Abstract
Bipolar disorder is one of the most severely debilitating of all medical illnesses. For a large number of patients, outcomes are quite poor. The illness results in tremendous suffering for patients and their families and commonly impairs functioning and workplace productivity. Risks of increased morbidity and mortality, unfortunately, are frequent occurrences as well.
Until recently, little has been known about the specific molecular and cellular underpinnings of bipolar disorder. Such knowledge is crucial for the prospect of developing specific targeted therapies that are more effective and that have a more rapid onset of action than currently available treatments. Exciting recent data suggest that regulation of certain signalling pathways may be involved in the aetiology of bipolar disorder and that these pathways may be profitably targeted to treat the disorder. In particular, mania is associated with overactive protein kinase C (PKC) intracellular signalling, and recent genome-wide association studies of bipolar disorder have implicated an enzyme that reduces the activation of PKC. Importantly, the current mainstays in the treatment of mania, lithium (a monovalent cation) and valproate (a small fatty acid) indirectly inhibit PKC. In addition, recent clinical studies with the relatively selective PKC inhibitor tamoxifen add support to the relevance of the PKC target in bipolar disorder.
Overall, a growing body of work both on a preclinical and clinical level indicates that PKC signalling may play an important role in the pathophysiology and treatment of bipolar disorder. The development of CNS-penetrant PKC inhibitors may have considerable benefit for this devastating illness.
Bipolar disorder is a serious medical illness that, unfortunately, is quite common, having a lifetime prevalence of approximately 4.4% in the US.[1] Bipolar disorder is characterized by recurrent disturbances of emotional states, hedonic drive, motoric behaviour, cognition, sleep and functioning (all of which tend to conglomerate in episodes) and residual symptoms that manifest across the lifespan.
Because of such varied clinical syndromes, in part for diagnostic and treatment purposes, bipolar disorder is broken down into discrete acute episodes (manic, mixed, hypomanic and depressive episodes). Therapies for bipolar disorder are usually first tested in the acute phases of the illness, particularly in manic episodes, and once efficacy is established for this pole of the illness, usually a maintenance phase study takes place. There are now several antimanic agents available for clinical use, although a sizable proportion of patients have a suboptimal response to them or have intolerable adverse effects.[2] A major problem with these ‘choices’ of antimanic therapies in terms of drug development is that, except for lithium, all of the currently marketed treatments for mania fall into the category of anticonvulsant or antipsychotic drugs.[3] It is remarkable that no drug has been developed specifically for this severe recurrent mood disorder since its original conception by Kraepelin over a century ago. We have yet to develop a new treatment expressly for bipolar disorder; this lack of new treatments most likely is a consequence of our lack of understanding of the relevant molecular and cellular substrates of this complex emotional, behavioural, activity disorder.
Several drug development strategies in bipolar disorder have been proposed.[4] One path results from our understanding that severe mood disorders, although not classical neurodegenerative disorders, are associated with regional impairments of structural plasticity and cellular resilience, and that drugs that enhance resilience will have therapeutic effects. Another strategy is based on understanding the therapeutically relevant biochemical targets of the currently effective medications lithium and valproate; their target, which is the subject of this review, is protein kinase C (PKC). The PKC story provides one of the few examples where a drug is specifically being developed for bipolar disorder based on an identified molecular target. Indeed, such development has gone from identifying a direct molecular target in 1990 to a positive proof-of-concept clinical study in humans with a modulator of the relevant target in 2007.
