Abstract
Background:
Severe obstructive sleep apnea (OSA), metabolic syndrome (Mets) and short sleep duration are all risk factors for cardiovascular events. There has been no report which has investigated this relationship in an age- and BMI-matched population-based study. The prevalence of OSA in Mets subjects has not been established, although the converse (i.e., the prevalence of Mets in OSA subjects) has been investigated several times.
Methods:
This home cardiorespiratory (type 3) sleep study, using an actigraph, was conducted in 275 males working for an urban company. Retrospective measurements of fasting blood parameters were obtained from the company's periodical inspection data. The mean duration between the sleep study and the measurement of blood parameters was 213 days.
Results:
Although there was a significant relationship between OSA severity and the prevalence of Mets (P < 0.001), the association between severity and Mets was not significant after adjustments were made for age and BMI. Severe OSA was 7.8 times as likely to be present in subjects with Mets (16.2% of all 68 Mets subjects) as those without (2.4% of 207 non-Mets) (P < 0.001). Subject with severe OSA had a significantly short sleep duration (P < 0.05). Sleep duration in Mets subjects was also significantly shorter than in those without (P < 0.05).
Conclusions:
Although increased BMI and age both had a significant effect on the prevalence of OSA in patients with Mets, one of 6 subjects with Mets, but only one of 40 without Mets had severe OSA in an urban male population in Japan. Physicians should take into account this high prevalence of severe OSA in patients with Mets. Sleep duration should be taken into consideration as an important factor in studies investigating the prevalence of severe OSA and Mets.
Citation:
Chin K; Oga T; Takahashi K; Takegami M; Nakayama-Ashida Y; Wakamura T; Sumi K; Nakamura T; Horita S; Oka Y; Minami I; Fukuhara S; Kadotani H. Associations between obstructive sleep apnea, metabolic syndrome, and sleep duration, as measured with an actigraph, in an urban male working population in Japan. SLEEP 2010;33(1):89-95.
Keywords: Obesity, risk factors, metabolic syndrome, sleep apnea, sleep duration
OBSTRUCTIVE SLEEP APNEA (OSA) IS CHARACTERIZED BY REPEATED EPISODES OF APNEA AND HYPOPNEA DURING SLEEP. THERE IS A GROWING BODY OF evidence to support the belief that severe OSA is a risk factor for cardiovascular diseases and death,1 not only in clinical cases2,3 but also in the general population.4,5
The National Cholesterol Education Program (NCEP) Adults Treatment Panel III (2001) proposed a clinically-based approach that establishes a diagnosis of metabolic syndrome (Mets), when an individual has 3 of 5 risk factors for cardiovascular disease.6 There appears to be considerable overlap in the clustering of cardiovascular disease mechanisms involved in Mets and OSA. Indeed, 5 recent studies have shown that OSA was significantly associated with Mets.7–11 However, 4 of the 5 were non-epidemiological studies, while subjects in the one epidemiological study were relatively obese for an Asian population.9 Another study has reported that obesity,12 and not OSA was responsible for metabolic abnormalities. Thus, it has not yet been established whether Mets is primarily determined by obesity or sleep apnea. In addition, although the prevalence of OSA in Mets subjects is not well understood,9 the converse (i.e., the prevalence of Mets in OSA subjects) has been investigated several times.7–11 We hypothesized that OSA would be an independent risk factor for Mets in a normal male population. We investigated the prevalence of sleep apnea in subjects with Mets in a Japanese male working urban city population (n = 275) whose body mass index (BMI) matched that of the general Japanese male population.13 In this study, we also looked at the prevalence of OSA in Mets subjects, as this is an area that has not been investigated extensively.
Recently, in addition to obese subjects, it has been reported that Mets subjects have a shortened sleep duration which has a significant association with morbidity and mortality.14–19 In these studies, the sleep duration was measured by a sleep diary, rather than with an actigraph.20 The sleep duration in bed as measured by an actigraph has been shown to be compatible with the duration as measured by polysomnography.21 We also investigated weekly mean sleep duration by means of an actigraph.
METHODS
Study methods have been provided in detail in a previous report.13
Subjects and Study Design
The subjects were male employees of an urban company. Male subjects only were chosen for the study, as the number of female workers was small. Questionnaires were distributed to the employees. Of 322 male employees (current smokers: 54.9% and habitual drinkers: 48.9%), who recorded their breathing during sleep using a home monitoring system, 275 were investigated further; their clinical characteristics and comorbidities are presented in Table 1. The reasons for exclusion of the other 47 subjects were as follows: blood parameters were not measured (n = 28); subjects whose measurements of some of the components of metabolic syndrome, such as blood parameters, and abdominal circumference were not measured, were omitted (n = 18); blood parameters were measured after the monitoring of breathing (n = 1). The relative proportions of the degrees of severity in the sleep disordered breathing was similar in these excluded subjects to the 275 subjects included in the study, namely: severe in 4, moderate in 7, mild in 15, and no-OSA in 20 males; in addition 1 male subject had a poor recording.
