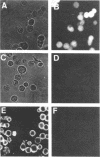
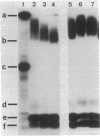
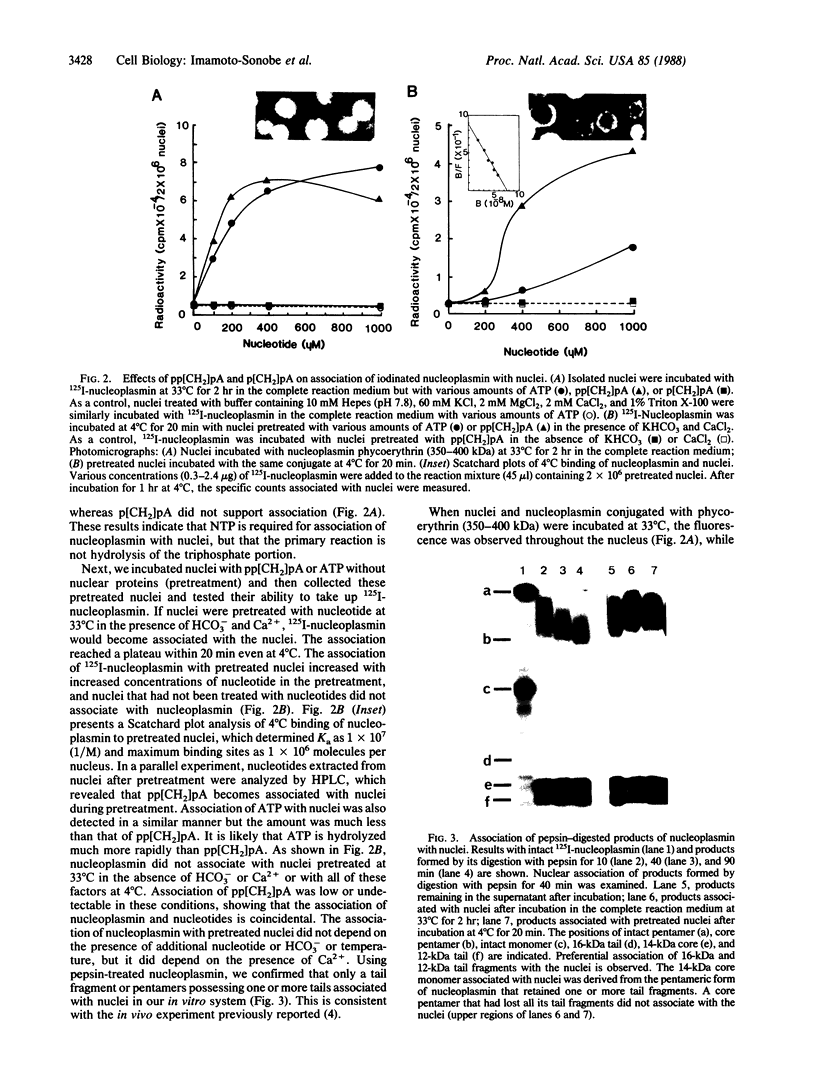
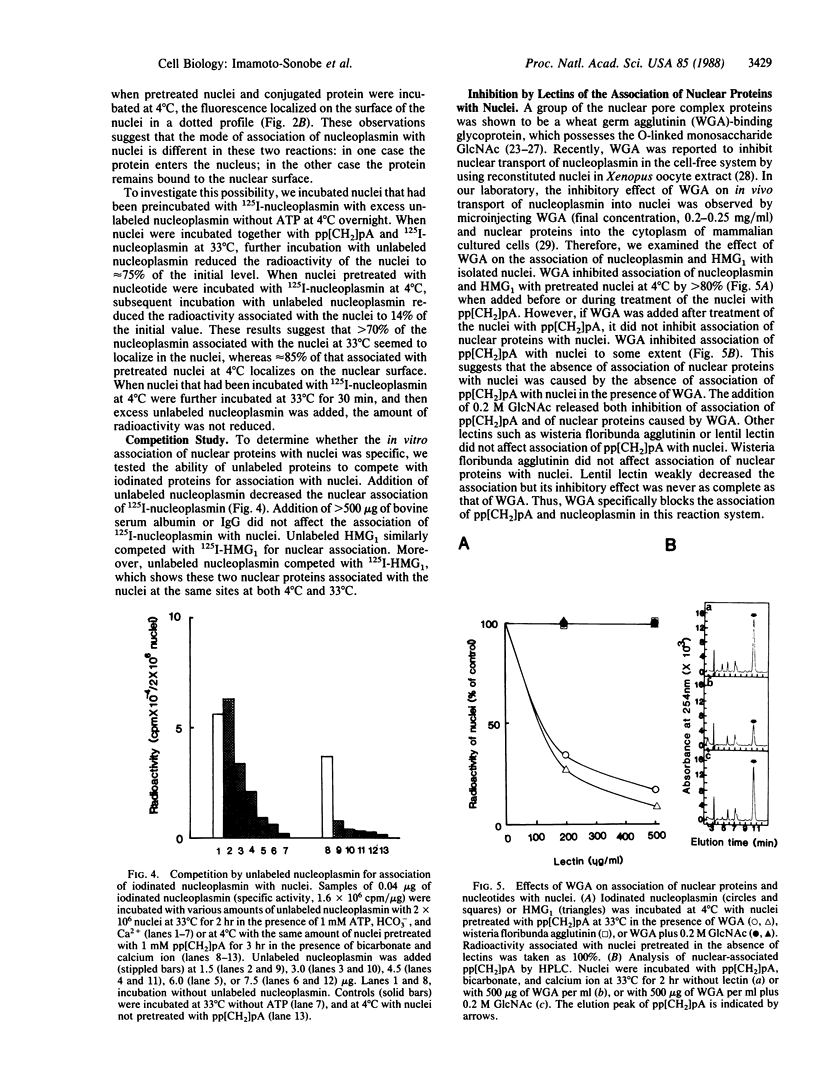
Abstract
In vitro association of Xenopus nucleoplasmin and mammalian nonhistone chromosomal high mobility group 1 (HMG1) protein with nuclei isolated from rat liver was examined. Efficient association of nuclear proteins with isolated nuclei requires ATP, HCO3-, and Ca2+. Association occurred at 33 degrees C but not at 4 degrees C. ATP could be replaced by adenosine 5'-[alpha,beta-methylene]triphosphate (pp[CH2]pA), a nonhydrolyzable ATP analog. pp[CH2]pA associated with nuclei at 33 degrees C and nucleoplasmin and HMG1 rapidly associated with the pp[CH2]pA-bound nuclei at 4 degrees C. Competition studies showed that these associations at both 33 degrees C and 4 degrees C were specific. More than 80% of the bindings of nuclear proteins to the nuclear surface were blocked by wheat germ agglutinin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson R. P., Blobel G. On the attachment of the nuclear pore complex. J Cell Biol. 1974 Sep;62(3):746–754. doi: 10.1083/jcb.62.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. I., Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986 Jun 6;45(5):699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Sharnick S. V., Laskey R. A. A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell. 1982 Sep;30(2):449–458. doi: 10.1016/0092-8674(82)90242-2. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Honda B. M., Laskey R. A., Thomas J. O. Assembly of nucleosomes: the reaction involving X. laevis nucleoplasmin. Cell. 1980 Sep;21(2):373–383. doi: 10.1016/0092-8674(80)90474-2. [DOI] [PubMed] [Google Scholar]
- Feldherr C. M., Kallenbach E., Schultz N. Movement of a karyophilic protein through the nuclear pores of oocytes. J Cell Biol. 1984 Dec;99(6):2216–2222. doi: 10.1083/jcb.99.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay D. R., Newmeyer D. D., Price T. M., Forbes D. J. Inhibition of in vitro nuclear transport by a lectin that binds to nuclear pores. J Cell Biol. 1987 Feb;104(2):189–200. doi: 10.1083/jcb.104.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A. Phycobilisomes of Porphyridium cruentum: pigment analysis. Biochemistry. 1974 Jul 2;13(14):2960–2966. doi: 10.1021/bi00711a027. