Abstract
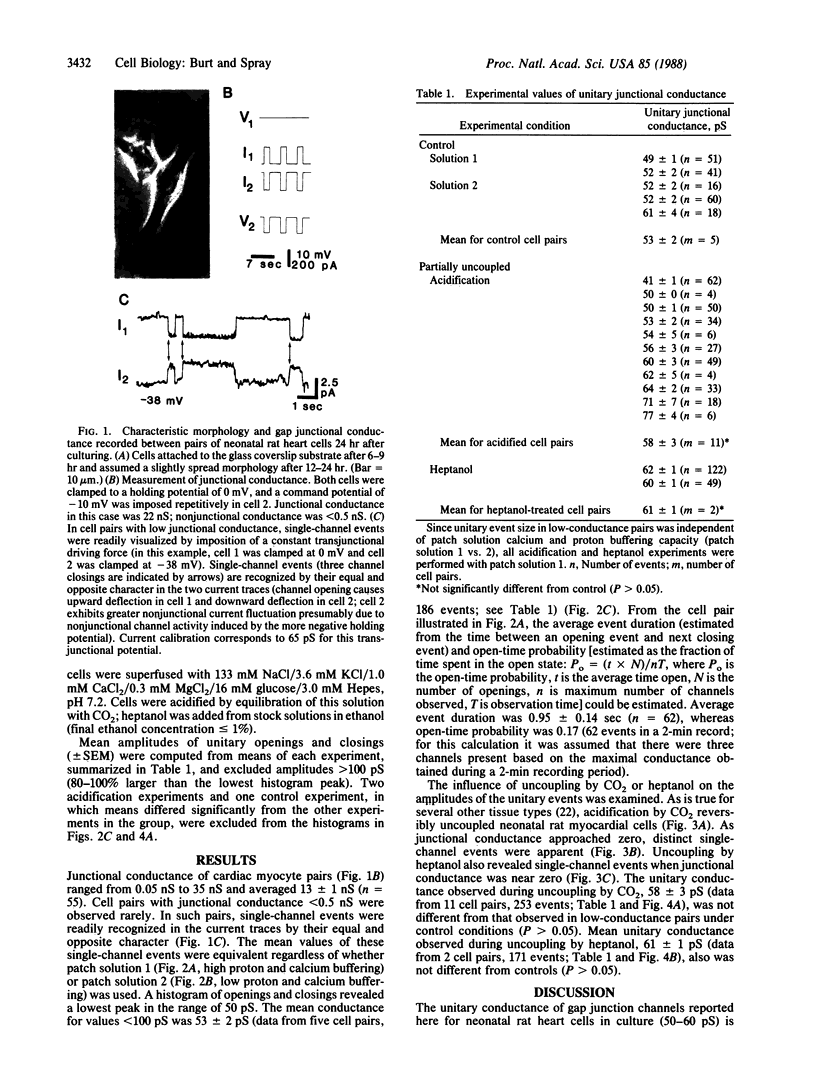
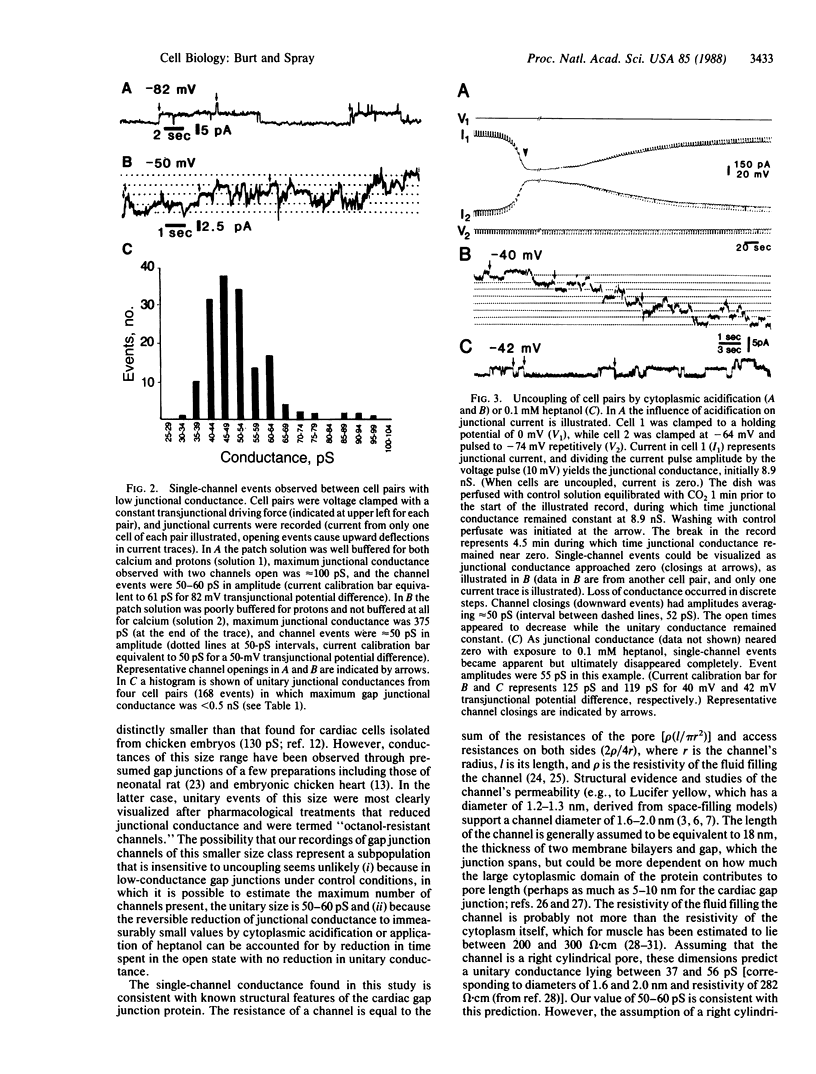
The activity of gap junction channels between pairs of neonatal rat heart cells in culture was studied under control conditions and during uncoupling procedures by using dual whole-cell voltage clamp techniques. Under control conditions gap junctional conductance ranged from 0.05 to 35 nS. In cell pairs exhibiting low gap junctional conductance (less than 500 pS), single-channel events with a unitary conductance of 53 +/- 2 pS (5 experiments; 186 events) were apparent. Event duration and open-time probability were estimated to be 0.95 sec and 0.17, respectively. When the junctional conductance in well-coupled cell pairs (with initial junctional conductance, greater than 5 nS) was reduced by cytoplasmic acidification or application of heptanol, single-channel events could be visualized. Compared to low-conductance controls, unitary channel conductance was unaltered (for acidification the conductance was 58 +/- 3 pS in 11 experiments with 253 events; for heptanol the conductance was 61 +/- 1 pS in 2 experiments with 171 events), while the probability of channels being open was decreased. The constancy of unitary channel conductance under control conditions and during uncoupling procedures suggests that opening and closing of the gap junction channel are all-or-none processes during which no stable subconductance states are formed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. V., Spira M. E., Spray D. C. Permeability of gap junctions between embryonic cells of Fundulus: a reevaluation. Dev Biol. 1978 Jul;65(1):114–125. doi: 10.1016/0012-1606(78)90184-7. [DOI] [PubMed] [Google Scholar]
- Burt J. M. Electrical and contractile consequences of Na+ or Ca2+ gradient reduction in cultured heart cells. J Mol Cell Cardiol. 1982 Feb;14(2):99–110. doi: 10.1016/0022-2828(82)90198-5. [DOI] [PubMed] [Google Scholar]
- Chapman R. A., Fry C. H. An analysis of the cable properties of frog ventricular myocardium. J Physiol. 1978 Oct;283:263–282. doi: 10.1113/jphysiol.1978.sp012499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow I., Young S. H. Opening of single gap junction channels during formation of electrical coupling between embryonic muscle cells. Dev Biol. 1987 Aug;122(2):332–337. doi: 10.1016/0012-1606(87)90298-3. [DOI] [PubMed] [Google Scholar]
