Abstract
A progressive reduction in β-cell mass occurs in the evolution of diabetes. Thus understanding the mechanisms responsible for this reduction in β-cell mass is important for understanding the pathogenesis of diabetes, and in developing novel approaches to prevention and treatment. Pancreatic duodenal homeobox 1 (Pdx1) is a transcription factor that plays a central role in pancreatic β-cell function and survival. Complete deficiency of Pdx1 is associated with pancreatic agenesis and partial deficiency leads to severe β-cell dysfunction, and increases β-cell death and diabetes both in rodent and human. Chronic hyperglycemia and dyslipidemia, which are major features of type 2 diabetes, cause β-cell dysfunction via reduced Pdx1 expression. Inhibition of insulin/insulin-like growth factor (Igf) signaling followed by reduced Pdx1 expression is a common pathway induced by the majority of the mechanisms in apoptotic β-cells. Although the report so far paid little attention about non-apoptotic β-cell death (autophagy and necrosis), we expect these are also involved in the pathogenesis of diabetes. The potential role of Pdx1 in non-apoptotic β-cell death should also be considered in future studies in diabetes, and in attempts to develop novel agents that target this process for prevention and treatment of the disorder.
Keywords: apoptosis, autophagy, β-cell mass, necrosis, Pdx1
Introduction
The endocrine pancreas is comprised of 4 cell types: β-cells produce insulin, α-cells produce glucagon, δ-cells produce somatostatin, and PP-cells produce pancreatic polypeptide. The pancreatic β-cells of the islets are solely responsible for the transcription, synthesis, and release of insulin in response to ambient blood glucose level. Although β-cells comprise 70-80% of islet mass, islets themselves comprise only about 1% of total pancreatic mass [1]. The central role played by such a small mass of cells most likely contributes to our susceptibility to β-cell function and to the development of diabetes. β-cell dysfunction and/or reductions in β-cell mass are central abnormalities in both type 1 and type 2 diabetes [2]. Thus, elucidation of the molecular mechanisms responsible for regulating death of β-cells is critical for our understanding of the pathogenesis of diabetes.
Pancreatic duodenal homeobox 1 (Pdx1) plays a central role in β-cell survival. Not only is Pdx1 a key regulator of insulin gene expression, but it is also essential for normal development of the pancreas, most probably by determining maturation and differentiation of common pancreatic precursor cells in the developing gut [1,3]. The gene encoding Pdx1 is a member of a mammalian Parahox gene cluster on mouse chromosome 5. This cluster is so-named because it represents a group of developmentally important genes in mammals that is found outside the classical Hox (homeobox) cluster of gene [4]. The Parahox cluster is comprised of three genes, Gsh1, Pdx1, and Cdx2/3, all of which are expressed in specific pancreatic cell type [5]. The importance of Pdx1 in the pancreas is underscored by the development of pancreatic agenesis in Pdx1 null mice [3]. This dramatic phenotype also occurs in humans with homozygous mutations of the human orthologue of the Pdx1 gene [6], thereby underscoring the relevance of this factor to human pancreas development. Pdx1 also appears to be crucial for the function of the mature β-cell. Heterozygous missense and frameshift mutations of the Pdx1 gene in humans (while not impairing pancreas formation) result in defective insulin secretion, and the development of one form of maturity onset diabetes of the young 4 (MODY4) in humans [7-10]. Similarly, animal models suggest that down regulation of Pdx1 expression in the β-cell may underlie the pathogenesis of β-cell failure and type 2 diabetes [11]. Thus Pdx1 plays both a broad role in pancreas development, and a much more specific role in β-cell survival and function in the adult mammal. Although the role of Pdx1 in the differentiation of specific cell types in the developing pancreas has been clearly defined, how Pdx1 regulates β-cell survival and function in adult pancreatic islet is less well understood. Therefore, this review describes how Pdx1 expression and function are regulated to maintain normal glucose homeostasis and β-cell mass especially in adult pancreatic islet.
Pancreas and islet development
In recent years there have been major advances in our understanding of pancreas development, particularly in the mouse and these have been summarized in excellent reviews. [12-14]. In Pdx1 knockout mice, the initial buds of the pancreas form but subsequent branching and morphogenesis of these buds is arrested [3,15]. On the other hand, Pdx1 haploinsufficiency (Pdx1+/-) does not appear physically or functionally impinge upon pancreas development in Pdx1+/− mice. Shortly after birth, the endocrine pancreas undergoes remodeling through a process that involves substantial apoptosis and β-cell replication [16]. In the adult under normal conditions, the β-cell has a slow turnover rate [17], whereby β-cell death is balanced by the replication of existing β-cells as demonstrated by in vivo lineage tracing using an insulin promoter driven Cre recombinase [18], and subsequently confirmed by using a DNA analogue based lineage tracing technique [19]. The overall mass can, however, be modulated to compensate for the increased metabolic demands.