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. S., Gariépy J., Schoolnik G., Kornberg R. D. Synthetic peptides as nuclear localization signals. Nature. 1986 Aug 14;322(6080):641–644. doi: 10.1038/322641a0. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Nicolas R. H., Johns E. W. An improved large scale fractionation of high mobility group non-histone chromatin proteins. Biochim Biophys Acta. 1975 Oct 20;405(2):280–291. doi: 10.1016/0005-2795(75)90094-x. [DOI] [PubMed] [Google Scholar]
- Hanover J. A., Cohen C. K., Willingham M. C., Park M. K. O-linked N-acetylglucosamine is attached to proteins of the nuclear pore. Evidence for cytoplasmic and nucleoplasmic glycoproteins. J Biol Chem. 1987 Jul 15;262(20):9887–9894. [PubMed] [Google Scholar]
- Holt G. D., Hart G. W. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem. 1986 Jun 15;261(17):8049–8057. [PubMed] [Google Scholar]
- Holt G. D., Snow C. M., Senior A., Haltiwanger R. S., Gerace L., Hart G. W. Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol. 1987 May;104(5):1157–1164. doi: 10.1083/jcb.104.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Richardson W. D., Markham A. F., Smith A. E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984 Sep 6;311(5981):33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kamiike W., Watanabe F., Hashimoto T., Tagawa K., Ikeda Y., Nakao K., Kawashima Y. Changes in cellular levels of ATP and its catabolites in ischemic rat liver. J Biochem. 1982 Apr;91(4):1349–1356. doi: 10.1093/oxfordjournals.jbchem.a133822. [DOI] [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Construction and characterization of an SV40 mutant defective in nuclear transport of T antigen. Cell. 1984 Jul;37(3):801–813. doi: 10.1016/0092-8674(84)90415-x. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Kanda P., Kennedy R. C. Induction of nuclear transport with a synthetic peptide homologous to the SV40 T antigen transport signal. Cell. 1986 Aug 15;46(4):575–582. doi: 10.1016/0092-8674(86)90883-4. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Honda B. M., Mills A. D., Finch J. T. Nucleosomes are assembled by an acidic protein which binds histones and transfers them to DNA. Nature. 1978 Oct 5;275(5679):416–420. doi: 10.1038/275416a0. [DOI] [PubMed] [Google Scholar]