Table 1.
Clinical features and comorbidities of 275 male subjects
| All subjects | No OSA (RDI < 5) | Mild OSA (5 ≤ RDI < 15) | Moderate OSA (15 ≤ RDI < 30) | Severe OSA (30 ≤ RDI) | p value | |
|---|---|---|---|---|---|---|
| No. of subjects | 275 | 114 | 103 | 42 | 16 | |
| RDI (/h) | 10.2 ± 10.7 | 2.5 ± 1.4 | 9.5 ± 2.6* | 20.4 ± 3.3*¶ | 43.1 ± 10.4*¶‡ | < 0.001 |
| Age (years) | 44 ± 8 | 41 ± 8 | 46 ± 8* | 47 ± 7* | 47 ± 7* | < 0.001 |
| BMI (kg/m2) | 23.9 ± 3.1 | 23.0 ± 2.8 | 23.5 ± 3.0 | 26.0 ± 2.8*¶ | 27.5 ± 2.4*¶ | < 0.001 |
| Sleep duration (h) | 6.0 ± 0.8 | 6.1 ± 0.8 | 6.0 ± 0.8 | 6.0 ± 0.7 | 5.4 ± 0.9*¶ | 0.004 |
| Epworth Sleepiness Scale | 6.7 ± 3.7 | 6.5 ± 3.6 | 6.6 ± 3.7 | 6.4 ± 3.6 | 8.8 ± 4.1 | 0.12 |
| Waist circumference (cm) | 83.6 ± 8.5 | 80.8 ± 7.9 | 83.3 ± 7.6 | 88.6 ± 8.3*¶ | 93.1 ± 6.3*¶ | < 0.001 |
| Blood parameters | ||||||
| TC (mg/dL) | 203 ± 32 | 198 ± 33 | 205 ± 30 | 209 ± 33 | 208 ± 27 | 0.22 |
| TG (mg/dL) | 123 ± 81 | 111 ± 62 | 118 ± 75 | 147 ± 112 | 181 ± 112*¶ | 0.002 |
| HDL-cho (mg/dL) | 57 ± 14 | 57 ± 15 | 60 ± 13 | 54 ± 12 | 49 ± 13¶ | 0.011 |
| Blood glucose (mg/dL) | 104 ± 22 | 100 ± 15 | 104 ± 18 | 108 ± 22 | 119 ± 56* | 0.004 |
| Dyslipidemia (n, [%]) | 91 (33.1) | 36 (31.6) | 32 (31.1) | 13 (31.0) | 10 (62.5) | 0.138 |
| Hypertension (n, [%]) | 156 (56.7) | 57 (50.0) | 56 (54.4) | 31 (73.8) | 12 (75.0) | 0.004 |
| Hyperglycemia (n, [%]) | 54 (19.6) | 17 (14.9) | 19 (18.5) | 13 (31.0) | 5 (31.3) | 0.017 |
| Systolic BP (mm Hg) | 129 ± 14 | 127 ± 14 | 130 ± 15 | 132 ± 12 | 132 ± 11 | 0.21 |
| Diastolic BP (mm Hg) | 81 ± 11 | 79 ± 11 | 81 ± 10 | 85 ± 11* | 84 ± 6 | 0.0018 |
| Prevalence of Mets, using: | ||||||
| NCEP III criteria (n, [%]) | 68 (24.7) | 19 (16.7) | 24 (23.3) | 14 (33.3) | 11 (68.8) | < 0.001 |
| Japanese criteria (n, [%]) | 58 (21.1) | 16 (14.0) | 19 (18.4) | 13 (31.0) | 10 (62.5) | < 0.001 |
P < 0.05, vs No OSA
P < 0.05, vs Mild OSA
P < 0.05, vs Moderate OSA.
OSA, obstructive sleep apnea; RDI, respiratory disturbance index; BMI, body mass index; TC, Total cholesterol; TG, triglycerides; HDL-cho, high-density lipoprotein cholesterol; BP, blood pressure; Mets, metabolic syndrome; NEP III, The National Cholesterol Education Program (NCEP) Adults Treatment Panel III (2001)
The study protocol was approved by the Kyoto University Graduate School and Faculty of Medicine Ethics Committee.
