Abstract
Study Objectives:
We studied the effects of sleep deprivation on executive functions using a task battery which included a modified Sternberg task, a probed recall task, and a phonemic verbal fluency task. These tasks were selected because they allow dissociation of some important executive processes from non-executive components of cognition.
Design:
Subjects were randomized to a total sleep deprivation condition or a control condition. Performance on the executive functions task battery was assessed at baseline, after 51 h of total sleep deprivation (or no sleep deprivation in the control group), and following 2 nights of recovery sleep, at fixed time of day (11:00). Performance was also measured repeatedly throughout the experiment on a control task battery, for which the effects of total sleep deprivation had been documented in previously published studies.
Setting:
Six consecutive days and nights in a controlled laboratory environment with continuous behavioral monitoring.
Participants:
Twenty-three healthy adults (age range 22–38 y; 11 women). Twelve subjects were randomized to the sleep deprivation condition; the others were controls.
Results:
Performance on the control task battery was considerably degraded during sleep deprivation. Overall performance on the modified Sternberg task also showed impairment during sleep deprivation, as compared to baseline and recovery and compared to controls. However, two dissociated components of executive functioning on this task—working memory scanning efficiency and resistance to proactive interference—were maintained at levels equivalent to baseline. On the probed recall task, resistance to proactive interference was also preserved. Executive aspects of performance on the phonemic verbal fluency task showed improvement during sleep deprivation, as did overall performance on this task.
Conclusion:
Sleep deprivation affected distinct components of cognitive processing differentially. Dissociated non-executive components of cognition in executive functions tasks were degraded by sleep deprivation, as was control task performance. However, the executive functions of working memory scanning efficiency and resistance to proactive interference were not significantly affected by sleep deprivation, nor were dissociated executive processes of phonemic verbal fluency performance. These results challenge the prevailing view that executive functions are especially vulnerable to sleep loss. Our findings also question the idea that impairment due to sleep deprivation is generic to cognitive processes subserved by attention.
Citation:
Tucker AM; Whitney P; Belenky G; Hinson JM; Van Dongen HPA. Effects of sleep deprivation on dissociated components of executive functioning. SLEEP 2010;33(1):47-57.
Keywords: Executive functions, cognitive performance, working memory, Sternberg task, probed recall, verbal fluency, psychomotor vigilance test (PVT), digit–symbol substitution task (DSST), subjective sleepiness, total sleep deprivation
SLEEP LOSS IS A GROWING THREAT TO SAFETY IN MODERN SOCIETIES, AS BOTH WORK HOURS AND COMMUTE TIMES ARE EXTENDED.1 SLEEP LOSS impairs performance on simple cognitive tasks such as signal detection and reaction time (RT) tests.2 Many occupational settings, however, require executive functioning—the ability to initiate, monitor, and stop actions so as to achieve goals3—in order to execute complex tasks such as interpersonal communication, creative problem solving, and decision making.4 Thus, an important question is to what extent executive functions are impaired by sleep loss.5 The real-world relevance of this question is illustrated by occupational disasters including the nuclear meltdown of Chernobyl, the grounding of the Exxon Valdez, and the disastrous launch decision of the Challenger space shuttle, all of which involved complex decision errors for which sleep loss has been cited to be a contributing factor.
Several studies have examined deficits in executive functioning during sleep deprivation.6–20 Between studies there has been considerable inconsistency as to whether and how executive functions were found to be impaired.21 For example, two recent studies found that sleep deprivation impaired performance on a go/no-go task,8,9 which is typically considered to measure the ability to inhibit a prepotent response. Another study, using Stroop task performance as an index of ability to inhibit a prepotent response, reported that this executive function was not impaired during sleep deprivation.19 Similarly, one study reported that sleep deprivation changed behavioral decisions involving risk on a lottery choice task,16 while another study using a different gambling task observed no significant differences in choices made after sleep loss.20 Inconsistencies like these have made it difficult to derive a uniform account of whether and how sleep deprivation affects executive functions.
Horne and colleagues12 have posited that sleep deprivation especially impairs performance on tasks tapping executive functions because these tasks selectively rely on the prefrontal cortex. A basis for this theory is provided by EEG-based and neuroimaging evidence that sleep loss affects the frontal lobes more than most other brain areas. For instance, studies have shown that sleep pressure, as operationalized by increased theta power density in the waking EEG, is most evident in frontal areas during total sleep deprivation (TSD).22,23 Using PET neuroimaging, which allows greater anatomical specificity, it has been documented that TSD decreases metabolism specifically in the prefrontal cortex.24 From findings like these it has been inferred that sleep loss would impair executive functioning and performance on tasks that rely on prefrontal cortical function more than non-executive task performance. In this vein, a parallel between the cognitive impairments seen in sleep deprivation and those seen in aging has been hypothesized,12 as both conditions seem to selectively involve reduced activity in the prefrontal cortex.
There is ample evidence, however, that sleep deprivation also impairs performance on cognitive tasks requiring relatively little executive control.25 This includes the psychomotor vigilance test (PVT), a simple RT task measuring sustained attention.26 Based in part on detailed analyses of RT data from the PVT, Dinges and colleagues postulated that performance impairment during sleep deprivation is caused by an increase in moment-to-moment variability of attention resulting from the interaction of the homeostatic drive for sleep, the circadian drive for wakefulness, and compensatory effort to perform.27 They hypothesized that the variability in performance due to difficulty sustaining attention would transfer to a wide variety of cognitive tasks since “attention is a requirement of many goal-directed activities.”27 According to this “state instability” theory, sleep deprivation does not necessarily cause impairments in executive functions tasks because of selective deficits in the prefrontal cortex, but at least in part due to deficits in the ability to sustain attention. The theory implies that through impairment of sustained attention, sleep deprivation affects cognitive performance globally, including not only executive functioning and other higher order cognitive processes, but many other aspects of performance as well.
One reason that different views exist as to how and why executive functioning may be degraded during sleep deprivation is that the tasks commonly used to measure executive functions do not allow dissociation of the various cognitive processes contributing to performance. By definition, executive functions operate on other cognitive processes, and any task that targets executive functions therefore also implicates non-executive cognitive processes (i.e., the task impurity problem).3 As such, a low score on an executive functions test does not necessarily arise from impairment of the target executive functions; it could also result from impairment of other component cognitive processes involved in the task.28
In the present laboratory study, we investigated sleep-deprived performance on an executive functions battery. The tasks in the battery were selected because they allow for the dissociation of some of the intertwined components of cognitive performance. Our battery made it possible to isolate 2 specific executive function components: working memory scanning efficiency, and suppression of irrelevant information that leads to proactive interference (that is, inhibition of information that is no longer relevant). Both of these executive function components involve the prefrontal cortex.29,30 They are associated with working memory capacity, and are fundamentally important to executive control during complex task performance.28 We investigated the extent to which working memory scanning efficiency, resistance to proactive interference, and other elements of task performance are affected by acute TSD.
METHODS
Subjects
Subjects eligible for participation in the study met the following criteria: age 22–40 years; physically and psychologically healthy, as assessed by physical examination and history; no clinically significant abnormalities in blood chemistry; free of traces of drugs, as assessed by urine screen and breathalyzer; no sleep or circadian disorder, as assessed by questionnaires and baseline polysomnography; no history of brain injury; not a current smoker by self-report; and not pregnant, as assessed by blood test. In addition, they reported to have good habitual sleep, between 6 h and 10 h in duration daily; regular bedtimes, getting up between 06:00 and 09:00; no shift work within 3 months of entering the study; and no travel across time zones within one month of entering the study. Furthermore, they had normal or corrected to normal vision and hearing, and were native English speakers.