Apoptosis in β-cell
There are three types of cell death, apoptosis, autophagy and necrosis [20]. It has generally been assumed that apoptosis is the major cause of β-cell death in animal models of diabetes and when islets or insulin secreting β-cells are exposed to various pathophysiologic states [2]. The mechanisms responsible for β-cell apoptosis are complex. Reduced signaling through the insulin/insulin-like growth factor (Igf) pathway appears to be an important common mechanism leading to the decreased Pdx1 expression that is consistently present in states of β-cell failure [21].
Insulin/Igf signaling
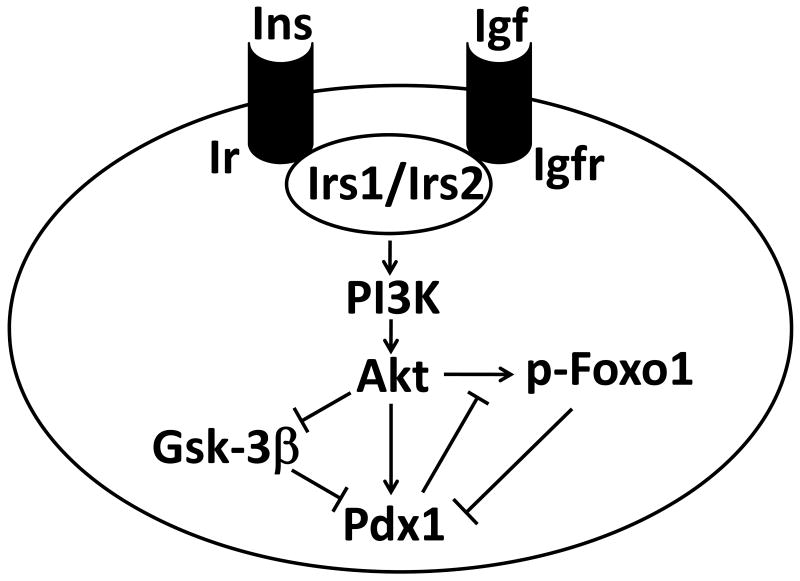
Insulin/Igf signaling is an important regulator of β-cell growth and proliferation acting through Irs1/Irs2-PI3K-Akt pathway (Figure). A mouse with a specific deletion of the β-cell insulin receptor (Ir) exhibits a progressively impaired glucose tolerance, reduced β-cell mass and islet number [22,23]. These data indicate that loss of functional receptors for insulin in β-cell leads primarily to profound defects in postnatal β-cell growth. Mice in which the Irs2 gene has been inactivated in β-cells develops β-cell failure due to decreased proliferation and an increased rate of apoptosis [24,25]. β-cell specific Igf1 knockout mice exhibited a delayed onset of type 2 diabetes induced by a high fat diet, accompanied by enlarged pancreatic islets, increased insulin mRNA levels, and preserved sensitivity to insulin [26]. Therefore, locally produced Igf1 within the pancreas plays a unique role and inhibits islet cell growth and insulin secretion. Thus, lots of mouse models in which reduced insulin/Igf signaling in the β-cell lead to impaired beta cell function and/or survival.
Figure. Insulin/Igf signaling in β-cell.
Insulin and Igf bind to insulin receptor and Igf receptor, respectively followed by phosphorylation of Irs1/Irs2. This promotes the activation of Akt in a PI3K-dependent fashion, resulting in inactivation of Gsk-3β and Foxo1. Gsk-3β and Foxo1 decrease Pdx1 expression at protein and transcription level, respectively. Akt can also directly regulate Pdx1 function. Ins; insulin, Igf; insulin-like growth factor, Ir; insulin receptor, Igfr; Igf receptor; Irs; insulin receptor substrate, PI3K; phosphatidylinositol 3-kinase, Gsk-3β; glycogen synthase kinase-3β, Foxo1; forkhead box o 1, Pdx1; pancreatic duodenal homeobox 1.