Questionnaire
A Japanese modification of the Epworth Sleepiness Scale (ESS) was used to assess subjective sleepiness.22,23 A separate sleep diary was filled out during the survey period.13
Examinations and Home Monitoring
Each subject was asked to wear an actigraph (Actiwatch AW-Light: Mini Mitter, OR, USA) for 7 days to estimate sleep/wake time, and a type 3 portable monitor (PM) (Morpheus: Teijin, Tokyo, Japan, which is the same as Somt́e: Compumedics, Victoria, Australia) for 2 nights at home.13,20,24
Actigraphy and PM Data Analysis
Sleep duration at night was estimated from analysis of the wrist actigraphy tracing, in conjunction with a sleep diary. 13 The respiratory disturbance index (RDI: number of apnea and hypopnea episodes per hour of the analyzed time length) was calculated from both actigraph and PM data. The PM records were visually inspected and scored by ≥ 2 medical doctors specialized in respiratory medicine. Apnea (cessation of breathing for ≥ 10 sec) and hypopnea ( > 50% reduction in the amplitude of nasal pressure or respiratory effort associated with more than 3% reduction in oxyhemoglobin saturation ≥ 10 sec) were scored blind to other information, except sleep/wake time estimated by the actigraphy. Data without oxygen saturation signals or indecipherable recordings were excluded from analysis. Data recorded for < 2 h were also excluded, because the Medicare guidelines require ≥ 2 h of documented sleep time. When data from both recorded nights were available, records from the second night were analyzed further. Subjects with an RDI of 5–14.9, 15–29.9, and ≥ 30 were considered to have mild, moderate, and severe sleep disordered breathing (SDB), respectively.
Anthropometric and Biochemical Measures
Measurements of waist circumference and blood pressure were measured at the same time as the subjects were trained in the use of the PM and actigraph. These measurements, and those of weight, were done by trained research staff.13 Measurements of fasting blood sugar, total cholesterol, HDL-cholesterol, triglyceride levels, and height were obtained, retrospectively, from the company's periodic inspection data.
Diagnosis of Metabolic Syndrome
Instead of using the definition of metabolic syndrome published by the International Diabetes Federation,25 NCEP criteria were used to compare the results from the current study with previously published data. This decision was based on the fact that the majority of previous studies had used NCEP criteria. Based on NCEP criteria, a diagnosis of Mets was made when an individual had 3 of the following 5 characteristics: increased waist circumference ( > 85 cm for men), systolic blood pressure > 130 mm Hg or diastolic blood pressure > 85 mm Hg, increased fasting glucose > 110 mg/dL, increased triglycerides > 150 mg/dL, and decreased HDL cholesterol ( < 40 mg/dL).6 The waist circumference was determined to take account of ethnicity.26 In addition, a diagnosis of Mets was made using the Japanese criteria,27 namely, if the individual had a waist circumference > 85 cm for men and ≥ 2 of the following risk factors: (1) increased triglycerides or decreased HDL cholesterol; (2) high blood pressure; (3) increased fasting plasma glucose. Subjects who took BP-lowering medications were included as hypertensive subjects. Eleven subjects took drugs for hyperlipidemia; as we could not obtain “natural” lipid profile data for these subjects because of this concomitant medication, they were considered to suffer from both increased triglycerides and decreased HDL cholesterol.
