participants were restricted to 75% of their habitual total sleep time, as determined by 7 days of sleep-wake diary entries. This was to provide a moderate increase of homeostatic sleep drive for the laboratory session. Bedtime restriction was achieved by shifting participants' bedtimes later and rise times earlier by equal amounts. Sleep restriction was conducted in the participant's home. Compliance with this sleep schedule was verified by having participants telephone the sleep laboratory at the designated bedtime and rise time. The laboratory session started at 19:00 and finished at approximately 02:00. The timing was selected to maximize within-subjects correlations by providing a large range of sleepiness values across the session. Participants were instructed to avoid alcohol and to restrict caffeinated drinks to a maximum of 1 consumed before 12 noon on the day of the laboratory session. They were also asked to refrain from napping during that day. To the authors' knowledge, this is the first study that has taken advantage of such a wide spectrum of the circadian variation in sleepiness when examining the relationship between subjective sleepiness and objective sleep latency measures.
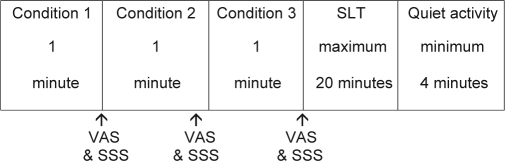
Figure 1 shows the schedule for each experimental trial. After 1 practice trial at 19:00, participants underwent twelve 30-minute trials starting at 19:30. In each trial, participants completed each of the 3 subjective sleepiness rating conditions followed by a sleep-latency trial. The eyes-closed condition involved participants sitting quietly for 1 minute with their eyes closed. The eyes-open condition involved participants sitting quietly for 1 minute with their eyes fixated on a point on an otherwise-blank computer screen. The performance task condition involved participants sitting for one minute with their eyes fixated on a point on a computer screen while they performed the psychomotor vigilance task (PVT).23 During the PVT, participants visually fixated on a black computer screen. They pressed the space bar on a computer keyboard as quickly as possible when the screen turned red. Interstimulus intervals occurred randomly, ranging from 2 seconds to 10 seconds.
Figure 1.
Schedule for each experimental trial. Order of conditions was completely counterbalanced. VAS refers to visual analog scale; SSS, Stanford Sleepiness Scale.
The interval between the 3 conditions was approximately 1 minute. After each condition, participants rated their subjective sleepiness on a VAS and then the SSS.24 The VAS consisted of a 10-cm line with an anchor of “not sleepy at all” on the left of the line and “extremely sleepy” on the right. Participants marked the place on the line that most accurately represented their personal feelings at that time. The score is the distance from the left anchor to the point marked on the line, in millimeters. The SSS is a 7-item scale consisting of 7 statements. These range from 1 “Feeling active and vital; alert; wide awake” to 7 “Almost in reverie; sleep onset soon; lost struggle to remain awake.” Participants selected the statement that most closely reflected their sleepiness. Posture was controlled throughout the protocol, with participants maintaining a seated position in bed for all conditions and subjective ratings. No food was consumed during the laboratory session. Order of conditions was completely counterbalanced for each participant using a Latin squares design. Complete counterbalancing was also done for order of presentation between participants at each time point of the session.
After all 3 subjective rating conditions, participants underwent a sleep latency trial in which they were given a 20-minute sleep opportunity. If, during this time, the participant met the electroencephalographic criteria for sleep onset, as determined by standard criteria,22 they were woken immediately. If 20 minutes elapsed without sleep onset, the trial was ended and the SOL was recorded as 20 minutes. The SOL could range from 0 to 20, indicating the number of minutes elapsed from the time of lights out to the beginning of the first epoch of any stage 1 or deeper sleep. Between trials, participants were able to read or watch videos. Bathroom visits, if required, occurred in the break between trials.
The strength of the relationship between subjective and objective sleepiness was analyzed using Pearson correlations. Before averaging the correlations for each of the 3 conditions, correlations were transformed to z-scores using Fisher r-to-z transformations.25 After averaging, scores were backtransformed to r values. One-sample t tests were used to determine whether the relationship between subjective and objective sleepiness was significant. Repeated-measures t tests were used to examine the difference in mean correlations between conditions.