The survival function of Pdx1 in insulin/Igf signaling acts through the forkhead transcription factor Foxo1 and glycogen synthase kinase-3β (Gsk-3β) (Figure). When diabetic Irs2−/− mice were crossed with Foxo1+/− mice, the reduced levels of Foxo1 were able to partially rescue the phenotype with a concomitant increase in Pdx1 expression levels, suggesting that insulin and/or Igf regulate β-cell mass by relieving Foxo1 inhibition of Pdx1 expression [27]. Insulin (or Igf1) inhibits the actions of Foxo1 through Akt-mediated phosphorylation of Foxo1, which induces movement of the transcription factor Pdx1 from the nucleus to the cytoplasm. Pdx1 also acts as a critical regulator of β-cell replication that occurs during the compensatory response to insulin resistance [28]. This was demonstrated by crossing insulin receptor deficient (Ir−/−) or Irs1 deficient (Irs1−/−) mice with Pdx1+/− mice. Evidence that Foxo1 was also involved in this process was provided by using mice that harbored a mutant Foxo1 transgene that is retained in the nucleus. Thus, in two models of insulin resistance, i.e. Ir−/− mice, and mice in which the elastase promoter is used to achieve local expression of Igf2, the presence of the mutant Foxo1 transgene that is retained in the nucleus (and thus inhibits expression of Pdx1) blocked Igf-induced β-cell proliferation [29]. Recently, Tanabe et al. reported the role of Gsk-3β in control of β-cell mass in insulin resistant diabetic models. Loss of one allele of Gsk-3β in wild type mice promotes insulin sensitivity, and in Ir+/− mice reduces insulin resistance and improves glucose tolerance by enhancing glucose disposal. Severely insulin resistant Irs2−/− mice were found to have elevated islet Gsk-3 activity associated with severe reduction of β-cell proliferation and elevated apoptosis. Loss of one allele of Gsk-3β in Irs2−/− mice reversed these findings, preserving β-cell mass and preventing diabetes. Additionally, Pdx1 expression levels were depressed and levels of p27Kip1, a cell cycle-dependent kinase inhibitor were increased in islets of Irs2−/− mice. Loss of one allele of Gsk-3β also preserved β-cell mass and prevented diabetes. β-cell specific deficiency of Gsk-3β reversed the diabetes of the Irs2−/− mice [30], indicating that Gsk-3β also negatively regulates β-cells mass and function. Phosphorylation of Ser61 and Ser66 of Pdx1 via Gsk-3β appears to target Pdx1 for proteosomal degradation, thereby decreasing its half-life. This modification could partially explain diminished β-cell function during endoplasmic reticulum stress and oxidative stress (both of which occur in the diabetic state), where Gsk-3β activity is enhanced [31,32]. The decrease in insulin/Igf signaling induces Foxo1 and Gsk-3β function. Thus, these results indicate that in insulin resistant animals, Foxo1 and Gsk-3β impair replication and enhances β-cell death at transcription and protein level respectively, ultimately leading to postnatal β-cell loss and diabetes.
Glucose and fatty acid
Glucose is the most extensively studied determinant of β-cell growth [33]. Among the islet cell types, it is principally the β-cell population that undergoes accelerated growth in response to hyperglycemia and insulin resistance. Pancreatic regeneration involves activation of Akt in both islets and endocrine cell clusters. Glucose infusion also leads to increased Akt activation in islet β-cells, as well as in insulin-positive and insulin-negative cells in the common duct epithelium and endocrine clusters. This correlates with strong Pdx1 expression in these same cells [34,35]. These results suggest that the mechanisms underlying the rapid increase in β-cell growth following a glucose infusion involve Akt-regulated enhanced β-cell survival as well as neogenesis from epithelial precursors.
In contrast to the effects of mild transient increases in glucose to increase insulin secretion, prolonged hyperglycemia induces glucotoxicity, resulting in β-cell dysfunction and decreases β-cell mass [36]. The effects of chronic hyperglycemia in β-cells have been assessed in animal models in vivo and using insulinoma cells and isolated islets in vitro. In the setting of chronic exposure to hyperglycemia, rat islets exhibit basal insulin hyper-secretion and defective glucose-stimulated insulin secretion [37]. Both in animal models and humans, chronic hyperglycemia is associated with alterations in β-cell mass and function [38]. The β-cell has an incredible ability to adapt and compensate for chronic hyperglycemia, as seen in the Zucker diabetic fatty (ZDF) rat, but ultimately, obesity, chronic hyperglycemia, and worsening insulin resistance lead to increased β-cell apoptosis [39]. Similarly, postmortem studies in human type 2 diabetic patients reveal low frequency of replication and reduced β-cell mass, mainly by increased apoptosis [40].
When β-cells are exposed to high glucose concentrations for increasingly prolonged periods of time, glucose saturates the normal route of glycolysis and increasingly is shunted to alternate pathways, such that reactive oxygen species (ROS) are generated from distinct metabolic processes within and outside the mitochondria. Reports also indicate that excessive levels of palmitate are associated with abnormal islet function (especially in the presence of high glucose concentrations), which leads to excessive lipid esterification that, in turn, can generate ceramide, thereby increasing oxidative stress. Treatment with a lipid lowering drug did not protect the animals from worsening of islet function and diabetes, whereas a glucose lowering drug was successful [41]. This suggested that lipotoxicity requires concomitant hyperglycemia to damage islet function, whereas glucose toxicity can exert harmful effects on the islet in the absence of elevated circulating triglyceride. One molecular mechanism of action through which chronic hyperglycemia can cause worsening β-cell function is via decreased protein expression of Pdx1 and MafA [42]. The possibility that oxidative stress is responsible for the decreased levels of Pdx1 and MafA was highlighted by studies showing that antioxidant treatment of β-cell lines and rodent models of type 2 diabetes protected against deterioration of insulin gene expression induced by exposure to high glucose concentrations [43-45]. N-acetylcysteine (NAC), a drug commonly used in these studies, provides direct antioxidant actions. Treatment with NAC protected against decreases in Pdx1 and MafA caused by high glucose concentrations in vitro, and also protected against loss of insulin gene expression and insulin secretion.