Statistical Analysis
Results are expressed as mean ± standard deviation (SD). The significance of intergroup differences based on the severity of OSA was determined by an analysis of variance (ANOVA). When a significant difference was observed, the Sheff́e method was performed to identify where the differences were significant. Univariate and multivariate logistic regression analyses were performed to assess the relationship between Mets or the components of Mets, and RDI, age, and BMI. Comparisons of the values between subjects with and without Mets were performed by a non-paired t or χ2 test. Univariate logistic regression analyses were performed to assess the relationship between OSA severity and the presence of Mets. The relationship between 2 sets of data was analyzed by the Pearson correlation coefficient tests, except for the Spearman correlation coefficient (rs) tests regarding RDI data and ESS scores, as the former data were skewed and the latter were ordinal variables. Since it is believed that BMI and age are significantly related to the occurrence of Mets, BMI- and age-matched subjects with and without Mets were compared. Comparisons of body weight data during the study were performed by a 2-tailed, paired t-test. P values < 0.05 were considered to be statistically significant. In a previous report,11 it was found that in the group with an AHI < 10, 40% had metabolic syndrome; in contrast, in the group with an AHI > 10, 60% had metabolic syndrome. Based on these data, the sample size was set to achieve 80% power at a 5% significance level. The calculated sample size was 97. Statistical analyses were performed using Statview 5.0 (SAS Institute, Inc. Cary, NC). The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Prevalence of SDB and Mets
Based on NCEP and Japanese criteria, respectively, 68 (24.7%) and 58 (21.1%) of 275 subjects had Mets (Table 1). This rate was similar to the previously published rate (23.0%) for the general public in Japan.28 In addition, in 2007 in Japan, it was reported that the prevalence of hyperglycemia in male subjects in their 40s was 18.6%.29 Therefore, the prevalence of hyperglycemia in this study (19.6%) is similar to the background prevalence in Japan.29
The mean duration between the measurements of the home cardiorespiratory sleep study and clinical data (waist circumference and blood pressure) was 7 days, while the mean duration between the sleep study and the measurement of blood parameters, such as blood sugar, cholesterol, and triglycerides, was 213 ± 138 days. The severity of OSA was significantly related with the prevalence of Mets (P < 0.001) (Table 2). After adjusting for age and BMI, the severity of OSA was not significantly related to the occurrence of Mets, or several other factors (Table 2). The unadjusted and adjusted odds ratios (OR) for having any severity of OSA (vs. non-OSA), the OR for severe, moderate, and mild OSA (vs. non-OSA), and the OR for severe and moderate OSA (vs. mild OSA) were examined. In all cases, the results were the same (Table 3). Similarly, the results were the same using the Japanese criteria for Mets diagnosis.
Table 2.
Logistic regression analyses with metabolic syndrome as an outcome for the severity of OSA
| Unadjusted |
Adjusted |
||||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| OSA | OSA | ||||
| none | reference | none | reference | ||
| mild | 1.52 (0.78-2.97) | 0.22 | mild | 1.00 (0.47-2.13) | 0.99 |
| moderate | 2.50 (1.11-5.61) | 0.026 | moderate | 0.77 (0.30-1.99) | 0.59 |
| severe | 11.0 (3.43-35.3) | < 0.001 | severe | 2.57 (0.68-9.69) | 0.16 |
| Age | 1.06 (1.02-1.11) | 0.0064 | |||
| BMI | 1.39 (1.24-1.57) | < 0.001 | |||
OSA, obstructive sleep apnea; CI, confidence intervals; BMI, body mass index.
Table 3.
Logistic regression analyses with primary components of metabolic syndrome as an outcome for the severity of OSA
| a) Hypertension | |||||
|---|---|---|---|---|---|
| Unadjusted |
Adjusted |
||||
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| OSA | OSA | ||||
| none | reference | none | reference | ||
| Mild | 1.19 (0.70-2.03) | 0.52 | mild | 0.94 (0.53-1.65) | 0.82 |
| moderate | 2.82 (1.29-6.15) | 0.0092 | moderate | 1.89 (0.81-4.43) | 0.14 |
| Severe | 3.00 (0.91-9.86) | 0.070 | severe | 1.82 (0.51-6.56) | 0.36 |
| Age | 1.05 (1.02-1.08) | 0.0043 | |||
| BMI | 1.05 (0.96-1.15) | 0.25 | |||
| b) Dyslipidemia | |||||
| Unadjusted |
Adjusted |
||||
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| OSA | OSA | ||||
| none | reference | none | reference | ||
| Mild | 0.98 (0.55-1.74) | 0.94 | mild | 0.94 (0.51-1.73) | 0.84 |
| moderate | 0.97 (0.45-2.09) | 0.94 | moderate | 0.65 (0.28-1.53) | 0.32 |
| Severe | 3.61 (1.22-10.7) | 0.021 | severe | 2.03 (0.62-6.68) | 0.25 |
| Age | 0.99 (0.96-1.03) | 0.64 | |||
| BMI | 1.16 (1.06-1.28) | 0.0016 | |||
| c) Hyperglycemia | |||||
| Unadjusted |
Adjusted |
||||
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| OSA | OSA | ||||
| none | reference | none | reference | ||
| mild | 1.29 (0.63-2.64) | 0.49 | mild | 0.68 (0.30-1.54) | 0.35 |
| moderate | 2.56 (1.11-5.88) | 0.027 | moderate | 0.73 (0.27-1.97) | 0.54 |
| severe | 2.59 (0.80-8.41) | 0.11 | severe | 0.46 (0.11-1.91) | 0.29 |
| Age | 1.11 (1.06-1.17) | < 0.001 | |||
| BMI | 1.31 (1.16-1.48) | < 0.001 | |||
| d) Waist circumference | |||||
| Unadjusted |
Adjusted |
||||
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| OSA | OSA | ||||
| none | reference | none | reference | ||
| mild | 1.56 (0.88-2.75) | 0.12 | mild | 1.20 (0.52-2.77) | 0.67 |
| moderate | 5.48 (2.54-11.8) | < 0.001 | moderate | 1.04 (0.35-3.06) | 0.94 |
| severe | 36.8 (4.67-290) | < 0.001 | severe | 6.73 (0.51-89.7) | 0.15 |
| Age | 1.08 (1.03-1.13) | 0.0025 | |||
| BMI | 2.57 (2.04-3.24) | < 0.001 | |||
OSA, obstructive sleep apnea; CI, confidence intervals; BMI, body mass index.
