Abstract
Chronic rhinosinusitis (CRS) is characterized by a chronic symptomatic inflammation of the nasal and paranasal sinus mucosae and is one of the most frequently reported chronic diseases in the United States, with an estimated prevalence of greater than 10% of the general population. Although the pathogenesis of CRS remains poorly understood, there is evidence for a role of bacteria and fungi, as well as the presence of a robust adaptive immune response in the upper airways and sinuses. Recent studies of CRS, as well as several other diseases in the skin and respiratory epithelium, have uncovered evidence that deficiencies in epithelial immune barrier function might compromise the interaction between the host and external immune stimuli. Recent studies suggest the hypothesis that reduced expression of antimicrobial S100 proteins, particularly psoriasin and calprotectin, might lead to increased susceptibility to bacterial and fungal colonization in patients with CRS. The main emphasis of this review will be to highlight the current literature that suggests that a defect in the expression of a broad set of epithelially derived genes might lead to barrier compromise and subsequently a dysfunctional host immune response to environmental agents in patients with CRS.
Keywords: Chronic rhinosinusitis, S100, epithelium, barrier hypothesis, inflammation
Chronic rhinosinusitis (CRS) is one of the most commonly reported diseases in the United States, with an estimated prevalence of greater than 10% of the general population. Defined as a persistent symptomatic inflammation of the nasal mucosa resulting from the interaction of multiple host and environmental factors, the cause of CRS remains elusive. Clinically, CRS might be suspected if 2 or more of the following symptoms are present and resistant to medical therapy: anterior, posterior, or both types of mucopurulent discharge; facial pain/pressure/fullness; decreased sense of smell; and nasal obstruction. Nasal endoscopy and sinus computed tomographic scans are recommended to verify the diagnosis because of the high false-positive rate with subjective criteria alone. CRS is commonly subdivided into 2 subtypes: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP).1 The tissue inflammatory response in patients with CRSsNP has been shown to be highly neutrophilic with a tendency toward TH1 polarization, whereas CRSwNP inflammatory responses are characterized by eosinophilia with a TH2 skewing.2 Although complex host genetic factors are widely believed to influence the pathogenesis of CRS, recent investigations have highlighted environmental factors, such as fungal or bacterial colonization, the presence of biofilms or superantigens, osteitis, and allergen exposure.1
Recent studies of allergic diseases in the skin and lungs, such as atopic dermatitis (AD), psoriasis, asthma, allergic rhinitis, and chronic obstructive pulmonary disease, have uncovered evidence that there might be intrinsic or induced deficiencies in the barrier function of the skin and airway mucosal epithelium in these diseases, underscoring the importance of the epithelial interface with the environment and the importance of the genes that influence barrier function.3 As one example, a line of investigation has implicated defects in the barrier gene filaggrin in patients with AD, as well as in atopic sensitization associated with asthma and rhinitis.4 As with the skin, the respiratory epithelium is a dynamic tissue involved in both innate and acquired host defense.5 In addition to providing a barrier to entry of pathogens through tight junctions, epithelial cells lining the airways have been shown to resist the presence of microorganisms by producing natural antimicrobial factors constitutively and after stimulation and by mounting an inflammatory response under intense stimulation. Because of the importance of the epithelium as a mediator of immune defense, defects in a broad set of epithelium-related genes, in theory, could contribute to a dysfunctional immune response to environmental agents in patients with CRS.6 This review will highlight the current literature on the pathogenesis of CRS, with an emphasis on emerging studies on epithelial barrier dysfunction.
PATHOGENIC TRIGGERS OF CRS
Many current theories on the cause of CRS and its exacerbations postulate that infectious agents and their products mediate the inflammatory mucosal changes observed. Ponikau et al7 have suggested that fungi, especially Alternaria species, are important sources of antigens that drive CRS. Their studies have demonstrated the presence of fungi and eosinophilic mucin in most patients with CRS, as well as the presence of fungi in healthy subjects. Corresponding in vitro studies have shown that lymphocytes from patients with CRS, but not control subjects, respond to fungal antigens in vitro and produce IL-5, a cytokine that most investigators find to be increased in patients with CRS.8 They have advanced the hypothesis that patients with CRS mount an eosinophilic response to environmental fungi. This eosinophilic response, which can be observed as both tissue eosinophils and clusters of activated eosinophils in mucus, is proposed to lead to damage to the mucosal surface. To date, studies evaluating antifungal treatment have met with mixed success in patients with CRS, and a role of chronic colonization with fungi is as yet uncertain. The failure of many studies with antifungal drugs to diminish CRS symptoms does not eliminate the possibility that sensitization to fungi and fungal products in ambient air leads to an IgE-independent eosinophilic inflammation that is important in CRS. Indeed, such a sensitization would appear to be more likely if there were an epithelial barrier dysfunction in this disease.