- Fischmeister R., Ayer R. K., Jr, DeHaan R. L. Some limitations of the cell-attached patch clamp technique: a two-electrode analysis. Pflugers Arch. 1986 Jan;406(1):73–82. doi: 10.1007/BF00582957. [DOI] [PubMed] [Google Scholar]
- Hall J. E. Access resistance of a small circular pore. J Gen Physiol. 1975 Oct;66(4):531–532. doi: 10.1085/jgp.66.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Hiraoka M. Effects of hypoxia on passive electrical properties of canine ventricular muscle. Pflugers Arch. 1982 Mar;393(1):45–50. doi: 10.1007/BF00582390. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981 Oct;61(4):829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Manjunath C. K., Nicholson B. J., Teplow D., Hood L., Page E., Revel J. P. The cardiac gap junction protein (Mr 47,000) has a tissue-specific cytoplasmic domain of Mr 17,000 at its carboxy-terminus. Biochem Biophys Res Commun. 1987 Jan 15;142(1):228–234. doi: 10.1016/0006-291x(87)90475-x. [DOI] [PubMed] [Google Scholar]
- Neyton J., Trautmann A. Single-channel currents of an intercellular junction. 1985 Sep 26-Oct 2Nature. 317(6035):331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- Schwarzmann G., Wiegandt H., Rose B., Zimmerman A., Ben-Haim D., Loewenstein W. R. Diameter of the cell-to-cell junctional membrane channels as probed with neutral molecules. Science. 1981 Jul 31;213(4507):551–553. doi: 10.1126/science.7244653. [DOI] [PubMed] [Google Scholar]
- Simpson I., Rose B., Loewenstein W. R. Size limit of molecules permeating the junctional membrane channels. Science. 1977 Jan 21;195(4275):294–296. doi: 10.1126/science.831276. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Saez J. C., Brosius D., Bennett M. V., Hertzberg E. L. Isolated liver gap junctions: gating of transjunctional currents is similar to that in intact pairs of rat hepatocytes. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5494–5497. doi: 10.1073/pnas.83.15.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin P. N., Zampighi G. Structure of the junction between communicating cells. Nature. 1980 Feb 7;283(5747):545–549. doi: 10.1038/283545a0. [DOI] [PubMed] [Google Scholar]