Prevalence of OSA in Subjects with Mets
While there was no significant difference in BMI or age, 16.2% of subjects with Mets had an RDI ≥ 30, compared with only 2.4% of subjects without Mets (P < 0.001) (Table 4). It seemed that Mets increased the risk of severe OSA (RDI ≥ 30). A similar relationship was not seen in subjects with an RDI < 30.
Table 4.
The relationship between severe OSA and the presence of metabolic syndrome
| Severe OSA (n = 16) | Mets (+) (n = 68) | Mets (−) (n = 207) | p |
|---|---|---|---|
| Age (y) | 48.5 ± 6.8 | 44.6 ± 6.6 | 0.56 |
| BMI (kg/m2) | 27.7 ± 2.1 | 26.9 ± 3.1 | 0.30 |
| Waist circumference (cm) | 95.0 ± 7.1 | 89.0 ± 6.8 | 0.08 |
| n (%) | 11 (16.2) | 5 (2.4) | <0.001 |
OSA, obstructive sleep apnea; BMI, body mass index
Weekly Mean Sleep Duration
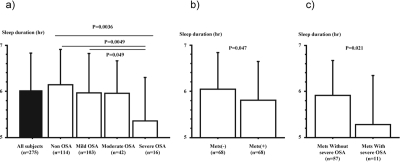
We found a significant correlation between the weekly mean sleep duration by actigraph and the duration of type 3 PM monitor (r = 0.52, P < 0.001), and a significant negative correlation between BMI and weekly mean sleep duration as measured by actigraph (r = −0.19, P = 0.0016). Subjects with severe OSA had significantly shorter sleep duration (Figure 1a and Table 1, P < 0.05).
Figure 1.
Weekly mean sleep duration in OSA and/or Mets subjects
a) The relationship between the severity of OSA and the weekly mean sleep duration in bed, as measured by an actigraph. Only severe OSA significantly shortened sleep duration.
b) BMI- and age-matched subjects with Mets (n = 68) and without Mets (n = 68)
c) Mets with severe OSA (n = 11) and Mets without severe OSA (n = 57).
OSA, obstructive sleep apnea; Mets, metabolic syndrome.
Weekly mean sleep duration in Mets subjects was significantly less than in non-Mets subjects (5.8 ± 0.8 h vs. 6.1 ± 0.8 h: P = 0.026). BMI- and age-matched subjects with (n = 68) and without (n = 68) Mets were compared, because BMI and age were significantly related with its occurrence: there was a significant difference in sleep duration between subjects with and without Mets, but not in RDI (Table 5, Figure 1b). Weekly mean sleep duration was 5.3 ± 1.1 h in Mets subjects with severe OSA and 5.9 ± 0.8 (P = 0.021) in Mets subjects without severe OSA (Figure 1c).
Table 5.
Comparison of the background between 68 patients with Mets and 68 patients without Mets, matched for age and BMI
| Non-Mets | Mets | P value | |
|---|---|---|---|
| No. of subjects | 68 | 68 | |
| RDI (/h) | 12.7 ± 10.2 | 15.7 ± 14.2 | 0.16 |
| Age (years) | 46 ± 8 | 46 ± 7 | 0.94 |
| BMI (kg/m2) | 26.0 ± 2.4 | 26.1 ± 2.6 | 0.78 |
| Sleep duration (h) | 6.1 ± 0.7 | 5.8 ± 0.8 | 0.047 |
| Epworth Sleepiness Scale | 8.2 ± 4.6 | 8.7 ± 4.3 | 0.46 |
RDI, respiratory disturbance index; BMI, body mass index
Body Weight Changes Between Two Check Points
All sleep studies were performed after the blood measurements. As previously shown, the mean duration of time between the sleep studies and blood parameter measurements was 213 ± 138 days (median 221 and interquartile range = 93 to 320 days). The mean duration of time between the sleep studies and blood parameter measurements was unaffected by severity of OSA (P = 0.12). Subjects had significant body weight gain during the period between the day blood parameters were measured and the day of type 3 PM monitoring (from 23.2 ± 3.1 to 23.9 ± 3.1kg/m2, P < 0.001). An association between weight gained and the severity of RDI was not significant (rs = 0.06, P = 0.35).