Another school of thought proposes a role for bacteria in CRS, a view supported but not proved by the fact that antibiotics remain the most commonly prescribed medications for management of CRS. The role of bacteria has been suggested by studies in which sinonasal cultures of patients with CRS were shown to yield results distinct from those seen in either healthy patients or those with acute rhinosinusitis. Specifically, CRS cultures have been shown to frequently include coagulase-negative Staphylococcus species, Staphylococcus aureus, anaerobes, and Streptococcus pneumoniae.9 Some recent studies have supported a superantigen hypothesis, suggesting that toxins secreted by S aureus, in some cases protected by biofilms or sequestered within epithelial cells, mediate inflammation in patients with CRS through direct stimulation of T cells, leading to cytokine release and local polyclonal IgE responses.10 In this model the inflammatory pathway in common with that of the fungal hypothesis is one in which TH2 lymphocytes, especially those producing IL-5, play a role in recruiting and activating eosinophils.
EPITHELIUM AND PATHOGEN RECOGNITION
There is considerable evidence for the occurrence of epithelial cell activation in patients with CRS; this activation could reflect the frequent colonization with bacteria or the inflammatory response to fungi and other environmental antigens discussed above. Increased levels of cytokines, such as GM-CSF; chemokines, such as CCL5 (RANTES) and eotaxins; growth factor receptors, such as epidermal growth factor receptor; costimulatory molecules, such as programmed death ligand 1 and 2; and other molecules involved in inflammation and immunity are observed in epithelium in patients with CRS. Airways epithelium plays an important role in innate host defense.11 Epithelial defense mechanisms are both constitutive and can be activated by membrane-bound and cytoplasmic pattern recognition receptors that recognize pathogen-associated molecular patterns commonly found in parasites, viruses, bacteria, yeast, and mycobacteria.5 One of the best characterized classes of pattern recognition receptors is the Toll-like receptor (TLR) family, which are transmembrane receptors expressed on multiple cell types, including respiratory epithelial cells.12-14 TLR2 and TLR4 play prominent roles in responses to fungi, gram-positive bacteria, and endotoxin. TLR3 recognizes viral double-stranded RNA, TLR5 recognizes bacterial flagellin, and TLR7 and TLR8 are activated by single-stranded RNA.15 Although there are some studies suggesting increased TLR expression or function in patients with CRS,16,17 one study reported the absence of TLR2, TLR3, and TLR4 in nasal polyps of patients whose nasal vestibules were positive for culture of either bacteria or fungi.18 Our unpublished studies on nasal epithelial cells suggest a modest and product-selective decrease in TLR2 responses and no decrease in TLR3 responses in epithelial cells derived from either the turbinates or uncinate process of patients with CRS compared with cells from healthy subjects, suggesting that any deficiency that might exist in vivo does not reflect a stable phenotype observed in cultured cells. Because exposure to TLR ligands can initiate production of chemokines, cytokines, and growth factors that trigger the inflammatory response, epithelial TLR, especially TLR3, which drives the strongest response in vitro, might be involved in triggering exacerbations of sinonasal disease.