RESULTS
Examination of the raw data revealed a robust variation of sleepiness across the night (19:30 to 01:30). Figure 2 (a,b) illustrates the change across the evening in SOL and subjective sleepiness for all 3 conditions. Across the evening, mean SOL ranged from over 17 minutes to less than 4 minutes, mean SSS values ranged from less than 3 to over 5.5, and mean VAS scores ranged from 38 to 80.
Figure 2.
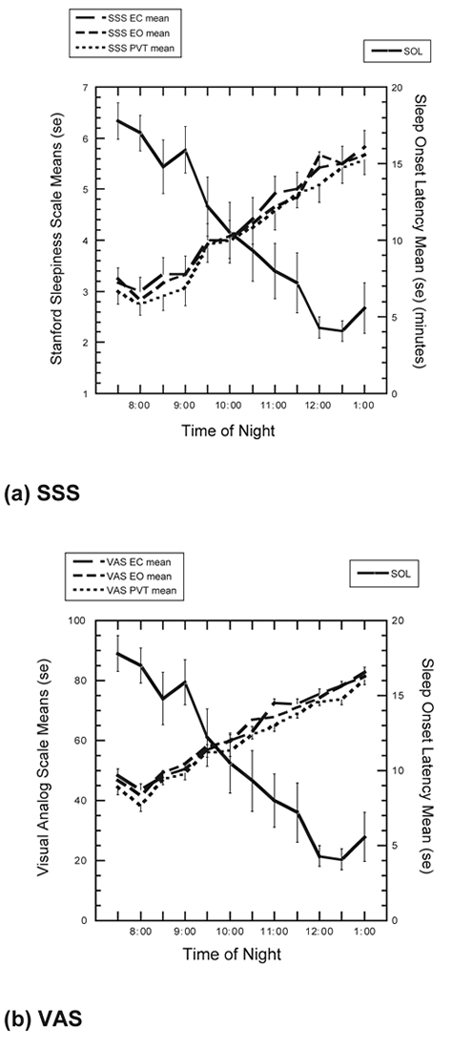
Mean subjective sleepiness scores and sleep-onset latency (SOL) in the eyes-closed (EC), eyes-open (EO), and psychomotor vigilance test (PVT) conditions across 12 trials on (a) Stanford Sleepiness Scale (SSS) and (b) visual analog scale (VAS).
Pearson correlation coefficients for subjective and objective sleepiness for each individual in each condition are displayed in Table 1. Correlations revealed generally strong negative relationships between subjective and objective sleepiness, as increased subjective sleepiness was related to shorter SOL. Of the 3 participants for whom lower correlations were observed, 2 of them recorded a severely restricted range of scores for 1 of the measures. Participant 8 did not fall asleep for 75% of the sleep-latency tests, and Participant 7 reported a very restricted range of subjective sleepiness values: over 90% of ratings on the SSS were the same value, and VAS scores ranged from only 21 to 39 across the evening. Overall, mean correlations were highly significant between subjective and objective sleepiness in all conditions, all P < 0.001.
Table 1.
Pearson correlation coefficients for subjective and objective sleepiness for each condition and participant, taken from the 12 time points across 1 night
| Participant | VAS/SOL |
SSS/SOL |
||||
|---|---|---|---|---|---|---|
| Eyes closed | Eyes open | PVT | Eyes closed | Eyes open | PVT | |
| 1 | −0.51 | −0.46 | −0.43 | −0.59a | −0.59a | −0.42 |
| 2 | −0.65a | −0.72b | −0.69a | −0.66a | −0.75b | −0.65a |
| 3 | −0.85b | −0.80b | −0.78b | −0.72b | −0.69a | −0.67a |
| 4 | −0.72b | −0.64a | −0.72b | −0.61a | −0.37 | −0.57a |
| 5 | −0.29 | −0.29 | −0.37 | −0.34 | −0.34 | −0.46 |
| 6 | −0.48 | −0.69a | −0.48 | −0.83b | −0.84b | −0.82b |
| 7 | −0.34 | −0.12 | −0.10 | −0.16 | −0.16 | −0.16 |
| 8 | −0.28 | −0.22 | −0.34 | −0.15 | −0.23 | −0.31 |
| 9 | −0.72b | −0.83b | −0.67a | −0.82b | −0.82b | −0.82b |
| 10 | −0.67a | −0.73b | −0.71a | −0.85b | −0.85b | −0.78b |
| 11 | −0.73b | −0.75b | −0.71a | −0.52 | −0.58a | −0.64a |
| 12 | −0.88b | −0.87b | −0.57 | −0.90b | −0.86b | −0.86b |
| Mean | −0.64c | −0.64c | −0.57c | −0.65c | −0.65c | −0.64c |
Note: VAS refers to visual analog scale; SOL, sleep-onset latency; SSS, Stanford Sleepiness Scale; PVT, Psychomotor Vigilance Test.