A total of 23 subjects (12 men, 11 women; age range 22–38 y) passed the screening criteria and completed the study. A power calculation performed in advance of the study indicated that this sample size should suffice to detect previously reported effects31 of TSD on PVT performance, and by implication on executive components of cognitive performance if these are particularly vulnerable to sleep deprivation as has been posited.12 To assess subjects' baseline intellectual functioning, they were asked to complete the Shipley Institute of Living Scale.32 There was no significant difference between the 2 groups in subjects' vocabulary score (t21 = 1.15, P = 0.26) and overall intellectual functioning score (t21 = 0.91, P = 0.37) on this instrument. The subjects had on average 14.3 years of education (minimally they completed high school), with no significant difference between the 2 groups (t21 = 0.03, P = 0.97).
The study was approved by the Institutional Review Board of Washington State University, and all subjects gave written informed consent.
Experimental Design
Subjects were in a laboratory for 6 consecutive days and nights (Figure 1). Subjects were randomized to either a TSD condition (N = 12; 7 men, 5 women; age range 22–37 y) or a control condition (N = 11; 5 men, 6 women; age range 22–38 y). In the TSD group, subjects first received 2 baseline nights, each with 10 h time in bed (TIB) for sleep. They were then kept awake for 62 h of TSD, which entailed missing the next 2 nights of sleep. Finally, they were allowed 2 recovery nights, each with 10 h TIB. In the control group, subjects received 10 h TIB for sleep every night. All TIB periods were from 22:00 until 08:00. Polysomnographically assessed total sleep time was 8.9 h on average during the first baseline night (not significantly varying by group: t21 = 0.48, P = 0.63) and 8.7 h on average during the second baseline night (not significantly varying by group: t21 = 1.10, P = 0.28).
Figure 1.
Laboratory study design. Subjects stayed inside the laboratory from 15:00 on day 1 until 22:00 on day 7. Black areas represent 10 h nocturnal periods of time in bed for sleep (22:00–08:00). Gray areas represent 10 h nocturnal periods (22:00–08:00) when subjects in the TSD group were kept awake while subjects in the control group were in bed to sleep. The subjects in the TSD group stayed awake continuously for a total of 62 h during the study. Diamonds indicate the 3 administrations of the executive functions task battery (11:00) at 48-h intervals: after 3 h of scheduled wakefulness at baseline; after 51 h of continuous wakefulness in the TSD group, or 3 h of scheduled wakefulness in the control group; and after 3 h of scheduled wakefulness following 2 nights of recovery sleep. Dots indicate the repeated administrations of the control task battery (bullets overlapping gray background denote test bouts performed in the TSD condition only). The first 2 administrations of the control task battery served as practice bouts. Tick marks denote time of day.
The experiment was conducted in the controlled laboratory environment of the Sleep and Performance Research Center at Washington State University Spokane. Up to 4 subjects were in the laboratory at one time; each had an isolated room for sleep and performance testing. The laboratory was temperature-controlled (21 ± 1°C). Light levels were fixed ( < 100 lux) during scheduled wakefulness, and lights were off during scheduled sleep periods. No visitors or phone calls were allowed. Meals were provided every 4 waking hours. Between performance test bouts and meals, subjects were permitted only non-vigorous activities. Subjects' behavior was monitored throughout the experiment by trained research assistants.
During the experiment, as well as in the 7 days leading up to the experiment, subjects were not allowed to use caffeine, alcohol, or tobacco products. During the 7 days before the study they were required to keep their regular bedtimes and to refrain from daytime napping. Compliance was assessed by wrist actigraphy, sleep diary, and a time-stamped voice recorder which subjects called at bedtime and upon awakening. Grand average daily sleep time during the pre-study week was 7.6 h as assessed by actigraphy, with a trend for the control group sleeping on average 0.8 h more than the TSD group (F1,96 = 3.6, P = 0.063).
Executive Functions Task Battery
The executive functions task battery was composed of tasks selected specifically for their properties allowing the dissociation of important executive functions from non-executive components of cognition. The battery consisted of a modified Sternberg task, a probed recall task, and a phonemic verbal fluency task, which are described below. The battery was administered during baseline, after 51 h of TSD (or no sleep deprivation in the control group), and following 2 nights of recovery sleep (see Figure 1). The baseline measurement was scheduled on day 3, after 2 nocturnal sleep periods in the laboratory, to ensure that subjects were fully acclimated and rested. The sleep deprivation measurement was scheduled after 51 h awake, so that subjects had accrued a high homeostatic drive for sleep and were close to the circadian nadir in alertness during TSD.33 The corresponding measurement in the control condition was scheduled after 3 h awake, which avoided the hours immediately after awakening when performance could be affected by sleep inertia. The recovery measurement was scheduled after two 10 h sleep opportunities, allowing recuperation of performance.31 The task battery was administered at the same time of day, 11:00, for all 3 test sessions in both experimental conditions, in order to preclude circadian confounds.
The executive functions performance tasks were administered only 3 times, to minimize practice effects and retain a high level of novelty. Because task novelty promotes the validity of tests of executive functioning,3 three different but equivalent versions of each of the 3 executive functions performance tasks were administered, in randomized order across the 3 test sessions. Furthermore, the order of the tasks within the battery was randomized over subjects, to control for any carry-over effects from one test to the next. The executive functions task battery took approximately 50 min to complete.
Modified Sternberg Task
In the classic Sternberg task,34 subjects are shown a set of items to be held in working memory, and then a probe item. They are asked to indicate, as quickly and accurately as possible, whether or not the probe item was in the memory set. The number of items in the memory set (i.e., set size) is varied across trials. The relationship between RT and set size, which is linear,34 reflects working memory scanning efficiency. Our version of the Sternberg task combined the original task with a modified version of the task,28,35,36 allowing us to separate out 2 distinct components of executive functioning (as outlined below). Each test bout contained 128 trials; for every trial, subjects were required to respond within a 2-s window. The test items were consonant letters.
Our modified Sternberg task contained memory sets of 2 items and 4 items (50% of each). Per standard procedure, the linear relationship between RT and memory set size (2 versus 4 items) was described in terms of a slope and intercept.37 The slope is a measure of the executive functions component of working memory scanning efficiency. The intercept captures the other, largely non-executive, component processes involved in performing the task, such as probe encoding, response selection, and motor execution of the response.
Fifty percent of probes were positive probes, meaning that they were in the memory set (i.e., the correct response was “yes”); and 50% of probes were negative (i.e., the correct response was “no”). To examine susceptibility to proactive interference in working memory, the recency of negative probes was manipulated: they were either recent (seen in the previous trial memory set) or non-recent (50% of each). The difference in RTs between recent and non-recent negative probes is a measure of the ability to resist proactive interference, an important executive function.38
For RT analyses, only data from trials in which a correct response was made were used. Using analyses equivalent to those used for RT, accuracy was also examined as an outcome variable, as was the number of errors of omission (failures to respond within the 2-s window permitted).
Probed Recall Task
We used a probed recall task developed by Bunting.39 In each trial of this task, subjects were shown 12 items, one at a time, and were then asked to recall either the first 4 items, middle 4 items, or last 4 items in the correct order. Subjects were instructed to respond as quickly and as accurately as possible. Each test bout contained 12 trials, in which the items could be either words or digits. Half of the trials were set up to induce interference—the item type was either all words or all digits throughout the list. The other half of the trials were set up to release interference—the item type switched for the last 4 items from words to digits or vice versa. The difference in recall scores for the last 4 items between interference-maximum and interference-release trials provides a measure of resistance to proactive interference.