ANTIMICROBIAL RESPONSES OF EPITHELIAL CELLS
In addition to their function in mucociliary clearance, sinonasal epithelial cells secrete a large array of molecules that are known to kill or neutralize microorganisms.5 These antimicrobial factors, first described by Sir Alexander Fleming in sputum and tears, are produced by both mucosal and glandular epithelium and probably play a critical role in the immediate host defense against invasion of potential pathogens at the epithelial interface. Epithelial host defense molecules include small peptides that lyse bacteria, such as defensins and cathelicidins, as well as enzymes and larger proteins, such as lysozyme, lactoferrin, and secretory leukocyte proteinase inhibitor. Lysozyme, a major antibacterial enzyme secreted by nasal and glandular epithelial cells, targets glycosidic bonds in the peptidoglycan cell wall of bacteria, typically leading to an enzymatic lysis, and is highly effective against many common upper airway gram-positive bacteria, such as streptococci. Lactoferrin sequesters iron, leading to inhibition of bacterial growth, and is produced by serous mucosal gland epithelium. Secretory leukocyte proteinase inhibitor kills both gram-positive and gram-negative bacteria. Defensins are small cysteine-rich cationic proteins that are directly toxic to many bacteria, fungi, and viruses. Most defensins function by binding microbial cell membrane and, once embedded, form pore-like membrane defects that allow efflux of essential ions and nutrients. Similarly, the cathelicidin LL-37 is an antimicrobial peptide that contributes to innate immune defense against invasive bacterial infection. Although this is not an exhaustive list of antimicrobials produced by epithelial cells, it should be clear that epithelial cells routinely protect the interface between the external environment and the mucosal surface and that disruption of this antimicrobial action might lead to the risk of colonization or infection by microorganisms.
FOCUS ON THE S100 GENES IN EPITHELIA
The S100 proteins comprise a multigene family consisting of more than 20 low-molecular-weight proteins that have numerous effects, including influences on cell differentiation and transformation, barrier function, and direct antimicrobial actions.19 The majority of the S100 protein genes are encoded on chromosome 1q21 among the genes of the epidermal differentiation complex. This region is of heightened interest because it encodes several other families of important genes that are expressed in epidermal keratinocytes. Of particular interest are the proteins S100A7, S100A8, and S100A9, which were initially found to be overex-pressed in patients with psoriasis.20 S100A7, also called psoriasin, is an S100 protein that has the calcium binding-properties typical of this family. It was originally identified in patients with psoriasis, in whom it was found to be highly upregulated.20 In normal human epidermis S100A7 is present at the cell periphery in terminally differentiated keratinocytes. S100A7 has been shown to function as a potent chemotactic agent that attracts CD4+ lymphocytes and neutrophils. Perhaps more importantly, S100A7 kills bacteria, and Meyer et al21 showed that S100A7 is the principal antimicrobial peptide that kills Escherichia coli in the human tongue. S100A7 has been shown to play a key antimicrobial role in human keratinocytes as well, particularly in the defense against E coli.22 Cutaneous levels of S100A7 are increased in patients with AD, in wound exudates, and after skin barrier disruption.23,24 Recently, S100A7 was detected in nasal lavage fluid from allergic and nonallergic individuals by using 2-dimensional gel electrophoresis and mass spectrometry.25 Interestingly, S100A7 levels in nasal lavage fluid were found to be reduced in lavage fluid from patients with allergic rhinitis compared with that from nonallergic individuals. It is not clear whether the presence of allergic inflammation diminishes its expression, although if that were true, one might expect reduced rather than increased levels in patients with AD. With regard to the antimicrobial effects of S100A7, recent evidence has suggested a strong similarity between the 3-dimensional structures of S100A7 and that of amoebapore A, an ancient antimicrobial pore-forming peptide from Entamoeba histolytica. S100A7 exerts its antimicrobial activity through disruption of microbial membranes at low pH.26 The varying roles of S100A7 in the epithelium, along with its implication in the allergic airway, served as a rationale to study a possible contributing role in the pathogenesis of CRS.
