Abstract
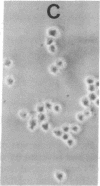
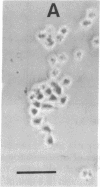
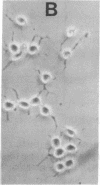
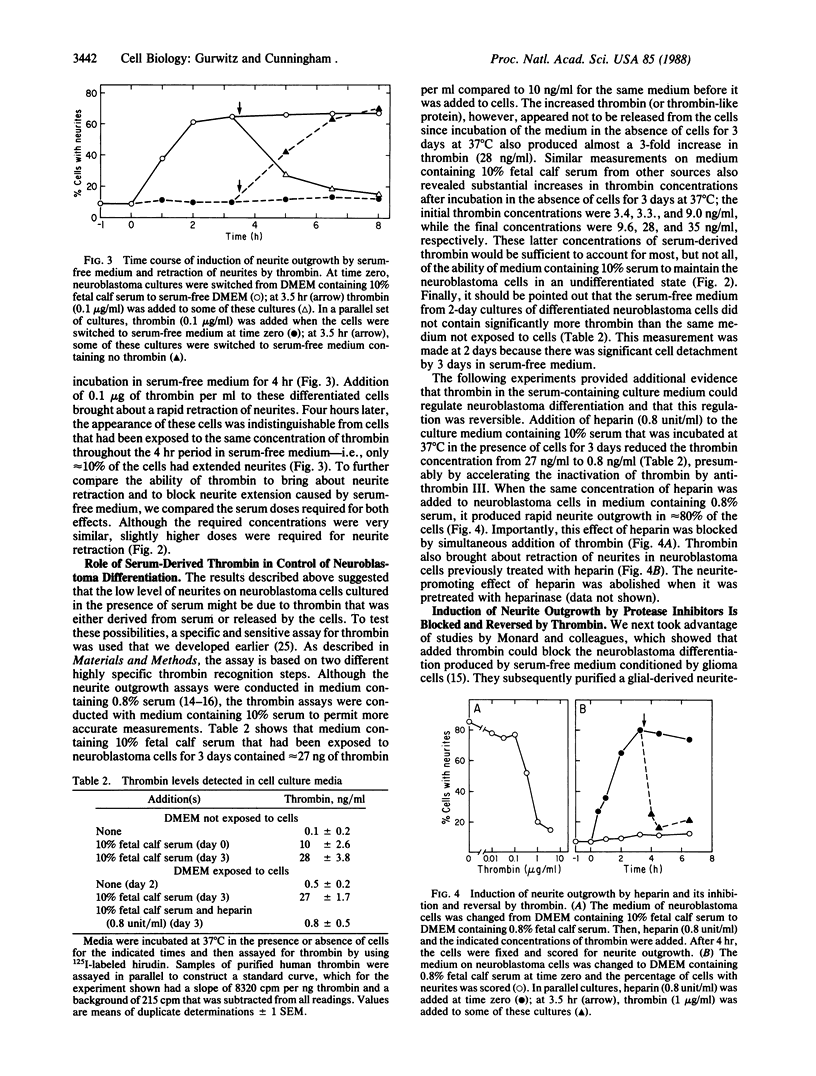
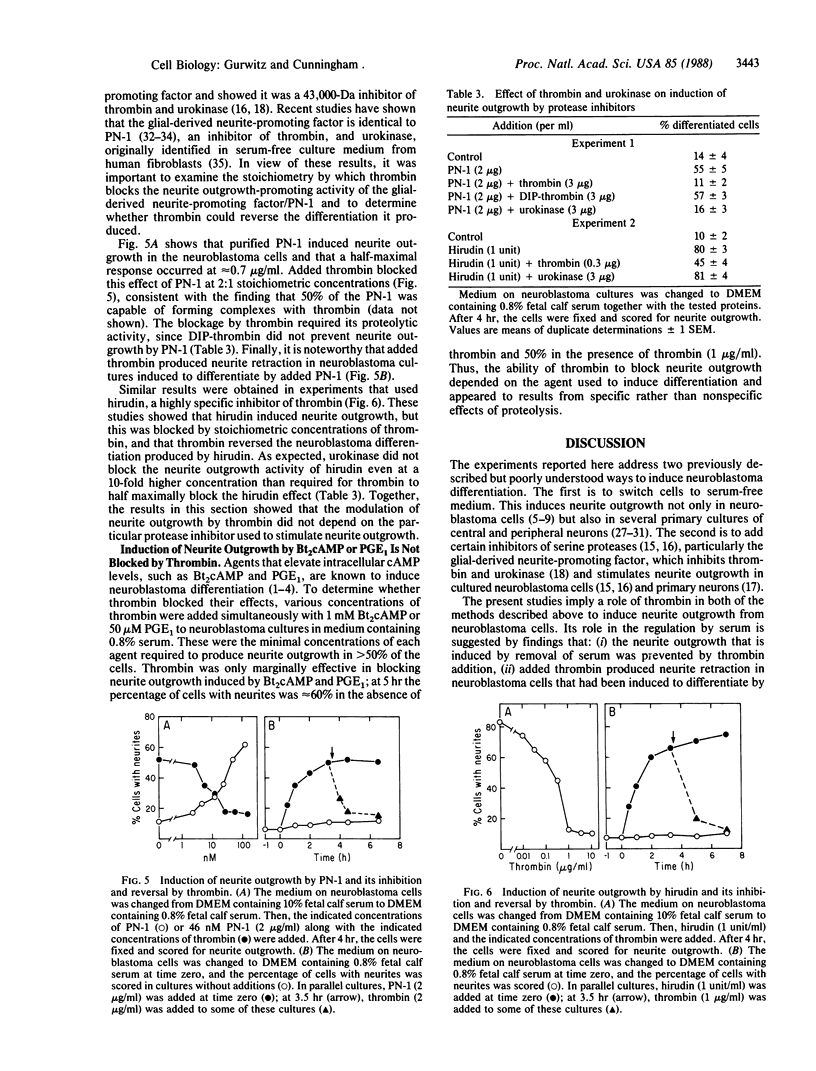
Previous studies have shown that neuroblastoma cells and several types of primary neuronal cells in culture rapidly extend neurites when switched from serum-containing to serum-free medium. The present studies on cloned neuroblastoma cells show that thrombin blocked this spontaneous differentiation at 2 nM with a half-maximal potency of 50 pM. This required the catalytic activity of thrombin and was reversed upon thrombin removal. Thrombin also caused cells in serum-free medium to retract their neurites at equally low concentrations. Two other serine proteases, urokinase and plasmin, did not block or reverse neurite extension even at 100-fold higher concentrations. A specific assay for thrombin indicated that thrombin detected in serum-containing medium from neuroblastoma cultures was derived from serum and that it was likely responsible for much of the known capacity of serum to maintain neuroblastoma cells in a nondifferentiated state. This was supported by the finding that heparin addition reduced the thrombin concentration in serum-containing medium and stimulated neurite outgrowth from neuroblastoma cells in serum-containing medium. Studies on the ability of thrombin to modulate neurite outgrowth by other agents showed that it blocked and reversed the neurite outgrowth activity of two thrombin inhibitors: protease nexin-1 (which is identical to glial-derived neurite-promoting factor) and hirudin. Thrombin, however, did not block the neurite-promoting activity of dibutyryl cAMP or prostaglandin E1. These results suggest a specific role for thrombin in control of neurite outgrowth.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R. Regulation of neurite growth in purified retina neuronal cultures: effects of PNPF, a substratum-bound, neurite-promoting factor. J Neurosci Res. 1982;8(2-3):165–177. doi: 10.1002/jnr.490080207. [DOI] [PubMed] [Google Scholar]