Phonemic Verbal Fluency Task
We used the Controlled Oral Word Association Test,40 a verbal fluency task used in previous sleep deprivation studies.6,41 Subjects were given a letter as a prompt and were then asked to generate as many words as possible that begin with this letter in a 1-min interval. The procedure was repeated for 2 additional letters, for a total of 3 trials per test bout. Three standard versions of this test were employed, with start letters of F-A-S, P-R-W, or C-F-L.42 At the beginning of each test bout, a trained research assistant read instructions following a standard script,43 which explained the task and indicated what constituted correct and incorrect responses. Subjects' verbal responses were recorded digitally and double scored afterwards by trained research assistants. Because of equipment problems, the data of 2 subjects in the control group were incomplete and had to be discarded.
As in previous studies, the total number of words generated was examined. In addition, 2 variables representing dissociable components of fluency performance were analyzed: average phonemic cluster size (defined as the average number of phonemically related words minus one), which is believed to represent automatic (non-executive) processing; and number of switches between phonemic clusters, which is believed to represent executive processing related to cognitive flexibility and mental set shifting.44 Two types of errors were also examined: perseverative errors, i.e., the number of times that a subject repeated the same word; and non-perseverative errors, which include the number of non-words, number of proper nouns, number of words repeated with a different ending, and number of words that began with a different (i.e., wrong) letter.
Control Task Battery
A battery of control tests was administered at 2-h intervals throughout most of the waking periods (Figure 1). This battery contained the following tests, for which the effects of TSD have been documented previously45: the Psychomotor Vigilance Test (PVT),46 the Digit–Symbol Substitution Task (DSST),47 and the Karolinska Sleepiness Scale (KSS).48 The KSS was administered both at the beginning and at the end of the test battery. Here, we report the results from the KSS administered at the end, where it shows increased sensitivity to sleep deprivation.33 It took approximately 15 min to complete each test bout.
Psychomotor Vigilance Test
We used the 10-min original version of the PVT,46 which is considered a standard measure of the effects of sleep deprivation on behavioral alertness.26,49 Subjects were required to respond as quickly as possible to a visual stimulus; presentations of this stimulus occurred randomly at intervals of 2 s to 10 s. Our primary outcome variable was the total number of lapses, defined as the number of RTs ≥ 500 ms.26
Digit Symbol Substitution Task
We used a 3-min computerized version of the cognitive performance test of the same name in the Wechsler Adult Intelligence Scale.47 In this task, subjects were shown a key whereby symbols are randomly associated with numbers. The key varied between test bouts but stayed the same within each test bout. Throughout the test bout, symbols were presented, one a time, and the subject typed the corresponding numbers as quickly and as accurately as possible. The total number of correct responses was used as the outcome measure. This task is known to display a practice effect continuing across dozens of repeated test bouts.45
Karolinska Sleepiness Scale
To measure subjective sleepiness, we used the KSS.48 This is a Likert-type rating scale on which subjects rated their sleepiness, ranging from 1 (very alert) to 9 (very sleepy).
Statistical Analyses
For the executive functions tasks, we used mixed-effects analysis of variance (ANOVA) of group (TSD, control) by session (baseline, deprivation/control, recovery). The result of primary interest was the interaction of group by session. Planned comparisons were made for the TSD group between the first (baseline) and second (deprivation) sessions and between the second and third (recovery) sessions. Further planned comparisons were made for the first (baseline) session between the TSD group and the control group, and for the second session between the 2 groups controlling for baseline. The task version (which was randomized across sessions within subjects) and the order of tasks in the battery (which was randomized between subjects) were included as covariates.
For the modified Sternberg task, mixed-effects ANOVA first focused on average RT, percent accurate, and number of errors of omission per test bout. In further analyses, components of cognitive performance were dissociated by considering the linear relationship between RT and memory set size. The slope and intercept of this relationship were calculated for each test bout, using only trials where the probe was non-recent. Separate mixed-effects ANOVAs were then run for the slope and for the intercept. An additional analysis of the modified Sternberg task focused on the trials inducing proactive interference through recency. Only memory set sizes of 4 were considered, as these produce the most robust recency interference effect. The average difference of RTs between recent and non-recent trials that were negative (i.e., where the probe was not in the memory set) was calculated. A mixed-effects ANOVA was run on this measure of the recency effect. The above analyses of the modified Sternberg task were repeated using accuracy instead of average RT as the dependent variable.
For the probed recall task, mixed-effects ANOVA first focused on percent recall overall. A subsequent analysis focused on recall of the last 4 items, which had been manipulated to induce or release interference. The difference in recall scores between interference-maximum and interference-release trials was calculated as a measure of resistance to proactive interference, and subjected to mixed-effects ANOVA.
For phonemic verbal fluency, the following outcome variables were assessed: total number of words generated, average phonemic cluster size, number of switches between phonemic clusters, number of perseverative errors, and number of non-perseverative errors. Separate mixed-effects ANOVAs were run for these 4 variables.
For the control task battery, we used mixed-effects ANOVA of group by test bout. Only the 29 test bouts that the 2 groups had in common were included (thus, the test bouts administered during sleep deprivation while the control group was asleep were omitted). The first 2 test bouts of the first day were practice bouts and were not included in the analysis. The last test bout of the study was also not included, to control for any end-of-study effects. The result of primary interest was the interaction of group by test bout.
Results are reported as mean ± standard error of the mean. Graphs show results derived from the mixed-effects ANOVAs. For the executive functions tasks, these are therefore controlled for the task version and task order covariates.
RESULTS
Control Task Battery
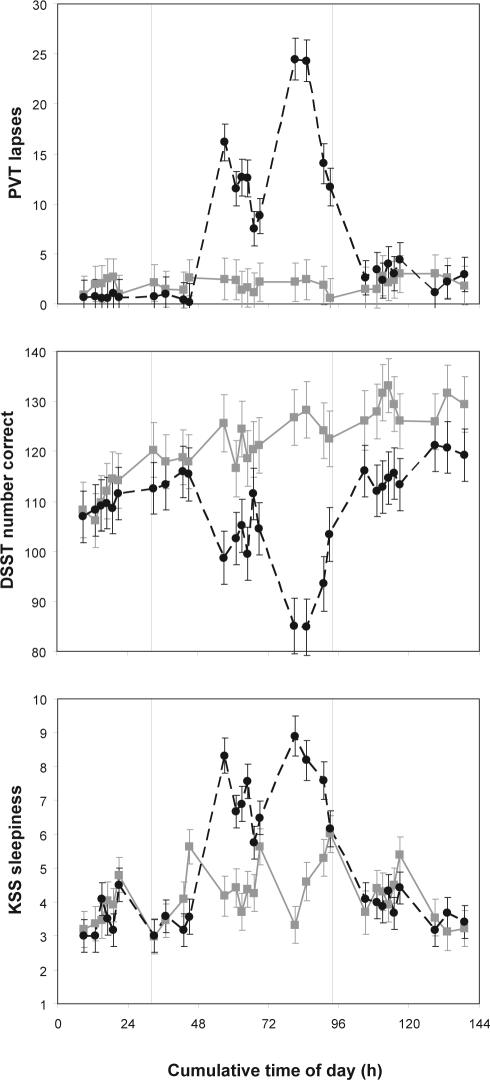
Figure 2 shows the response profiles in the TSD group and in the control group across the test bouts for the control task battery. These profiles show the expected homeostatic and circadian responses to sleep deprivation and the recuperative effect of recovery sleep in the TSD group, in close agreement with published data,31,33 and the expected absence of such progressive changes in the control group.