A recent study from our laboratory demonstrated that S100A7 mRNA is profoundly reduced in epithelial scrapings taken from uncinate tissue of patients with either CRSsNP or CRSwNP.27 We have recently studied S100A7 in patients with CRS more comprehensively and found that levels of S100A7 protein in nasal lavage fluid of patients with CRS are lower than levels in control subjects. Furthermore, immunohistochemical staining indicates a large reduction in epithelial expression of this antimicrobial peptide in both mucosal and glandular epithelium (Tieu et al, unpublished observations). In cultured keratinocytes the TLR5 ligand flagellin is the most potent inducer of S100A7.28 If this is also true in the upper airways, our recent results might possibly reflect a deficient response of nasal epithelium to flagellated bacteria in patients with CRS. The expression of S100 proteins is regulated by the T-cell cytokine IL-22 and its receptor, IL-22R.29 It will be worthwhile to determine whether the reduced levels of S100 proteins in patients with CRS might reflect a defect in the production of IL-22 and related IL-10 family cytokines (eg, IL-19, IL-20, and IL-24) or a defect in their signaling.30
S100A8 and S100A9 are produced by epithelial cells, neutrophils, and other cells and are important participants in the innate immune response. S100A8 and S100A9 are produced primarily as a heterodimeric complex (S100A8/A9) known as calprotectin, and the subunits are not readily found individually.31 Calprotectin is released into inflammatory exudates and saliva and is a potent proinflammatory chemoattractant for neutrophils and monocytes. Calprotectin is involved in transendothelial migration of leukocytes by inducing neutrophil adhesion to fibrinogen through activation of the β2-intergrin Mac-1 in response to LPS and monosodium urate crystals.32,33 Calprotectin manifests both antibacterial and antifungal properties. Squamous mucosal epithelial cells have been shown to constitutively express cytoplasmic calprotectin, and it confers resistance to intracellular invading bacteria, such as Listeria monocytogenes, Porphyromonas gingivalis, and Salmonella enterica.34 Calcium-binding loops I and II within the molecule are essential for bacterial resistance in keratinocytes.35 Given its expression by epithelium, abundance in neutrophils, and upregulation in various inflammatory diseases, there is a considerable amount of evidence supporting a role for calprotectin as a first-line defense in innate immune responses. Although data suggest that calprotectin is important in dermal inflammation and wound repair and critical in pancreatic epidermal cell-cell contacts, the role of this protein in the epithelium remains elusive.36,37 In our studies of patients with CRS, we found greater than 80% reductions in the levels of mRNA for S100A8 and S100A9 in freshly collected uncinate epithelial cells.27 More recently, we have found that nasal lavage fluids taken from patients with either CRSsNP or CRSwNP have dramatic reductions in levels of these proteins and that reduced epithelial expression is supported by immunohistochemical evaluation (Tieu et al, unpublished observations). Interestingly, detergent extracts of surgical samples of nasal polyps had large increases in levels of these proteins, and the levels correlated with increased presence of neutrophil elastase. Taken together, these findings suggest that epithelium from patients with CRS expresses diminished levels of calprotectin and that the influx of neutrophils might represent a compensatory response. Although reduced calprotectin levels might compromise innate immunity, a recent study indicates that these proteins bind to TLR4 and are necessary for endotoxic shock in mice, suggesting that they might have diverse roles in the innate immune response.38 Clearly, further studies are necessary to determine how important constitutive calprotectin is in innate immunity in the upper airways.
EPITHELIAL BARRIER FUNCTION AND CRS
The importance of barrier maintenance in a variety of allergic diseases has recently become elucidated. Studies on filaggrin strongly support the necessity of full function of this barrier protein in the skin to avoid allergic sensitization in patients with AD, as well as in patients with allergic rhinitis and asthma.4 Considerable evidence supports the concept that sensitization in patients with AD is related to dysfunction of the epidermal barrier.39 One of many proteins involved in barrier maintenance is serine peptidase inhibitor Kazal type 5 (SPINK5), a serine protease inhibitor thought to regulate the function of numerous proteases that might compromise barrier. Although it appears that polymorphisms of SPINK5 are only weakly associated with AD, mutations in this protein have been identified in Netherton syndrome, an autosomal recessive disorder characterized by trichorrhexis invaginata (“bamboo hair”) and congenital ichthyosis.40,41 In patients with asthma, several studies suggest that the formation, maintenance, or both of epithelial tight junctions is compromised.42 This probably results from poor proliferative epithelial repair responses and improperly formed tight junctions having reduced Zonula Occludens-1 (ZO-1). Anchorage of epithelial cells is also disrupted in patients with asthma and is in agreement with the long known observation of Creola bodies (ie, clusters of shed epithelial cells) found in the sputum of asthmatic patients.