- Mills A. D., Laskey R. A., Black P., De Robertis E. M. An acidic protein which assembles nucleosomes in vitro is the most abundant protein in Xenopus oocyte nuclei. J Mol Biol. 1980 May 25;139(3):561–568. doi: 10.1016/0022-2836(80)90148-5. [DOI] [PubMed] [Google Scholar]
- Newmeyer D. D., Finlay D. R., Forbes D. J. In vitro transport of a fluorescent nuclear protein and exclusion of non-nuclear proteins. J Cell Biol. 1986 Dec;103(6 Pt 1):2091–2102. doi: 10.1083/jcb.103.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner M., Kuehl L. Microinjection of the nonhistone chromosomal protein HMG1 into bovine fibroblasts and HeLa cells. Cell. 1979 Apr;16(4):901–908. doi: 10.1016/0092-8674(79)90105-3. [DOI] [PubMed] [Google Scholar]
- Roth J. Methods for assessing immunologic and biologic properties of iodinated peptide hormones. Methods Enzymol. 1975;37:223–233. doi: 10.1016/s0076-6879(75)37018-3. [DOI] [PubMed] [Google Scholar]
- Snow C. M., Senior A., Gerace L. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J Cell Biol. 1987 May;104(5):1143–1156. doi: 10.1083/jcb.104.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawa H., Imamoto N., Wataya-Kaneda M., Uchida T. Foreign protein can be carried into the nucleus of mammalian cell by conjugation with nucleoplasmin. Exp Cell Res. 1985 Aug;159(2):419–429. doi: 10.1016/s0014-4827(85)80015-x. [DOI] [PubMed] [Google Scholar]
- Tsuneoka M., Imamoto N. S., Uchida T. Monoclonal antibody against non-histone chromosomal protein high mobility group 1 Co-migrates with high mobility group 1 into the nucleus. J Biol Chem. 1986 Feb 5;261(4):1829–1834. [PubMed] [Google Scholar]
- Yamaizumi M., Uchida T., Okada Y., Furusawa M., Mitsui H. Rapid transfer of non-histone chromosomal proteins to the nucleus of living cells. Nature. 1978 Jun 29;273(5665):782–784. doi: 10.1038/273782a0. [DOI] [PubMed] [Google Scholar]
- Yoneda Y., Arioka T., Imamoto-Sonobe N., Sugawa H., Shimonishi Y., Uchida T. Synthetic peptides containing a region of SV 40 large T-antigen involved in nuclear localization direct the transport of proteins into the nucleus. Exp Cell Res. 1987 Jun;170(2):439–452. doi: 10.1016/0014-4827(87)90319-3. [DOI] [PubMed] [Google Scholar]
- Yoneda Y., Imamoto-Sonobe N., Yamaizumi M., Uchida T. Reversible inhibition of protein import into the nucleus by wheat germ agglutinin injected into cultured cells. Exp Cell Res. 1987 Dec;173(2):586–595. doi: 10.1016/0014-4827(87)90297-7. [DOI] [PubMed] [Google Scholar]