Figure 2.
Performance on the control task battery. The top panel displays number of lapses on the PVT (upwards is worse performance). The middle panel shows number of correct responses on the DSST (downwards is worse performance). The bottom panel shows subjective sleepiness as rated on the KSS (upwards is greater sleepiness). Means ± standard errors are shown for the TSD group (black circles) and the control group (gray boxes), across all test bouts that the 2 groups had in common (i.e., test bouts administered at night for the sleep-deprived subjects are not shown). The abscissa shows cumulative clock time (e.g., 24 is midnight of the second day, 48 is midnight of the third day). The 62-h period of TSD took place between hours 32 and 94 (vertical dashed lines).
For the number of lapses on the PVT, there was a significant interaction of group by test bout (F28,511 = 9.1, P < 0.001). Subjects in the TSD group exhibited significantly more lapses during the sleep deprivation portion of the experiment. For the number of correct responses on the DSST, there was also a significant interaction of group by test bout (F28,511 = 8.1, P < 0.001). Compared to controls, subjects in the TSD group produced significantly fewer correct responses during the sleep deprivation portion of the experiment. This effect was superimposed on the practice effect normally seen for this task.45 For sleepiness ratings on the KSS, there was likewise a significant interaction of group by test bout (F28,511 = 7.5, P < 0.001). Subjects in the TSD group reported significantly greater sleepiness during the sleep deprivation portion of the experiment. These results confirm that our experimental sleep deprivation manipulation was successful.
Executive Functions Task Battery
Modified Sternberg Task
TSD significantly slowed overall RTs (F2,40 = 13.2, P < 0.001), significantly decreased accuracy (F2,40 = 4.4, P = 0.019), and significantly increased errors of omission (F2,40 = 12.1, P < 0.001), relative to baseline and recovery and relative to the control group. Errors of omission constituted less than 1% of the data during all sessions in both groups, except during sleep deprivation in the TSD group, when they increased to 8% ± 1%. Accuracy was in the 95% to 97% range during all sessions in both groups, except during sleep deprivation in the TSD group, when it was 92% ± 1%. Thus, overall performance on the modified Sternberg task, which taps important executive functions, was significantly degraded during sleep deprivation. However, as noted above, degradation of performance on a task that involves executive functions could be due to non-executive components of the task. Therefore, we examined dissociated components of the modified Sternberg task to determine the sources of the overall impairment.
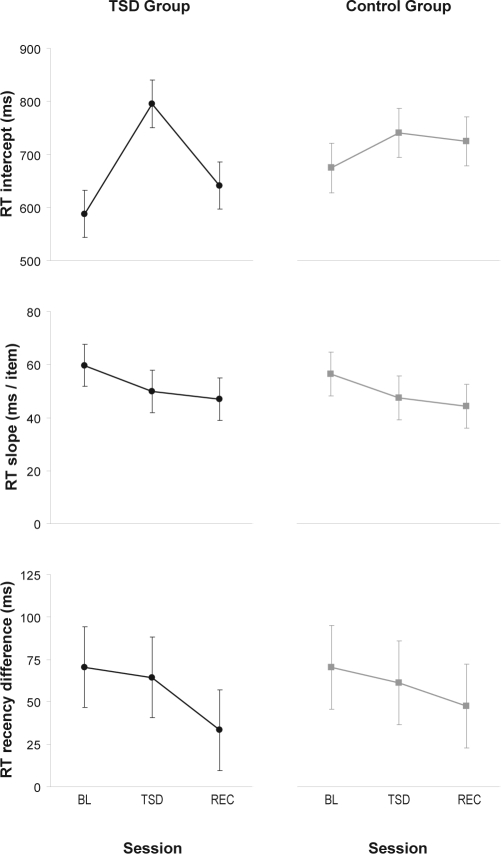
The slope of the linear relationship between RT and memory set size dissociates working memory scanning efficiency, an executive function, from the largely non-executive component processes reflected in the intercept.34 The intercept at baseline was 588 ± 45 ms in the TSD group and 675 ± 46 ms in the control group, which was not significantly different (t40 = –1.3, P = 0.19). The slope (RT increase per item) at baseline was 60 ± 8 ms in the TSD group and 56 ± 8 ms in the control group, which also was not significantly different (t40 = 0.3, P = 0.78). These values are close to those reported in another study using a similar implementation of the task.50
For the RT intercept, there was a significant interaction of group by session (F2,40 = 10.6, P < 0.001). Within the TSD group, in the second (sleep deprivation) session the intercept was 207 ± 24 ms longer than at baseline (t40 = 8.5, P < 0.001), and 154 ± 24 ms longer than after recovery (t40 = −6.3, P < 0.001). Also, in the second session, the intercept was significantly longer in the TSD group than in the control group by 141 ± 35 ms (controlling for baseline, t40 = 4.0, P < 0.001). See Figure 3, top panels.
Figure 3.
Performance on the modified Sternberg task. The top panels show the intercept of the linear relationship between memory set size and RT, which measures the non-executive component processes involved in performing the task (e.g., encoding the probe, deciding on a response, and executing the motor response). The middle panels display the slope of the linear relationship between memory set size and RT, which measures the executive functions component of working memory scanning efficiency. The bottom panels display the difference in RT between recent and non-recent negative probes, which measures the executive function of resistance to proactive interference. Means ± standard errors are shown for baseline (BL), total sleep deprivation or control (TSD/CTRL), and recovery (REC), in the TSD group (left panels) and the control group (right panels).
For the RT slope variable, there was no significant interaction of group by session (F2,40 < 0.01, P > 0.99). Planned comparisons revealed no significant difference in the TSD group between baseline and sleep deprivation (t40 = −1.2, P = 0.24) and between sleep deprivation and recovery (t40 = −0.4, P = 0.72), and in the second session between the TSD group and the control group (contolling for baseline, t40 = −0.1, P = 0.94). See Figure 3, middle panels.
The difference in RTs between recent and non-recent negative probes with a memory set size of 4 was examined to assess resistance to proactive interference.38 At baseline, this RT difference was 70 ± 24 ms in the TSD group, and 70 ± 25 ms in the control group (|t40| < 0.01, P > 0.99). These values are in line with the RT increase associated with proactive interference as reported in other studies using similar tasks.36,38 There was no significant interaction of group by session (F2,40 = 0.1, P = 0.92). Planned comparisons revealed no significant difference in the TSD group between baseline and sleep deprivation (t40 = −0.2, P = 0.85) and between sleep deprivation and recovery (t40 = −1.0, P = 0.34), and in the second session between the 2 groups (controlling for baseline, t40 = 0.1, P = 0.94). See Figure 3, bottom panels.
Analogous to the RT analyses, the intercept and slope of the linear relationship between accuracy and memory set size were determined. There were no significant interactions of group by session for the accuracy intercept (F2,40 = 0.1, P = 0.95) and slope (F2,40 = 1.7, P = 0.21). In addition, the difference in accuracy between recent and non-recent negative probes with a memory set size of 4 was assessed. There was no significant interaction of group by session for this outcome variable either (F2,40 = 0.6, P = 0.54).