Association Between Sleepiness, RDI, and Actigraphic Sleep Duration
Although ESS scores in severe OSA were significantly higher than any other group, the differences disappeared using an analysis of variance (ANOVA). The relationship between ESS scores and sleep duration was significant (rs = −0.23, P < 0.001), but the relationship between ESS scores and RDI was not (rs = 0.04, P = 0.51).
DISCUSSION
In this first, body weight-matched epidemiological study based on a Japanese urban population of businessmen, we found the following: (1) although there was a significant association between an increase in the proportion of Mets and the severity of OSA, the severity of OSA had no significant effect on the occurrence of Mets after data were adjusted for age and BMI; (2) although BMI and age both had a significant effect, the prevalence of severe OSA in Mets subjects was higher than that in non-Mets subjects; (3) severe OSA patients and subjects with Mets had a shorter sleep duration in bed.
Several studies have considered the relationship between OSA and Mets, five of which concluded that OSA was independently associated with an increase in the prevalence of Mets.7–11 Another study, which did not find an association between OSA and Mets prevalence, found that obesity was the major determinant of metabolic abnormalities,12 our findings support the latter. The mean BMI in our study was comparable to the normal Japanese male population.13 Therefore, we consider that this current study is the second epidemiological study, but the first population-based one, to investigate the relationship between OSA and Mets in adults using a population of subjects with a BMI comparable to the relevant normal male population. From our data, the presence of Mets was primarily determined by obesity and age, and to a lesser extent, by sleep apnea. However, this urban male working population study also showed that the prevalence of Mets in severe OSA patients was nearly 70%, which is the same as that reported in previous studies.7–11 Therefore, it appears that over two-thirds of severe OSA (RDI ≥ 30) patients have Mets in both Eastern and Western countries. Redline et al. have emphasized that this association is also important in the prevalence of Mets in adolescents.30
There have been few previous reports concerning the prevalence of OSA in Mets subjects.9 Lam et al. showed that 62.5% subjects with Mets had OSA (RDI > 5), while only 24% subjects without Mets had OSA (P < 0.001).9 However, they did not investigate what proportion of patients with or without Mets had severe OSA (RDI ≥ 30), which is linked to cardiovascular disease and death.1–5 We have shown that severe OSA had a significantly higher prevalence in Mets subjects than subjects without Mets (Table 4). Although BMI and age are the most important factors (Tables 2 and 3), this result suggests that Mets components, such as visceral fat, might induce severe OSA. Indeed, it has been reported that continuous positive airway pressure (CPAP) treatment for OSA significantly reduced visceral fat accumulation.31,32 Recently, a positive, independent relationship has been shown between lung function impairment and metabolic syndrome.33 Thus, the mechanism why Mets might induce severe OSA should be further studied.
In this study, BMI and weekly mean sleep duration, as measured by actigraph, had a significant negative correlation (r = −0.19, P = 0.0016). Although BMI and age had a significant association with the prevalence of Mets, BMI- and age-matched subjects with Mets had shorter sleep duration than that of control subjects without Mets (Table 5). Several published reports have shown that sleep duration has a significant effect on BMI,14–16 mortality,17 and diabetes.34 Recently, the relationship between Mets and sleep duration has been reported as shown in this study.18,19 Sleep duration in these reports was calculated by sleep diary and/or questionnaire,14–19,34 while in our study it was calculated by sleep diary and actigraphy.13,20 However, we feel that our method of measuring sleep duration during a night in bed, was a more reliable method, as the validity of actigraphy in subjects with SDB has been proven in a recent report.21 In our study, we showed that the weekly mean sleep duration was shorter (5.4 h) in severe OSA subjects than in subjects with other OSA grades (≥ 6 h); severe OSA is associated with a significantly high mortality rate from cardiovascular disease.1–5 Thus, sleep duration of subjects with severe OSA or Mets was less than 6 h, while that in subjects with both Mets and severe OSA was only 5.3 h (Figure 1c). The percentage of adults sleeping ≤ 6 h per day has increased markedly between 1985 and 2004, in parallel with a nationwide substantial increase in BMI.35 It is well known that severe OSA subjects have excessive daytime sleepiness, which is believed to be due to the intermittent awaking caused by sleep apnea. We found that the sleep duration in bed of such severe OSA subjects was short, which, therefore, may be one of the factors for the hypersomnolence observed in such subjects. Pack et al. reported a greater association between increases in subjective sleepiness in commercial drivers and shorter sleep duration, as measured by an actigraph, than with increases in severity of apnea.21 We can draw similar conclusions from an urban male working population in Japan. Based on our results, in order to decrease the mortality rate in subjects with severe OSA or Mets, it would be best to improve the length and quality of sleep. This could be partly achieved through the use of active treatments, such as CPAP for OSA., At the very least, the differences in sleep duration before and after CPAP treatment warrant further study.