Some early studies with nasal airway epithelial cells have indicated that the epithelial barrier might be compromised in patients with CRS. Bernstein et al43 demonstrated that cultured epithelial cells from nasal polyps manifest a greater absorption of sodium and water than cells from turbinate and propose that this is cytokine induced and contributes to pathogenesis. More recently, Kejima et al44 generated air-liquid interface monolayers of sinonasal epithelial cells from control subjects and patients with CRS and found that basal and amiloride-sensitive short-circuit currents were greater in the CRS cultures. Although they concluded that increased rates of ion transport might be pathophysio-logically relevant in patients with CRS, the molecular mechanism of the increased transport/permeability in patients with CRS is not clear. We recently studied the protease inhibitor SPINK5 in patients with CRS and found highly reduced levels expressed in the epithelial cells taken from patients with CRS.27 Immunohisto-chemistry supported the observation that this protein, previously observed primarily in skin, is indeed expressed in the airways and confirmed reduced levels of SPINK5 (Lympho-epithelial Kazal-type-related inhibitor, LEKTI) in CRS. SPINK5 contains 15 distinct protease inhibitory domains that are cleaved by complex processes in the skin. Many of the fragments generated by cleavage of SPINK5 inhibit a host of kallikrein enzymes that are important in cleaving desmosomal proteins during formation of the cornified layer of the skin, especially kallikrein-related peptidase (KLK) 1, KLK5, KLK6, KLK13, and KLK14.40 Thus it is quite possible that SPINK5 also inhibits host proteases that regulate desmosomal integrity in the nasal cavity as well. Although the enzymes that might be targeted by SPINK5 in the airways are unknown, based on the wide specificity of this molecule as an inhibitor of diverse proteases, our finding of substantial levels of expression of SPINK5 in the nose and sinuses, as well as in airway epithelial cells, suggest that it also functions in the respiratory tract. Also, our finding of reduced expression of SPINK5 in patients with CRS is notable, considering the essential role of this protein in normal formation and function of the skin. SPINK5 might protect gap junctions from attack by proteases derived from microbes and allergens, as well as host proteases. Additionally, SPINK5 might also decrease the effect of exogenous proteases on protease-activated receptors (PARs), which are present on multiple cell types in the nasal mucosa.
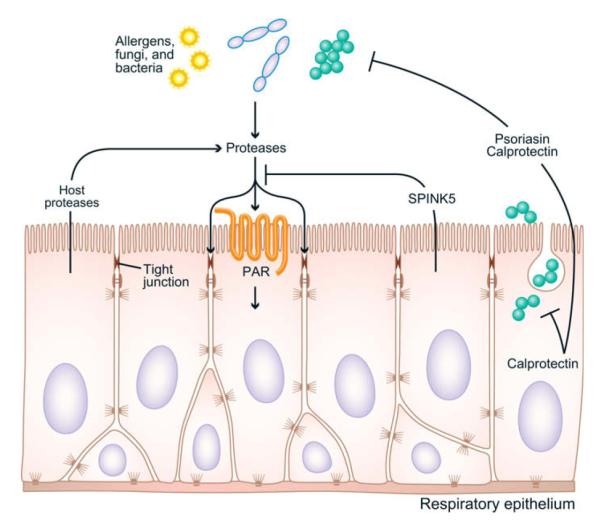
The influence of S100 proteins on barrier function is not clear. Certainly, any failure of innate immunity leading to bacterial or fungal colonization would influence the epithelial barrier (Fig 1). To the extent that these proteins are centrally important antimicrobials in the upper airways, as has been shown for S100A7 in the tongue, a deficiency in their production would promote microbial growth and have indirect effects on the barrier.21 As to direct effects on epithelial maintenance and repair, several S100 proteins have been implicated in cancer progression and have been found to promote tumor cell growth, possibly through enhancing epidermal growth factor receptor signaling in some cases. Recently, calprotectin was found to be critical for inflammatory cell transmigration into epithelial tissues in pancreatitis and directly dissociated epithelial cell-cell contacts in a highly calcium-dependent manner.36 Thus based on presently available information, a reduction in S100 proteins in patients with CRS could (1) lead to increased susceptibility to infection by organisms that are directly sensitive to these proteins, (2) diminish transepithelial migration of leukocytes, and (3) lead to reduced repair and proliferation of epithelium. Although studies on short-circuit current suggest a dysfunction in barrier function of epithelial cells from patients with CRS with respect to transport of water and ions, it is not clear whether this defect and the reduced expression of S100 proteins are mechanistically related. In patients with psoriasis, barrier function is believed to be poor, and yet S100 proteins are expressed at high quantities. Further studies will be needed to determine the role of S100 proteins in epithelial repair, differentiation, and proliferation in the upper airways.
FIG 1.