P < 0.05, 2-tailed;
P < 0.01, 2-tailed;
P < 0.001
Contrary to expectation, the strength of the relationship between subjective and objective sleepiness was not greater in the eyes-closed condition than in the eyes-open condition, nor was it greater in the nonresponding eyes-open condition than in the PVT condition. Differences between correlation means were analyzed using repeated-measures t tests; however, none were significant, all P > 0.14.
An alternative explanation for the failure to find differences among the conditions derives from the close temporal proximity of the 3 judgment conditions and the possible expectation of consistency in judgments at any one time. If participants were biased to maintain consistent subjective sleepiness ratings following the first of the 3 ratings over a limited judgment period of less than 5 minutes, then we would expect that only the first of the 3 ratings would accurately reflect their subjective sleepiness. To test whether this may have had an impact upon our findings, we correlated only the first subjective sleepiness ratings with SOL for each participant. Because order of presentation of the 3 pre-subjective rating conditions was counter balanced, each condition was the first presented on 4 occasions for each participant. The eyes-closed condition showed no advantage over the 2 eyes-open conditions. Using the VAS, the eyes-open fixated condition resulted in a larger correlation (-0.76) between subjective and objective sleepiness than did the eyes-open PVT (-0.53) or the eyes-closed condition (-0.50). Using the SSS, the eyes-open fixated condition resulted in a larger correlation (-0.77) than both the eyes-closed (-0.69) and the eyes-open PVT conditions (-0.60). However, there were no significant (P > 0.10) differences between any of these means. Thus, after eliminating the data that could have been influenced by expectation effects, our conclusions remain the same regarding the lack of differences between conditions in subjective and objective sleepiness correlations.
Unlike Yang, Lin, and Speilman,14 the present study compared conditions within subjects. To help to tease apart the relative contribution of circadian time and within-subjects versus between-subjects results, 2 further analyses were conducted. Firstly, between-subjects correlations across the range of circadian time were calculated. This allowed for the comparison of the within- and between-subjects results across a range of testing times, to determine whether a different pattern of results would be observed using a between-subjects approach, which is the approach taken by Yang, Lin, and Speilman.
Secondly, the effect of circadian time versus collecting data at 1 time point was examined by calculating correlations between subjective and objective sleepiness for each condition, at each time point. Comparing these results with the between-subjects results across a range of time points will help to reveal the impact and relative importance of capturing the circadian variation in sleepiness to accurately reveal the relationship between subjective and objective sleepiness.
As shown in Table 2, collecting data between subjects and across the circadian variation of sleepiness resulted in moderate negative correlations, approximately 20% of which were significant. Although mean correlations indicated a highly significant relationship between subjective and objective sleepiness in all conditions, the difference in mean correlations between conditions remained nonsignificant (all P values > 0.69).
Table 2.