Sterol regulatory element-binding protein (SREBP)-1c is a transcription factor that controls hepatic fatty acid synthesis. Activation of SREBP-1c by over nutrition also inhibits Irs2 expression and induces insulin resistance in the liver. In mouse pancreatic islets, nuclear SREBP-1c has a negative impact on both glucose- and potassium-stimulated insulin secretions as determined in islets from β-cell specific SREBP-1c transgenic mice as well as SREBP-1c knockout mice [46]. Activation of SREBP-2 in β-cells also causes severe diabetes by loss of β-cell mass with accumulation of cholesterol [47]. Although the precise mechanism of SREBPs on β-cell is unknown, repressed Irs2 and Pdx1 expressions and increased pro-apoptotic genes seem to be common feature of increased SREBPs expression [48].
Insulin
We have previously reported that exogenous insulin completely prevented apoptosis induced by serum withdrawal when given at picomolar or low nanomolar concentrations, but not at higher concentrations, indicating that physiological concentrations of insulin are anti-apoptotic, and that insulin signaling is self-limiting in islets. Insulin treatment was associated with the nuclear localization of Pdx1 and the pro-survival effects of insulin were largely absent in islets from Pdx1+/- mice, providing direct evidence that Pdx1 is a signaling target of insulin [49]. Thus, insulin can also act as a regulator of islet survival by regulating Pdx1. Since it is very difficult to differentiate the effects of glucose and insulin on β-cells in vivo, it is still uncertain whether the major effects on β-cell survival and function are mediated by glucose and/or insulin.
Glucagon-like peptide-1
Studies of Pdx1+/- mice demonstrated either relatively preserved [50] or modestly reduced glucose-stimulated insulin secretion [51] and a paradoxically increased insulin secretory response to glucagon-like peptide-1 (GLP-1) [51]. The reason that the insulin secretory response to GLP-1 is preserved in Pdx1+/- mice is not clear but may be related to activation of a K+ATP channel independent stimulus secretion coupling mechanism in the β-cell that is not dependent on Pdx-1. In contrast, glucose-stimulated insulin secretion was abnormal in β-cell specific Pdx1 knockout mice, and isolated islets from these mice exhibited defects in both glucose-stimulated insulin secretion and islet insulin content. In contrast to the enhanced secretory response to GLP-1 in Pdx1+/- mice, the GLP-1 analogue exendin-4 or forskolin, both of which increase cAMP in β-cells failed to stimulate insulin secretion in β-cell specific Pdx1 knockout mice. This result was unexpected in view of the fact that GLP-1 also increases cAMP in β-cells. Nevertheless, β-cell specific Pdx1 knockout islets retain responsiveness to sulfonylurea tolbutamide and potassium, indicating that the β-cell specific Pdx1 knockout islets exhibit highly selective rather than generalized defects in insulin secretion [52]. Thus, Pdx1 expression is essential for integrating GLP-1 receptor-dependent signals for the growth, differentiated function, and survival of islet β-cells.
Pregnancy and intrauterine growth retardation
During pregnancy, maternal pancreatic islets grow to match dynamic physiological demands. Menin, a protein previously characterized as an endocrine tumor suppressor and transcriptional regulator, controls islet growth in pregnant mice. Pregnancy stimulated proliferation of maternal pancreatic islet β-cells that was accompanied by reduced islet levels of menin and its targets. Transgenic expression of menin in maternal β-cells prevented islet expansion and led to hyperglycemia and impaired glucose tolerance, hallmark features of gestational diabetes. Prolactin, whose levels are increased in pregnancy, repressed islet menin levels and stimulated β-cell proliferation. mRNAs encoding insulin1, insulin2, Glut2, and Pdx1 were unchanged [53]. Thus, it seems that menin does not regulate β-cell mass through Pdx1 signaling.
Intrauterine growth retardation (IUGR) has been linked to the onset of diseases in adulthood, including type 2 diabetes, and has been proposed to result from altered gene regulation patterns due to epigenetic modifications of developmental genes. Rodent models of IUGR that lead to lower levels of expression of Pdx1 in the islet develop diabetes in adulthood. Expression of Pdx1 was permanently reduced in IUGR β-cells, and underwent epigenetic modifications throughout development. The fetal IUGR state was characterized by loss of USF-1 binding at the proximal promoter of Pdx1, recruitment of the histone deacetylase 1 (HDAC1) and the co-repressor Sin3A, and deacetylation of histones H3 and H4. Following birth, histone 3 lysine 4 (H3K4) was demethylated and histone 3 lysine 9 (H3K9) was methylated. After the onset of diabetes in adulthood, the CpG island in the proximal promoter of Pdx1 was methylated, resulting in permanent silencing at this locus [54]. These results indicate that chromatin remodeling induces silencing of Pdx1 gene in the development of diabetes following IUGR.