There were several limitations in this study. Firstly, we did not carry out polysomnography. However, as previously reported, the inter-scorer and night-to-night reliability of RDI were excellent (interclass correlation coefficients (ICC) of 0.98 and 0.95, respectively).13 In addition, it has been reported recently that non-attended type 3 monitoring is reliable under the specified conditions in which our study was conducted.36 Secondly, our study population was based on an urban male working population in Japan. As reported previously, 58.5% of the Japanese population lives in the 7 largest metropolitan areas of Japan, and 67.2% of Japanese workers are employed in the tertiary sector of industry (also known as the service sector or the service industry). Thus, approximately 40% of Japanese workers may be in the tertiary sector of industry in the metropolitan areas, in which our study population was based.13 Although ethnicity issues might have impacted our results, the criterion of waist circumference was determined to take account of ethnicity.26 In addition, the prevalence of SDB in this study was comparable with that of Western countries.13,37 Furthermore, the mean prevalence of Mets with severe OSA using NCEP criteria was 69% in our study and 71% in 4 of 5 previous studies that included Western and Asian countries.7,9>–11 Therefore, our data might be applicable to Western populations, although this requires further study. Thirdly, we did not gather sleep related data with waist circumference at the same time as several blood parameters. However, there was no significant difference in the duration between the timing of the two measurements in any group. The prevalence of Mets in our subjects may have increased due to the significant body weight gain having a significant effect on several blood markers. Fourthly, the number of subjects in our study was limited due to the small number of employees. Future studies should use a greater number of subjects in order to meet the predicted sample size.
This study has shown that almost 70% of severe OSA subjects also had Mets in an urban male working population in Japan. Furthermore, it also demonstrated that 16% of subjects with Mets had severe OSA, while only 2.4% of subjects without Mets had severe OSA, although, in addition to BMI and age, factors which induce severe OSA in Mets subjects were not clearly investigated in this study, because of a shortage of severe OSA patients (11 patients with Mets and 5 patients without Mets). It will be important to investigate the reasons why short sleep duration, which has been shown in patients with Mets, obesity, and several comorbidities in recent reports,14–16,18,19 and in patients with severe OSA and/or Mets in this report, might be induced in these patients. In addition, short sleep duration increased mortality.17 This knowledge should allow the successful treatment of sleep disturbances in subjects, including those with OSA or Mets, which might have a significant and beneficial effect on sleep duration in bed and hence mortality.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank Dr. Motoharu Ohi for his valuable suggestions. We also thank Tomoko Toki and Miho Sotoda for assistance with manuscript preparation. This work was supported in part by a grant from the Japanese Ministry of Education, Culture, Sports, Science and Technology (no. 20590921), Respiratory Failure Research Group from the Japanese Ministry of Health, Labor and Welfare, the Japan Vascular Disease Research Foundation, and research grants from PRESTO JST, Suzuken Memorial Foundation, Takeda Science Foundation, Mitsui Life Social Welfare Foundation, Chiyoda Kenko Kaihatsu Jigyodan Foundation, and Health Science Center Foundation.