Probed Recall Task
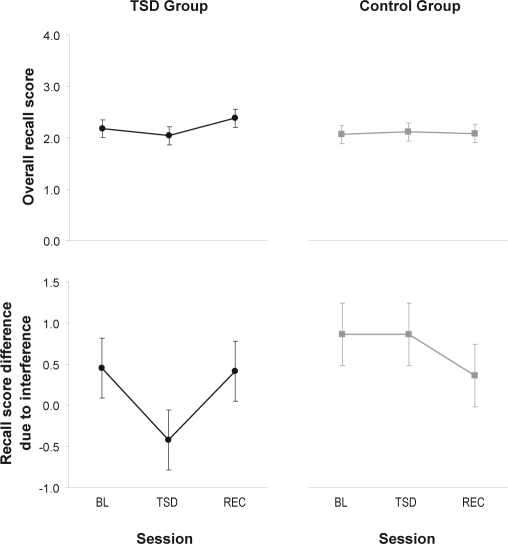
There was no statistically significant group by session interaction for overall accuracy on the probed recall task (F2,40 = 1.3, P = 0.29). However, planned comparisons showed that performance during sleep deprivation was significantly reduced compared to performance following recovery sleep in the TSD group (t40 = 2.14, P = 0.039), although no statistical significance was found for the comparison with baseline (t40 = –0.9, P = 0.39) or with the control group (t40 = –0.8, P = 0.42). See Figure 4, top panels.
Figure 4.
Performance on the probed recall task. The top panels show overall number of items recalled accurately. Higher on the ordinate indicates greater accuracy. The bottom panels show the difference in the number of items recalled between interference-release trials and interference-maximum trials. Lower on the ordinate represents greater resistance to proactive interference. Negative difference scores indicate that, paradoxically, more items were recalled in the trials set up to induce interference. Means ± standard errors are shown for baseline (BL), total sleep deprivation or control (TSD/CTRL), and recovery (REC), in the TSD group (left panel) and the control group (right panel).
At baseline, subjects in the TSD group recalled 0.45 ± 0.36 items more from the last 4 items of the interference-release trials than from those of the interference-maximum trials. Subjects in the control group recalled 0.86 ± 0.38 items more in the interference-release trials than in the interference-maximum trials. Thus, the phenomenon of release of proactive interference that should be seen in this task39 was confirmed at baseline (t40 = 2.5, P = 0.017), and there was no significant difference between the 2 groups (t40 = –0.78, P = 0.44).
There was no significant group by session interaction for this interference effect (F2,40 = 1.8, P = 0.19). In the TSD group, a planned comparison revealed a trend towards significance between baseline and sleep deprivation (t40 = −1.8, P = 0.09). The difference in recall between interference-maximum trials and interference-release trials was reduced by 0.88 ± 0.50 in the sleep deprivation session compared to the baseline session, such that subjects actually recalled 0.42 ± 0.36 items more from the last 4 items of the interference-maximum trials than from those of the interference-release trials when they were sleep deprived. There was a reverse trend in the comparison between the sleep deprivation session and the recovery sessions (t40 = 1.7, P = 0.10). However, relative to baseline, the TSD group was not significantly different from the control group during the second test session (t40 = −1.2, P = 0.24). See Figure 4, bottom panels.
Phonemic Verbal Fluency Task
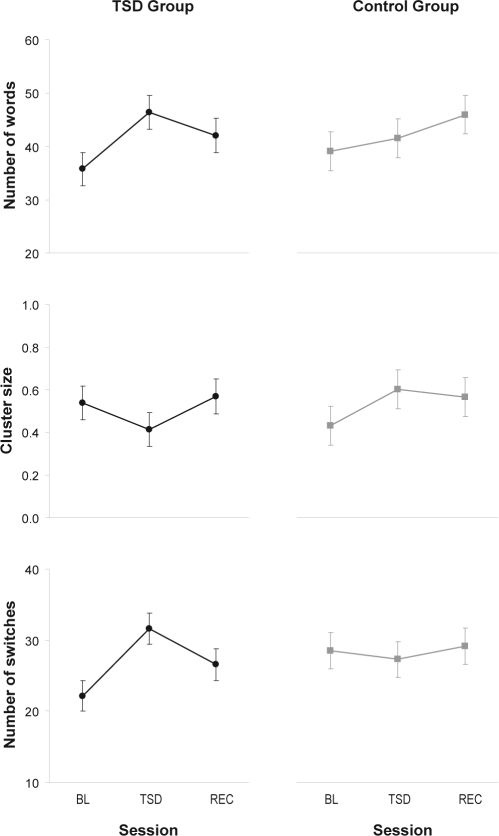
At baseline, subjects in the TSD group generated 39.9 ± 7.6 words, and subjects in the control group generated 42.3 ± 10.1 words (not significantly different: t35 = –0.7; P = 0.49); this is in line with published reports.44 For the number of words generated, there was an interaction of group by session (F2,35 = 5.1, P = 0.011). In both groups, there was a practice effect. In the TSD group, planned comparisons revealed a significant increase in number of words from the baseline session to the sleep deprivation session (t35 = 5.3, P < 0.001). There was a smaller but significant decrease from the sleep deprivation session to the recovery session (t35 = −2.1, P = 0.044). Relative to baseline, the number of words generated in the second session was 8.1 ± 3.0 higher in the TSD group than in the control group (t35 = 2.7, P = 0.011), indicating that the increase in the number of words during the sleep deprivation session occurred on top of the increase already expected because of the practice effect. See Figure 5, top panels.
Figure 5.
Performance on the phonemic verbal fluency task. The top panels display the number of words generated per test bout, representing global performance on the task. The middle panels display the average phonemic cluster size (where cluster size is defined as the number of phonemically related words minus 1), representing automatic (non-executive) processing. The bottom panels display the number of switches between phonemic clusters, which measures executive processing related to cognitive flexibility and mental set shifting. Means ± standard error are shown for baseline (BL), total sleep deprivation or control (TSD/CTRL), and recovery (REC), in the TSD group (left panels) and the control group (right panels).
We also examined dissociated components of cognition by considering average phonemic cluster size, which represents automatic processing, and number of switches between phonemic clusters, which represents executive processing.44 For average phonemic cluster size, there was no significant interaction of group by session (F2,35 = 2.0, P = 0.15). However, there was a trend for a difference between the 2 groups during the second session, controlling for baseline, with sleep deprivation resulting in somewhat smaller average cluster size (t35 = –2.0, P = 0.054). See Figure 5, middle panels. For the number of switches between phonemic clusters, there was a significant interaction of group by session (F2,35 = 5.7, P = 0.007). In the TSD group, subjects made significantly more switches between phonemic clusters when sleep deprived than during baseline (t35 = 4.5, P < 0.001) and after recovery (t35 = –2.3, P = 0.026). Additionally, during the second (sleep deprivation) session, subjects in the TSD group made significantly more switches than rested control as expressed relative to baseline (t35 = 3.3, P = 0.002). See Figure 5, bottom panels.
There was no significant group by session interaction for perseverative errors (F2,35 = 0.1, P = 0.95). There was similarly no significant group by session interaction for non-perseverative errors (F2,35 = 0.5, P = 0.61).
DISCUSSION
Experimental Findings
We investigated the effects of 51 h of sleep deprivation on a modified Sternberg task,28 a probed recall task,39 and a phonemic verbal fluency task.40 Each task was selected specifically to dissociate important executive functions from non-executive components of cognition. We analyzed global performance outcomes as well as dissociable components of cognition.