Autophagy and necrosis in β-cell
Autophagy is a ubiquitous process in eukaryotic cells that involves sequestration of cytoplasmic material within a double-membrane autophagosome, and fusion of the autophagosome with a lysosome (autolysosomes) that supplies acid hydrolases that break down the inner membrane of the autolysosomes and degrade the contents. In some contexts autophagy can be considered to be a catabolic, energy generating pathway that allows the cell to adapt to environmental stress or developmental changes [55-57]. Conversely, it is becoming increasing clear that autophagy may play a broader role in biology and in disease pathogenesis. There is evidence that autophagy contributes to the development of cancer, infectious and autoimmune diseases [55,56], and it has also been suggested that autophagy if excessive may lead to cell death [58,59]. Substantial insights have been gained into the genes and signaling pathways that regulate autophagy. Over thirty Atg genes have been identified in this pathway. Critical roles in the regulation of autophagy have been defined for the target of rapamycin gene, TOR and a class III PI3K [55,56].
A potential role for autophagy in the pathogenesis of diabetes has received little attention. Marsh et al have suggested that the rate of autophagy within the insulin secretory granule determines the rate of turnover of these granules and this is an important mechanism for ensuring that the pool of insulin secretory granules is adequate to meet the demands of the body for insulin [60]. β-cell specific knockout of Atg7 resulted in degradation of islets and impaired glucose-stimulated insulin secretion because of abnormal turnover and function of cellular organelles [61,62], indicating basal autophagy is indispensable for maintenance of normal β-cell function and survival. β-cells in db/db and C57BL/6 mice that had been fed high fat diet showed autophagosome formation, probably due to increased insulin resistance caused by high calorie intake or obesity [61]. Autophagy has also been noted in high glucose-induced ubiquitination of proteins into cytoplasmic aggregates in a diabetic model [63]. Evidence of autophagy induced by high glucose was assessed by transfecting GFP-LC3 in INS1 β-cells. Since transfected GFP-LC3 associates with protein aggregates and this aggregation is thought to be independent of autophagy [64], at present it is not clear if high glucose induces autophagy in β-cells in vivo. Pdx1+/- β-cell and Pdx1 reduced mouse insulinoma MIN6 cells have increased autophagy and inhibition of autophagy delays the onset of β-cell death induced by reduced Pdx1 (unpublished data), indicating that Pdx1 also regulates β-cell survival by autophagy. Further studies are needed to determine how autophagy regulates β-cell survival and function.
There are only a few reports concerning necrotic β-cell death, probably due to scarcity of method to detect this process. Steer et al. reported that interleukin-1 induces necrosis in β-cell lines and pancreatic islets as assessed by release of high mobility group box 1 (HMGB1) protein from β-cells [65]. HMGB1 was originally reported as a biochemical marker of necrosis that only released from necrotic but not from apoptotic cells. Recently, however, HMGB1 was re-recognized as an innate danger signal (alarmin) adopted by the innate immune system during evolution of adaptive immune responses. HMGB1 can be either passively released from damaged pancreatic β-cells or released by islet infiltrated auto-reactive immune cells, such as dendritic cells [66]. Thus, it is difficult to conclude at present whether necrosis is associated in β-cell death.
Translocation of Pdx1
As a member of the homeodomain class of proteins, Pdx1 is believed to exert its actions almost exclusively within the nucleus, as a result of regulating gene transcription. Although some reports in the literature have described physiologic circumstances where the distribution of Pdx1 might be cytoplasmic, it is believed that cytoplasmic sequestration more likely represents a mechanism to attenuate the nuclear action of Pdx1 under physiologic or pathologic conditions, rather than to promote a specific cytoplasmic function. For example, it has been suggested that the negative effects of fatty acids on β-cell function may be related to their eventual sequestration of Pdx1 in the cytoplasm [67], whereas the positive effects of glucose on insulin transcription may be a result of its enhancement of Pdx1 nuclear translocation [68]. Under oxidative stress in β-cell, nuclear retention of Foxo1 protects β-cell from oxidative stress-induced damage. Acetylated Foxo1 is retained in the nucleus, where it engages Sirt1. Deacetylation of Foxo1 by Sirt1 promotes Foxo1-dependent transcription and accelerates Foxo1 degradation. Because Foxo1 becomes rapidly degraded when deacetylated, this mechanism has the potential to protect β-cell against acute metabolic distress [69]. A basic amino acid sequence within the homeodomain of Pdx1, RRMKWKK, is believed to function as the nuclear targeting sequence [70]. Teleologically, regulation of Pdx1 protein at the level of compartmentation (i.e. nuclear-cytoplasmic shuttling) would allow for acute alterations in target gene transcription, where control on the order of seconds to minutes might be crucial.