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusti-Tocco G., Sato G. Establishment of functional clonal lines of neurons from mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):311–315. doi: 10.1073/pnas.64.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. B., Low D. A., Simmer R. L., Cunningham D. D. Protease-nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell. 1980 Aug;21(1):37–45. doi: 10.1016/0092-8674(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G. E., Skaper S. D., Manthorpe M., Moonen G., Varon S. Fetal calf serum-mediated inhibition of neurite growth from ciliary ganglion neurons in vitro. J Neurosci Res. 1984;12(1):29–39. doi: 10.1002/jnr.490120104. [DOI] [PubMed] [Google Scholar]
- Farrell D. H., Van Nostrand W. E., Cunningham D. D. A simple two-step purification of protease nexin. Biochem J. 1986 Aug 1;237(3):907–912. doi: 10.1042/bj2370907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furmanski P., Silverman D. J., Lubin M. Expression of differentiated functions in mouse neuroblastoma mediated by dibutyryl-cyclic adenosine monophosphate. Nature. 1971 Oct 8;233(5319):413–415. doi: 10.1038/233413a0. [DOI] [PubMed] [Google Scholar]
- Gloor S., Odink K., Guenther J., Nick H., Monard D. A glia-derived neurite promoting factor with protease inhibitory activity belongs to the protease nexins. Cell. 1986 Dec 5;47(5):687–693. doi: 10.1016/0092-8674(86)90511-8. [DOI] [PubMed] [Google Scholar]
- Guenther J., Nick H., Monard D. A glia-derived neurite-promoting factor with protease inhibitory activity. EMBO J. 1985 Aug;4(8):1963–1966. doi: 10.1002/j.1460-2075.1985.tb03878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystosek A., Seeds N. W. Plasminogen activator release at the neuronal growth cone. Science. 1981 Sep 25;213(4515):1532–1534. doi: 10.1126/science.7197054. [DOI] [PubMed] [Google Scholar]
- Krystosek A., Seeds N. W. Plasminogen activator secretion by granule neurons in cultures of developing cerebellum. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7810–7814. doi: 10.1073/pnas.78.12.7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low D. A., Cunningham D. D. A novel method for measuring cell surface-bound thrombin. Detection of iodination-induced changes in thrombin-binding affinity. J Biol Chem. 1982 Jan 25;257(2):850–858. [PubMed] [Google Scholar]
- Ludueña M. A. The growth of spinal ganglion neurons in serum-free medium. Dev Biol. 1973 Aug;33(2):470–476. doi: 10.1016/0012-1606(73)90152-8. [DOI] [PubMed] [Google Scholar]