REFERENCES
- 1.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease. An American heart association/American college of cardiology foundation scientific statement from the American heart association council for high blood pressure research professional education committee, council on clinical cardiology, stroke council, and council on cardiovascular nursing. Circulation. 2008;118:1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 2.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep Apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 3.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall NS, Wong KKH, Liu PY, Cullen SRJ, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton health study. Sleep. 2008;31:1079–85. [PMC free article] [PubMed] [Google Scholar]
- 6.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation and treatment of high blood cholesterol in adults (adult treatment panel) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 7.Coughlin SR, Mawdsley L, Mugarza JA, Calverley PMA, Wilding JP. Obstructive sleep Apnea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–41. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Gruber A, Horwood F, Sithole J, Ali NJ, Idris I. Obstructive sleep apnoea is independently associated with the metabolic syndrome but not insulin resistance state. Cardiovasc Diabetol. 2006;5:22. doi: 10.1186/1475-2840-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam JCM, Lam B, Lam C, et al. Obstructive sleep apnea and metabolic syndrome in community-based Chinese adults in Hong Kong. Respir Med. 2006;100:980–7. doi: 10.1016/j.rmed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Sasanabe R, Banno K, Otake K, et al. Metabolic syndrome in Japanese patients with obstructive sleep apnea syndrome. Hypertens Res. 2006;29:315–22. doi: 10.1291/hypres.29.315. [DOI] [PubMed] [Google Scholar]
- 11.Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;13:355–362. [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma SK, Kumpawat S, Goel A, Banga A, Ramakrishnan L, Chaturvedi P. Obesity, and not obstructive sleep apnea, is responsible for metabolic abnormalities in a cohort with sleep-disordered breathing. Sleep Med. 2007;8:12–7. doi: 10.1016/j.sleep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama-Ashida Y, Takegami M, Chin K, et al. Sleep-disordered breathing in the usual lifestyle setting as detected with home monitoring in a Japanese male working population. Sleep. 2008;31:419–25. doi: 10.1093/sleep/31.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohatsu ND, Tsai R, Young T, et al. Sleep duration and body mass index in a rural population. Arch Intern Med. 2006;166:1701–5. doi: 10.1001/archinte.166.16.1701. [DOI] [PubMed] [Google Scholar]
- 15.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 16.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in-young adults: a 13-year prospective study. Sleep. 2004;27:661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 17.Ferrie JE, Shipley MJ, Cappuccio FP, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30:1659–66. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory JD, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31:635–43. doi: 10.1093/sleep/31.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi KM, Lee JS, Park HS, Baik SH, Choi DS, Kim SM. Relationship between sleep duration and the metabolic syndrome: Korean National Health and Nutrition Survey 2001. Int J Obes (Lond) 2008;32:1091–7. doi: 10.1038/ijo.2008.62. [DOI] [PubMed] [Google Scholar]
- 20.Littner M, Kushida CA, Anderson WM, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–41. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 21.Pack AI, Maislin G, Staley B, et al. Impaired performance in commercial Drivers Role of sleep apnea and short sleep duration. Am J Respir Crit Care Med. 2006;174:446–54. doi: 10.1164/rccm.200408-1146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takegami M, Suzukamo Y, Wakita T, et al. Development of a Japanese version of the Epworth Sleepiness Scale (JESS) based on Item Response Theory. Sleep Med. 2009;10:556–65. doi: 10.1016/j.sleep.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 24.Chesson AL, Jr, Berry RB, Pack A. Practice parameters for the use of portable monitoring devices in the investigation of suspected obstructive sleep apnea in adults. Sleep. 2003;26:907–13. doi: 10.1093/sleep/26.7.907. [DOI] [PubMed] [Google Scholar]
- 25.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome-a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 26.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome: a new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 27.Committee on Evaluation of Diagnosis Standards for Metabolic Syndrome. Definition and diagnostic standards for metabolic syndrome. Nippon Naika Gakkai Zasshi. 2005;94:794–809. (in Japanese) [PubMed] [Google Scholar]
- 28. The national health and nutrition survey in Japan, 2004. Ministry of Health, Labor and welfare of Japan. (in Japanese)
- 29. Ministry of Health, Labor and Welfare (in Japanese). http://www.mhlw.go.jp/houdou/2008/12/h1225-5.html.
- 30.Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med. 2007;176:401–8. doi: 10.1164/rccm.200703-375OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chin K, Shimizu K, Nakamura T, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure. Circulation. 1999;100:706–12. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 32.Trenell MI, Ward JA, Yee BJ, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab. 2007;9:679–87. doi: 10.1111/j.1463-1326.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 33.Leone N, Courbon D, Thomas F, et al. Lung function impairment and metabolic syndrome. The critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179:509–16. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 34.Gangwisch JE, Heymsfield SB, Boden-Albalba B, et al. Sleep duration as a risk factor for diabetes incidence in a large US sample. Sleep. 2007;30:1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Center for Health Statistics. QuickStats: Percentage of adults who reported an average < 6 hours of sleep per 24-hour period, by sex and age group: United States, 1985 and 2004. MMWR Morb Mortal Wkly Rep. 2005;54:933. [Google Scholar]
- 36.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 37.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. A population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 38.Peppard PF, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of modest weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]