Conclusion
Not surprisingly, given its importance in the development of the pancreas, the differentiation of β-cells and in gene expression in the mature β-cell, Pdx1 turns out also to be a major player in the maintenance of an adequate pool of healthy β-cells in adults. The developmental role of Pdx1 persists into adulthood through the lifelong maintenance of islet mass, architecture, and plasticity, processes that involve the interaction of β-cell neogenesis, differentiation, and apoptosis. Although many diabetic animal models have decreased Pdx1 expression levels, and many reports attributed to decreased Pdx1 expression as a cause of diabetes, little is known about how Pdx1 regulates β-cell survival and function. The potential role of Pdx1 should be considered in future studies of pancreatic β-cell death in diabetes and in attempts to develop novel agents that target this process for prevention and treatment of the disorder.
Acknowledgments
This work was supported by the Clinical and Translational Science Award to Washington University (UL1RR024992) and the Blum Kovler Foundation.
Footnotes
Conflicts of interest
The authors have declared no conflicts of interest
References
- 1.Kim SK, Hebrok M. Intercellular signals regulating pancreas development and function. Genes Dev. 2001;15:111–127. doi: 10.1101/gad.859401. [DOI] [PubMed] [Google Scholar]
- 2.Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of beta cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab. 2007;3:758–768. doi: 10.1038/ncpendmet0647. [DOI] [PubMed] [Google Scholar]
- 3.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 4.Brooke NM, Garcia-Fernàndez J, Holland PW. The ParaHox gene cluster is an evolutionary sister of the Hox gene cluster. Nature. 1998;392:920–922. doi: 10.1038/31933. [DOI] [PubMed] [Google Scholar]
- 5.Rosanas-Urgell A, Marfany G, Garcia-Fernàndez J. Pdx1-related homeodomain transcription factors are distinctly expressed in mouse adult pancreatic islets. Mol Cell Endocrinol. 2005;237:59–66. doi: 10.1016/j.mce.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- 7.Stoffers DA, Stanojevic V, Habener JF. Insulin promoter factor-1 gene mutation linked to early-onset type 2 diabetes mellitus directs expression of a dominant negative isoprotein. J Clin Invest. 1998;102:232–241. doi: 10.1172/JCI2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hani EH, Stoffers DA, Chevre JC, et al. Defective mutations in the insulin promoter factor-1 (ipf-1) gene in late-onset type 2 diabetes mellitus. J Clin Invest. 1999;104:41–48. doi: 10.1172/JCI7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clocquet AR, Egan JM, Stoffers D, et al. Impaired insulin secretion and increased insulin sensitivity in familial maturity-onset diabetes of the young 4 (insulin promoter factor 1 gene) Diabetes. 2000;49:1856–1864. doi: 10.2337/diabetes.49.11.1856. [DOI] [PubMed] [Google Scholar]
- 10.Macfarlane WM, Frayling TM, Ellard S, et al. Missense mutations in the insulin promoter factor-1 gene predispose to type 2 diabetes. J Clin Invest. 1999;104:33–39. doi: 10.1172/JCI7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weir GC, Sharma A, Zangen DH, Bonner-Weir S. Transcription factor abnormalities as a cause of beta cell dysfunction in diabetes: a hypothesis. Acta Diabetol. 1997;34:177–184. doi: 10.1007/s005920050071. [DOI] [PubMed] [Google Scholar]
- 12.Bernardo AS, Hay CW, Docherty K. Pancreatic transcription factors and their role in the birth, life and survival of the pancreatic beta cell. Mol Cell Endocrinol. 2008;294:1–9. doi: 10.1016/j.mce.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Wilson ME, Scheel D, German MS. Gene expression cascades in pancreatic development. Mechanisms of Development. 2003;120:65–80. doi: 10.1016/s0925-4773(02)00333-7. [DOI] [PubMed] [Google Scholar]
- 14.Servitja JM, Ferrer J. Transcriptional networks controlling pancreatic development and beta cell function. Diabetologia. 2004;47:597–613. doi: 10.1007/s00125-004-1368-9. [DOI] [PubMed] [Google Scholar]
- 15.Guz Y, Montminy MR, Stein R, et al. Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121:11–18. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- 16.Bonner-Weir S. Life and death of the pancreatic beta cells. Trends Endocrinol Metab. 2000;11:375–358. doi: 10.1016/s1043-2760(00)00305-2. [DOI] [PubMed] [Google Scholar]
- 17.Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes. 1995;44:249–256. doi: 10.2337/diab.44.3.249. [DOI] [PubMed] [Google Scholar]
- 18.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 19.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Scarlatti F, Granata R, Meijer AJ, Codogno P. Does autophagy have a license to kill mammalian cells. Cell Death Differ. 2008;16:12–20. doi: 10.1038/cdd.2008.101. [DOI] [PubMed] [Google Scholar]