- Marchisio P. C., Osborn M., Weber K. The intracellular organization of actin and tubulin in cultured C-1300 mouse neuroblastoma cells (clone NB41A3). J Neurocytol. 1978 Oct;7(5):571–582. doi: 10.1007/BF01260890. [DOI] [PubMed] [Google Scholar]
- McKinney M., Snider R. M., Richelson E. Thrombin binding to human brain and spinal cord. Mayo Clin Proc. 1983 Dec;58(12):829–831. [PubMed] [Google Scholar]
- Means E. D., Anderson D. K. Thrombin interactions with central nervous system tissue and implications of these interactions. Ann N Y Acad Sci. 1986;485:314–322. doi: 10.1111/j.1749-6632.1986.tb34593.x. [DOI] [PubMed] [Google Scholar]
- Monard D., Niday E., Limat A., Solomon F. Inhibition of protease activity can lead to neurite extension in neuroblastoma cells. Prog Brain Res. 1983;58:359–364. doi: 10.1016/S0079-6123(08)60037-0. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Tubulin pools in differentiating neuroblastoma cells. J Cell Biol. 1981 Jun;89(3):418–423. doi: 10.1083/jcb.89.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K. N., Hsie A. W. Morphologic differentiation of mouse neuroblastoma cells induced in vitro by dibutyryl adenosine 3':5'-cyclic monophosphate. Nat New Biol. 1971 Sep 29;233(39):141–142. doi: 10.1038/newbio233141a0. [DOI] [PubMed] [Google Scholar]
- Prasad K. N. Morphological differentiation induced by prostaglandin in mouse neuroblastoma cells in culture. Nat New Biol. 1972 Mar 15;236(63):49–52. doi: 10.1038/newbio236049a0. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Sahu S. K., Sinha P. K. Cyclic nucleotides in the regulation of expression of differentiated functions in neuroblastoma cells. J Natl Cancer Inst. 1976 Sep;57(3):619–631. doi: 10.1093/jnci/57.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt D. E., Cotman C. W., Nieto-Sampedro M., Rowe J. W., Knauer D. J. Identification of a protease inhibitor produced by astrocytes that is structurally and functionally homologous to human protease nexin-I. Brain Res. 1987 Jul 7;415(1):40–48. doi: 10.1016/0006-8993(87)90267-8. [DOI] [PubMed] [Google Scholar]