Overall performance on the modified Sternberg task deteriorated significantly during sleep deprivation in terms of RTs, response accuracy, and errors of omission. This provided evidence that the task was globally responsive to sleep deprivation, as was also consistently observed in earlier studies examining performance on Sternberg tasks during sleep deprivation.11,17,50 Accuracy remained above 90%, and errors of omission stayed below 10% of total responses during sleep deprivation in our study. The RTs of correct responses were dissociated into non-executive cognitive processes, represented by the intercept of the linear relationship between RT and memory set size, and working memory scanning efficiency, represented by the slope of that linear relationship.34 Furthermore, the RT difference between recent and non-recent probes was extracted as a measure of resistance to proactive interference.35,38 Working memory scanning efficiency and resistance to proactive interference are both executive function components involving the prefrontal cortex.29,30
Only the primarily non-executive components of cognitive processing represented by the RT intercept were significantly adversely affected by sleep deprivation (Figure 3). Working memory scanning efficiency and resistance to proactive interference showed no change relative to baseline and recovery and relative to controls. Response accuracy did not vary as a function of sleep deprivation for any of the dissociated components of cognition, indicating that results were not confounded by a speed/accuracy trade-off. Thus, executive components of cognition were preserved on this task during sleep deprivation, even as overall performance was impaired. The absence of an effect of sleep deprivation on working memory scanning efficiency in a Sternberg paradigm has been reported previously.11 We believe that the absence of a sleep deprivation effect for resistance to proactive interference in the modified Sternberg task is a new result. The finding suggests that the cognitive deficits associated with sleep loss are fundamentally different from those associated with aging,51 where reduced ability to overcome proactive interference causes irreversible disruptions in overall cognitive ability.
The absence of an effect of sleep deprivation on resistance to proactive interference was corroborated by results for the probed recall task, which allowed for the dissociation of susceptibility to proactive interference from immediate recall ability. The reduction of recall for interference-maximum trials relative to interference-release trials (i.e., the dissociated effect of proactive interference) was not significantly altered by sleep deprivation (Figure 4). It is noteworthy that overall performance on the probed recall task did not significantly deteriorate during sleep deprivation (although a significant improvement of recall was observed after 2 nights of recovery sleep). Previous studies employing other probed recall tasks reported significant performance deficits due to sleep deprivation,52,53 but these task implementations involved a delay between memorization and recall. It is possible that our version of the probed recall task, which measured immediate recall in subject-paced responses, was overall less susceptible to the effects of TSD. However, the task displayed proactive interference on the interference-maximum trials, confirming that the task was responsive to cognitive challenge. Sleep deprivation had no significant effect on the ability to resist the proactive interference. Thus, the probed recall task provided another example in which an executive component of cognition was not degraded by sleep deprivation.
Remarkably, overall performance on the phonemic verbal fluency task improved significantly during sleep deprivation, compared to baseline and recovery and compared to controls. This improvement occurred above and beyond the practice effect that was seen in both the TSD group and the control group (Figure 5). Performance on the phonemic verbal fluency task has been reported to be affected by vocabulary knowledge, speed of processing (as measured with tasks such as the DSST), education, sex, and native language.54,55 The TSD and control groups were comparable on these criteria as quantified by vocabulary score on the Shipley Institute of Living Scale, DSST baseline performance, years of education, male/female sample distribution, and the inclusion criterion to be a native English speaker. Moreover, baseline performance on the verbal fluency task was equivalent between the 2 groups. Thus, the performance improvement associated with TSD on this task does not appear to result from any confound associated with the study sample.
Previous studies of the effects of sleep deprivation on overall performance for similar versions of the phonemic verbal fluency task documented mixed findings of impairment,41 no impairment,6 or improvement that was less than that seen for control subjects.56 The present study was the first to examine phonemic verbal fluency performance during sleep deprivation under standardized, controlled laboratory conditions with continuous monitoring of subjects, and to include comparisons with performance both at baseline and after recovery as well as with a control condition. Lack of some experimental controls in the earlier studies, along with shorter durations of sleep deprivation, different circadian timings of task administration, and other variations in study design, may have contributed to the differences among reported results.
Our focus in this study was not on global performance, however, but specifically on executive and non-executive components of cognition. The phonemic verbal fluency task allowed for the decomposition of overall cognitive performance into automatic (non-executive) processing, represented by average phonemic cluster size, and executive processing, represented by number of switches between phonemic clusters.44 There was a trend towards significance for a reduction in cluster size during sleep deprivation compared to controls, suggesting there might have been a modest deterioration of non-executive performance due to sleep loss. On the other hand, there was a significant increase in the number of switches between phonemic clusters during sleep deprivation, indicating an improvement in executive functioning after 51 h of TSD. Number of switches and average cluster size are inversely related, so these results should be interpreted as a shift in relative balance between executive and non-executive functioning. Sleep-deprived subjects thus relied more on executive processing and less on automatic processing to perform the task. There was no increase in errors during sleep deprivation. As such, the automatic-to-executive balance shift might be a reflection of a successful change in performance strategy, with greater emphasis on still functional executive processes in order to maintain performance in the face of potentially compromised non-executive processes.
In designing the laboratory experiment (Figure 1), we took steps to avoid confounds known to interfere with the interpretability of results in sleep deprivation studies.33 A within-subject, repeated-measures study design was employed. The experiment was conducted in a controlled laboratory environment with a fixed level of light ( < 100 lux) during scheduled wakefulness, and constant ambient temperature. Subjects were healthy, good sleepers who were carefully screened. They maintained their habitual bedtime schedule in the week before the experiment, and were given 2 days to acclimate to the laboratory procedures and get fully rested prior to the first executive functions test bout. They slept in their own private bedroom and performed cognitive tasks in isolation. Half the sample was randomized to a control group, which was included to account for any study effects unrelated to sleep deprivation. Subjects were behaviorally monitored at all times during the experiment.
The circadian timing of the administration of the executive functions task battery was selected to coincide with the circadian nadir in alertness during sleep deprivation,33 and was standardized across test bouts while potential confounds from sleep inertia were avoided. Equivalent versions of each task were administered in randomized order, and the order of the tasks in the test battery was also randomized. A battery of control tasks (PVT, DSST, KSS), for which the effects of TSD are well documented,45 was administered frequently throughout the study, and displayed the expected effects of sleep deprivation and recovery sleep (Figure 2). Performance on all cognitive tasks, including the executive functions task battery, was equivalent between the 2 groups at baseline, and in the control group continued to show baseline levels or characteristic practice effects across the experiment. After recovery sleep in the TSD group, performance consistently converged to the level of the control group at the corresponding time in study. Thus, the experiment provided a solid platform for evaluating the effects of sleep deprivation on cognitive performance and its dissociated components.
Implications and Limitations
The results of our study showed that overall performance on executive functions performance tasks may decline with TSD, but the source of the decline can be—and indeed appeared to be—in the non-executive cognitive components of the tasks, as has also been pointed out by others.17 Because we did not set out to determine the effects of sleep deprivation on every possible aspect of executive functioning, we do not conclude that all executive functions—which are diverse57—are resilient to sleep deprivation. In addition, since we examined executive functioning at one fixed time of day, we do not know to what extent results would vary across the circadian cycle. Even so, our data strongly suggest that reports of TSD effects on an executive functions performance task should be regarded with caution, unless the data address the task impurity problem3,28 by allowing for discrimination of the source of the performance deficits within the executive task.