- 21.Chang-Chen KJ, Mullur R, Bernal-Mizrachi E. Beta-cell failure as a complication of diabetes. Rev Endocr Metab Disord. 2008;9:329–343. doi: 10.1007/s11154-008-9101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 23.Otani K, Kulkarni RN, Baldwin AC, et al. Reduced beta-cell mass and altered glucose sensing impair insulin-secretory function in betaIRKO mice. Am J Physiol Endocrinol Metab. 2004;286:E41–E49. doi: 10.1152/ajpendo.00533.2001. [DOI] [PubMed] [Google Scholar]
- 24.Withers DJ, Gutierrez JS, Towery H, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 25.Kubota N, Tobe K, Terauchi Y, et al. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes. 2000;49:1880–1889. doi: 10.2337/diabetes.49.11.1880. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y, Herrera PL, Guo Y, et al. Pancreatic-specific inactivation of IGF-I gene causes enlarged pancreatic islets and significant resistance to diabetes. Diabetes. 2004;53:3131–3141. doi: 10.2337/diabetes.53.12.3131. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura T, Nakae J, Kitamura Y, et al. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J Clin Invest. 2002;110:1839–1847. doi: 10.1172/JCI200216857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulkarni RN, Jhala US, Winnay JN, Krajewski S, Montminy M, Kahn CR. PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest. 2004;114:828–836. doi: 10.1172/JCI21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamoto H, Hribal ML, Lin HV, Bennett WR, Ward A, Accili D. Role of the forkhead protein FoxO1 in beta cell compensation to insulin resistance. J Clin Invest. 2006;116:775–782. doi: 10.1172/JCI24967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanabe K, Liu Z, Patel S, et al. Genetic deficiency of glycogen synthase kinase-3beta corrects diabetes in mouse models of insulin resistance. PLoS Biol. 2008;6:307–318. doi: 10.1371/journal.pbio.0060037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boucher MJ, Selander L, Carlsson L, Edlund H. Phosphorylation marks ipf1/pdx1 protein for degradation by glycogen synthase kinase 3-dependent mechanisms. J Biol Chem. 2006;281:6395–6403. doi: 10.1074/jbc.M511597200. [DOI] [PubMed] [Google Scholar]
- 32.Robertson LA, Kim AJ, Werstuck GH. Mechanisms linking diabetes mellitus to the development of atherosclerosis: A role for endoplasmic reticulum stress and glycogen synthase kinase-3. Can J Physiol Pharmacol. 2006;84:39–48. doi: 10.1139/Y05-142. [DOI] [PubMed] [Google Scholar]
- 33.Kahn SE, Carr DB, Faulenbach MV, Utzschneider KM. An examination of beta-cell function measures and their potential use for estimating beta-cell mass. Diabetes Obes Metab. 2008;4:63–76. doi: 10.1111/j.1463-1326.2008.00945.x. [DOI] [PubMed] [Google Scholar]
- 34.Hagman DK, Latour MG, Chakrabarti SK, et al. Cyclical and alternating infusions of glucose and intralipid in rats inhibit insulin gene expression and Pdx-1 binding in islets. Diabetes. 2008;57:424–431. doi: 10.2337/db07-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fornoni A, Pileggi A, Molano RD, et al. Inhibition of c-jun N terminal kinase (JNK) improves functional beta cell mass in human islets and leads to AKT and glycogen synthase kinase-3 (GSK-3) phosphorylation. Diabetologia. 2008;51:298–308. doi: 10.1007/s00125-007-0889-4. [DOI] [PubMed] [Google Scholar]
- 36.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khaldi MZ, Guiot Y, Gilon P, Henquin JC, Jonas JC. Increased glucose sensitivity of both triggering and amplifying pathways of insulin secretion in rat islets cultured for 1 wk in high glucose. Am J Physiol Endocrinol Metab. 2004;287:E207–E217. doi: 10.1152/ajpendo.00426.2003. [DOI] [PubMed] [Google Scholar]
- 38.Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28:187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]
- 39.Finegood DT, McArthur MD, Kojwang D, et al. Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes. 2001;50:1021–1029. doi: 10.2337/diabetes.50.5.1021. [DOI] [PubMed] [Google Scholar]
- 40.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 41.Harmon JS, Gleason CE, Tanaka Y, Poitout V, Robertson RP. Antecedent hyperglycemia, not hyperlipidemia, is associated with increased islet triacylglycerol content and decreased insulin gene mRNA level in Zucker diabetic fatty rats. Diabetes. 2001;50:2481–2486. doi: 10.2337/diabetes.50.11.2481. [DOI] [PubMed] [Google Scholar]
- 42.Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279:42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci USA. 1999;96:10857–10862. doi: 10.1073/pnas.96.19.10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka Y, Tran PO, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci USA. 2002;99:12363–12368. doi: 10.1073/pnas.192445199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaneto H, Kajimoto Y, Miyagawa J, et al. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes. 1999;48:2398–2406. doi: 10.2337/diabetes.48.12.2398. [DOI] [PubMed] [Google Scholar]