Potential points of influence of proteases, SPINK5, and S100 proteins on the immune barrier in the upper airways. See text for discussion. PAR, Protease-activated receptor.
CONCLUDING REMARKS
In patients with asthma, disruption of the epithelial barrier through reduced gap junction integrity has been suggested to result in increased epithelial cell death, leading to a heightened inflammatory cascade with exposure of tissue TLR receptors to pathogen-associated molecular patterns (PAMPs).42 In patients with CRS, diminished innate immune responses and diminished barrier function can have several consequences. Observations of diminished barrier and reduced levels of antimicrobial peptides in CRS might be compatible with both leading theories of the pathogenesis of CRS discussed above. If the immune barrier is indeed disrupted (and this has yet to be formally demonstrated in patients with CRS), then sensitization to ambient fungi, such as Alternaria species, would be more likely to occur as a result of increased penetration of fungal allergens. This would be particularly true if SPINK5 is involved in the inhibition of fungal or host proteases that activate epithelial cells and increase their permeability. Likewise, reduced levels of key antimicrobial peptides, such as S100A7 and calprotectin, could increase the likelihood of bacterial colonization in the upper airways, even if this does not lead to acute sinus disease. Interestingly, calprotectin is more toxic to S aureus than E coli, whereas S100A7 is more toxic to E coli. Poor expression of both S100A7 and calprotectin might lead to inadequate innate resistance to diverse strains of extracellular and intracellular bacteria. Increased colonization of the upper airways with bacteria and fungi or increased epithelial activation and permeability by the products of bacteria and fungi might explain the robust adaptive immune response that is observed in patients with CRS.45 Because levels of S100A7 and S100A8/S100A9 appear to be reduced in both patients with CRSsNP and patients with CRSwNP, there might be some common pathogenic mechanisms in these 2 related diseases. The well-known observation that CRS tissue, especially nasal polyps, contains large numbers of B lymphocytes that produce IgA and IgE might reflect increased access of antigenic material to the lamina propria. In future studies, it will be important to determine whether defects in barrier function serve as one of the primary causative mechanisms in the pathogenesis of CRS and whether this renders the host susceptible to colonization by pathogens, leading to a heightened inflammatory response.
Abbreviations used
- AD
Atopic dermatitis
- CRS
Chronic rhinosinusitis
- CRSsNP
Chronic rhinosinusitis without nasal polyps
- CRSwNP
Chronic rhinosinusitis with nasal polyps
- KLK
Kallikrein-related peptidase
- SPINK5
Serine peptidase inhibitor Kazal type 5
- TLR
Toll-like receptor
Footnotes
Disclosure of potential conflict of interest: R. C. Kern has served as a consultant for the National Institutes of Health and has provided legal consultation or expert witness testimony on the topic of zinc toxicity. The rest of the authors have declared that they have no conflict of interest.
REFERENCES
- 1.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114(suppl):155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–9. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 3.Cookson W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat Rev Immunol. 2004;4:978–88. doi: 10.1038/nri1500. [DOI] [PubMed] [Google Scholar]
- 4.Weidinger S, O’Sullivan M, Illig T, Baurecht H, Depner M, Rodriguez E, et al. Filaggrin mutations, atopic eczema, hay fever, and asthma in children. J Allergy Clin Immunol. 2008;121:1203–9. e1. doi: 10.1016/j.jaci.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Avila PC, Schleimer RP. Airway epithelium. In: Kay AB, Kaplan AP, Bousquet J, Holt P, editors. Allergy and allergic diseases. 2nd ed Blackwell Publishing; Oxford: 2008. pp. 366–97. [Google Scholar]
- 6.Kern R, Conley D, Walsh W, Chandra R, Kato A, Tripathi-Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol. 2008;22:549–59. doi: 10.2500/ajr.2008.22.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponikau JU, Sherris DA, Kephart GM, Adolphson C, Kita H. The role of ubiquitous airborne fungi in chronic rhinosinusitis. Clin Rev Allergy Immunol. 2006;30:187–94. doi: 10.1385/CRIAI:30:3:187. [DOI] [PubMed] [Google Scholar]