Our results pose difficulties for two dominant views of how sleep loss affects cognitive functioning, and challenge the generalizability of these theories. One theory posits that sleep deprivation should specifically impair executive functioning because of the particularly large effect of sleep loss on the prefrontal cortex.5,12 If this were the case, then we should have seen clear evidence of executive functions impairment as we disentangled executive and non-executive task components. Instead, our results showed that two executive functions processes localized to the prefrontal cortex—working memory scanning efficiency and resistance to proactive interference—are not especially vulnerable to impairment due to sleep deprivation. Thus, our findings are inconsistent with this theory.
Another theory, referred to as state instability theory,25 postulates that sleep deprivation should induce more general performance deficits because it impairs attention, a prerequisite for many other cognitive processes.27 Beyond the critical impact of sleep loss on vigilant attention, state instability theory does not explicitly predict whether any aspects of cognitive performance would be more vulnerable to TSD than others. The theory does explain in detail why performance tasks measuring sustained attention (such as the PVT46) are sensitive to sleep deprivation.26 Working memory is an aspect of cognition believed to depend substantially on attention as well.58 Yet, in the present study, two components of working memory function did not show the adverse effects of sleep deprivation that would have been expected accordingly. Thus, the theory of state instability may need to be refined to account for how TSD-induced general deficits in attention can lead to selective decrements in some components of cognition and not in others.
Another theory, based on the idea that sleep and wakefulness are fundamentally regulated at the level of individual neuronal groups, suggests that the magnitude of the effects of sleep deprivation on distinct components of cognition is a reflection of the activity in the neuronal groups subserving these components.59 This idea would be compatible with state instability theory, but would predict variations in the impact of sleep deprivation on cognitive functions depending on the extent to which these functions are taxed by the performance task at hand. While our experiment was not designed to test this prediction, our data are not at odds with the concept.
Insufficient statistical power could be an important potential limitation of our study. However, it would be hard to argue that the study lacked statistical power, since we observed statistically significant effects of sleep deprivation on all performance tasks considered. Contradicting the idea that executive components of cognitive performance should be especially vulnerable to sleep deprivation,5,12 we found that these effects of sleep deprivation were not attributable to impairments in the executive function processes we examined. Likewise, in contrast to the hypothesis that sleep deprivation should degrade cognitive performance globally,27 we found that executive function processes could show relative improvements under conditions of TSD. These conclusions are derived from statistically significant findings, and therefore cannot be dismissed on the basis of a lack of statistical power.
In conclusion, our study exposed several cases where 51 h of acute TSD had no significant adverse effects on dissociated executive functions, while concurrently the deficits in non-executive functions were significant and considerable. These data suggest that the inconsistencies in published effects of sleep deprivation on executive functions performance tasks5–21,41,52,53,56,60 may be due in part to a focus on global performance outcomes that represent a mixture of different cognitive processes. The results of the present study indicate that these underlying cognitive processes are not all equally affected by sleep deprivation, which has critical consequences for understanding the effects of sleep deprivation on global performance outcomes. This also has important implications for better understanding the effects of sleep deprivation on cognitive performance in the workplace, and for improving mathematical models seeking to predict occupational task performance.61–63 Classification of a job task using categories like “executive” or “non-executive” will not readily reveal the extent to which such a task is susceptible to adverse effects from sleep deprivation. It is necessary also to consider the dissociated components of cognition that make up performance on the task, in order to more fully comprehend and more accurately predict decrements in real-world performance due to sleep loss.11,13,14,60
DISCLOSURE STATEMENT
This was not an industry supported study. Financial support was received from the U.S. Army Medical Research and Materiel Command and from the Department of Defense.
Drs. Van Dongen and Belenky have received research support from Pulsar Informatics Inc., Institute for Behavior Resources, and Continental Airlines. Dr. Van Dongen has made a paid presentation to Clayton Sleep Institute in St. Louis, MO. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We are grateful to the staff and research assistants of the Sleep and Performance Research Center at Washington State University Spokane for their help conducting the study. We also thank David Dinges and John Powell for providing the computerized tests constituting the control task battery. This study was supported by USAMRMC award W81XWH-05-1-0099 and DURIP grant FA9550-06-1-0281.
REFERENCES
- 1.Basner N, Fomberstein KM, Razavi FM, Banks S, William JH, Rosa RR. American time use survey: sleep time and its relationship to waking activities. Sleep. 2007;30:1085–95. doi: 10.1093/sleep/30.9.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–29. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 3.Phillips LH. Do “frontal tests” measure executive functions? Issues of assessment and evidence from fluency tests. In: Rabbitt P, editor. Methodology of frontal and executive function. Hove: Psychology Press; 1997. pp. 191–213. [Google Scholar]
- 4.Overtoon C. Employability skills: An update. Columbus: Clearinghouse on Adult, Career, and Vocational Education; 2000. [Google Scholar]
- 5.Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6:236–49. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- 6.Binks PG, Waters WF, Hurry M. Short-term total sleep deprivations does not selectively impair higher cortical functioning. Sleep. 1999;22:328–34. doi: 10.1093/sleep/22.3.328. [DOI] [PubMed] [Google Scholar]
- 7.Choo WC, Lee WW, Venkatraman V, Sheu FS, Chee MWL. Dissociation of cortical regions modulated by both working memory load and sleep deprivation and by sleep deprivation alone. NeuroImage. 2005;25:579–87. doi: 10.1016/j.neuroimage.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 8.Chuah YM, Venkatraman V, Dinges DF, Chee MWL. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci. 2006;26:7156–62. doi: 10.1523/JNEUROSCI.0906-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drummond SPA, Paulus MP, Tapert SF. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J Sleep Res. 2006;15:261–5. doi: 10.1111/j.1365-2869.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- 10.Gottselig JM, Adam M, Rétey JV, Khatami R, Achermann P, Landolt HP. Random number generation during sleep deprivation: Effects of caffeine on response maintenance and stereotypy. J Sleep Res. 2006;15:31–40. doi: 10.1111/j.1365-2869.2006.00497.x. [DOI] [PubMed] [Google Scholar]
- 11.Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T. An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-to-sample task. Cogn Brain Res. 2004;18:306–21. doi: 10.1016/j.cogbrainres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Harrison Y, Horne JA, Rothwell A. Prefrontal neuropsychological effects of sleep deprivation in young adults—a model for healthy aging? Sleep. 2000;23:1–7. [PubMed] [Google Scholar]
- 13.Heuer H, Kohlisch O, Klein W. The effects of sleep deprivation on the generation of random sequences of key-presses, numbers and nouns. Quart J Exp Psychol. 2005;58A:275–307. doi: 10.1080/02724980343000855. [DOI] [PubMed] [Google Scholar]
- 14.Jennings JR, Monk TH, van der Molen MW. Sleep deprivation influences some but not all processes of supervisory attention. Psychol Sci. 2003;14:473–9. doi: 10.1111/1467-9280.02456. [DOI] [PubMed] [Google Scholar]