- 46.Shimano H. SREBPs: physiology and pathophysiology of the SREBP family. FEBS J. 2009;276:616–621. doi: 10.1111/j.1742-4658.2008.06806.x. [DOI] [PubMed] [Google Scholar]
- 47.Ishikawa M, Iwasaki Y, Yatoh S, et al. Cholesterol accumulation and diabetes in pancreatic beta-cell-specific SREBP-2 transgenic mice: a new model for lipotoxicity. J Lipid Res. 2008;49:2524–2534. doi: 10.1194/jlr.M800238-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Kouri G, Wollheim CB. ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. J Cell Sci. 2005;118:3905–3915. doi: 10.1242/jcs.02513. [DOI] [PubMed] [Google Scholar]
- 49.Johnson JD, Bernal-Mizrachi E, Alejandro EU, et al. Insulin protects islets from apoptosis via Pdx1 and specific changes in the human islet proteome. Proc Natl Acad Sci U S A. 2006;103:19575–19580. doi: 10.1073/pnas.0604208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson JD, Ahmed NT, Luciani DS, et al. Increased islet apoptosis in Pdx1+/− mice. J Clin Invest. 2003;111:1147–1160. doi: 10.1172/JCI16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brissova M, Shiota M, Nicholson WE, et al. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem. 2002;277:11225–11232. doi: 10.1074/jbc.M111272200. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Cao X, Li LX, Brubaker PL, Edlund H, Drucker DJ. beta-cell Pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1. Diabetes. 2005;54:482–491. doi: 10.2337/diabetes.54.2.482. [DOI] [PubMed] [Google Scholar]
- 53.Karnik SK, Chen H, McLean GW, et al. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318:806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 54.Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest. 2008;118:2316–2324. doi: 10.1172/JCI33655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klonsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 56.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mortimore GE, Pösö AR. Intracellular protein catabolism and its control during nutrient deprivation and supply. Annu Rev Nutr. 1987;7:539–564. doi: 10.1146/annurev.nu.07.070187.002543. [DOI] [PubMed] [Google Scholar]
- 58.Shimizu S, Kanaseki T, Mizushima N, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 59.Yu L, Alva A, Su H, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 60.Marsh BJ, Soden C, Alarcón C, et al. Regulated autophagy controls hormone content in secretory-deficient pancreatic endocrine beta-cells. Mol Endocrinol. 2007;21:2255–2269. doi: 10.1210/me.2007-0077. [DOI] [PubMed] [Google Scholar]
- 61.Ebato C, Uchida T, Arakawa M, et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008;8:325–332. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 62.Jung HS, Chung KW, Won Kim J, et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8:318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 63.Kaniuk NA, Kiraly M, Bates H, Vranic M, Volchuk A, Brumell JH. Ubiquitinated-protein aggregates form in pancreatic beta-cells during diabetes-induced oxidative stress and are regulated by autophagy. Diabetes. 2007;56:930–939. doi: 10.2337/db06-1160. [DOI] [PubMed] [Google Scholar]
- 64.Shvets E, Elazar Z. Autophagy-independent incorporation of GFP-LC3 into protein aggregates is dependent on its interaction with p62/SQSTM1. Autophagy. 2008;4:1054–1056. doi: 10.4161/auto.6823. [DOI] [PubMed] [Google Scholar]
- 65.Steer SA, Scarim AL, Chambers KT, Corbett JA. Interleukin-1 stimulates beta-cell necrosis and release of the immunological adjuvant HMGB1. PLoS Med. 2006;3:253–266. doi: 10.1371/journal.pmed.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han J, Zhong J, Wei W, et al. Extracellular high-mobility group box 1 acts as an innate immune mediator to enhance autoimmune progression and diabetes onset in NOD mice. Diabetes. 2008;57:2118–2127. doi: 10.2337/db07-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hagman DK, Hays LB, Parazzoli SD, Poitout V. Palmitate inhibits insulin gene expression by altering pdx-1 nuclear localization and reducing mafa expression in isolated rat islets of langerhans. J Biol Chem. 2005;280:32413–32418. doi: 10.1074/jbc.M506000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Macfarlane WM, McKinnon CM, Felton-Edkins ZA, Cragg H, James RF, Docherty K. Glucose stimulates translocation of the homeodomain transcription factor pdx1 from the cytoplasm to the nucleus in pancreatic beta-cells. J Biol Chem. 1999;274:1011–1016. doi: 10.1074/jbc.274.2.1011. [DOI] [PubMed] [Google Scholar]
- 69.Kitamura YI, Kitamura T, Kruse JP, et al. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 70.Moede T, Leibiger B, Pour HG, Berggren P, Leibiger IB. Identification of a nuclear localization signal, rrmkwkk, in the homeodomain transcription factor pdx-1. FEBS Lett. 1999;461:229–234. doi: 10.1016/s0014-5793(99)01446-5. [DOI] [PubMed] [Google Scholar]