Pearson correlation coefficients for subjective and objective sleepiness for each condition and between participants, with 12 groups of between-groups data taken for each condition from the 12 time points across 1 night
| Group | VAS/SOL |
SSS/SOL |
||||
|---|---|---|---|---|---|---|
| Eyes closed | Eyes open | PVT | Eyes closed | Eyes open | PVT | |
| 1 | −0.17 | −0.09 | −0.39 | −0.64a | −0.39 | −0.11 |
| 2 | −0.32 | −0.66a | −0.74b | −0.27 | −0.44 | −0.56 |
| 3 | −0.07 | −0.48 | −0.60a | −0.55 | −0.25 | −0.61a |
| 4 | −0.39 | −0.39 | −0.61a | −0.52 | −0.60a | −0.50 |
| 5 | −0.62a | −0.50 | −0.30 | −0.66a | −0.34 | −0.54 |
| 6 | −0.86b | −0.33 | −0.01 | −0.48 | −0.52 | −0.29 |
| 7 | −0.37 | −0.20 | 0.16 | −0.20 | −0.74b | −0.55 |
| 8 | −0.56 | −0.34 | −0.37 | −0.37 | −0.58 | −0.63a |
| 9 | −0.18 | 0.05 | −0.24 | −0.50 | −0.24 | −0.16 |
| 10 | −0.37 | −0.47 | −0.63a | −0.70a | −0.32 | −0.51 |
| 11 | −0.27 | −0.41 | −0.45 | −0.38 | −0.55 | −0.49 |
| 12 | −0.21 | −0.41 | −0.14 | −0.30 | −0.21 | −0.44 |
| Mean | −0.40c | −0.36c | −0.39c | −0.48c | −0.45c | −0.46c |
Note: VAS refers to visual analog scale; SOL, sleep-onset latency; SSS, Stanford Sleepiness Scale; PVT, Psychomotor Vigilance Test.
P < 0.05, 2-tailed;
P < 0.01, 2-tailed;
P < 0.001
As shown in Table 3, collecting data between subjects and at 1 time point resulted in generally low correlations, with an almost equal number being positive and negative. Only 3 correlations out of 72 (4%) were significant at P < 0.05, which we interpret as a chance variation. Correlation means were close to 0 and nonsignificant (all P > 0.36).
Table 3.
Pearson correlation coefficients for subjective and objective sleepiness for the eyes-closed, eyes-open and PVT conditions for each trial across the 12 participants
| Trial | VAS/SOL |
SSS/SOL |
||||
|---|---|---|---|---|---|---|
| Eyes closed | Eyes open | PVT | Eyes closed | Eyes open | PVT | |
| 1 | 0.03 | 0.07 | 0.14 | −0.08 | 0.16 | 0.08 |
| 2 | 0.66a | 0.67a | 0.73b | 0.36 | 0.22 | 0.46 |
| 3 | 0.21 | 0.12 | 0.24 | 0.10 | −0.03 | 0.29 |
| 4 | 0.01 | 0.12 | 0.09 | −0.06 | 0.10 | −0.02 |
| 5 | 0.10 | −0.02 | 0.04 | 0.02 | −0.09 | −0.07 |
| 6 | −0.36 | −0.37 | −0.13 | −0.13 | −0.31 | −0.12 |
| 7 | −0.32 | −0.44 | −0.13 | −0.16 | −0.16 | −0.10 |
| 8 | −0.13 | −0.11 | −0.28 | −0.22 | −0.25 | −0.16 |
| 9 | −0.08 | −0.06 | −0.07 | 0.15 | 0.06 | 0.16 |
| 10 | −0.45 | −0.41 | −0.42 | −0.47 | −0.38 | −0.32 |
| 11 | 0.19 | 0.17 | 0.25 | −0.08 | 0.02 | 0.13 |
| 12 | −0.14 | −0.06 | −0.19 | −0.05 | 0.04 | −0.19 |
| Mean | −0.02 | −0.03 | 0.02 | −0.05 | −0.05 | 0.01 |
Note: VAS refers to visual analog scale; SOL, sleep-onset latency; SSS, Stanford Sleepiness Scale; PVT, Psychomotor Vigilance Test.
P < 0.05;
P < 0.01
DISCUSSION
The Yang, Lin, and Spielman14 study suggested the interesting and potentially important possibility that the judgment of subjective sleepiness could more closely reflect objective sleepiness measures of sleep latency if the subjective judgment followed a brief 1-minute period with eyes closed. The present study did not find this to be the case. There were no significant differences in the strength of the relationship between subjective and objective sleepiness between the eyes-closed and the eyes open conditions. Similarly, no differences were observed in the strength of this relationship between the eyes open and PVT conditions. Instead, the general pattern of results across all conditions were similarly strong and significant, with mean correlation coefficients for the 3 conditions ranging from −0.57 to −0.65.