- 15.Killgore WDS, Balkin TJ, Wesensten NJ. Impaired decision making following 49h of sleep deprivation. J Sleep Res. 2006;15:7–13. doi: 10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- 16.McKenna BS, Dickinson DL, Orff HJ, Drummond SPA. The effects of one night of sleep deprivation on known-risk and ambiguous-risk decisions. J Sleep Res. 2007;16:245–52. doi: 10.1111/j.1365-2869.2007.00591.x. [DOI] [PubMed] [Google Scholar]
- 17.Mu Q, Mishory A, Johnson KA, Nahas Z, Kozel FA, Yamanaka K. Decreased brain activation during a working memory task at rested baseline is associated with vulnerability to sleep deprivation. Sleep. 2005;28:433–46. doi: 10.1093/sleep/28.4.433. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson JP, Söoderströom M, Karlsson AU, Lekander M, Åkerstedt T, Lindroth NE. Less effective executive functioning after one night's sleep deprivation. J Sleep Res. 2005;14:1–6. doi: 10.1111/j.1365-2869.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 19.Sagaspe P, Sanchez-Ortuno M, Charles A, et al. Effects of sleep deprivation on Color-Word, Emotional, and Specific Stroop interference and on self-reported anxiety. Brain Cogn. 2006;60:76–87. doi: 10.1016/j.bandc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Venkatraman V, Eng M, Chuah YML, Huettel SA, Chee MWL. Sleep deprivation elevates expectation of gains and attenuates responses to losses following risky decisions. Sleep. 2007;30:603–9. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- 21.Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med Rev. 2001;5:463–75. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- 22.Cajochen C, Knoblauch V, Kröauchi K, Renz C, Wirz-Justice A. Dynamics of frontal EEG activity, sleepiness and body temperature under high and low sleep pressure. NeuroReport. 2001;12:2277–81. doi: 10.1097/00001756-200107200-00046. [DOI] [PubMed] [Google Scholar]
- 23.Finelli LA, Baumann H, Borbély AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neurosci. 2000;101:523–9. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 24.Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 25.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 26.Dorrian J, Rogers N, Dinges DF. Psychomotor vigilance performance: Neurocognitive assay sensitive to sleep loss. In: Kushida CA, editor. Sleep deprivation: clinical issues, pharmacology and sleep loss effects. New York: Marcel Dekker; 2005. pp. 39–70. [Google Scholar]
- 27.Doran SM, Van Dongen HPA, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- 28.Whitney P, Jameson T, Hinson JM. Impulsiveness and executive control of working memory. Pers Indiv Diff. 2004;37:417–28. [Google Scholar]
- 29.Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neurosci. 2006;139:181–93. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 30.Rypma B, Berger JS, D'Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. J Cogn Neurosci. 2002;14:721–31. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- 31.Van Dongen HPA, Dinges DF. Sleep, circadian rhythms, and psychomotor vigilance. Clin Sports Med. 2005;24:237–49. doi: 10.1016/j.csm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Zachary R. Shipley Institute for Living Scale: Revised Manual. Los Angeles: Western Psychological Services; 1986. [Google Scholar]
- 33.Van Dongen HPA, Dinges DF. Circadian rhythms in fatigue, alertness and performance. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: WB Saunders; 2005. pp. 435–43. [Google Scholar]
- 34.Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–4. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- 35.Monsell S. Recency, immediate recognition memory, and reaction time. Cogn Psychol. 1978;10:465–501. [Google Scholar]
- 36.Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JDE. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–86. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- 37.Sternberg S. Memory-scanning: Mental processes revealed by reaction-time experiments. Am Sci. 1969;57:421–57. [PubMed] [Google Scholar]
- 38.Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter-Lorenz PA. Inhibition in verbal working memory revealed by brain activation. Proc Natl Acad Sci USA. 1998;95:8410–3. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bunting M. Proactive interference and item similarity in working memory. J Exp Psychol: Learn Mem Cogn. 2006;32:183–96. doi: 10.1037/0278-7393.32.2.183. [DOI] [PubMed] [Google Scholar]
- 40.Benton AL, Hamsher KS, Sivan AB. Multilingual Aphasia Examination. 3rd ed. Iowa City: AJA Associates; 1994. [Google Scholar]
- 41.Harrison Y, Horne JA. Sleep deprivation affects speech. Sleep. 1997;20:871–7. doi: 10.1093/sleep/20.10.871. [DOI] [PubMed] [Google Scholar]
- 42.Ross TP, Furr AE, Carter SE, Weinberg M. The psychometric equivalence of two alternate forms of the Controlled Oral Word Association Test. Clin Neuropsychol. 2006;20:414–31. doi: 10.1080/13854040590967153. [DOI] [PubMed] [Google Scholar]
- 43.Spreen O, Strauss E. A compendium of neuropsychological tests: administration, norms, and commentary. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 44.Troyer AK, Moscovitch M, Winocur G. Clustering and switching as two components of verbal fluency: Evidence from younger and older healthy adults. Neuropsychology. 1997;11:138–46. doi: 10.1037//0894-4105.11.1.138. [DOI] [PubMed] [Google Scholar]
- 45.Van Dongen HPA, Maislin G, Mullington J, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 46.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Meth Instr Comp. 1985;17:652–5. [Google Scholar]
- 47.Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 48.Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 49.Rupp TL, Acebo C, Seifer R, Carskadon MA. Effects of a moderate evening alcohol dose on performance. II: Performance. Alcohol Clin Exp Res. 2007;31:1365–71. doi: 10.1111/j.1530-0277.2007.00434.x. [DOI] [PubMed] [Google Scholar]
- 50.Jensen O, Gelfand J, Kounious K, Lisman JE. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex. 2002;12:877–82. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- 51.Jonides J, Marshuetz C, Smith EE, Reuter-Lorenz PA, Koeppe RA, Hartley A. Age differences in behavior and PET activation reveal differences in interference resolution in verbal working memory. J Cogn Neurosci. 2000;12:188–96. doi: 10.1162/089892900561823. [DOI] [PubMed] [Google Scholar]
- 52.Drake CL, Roehrs TA, Burduvali E, Bonahoom A, Rosekind M, Roth T. Effects of rapid versus slow accumulation of eight hours of sleep loss. Psychophysiology. 2001;38:979–87. doi: 10.1111/1469-8986.3860979. [DOI] [PubMed] [Google Scholar]
- 53.Cajochen C, Khalsa SBS, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277:R640–9. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 54.Salthouse T. Speed and knowledge as determinants of adult age differences in verbal tasks. J Gerontol. 1993;48:P29–36. doi: 10.1093/geronj/48.1.p29. [DOI] [PubMed] [Google Scholar]
- 55.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14:167–77. [PubMed] [Google Scholar]
- 56.Horne JA. Sleep loss and “divergent” thinking ability. Sleep. 1988;11:528–36. doi: 10.1093/sleep/11.6.528. [DOI] [PubMed] [Google Scholar]
- 57.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 58.Baddeley A. Working memory. Science. 1992;255:556–9. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 59.Krueger JM, Rector DM, Roy S, Van Dongen HPA, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9:910–9. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turner TH, Drummond SPA, Salamat JS, Brown GG. Effects of 42 hr of total sleep deprivation on component processes of verbal working memory. Neuropsychology. 2007;21:787–95. doi: 10.1037/0894-4105.21.6.787. [DOI] [PubMed] [Google Scholar]
- 61.Mallis MM, Mejdal S, Nguyen TT, Dinges DF. Summary of the key features of seven biomathematical models of human fatigue and performance. Aviat Space Environ Med. 2004;75:A4–14. [PubMed] [Google Scholar]
- 62.Friedl KE, Mallis MM, Ahlers ST, Popkin SM, Larkin W. Research requirements for operational decision-making using models of fatigue and performance. Aviat Space Environ Med. 2004;75:A192–9. [PubMed] [Google Scholar]
- 63.Dinges DF. Critical research issues in development of biomathematical models of fatigue and performance. Aviat Space Environ Med. 2004;75:A181–91. [PubMed] [Google Scholar]