- 8.Shin SH, Ponikau JU, Sherris DA, Congdon D, Frigas E, Homburger HA, et al. Chronic rhinosinusitis: an enhanced immune response to ubiquitous airborne fungi. J Allergy Clin Immunol. 2004;114:1369–75. doi: 10.1016/j.jaci.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Benninger MS, Anon J, Mabry RL. The medical management of rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(suppl):S41–9. doi: 10.1016/S0194-59989770006-8. [DOI] [PubMed] [Google Scholar]
- 10.Patou J, Gevaert P, Van Zele T, Holtappels G, van Cauwenberge P, Bachert C. Staphylococcus aureus enterotoxin B, protein A, and lipoteichoic acid stimulations in nasal polyps. J Allergy Clin Immunol. 2008;121:110–5. doi: 10.1016/j.jaci.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 11.Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol. 2007;120:1279–84. doi: 10.1016/j.jaci.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev. 2000;173:27–38. doi: 10.1034/j.1600-065x.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- 13.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–64. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 14.Vandermeer J, Sha Q, Lane AP, Schleimer RP. Innate immunity of the sinonasal cavity: expression of messenger RNA for complement cascade components and toll-like receptors. Arch Otolaryngol Head Neck Surg. 2004;130:1374–80. doi: 10.1001/archotol.130.12.1374. [DOI] [PubMed] [Google Scholar]
- 15.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 16.Claeys S, de Belder T, Holtappels G, Gevaert P, Verhasselt B, van Cauwenberge P, et al. Human beta-defensins and toll-like receptors in the upper airway. Allergy. 2003;58:748–53. doi: 10.1034/j.1398-9995.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang CS, Dong Z. [Expression of toll-like receptor mRNA in epithelial cell of nasal mucosa] Zhonghua Er Bi Yan Hou Ke Za Zhi. 2003;38:243–6. [PubMed] [Google Scholar]
- 18.Pitzurra L, Bellocchio S, Nocentini A, Bonifazi P, Scardazza R, Gallucci L, et al. Antifungal immune reactivity in nasal polyposis. Infect Immun. 2004;72:7275–81. doi: 10.1128/IAI.72.12.7275-7281.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637–68. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 20.Madsen P, Rasmussen HH, Leffers H, Honore B, Celis JE. Molecular cloning and expression of a novel keratinocyte protein (psoriasis-associated fatty acid-binding protein [PA-FABP]) that is highly up-regulated in psoriatic skin and that shares similarity to fatty acid-binding proteins. J Invest Dermatol. 1992;99:299–305. doi: 10.1111/1523-1747.ep12616641. [DOI] [PubMed] [Google Scholar]
- 21.Meyer JE, Harder J, Sipos B, Maune S, Kloppel G, Bartels J, et al. Psoriasin (S100A7) is a principal antimicrobial peptide of the human tongue. Mucosal Immunol. 2008;1:239–43. doi: 10.1038/mi.2008.3. [DOI] [PubMed] [Google Scholar]
- 22.Glaser R, Harder J, Lange H, Bartels J, Christophers E, Schroder JM. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol. 2005;6:57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- 23.Lee KC, Eckert RL. S100A7 (psoriasin)—mechanism of antibacterial action in wounds. J Invest Dermatol. 2007;127:945–57. doi: 10.1038/sj.jid.5700663. [DOI] [PubMed] [Google Scholar]
- 24.Glaser R, Meyer-Hoffert U, Harder J, Cordes J, Wittersheim M, Kobliakova J, et al. The antimicrobial protein psoriasin (S100A7) is upregulated in atopic dermatitis and after experimental skin barrier disruption. J Invest Dermatol. 2009;129:641–9. doi: 10.1038/jid.2008.268. [DOI] [PubMed] [Google Scholar]
- 25.Bryborn M, Adner M, Cardell LO. Psoriasin, one of several new proteins identified in nasal lavage fluid from allergic and non-allergic individuals using 2-dimensional gel electrophoresis and mass spectrometry. Respir Res. 2005;6:118. doi: 10.1186/1465-9921-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michalek M, Gelhaus C, Hecht O, Podschun R, Schroder JM, Leippe M, et al. The human antimicrobial protein psoriasin acts by permeabilization of bacterial membranes. Dev Comp Immunol. 2009;33:740–6. doi: 10.1016/j.dci.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Richer SL, Truong-Tran AQ, Conley DB, Carter R, Vermylen D, Grammer LC, et al. Epithelial genes in chronic rhinosinusitis with and without nasal polyps. Am J Rhinol. 2008;22:228–34. doi: 10.2500/ajr.2008.22.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abtin A, Eckhart L, Mildner M, Gruber F, Schroder JM, Tschachler E. Flagellin is the principal inducer of the antimicrobial peptide S100A7c (psoriasin) in human epidermal keratinocytes exposed to Escherichia coli. FASEB J. 2008;22:2168–76. doi: 10.1096/fj.07-104117. [DOI] [PubMed] [Google Scholar]