Yang, Lin, and Spielman14 suggest that closing one's eyes may reduce transient activation. Although there was a trend for subjective sleepiness to be greater in the eyes-closed condition than in the eyes-open condition and greater in the eyes-open condition than in the PVT, as apparent in Figure 2, these were mostly nonsignificant. This suggests that transient activation was not reliably altered by the experimental manipulation. Previously, subjective sleepiness has been explained as physiologic (objective) sleepiness combined with other activating factors such as physical and mental activity, motivation, and context. The broader context in which participants made subjective sleepiness ratings may be important when considering these results: participants were in a dimly lit warm bedroom and they were in bed, wearing comfortable clothing, and had been in this environment between 45 minutes and 6 hours attempting sleep onset at half-hourly occasions during the MSLT. This environment was intentionally a relaxing sleep-conducive environment. The manipulation of condition into eyes closed, eyes open, or PVT may not have been of a magnitude sufficient to alter or induce transient activation to any meaningful degree in this environment.
Correlations calculated between subjects and across the range of circadian variation in sleepiness ranged from −0.36 to −0.48. Analyzing sleepiness data between participants led to weaker correlations by approximately 0.21, as compared with the same analysis conducted within participants, with 22% less of the variance explained.
Mean between-subjects correlations calculated at each time point ranged from −0.05 to 0.02, which is markedly different than the results obtained when taking advantage of the circadian variation in sleepiness. Controlling for clock time resulted in correlations that were weaker than the between-subjects correlations calculated across the evening by approximately 0.39, with 17% less of the variance explained. Examination of scatter plots for each correlation, together with the standard deviation of mean scores at each time point, revealed a restriction of range in the spread of scores reported at any 1 time point for the SSS, VAS, and SOL. Correlating data taken from 1 time point largely controls for the effect of circadian rhythm on sleepiness. Instead, the intrinsic variation between subjects in subjective and objective sleepiness is relied upon to provide a range of sleepiness values. The low correlations between objective and subjective sleepiness in some previous research could be a function of truncated ranges in either measure, and, thus, they may not reflect the strength of the relationship occurring within any individual across a greater range of sleepiness.
The findings of the present study have the potential to inform future studies that seek to assess this relationship. Correlations between subjective and objective sleepiness may be best calculated using within-subjects data. This would help to control for individual differences, such as the way people interpret subjective sleepiness scales in relation to their objective sleep propensity. In situations in which multiple within-subjects ratings are not possible, such as in patient populations, there are a number of methodologic considerations that could help best reveal this relationship. These include collecting sleepiness ratings at clock times that span as much of the circadian variation in sleepiness as possible, using an environment of low stimulation, and using measures of subjective sleepiness that are sensitive to circadian variations in sleepiness, such as the SSS or VAS, as opposed to measures, such as the Epworth Sleepiness Scale, that measures trait-like sleepiness.8
It is important to note that, even in relatively “ideal” testing conditions, subjective ratings of sleepiness explained an average of only 40% of the variance in objective sleep propensity. It would be beneficial for future research to explore other factors that may contribute to this variation. A study by Van Dongen and colleagues13 revealed that, under conditions of chronic sleep restriction, subjective ratings of sleepiness tended to regress toward the mean over time, whereas objective performance deficits continued to increase. It is possible that a degree of habituation to feelings of sleepiness occurs with ongoing sleep restriction over several days in their study. However, in the present study, there is no evidence of a putative habituation of subjective sleepiness over time. Unfortunately, MSLTs were not conducted in the Van Dongen study, so it is unclear whether sleep propensity would show a similar truncation over time.