- Veenstra R. D., DeHaan R. L. Cardiac gap junction channel activity in embryonic chick ventricle cells. Am J Physiol. 1988 Jan;254(1 Pt 2):H170–H180. doi: 10.1152/ajpheart.1988.254.1.H170. [DOI] [PubMed] [Google Scholar]
- Veenstra R. D., DeHaan R. L. Measurement of single channel currents from cardiac gap junctions. Science. 1986 Aug 29;233(4767):972–974. doi: 10.1126/science.2426781. [DOI] [PubMed] [Google Scholar]
- Verselis V., White R. L., Spray D. C., Bennett M. V. Gap junctional conductance and permeability are linearly related. Science. 1986 Oct 24;234(4775):461–464. doi: 10.1126/science.3489990. [DOI] [PubMed] [Google Scholar]
- Weidmann S. Electrical constants of trabecular muscle from mammalian heart. J Physiol. 1970 Nov;210(4):1041–1054. doi: 10.1113/jphysiol.1970.sp009256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann S. The diffusion of radiopotassium across intercalated disks of mammalian cardiac muscle. J Physiol. 1966 Nov;187(2):323–342. doi: 10.1113/jphysiol.1966.sp008092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. L., Spray D. C., Campos de Carvalho A. C., Wittenberg B. A., Bennett M. V. Some electrical and pharmacological properties of gap junctions between adult ventricular myocytes. Am J Physiol. 1985 Nov;249(5 Pt 1):C447–C455. doi: 10.1152/ajpcell.1985.249.5.C447. [DOI] [PubMed] [Google Scholar]
- Young J. D., Cohn Z. A., Gilula N. B. Functional assembly of gap junction conductance in lipid bilayers: demonstration that the major 27 kd protein forms the junctional channel. Cell. 1987 Mar 13;48(5):733–743. doi: 10.1016/0092-8674(87)90071-7. [DOI] [PubMed] [Google Scholar]
- Zampighi G. A., Hall J. E., Kreman M. Purified lens junctional protein forms channels in planar lipid films. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8468–8472. doi: 10.1073/pnas.82.24.8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A. L., Rose B. Permeability properties of cell-to-cell channels: kinetics of fluorescent tracer diffusion through a cell junction. J Membr Biol. 1985;84(3):269–283. doi: 10.1007/BF01871390. [DOI] [PubMed] [Google Scholar]