- Schubert D., Heinemann S., Carlisle W., Tarikas H., Kimes B., Patrick J., Steinbach J. H., Culp W., Brandt B. L. Clonal cell lines from the rat central nervous system. Nature. 1974 May 17;249(454):224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Baroni C., Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Jacob F., de Vitry F. Induced differentiation of a neuroblastoma. Dev Biol. 1971 Aug;25(4):514–546. doi: 10.1016/0012-1606(71)90004-2. [DOI] [PubMed] [Google Scholar]
- Schurch-Rathgeb Y., Mongard D. Brain development influences the appearance of glial factor-like activity in rat brain primary cultures. Nature. 1978 May 25;273(5660):308–309. doi: 10.1038/273308a0. [DOI] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider R. M., McKinney M., Fenton J. W., 2nd, Richelson E. Activation of cyclic nucleotide formation in murine neuroblastoma N1E-115 cells by modified human thrombins. J Biol Chem. 1984 Jul 25;259(14):9078–9081. [PubMed] [Google Scholar]
- Snider R. M., McKinney M., Richelson E. Thrombin binding and stimulation of cyclic guanosine monophosphate formation in neuroblastoma cells. Semin Thromb Hemost. 1986 Jul;12(3):253–262. doi: 10.1055/s-2007-1003563. [DOI] [PubMed] [Google Scholar]
- Snider R. M. Thrombin effects on cultured nerve cells: clinical implications and evidence for a novel mechanism of neuronal activation. Ann N Y Acad Sci. 1986;485:310–313. doi: 10.1111/j.1749-6632.1986.tb34592.x. [DOI] [PubMed] [Google Scholar]
- Soreq H., Miskin R., Zutra A., Littauer U. Z. Modulation in the levels and localization of plasminogen activator in differentiating neuroblastoma cells. Brain Res. 1983 Apr;283(2-3):257–269. doi: 10.1016/0165-3806(83)90182-7. [DOI] [PubMed] [Google Scholar]
- Stone S. R., Nick H., Hofsteenge J., Monard D. Glial-derived neurite-promoting factor is a slow-binding inhibitor of trypsin, thrombin, and urokinase. Arch Biochem Biophys. 1987 Jan;252(1):237–244. doi: 10.1016/0003-9861(87)90028-2. [DOI] [PubMed] [Google Scholar]
- Ziller C., Dupin E., Brazeau P., Paulin D., Le Douarin N. M. Early segregation of a neuronal precursor cell line in the neural crest as revealed by culture in a chemically defined medium. Cell. 1983 Feb;32(2):627–638. doi: 10.1016/0092-8674(83)90482-8. [DOI] [PubMed] [Google Scholar]
- Ziller C., Le Douarin N. M. Neuronal differentiation in cultured neural crest cells: the effect of serum on neurite outgrowth. Birth Defects Orig Artic Ser. 1983;19(4):251–261. [PubMed] [Google Scholar]