- 29.Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–23. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 30.Commins S, Steinke JW, Borish L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J Allergy Clin Immunol. 2008;121:1108–11. doi: 10.1016/j.jaci.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Ross KF, Herzberg MC. Calprotectin expression by gingival epithelial cells. Infect Immun. 2001;69:3248–54. doi: 10.1128/IAI.69.5.3248-3254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandal K, Rouleau P, Boivin A, Ryckman C, Talbot M, Tessier PA. Blockade of S100A8 and S100A9 suppresses neutrophil migration in response to lipopolysaccharide. J Immunol. 2003;171:2602–9. doi: 10.4049/jimmunol.171.5.2602. [DOI] [PubMed] [Google Scholar]
- 33.Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemo-taxis and adhesion. J Immunol. 2003;170:3233–42. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- 34.Nisapakultorn K, Ross KF, Herzberg MC. Calprotectin expression in vitro by oral epithelial cells confers resistance to infection by Porphyromonas gingivalis. Infect Immun. 2001;69:4242–7. doi: 10.1128/IAI.69.7.4242-4247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Champaiboon C, Sappington KJ, Guenther BD, Ross KF, Herzberg MC. Calprotectin S100A9 calcium-binding loops I and II essential for keratinocyte resistance to bacterial invasion. J Biol Chem. 2009;284:7078–90. doi: 10.1074/jbc.M806605200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnekenburger J, Schick V, Kruger B, Manitz MP, Sorg C, Nacken W, et al. The calcium binding protein S100A9 is essential for pancreatic leukocyte infiltration and induces disruption of cell-cell contacts. J Cell Physiol. 2008;216:558–67. doi: 10.1002/jcp.21433. [DOI] [PubMed] [Google Scholar]
- 37.Thorey IS, Roth J, Regenbogen J, Halle JP, Bittner M, Vogl T, et al. The Ca2+-binding proteins S100A8 and S100A9 are encoded by novel injury-regulated genes. J Biol Chem. 2001;276:35818–25. doi: 10.1074/jbc.M104871200. [DOI] [PubMed] [Google Scholar]
- 38.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–9. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 39.Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, et al. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J Allergy Clin Immunol. 2006;118:3–23. doi: 10.1016/j.jaci.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 40.Weidinger S, Baurecht H, Wagenpfeil S, Henderson J, Novak N, Sandilands A, et al. Analysis of the individual and aggregate genetic contributions of previously identified serine peptidase inhibitor Kazal type 5 (SPINK5), kallikrein-related peptidase 7 (KLK7), and filaggrin (FLG) polymorphisms to eczema risk. J Allergy Clin Immunol. 2008;122:560–8. e4. doi: 10.1016/j.jaci.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 41.Chavanas S, Bodemer C, Rochat A, Hamel-Teillac D, Ali M, Irvine AD, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141–2. doi: 10.1038/75977. [DOI] [PubMed] [Google Scholar]
- 42.Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol. 2007;120:1233–46. doi: 10.1016/j.jaci.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 43.Bernstein JM, Gorfien J, Noble B, Yankaskas JR. Nasal polyposis: immunohisto-chemistry and bioelectrical findings (a hypothesis for the development of nasal polyps) J Allergy Clin Immunol. 1997;99:165–75. doi: 10.1016/s0091-6749(97)70091-5. [DOI] [PubMed] [Google Scholar]
- 44.Dejima K, Randell SH, Stutts MJ, Senior BA, Boucher RC. Potential role of abnormal ion transport in the pathogenesis of chronic sinusitis. Arch Otolaryngol Head Neck Surg. 2006;132:1352–62. doi: 10.1001/archotol.132.12.1352. [DOI] [PubMed] [Google Scholar]
- 45.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121:1385–92. e1–2. doi: 10.1016/j.jaci.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]