Differences in how and when data have been collected may help to explain why previous studies have reported such wide variations in the strength of the relationship between subjective and objective sleepiness. The protocol utilized by Yang, Lin, and Spielman14 required participants to complete 1 trial at 09:00, 14:00, or 18:00. The time at which the subjects participated was self-selected, and there were differences in the numbers of participants from the eyes-open and eyes-closed groups that participated at any 1 of those times. The authors examined mean sleep latencies among the 3 times, which were not significantly different. Therefore, they may have found generally higher correlations had they used different times with predictably different sleepiness. However, they did not examine differences in mean subjective sleepiness across the 3 time points. Systematic differences in subjective sleepiness at each time point, coupled with the differences in the proportions of participants in the experimental and control groups at each time point, may have impacted upon their findings.
The inability of the different subjective-sleepiness judgment conditions (eyes-closed, eyes-open fixated, and eyes-open PVT) to induce differences in subjective sleepiness was a limitation of the present study. This could be remedied by conducting the eyes-open and eyes-closed conditions when the subject is in an environment more stimulating than a sleep laboratory bedroom. The only disadvantage of this would be the delay and change of context required to test sleep latency in a bedroom environment. In an applied or practical context, it could be argued that it is more relevant for an individual to be accurate in subjectively evaluating their sleep propensity than it is to be able to determine who of several different individuals is objectively sleepier, based on their subjective rating. The results of the present study show that, at least over a range of different clock times, there is significant validity in these subjective estimates of sleepiness for the large majority of individuals.
Conclusion
Assessing the accuracy of individuals' subjective sleepiness ratings has importance in both research and applied settings. The current results indicate that, under laboratory conditions of low stimulation, instituting an eyes-closed condition did not increase the correlation between subjective and objective sleepiness measures. Further research in which the eyes-closed procedure is used under more naturalistic and stimulating conditions is needed to evaluate whether this manipulation will lower transient activation and increase the accuracy of subjectively assessing sleep propensity in these contexts.
An important, yet unexpected finding is that, when circadian variation is controlled, the intrinsic individual differences in sleepiness in laboratory conditions did not yield significant subjective-objective correlations. In other words, individuals were reasonably accurate in detecting changes of objective sleepiness within themselves across a range of sleepiness. With somewhat diminished reliability, it is also possible to significantly identify objective sleepiness from the subjective sleepiness of different individuals as long as the judgments span across a considerable range of circadian sleepiness. However, when time of day is controlled, it is not possible to reliably identify those who are at greatest risk of falling asleep on the basis of their subjective rating of sleepiness. Thus, the variability of prior research findings may be due, at least partly, to the way in which correlations were derived. These results indicate that careful methodologic procedures are needed to accurately assess this relationship. It may be important for researchers examining this relationship to collect sleepiness data within participants, where possible, and across the spectrum of circadian variation or at varying levels of sleep loss or homeostatic sleep drive.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors wish to thank 2 anonymous reviewers for their thoughtful and generous suggestions.
REFERENCES
- 1.Benbadis SR, Mascha E, Perry MC, Wolgamuth BR, Smolley LA, Dinner DS. Association between the Epworth Sleepiness Scale and the Multiple Sleep Latency Test in a clinical population. Ann Intern Med. 1999;130:289–92. doi: 10.7326/0003-4819-130-4-199902160-00014. [DOI] [PubMed] [Google Scholar]
- 2.Chervin RD, Aldrich MS, Pickett R, Guilleminault C. Comparison of the results of the Epworth Sleepiness Scale and the Multiple Sleep Latency Test. J Psychosom Res. 1997;42:145–55. doi: 10.1016/s0022-3999(96)00239-5. [DOI] [PubMed] [Google Scholar]
- 3.Devoto A, Lucidi F, Violani C, Bertini M. Effects of different sleep reductions on daytime sleepiness. Sleep. 1999;22:336–43. doi: 10.1093/sleep/22.3.336. [DOI] [PubMed] [Google Scholar]
- 4.Johnson LC, Freeman CR, Spinweber CL, Gomez SA. Subjective and objective measures of sleepiness: Effect of benzodiazepine and caffeine on their relationship. Psychophysiology. 1991;28:65–71. doi: 10.1111/j.1469-8986.1991.tb03388.x. [DOI] [PubMed] [Google Scholar]
- 5.Carskadon MA, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107–13. doi: 10.1111/j.1469-8986.1981.tb02921.x. [DOI] [PubMed] [Google Scholar]
- 6.Chervin RD, Weatherley RA, Ruzicka DL, et al. Subjective sleepiness and polysomnographic correlates in children scheduled for adenotonsillectomy vs. other surgical care. Sleep. 2006;29:495–503. [PMC free article] [PubMed] [Google Scholar]
- 7.Gillberg M, Kecklund G, Akerstedt T. Relations between performance and subjective ratings of sleepiness during a night awake. Sleep. 1994;17:236–41. doi: 10.1093/sleep/17.3.236. [DOI] [PubMed] [Google Scholar]
- 8.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 9.Kaida K, Takahashi M, Akerstedt T, et al. Validation of the Karolinska sleepiness scale against performance and EEG variables. Clin Neurophysiol. 2006;117:1574–81. doi: 10.1016/j.clinph.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 10.LaFrance C, Dumont M. Diurnal variations in the waking EEG: comparisons with sleep latencies and subjective alertness. J Sleep Res. 2000;9:243–8. doi: 10.1046/j.1365-2869.2000.00204.x. [DOI] [PubMed] [Google Scholar]
- 11.Leproult R, Colecchia EF, Berardi AM, Stickgold R, Kosslyn SM, Van Cauter E. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am J Physiol Integr Comp Physiol. 2003;284:280–90. doi: 10.1152/ajpregu.00197.2002. [DOI] [PubMed] [Google Scholar]
- 12.Danker-Hopfe H, Kraemer S, Dorn H, Schmidt A, Ehlert I, Herrmann WM. Time of day variations in different measures of sleepiness (MSLT, pupillography, and SSS) and their interrelations. Psychophysiology. 2001;38:828–35. [PubMed] [Google Scholar]
- 13.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioural functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;2:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 14.Eriksen CA, Akerstedt T, Kecklund G, Akerstedt A. Comment on short-term variation in subjective sleepiness. Percept Mot Skills. 2005;101:943–8. doi: 10.2466/pms.101.3.943-948. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson JP, Soderstrom M, Karlsson AU, et al. Less effective executive functioning after one night's sleep deprivation. J Sleep Res. 2005;14:1–6. doi: 10.1111/j.1365-2869.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 16.Bonnet MH, Arand DL. Level of arousal and the ability to maintain wakefulness. J Sleep Res. 1999;8:247–54. doi: 10.1046/j.1365-2869.1999.00168.x. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto Y, Mishima K, Satoh K, Shimizu T, Hishikawa Y. Physical activity increases the dissociation between subjective sleepiness and objective performance levels during extended wakefulness in human. Neurosci Lett. 2002;326:133–6. doi: 10.1016/s0304-3940(02)00335-x. [DOI] [PubMed] [Google Scholar]
- 18.Yang C, Lin F, Spielman AJ. A standard procedure enhances the correlation between subjective and objective measures of sleepiness. Sleep. 2004;27:329–32. doi: 10.1093/sleep/27.2.329. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 20.Lovibond SH, Lovibond PF. Manual for the DASS. 2nd ed. Sydney, Australia: Psychology Foundation of Australia; 1995. [Google Scholar]
- 21.Carskadon MA, Dement WC, Mitter MM, Roth T, Westbrook PR, Keenan S. Guidelines for the Multiple Sleep Latency Test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 22.Rechtschaffen A, Kales A. Los Angeles, CA: Brain Information Service/Brain Research Institute; 1968. A Manual of Standardized Terminology, Techniques, and Scoring Systems for Sleep Stages in Human Subjects. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson RT, Houghton D. Field test of arousal: a portable reaction timer with data storage. Hum Factors. 1982;24:487–93. doi: 10.1177/001872088202400409. [DOI] [PubMed] [Google Scholar]
- 24.Hoddes E, Dement W, Zarcone V. The development and use of the Stanford Sleepiness Scale (SSS) Psychophysiology. 1972;9:150. [Google Scholar]
- 25.Silver NC, Dunlap WP. Averaging correlation coefficients: should Fisher's z transformation be used? J App Psyc. 1987;72:146–8. [Google Scholar]