1. Protein Kinase C (PKC)
PKC is a family of structurally related isozyme subspecies with a heterogeneous distribution throughout the body.[5,6] There are at least 12 isoforms that differ in structure, subcellular localization, tissue specificity, mode of activation and substrate specificity.[7] The isoforms are subdivided into three classes (classical/conventional, novel and atypical) on the basis of activation requirements. Conventional PKC isoforms (α, βI, βII, γ) require calcium and diacylglycerol (DAG) for activation, whereas novel PKC isoforms (δ, ε, η, θ, μ), which lack the C2 calcium-binding domain, only require DAG for activation. Atypical PKC isoforms (ζ, λ/ι) lack both C2 and DAG-binding C1 domains and, thus, are not responsive to calcium or DAG, but respond to lipidic mediators such as phosphatidylinositol 3,4,5-triphosphate.[8] Such isoforms are relevant to drug development, as directly targeting certain isoforms could bring about a therapeutic effect (e.g. antimanic) and the targeting of isozymes in a discrete region rather than ubiquitously may minimize adverse effects. The development of isozyme-specific compounds for therapeutic use has led to progress in the management of certain conditions (see section 2).
Activation of PKC results in its translocation, and subcellular localization is thought to regulate accessibility to activators and substrates.[7] PKC is activated by such varied upstream signals as G protein-coupled receptors (GPDRs), receptor tyrosine kinases (RTKs), and non-RTKs via DAG activation. Several PKC isoforms are independently activated by the phospholipase C (PLC) and phosphoinositide-3 kinase (P13 K) pathways.[7]
2. PKC Signalling Cascade in Disease
PKC is implicated in a diversity of cellular functions, including cell cycle progression, proliferation, differentiation and apoptosis (reviewed in Mellor and Parker[9]). In addition, PKC appears to have a major role in regulating survival signals in a variety of cell types, including neurons. PKC was first recognized as a proteolytically activated serine/threonine kinase in the late 1970s[10] and a decade later its first isoforms that were calcium dependent were discovered.[11] Since then, 12 isoforms that are expressed by mammalian cells have been identified.[12]
The first disease condition in which PKC isoforms and their modulation were demonstrated to have a major role was cancer.[12] Since then, the role of PKC has been examined in may other diseases including cardiovascular,[13] pulmonary,[14] immune and infectious disease,[15] diabetes mellitus[16] and, more recently, bipolar disorder.
3. PKC Signalling and the Brain
In the brain, PKC is highly enriched with a heterogeneous distribution. It has an important function in modulating both pre- and post-synaptic facets of neurotransmission and it is present in particularly high levels in presynaptic nerve terminals.[4] It has a number of additional functions including regulation of neuronal excitability, neurotransmitter release and long-term alterations in gene expression and plasticity. Regarding the latter, PKC signalling pathways regulate dendritic spine morphology in brain slices and in cultured neuronal synaptic preparations.[17,18] A recent study reported what was the structural basis for enhancement of long-term associated memory in single dendritic spines regulated by PKC.[19] Perhaps one of its most important functions is in modulating the major intracellular mediators of signals generated upon external stimulation of cells via a variety of neurotransmitter receptors (including muscarinic M1 and M3, noradrenergic α1, serotonergic 5-HT2A and metabotropic glutamatergic receptors), which induce the hydrolysis of various membrane phospholipids.[20]
PKC activity is increased in the prefrontal cortex following exposure to even mild, uncontrollable stress.[21] Stress exposure increases noradrenaline (norepinephrine) release in the pre-frontal cortex,[22] which stimulates α1-receptors and activates the phosphatidyl inositol signalling pathway.[23] These actions greatly impair pre-frontal cortex function. Thus, prefrontal cortical cognitive deficits are observed following exposure to stress, stimulation of α1-receptors, or direct activation of PKC with phorbol esters in the prefrontal cortex.[23,24] Conversely, inhibition of PKC restores prefrontal cognitive function following all of these conditions.[23,24] The detrimental effects of PKC activation are also observed at the level of single cells, where the firing of prefrontal cortical neurons during cognitive tasks is markedly reduced by activation of PKC, and restored by inhibition of PKC.[23] Thus, the ability of the prefrontal cortex to regulate emotion, thought and action is markedly impaired by overactivity of PKC signalling. These findings may be particularly important for bipolar disorder, with a number of neuroimaging studies highlighting structural and functional deficits in the prefrontal cortex in the disorder (see review by Arnsten and Manji[25]).
4. PKC Signalling Cascade in Bipolar Disorder: A Joint Biochemical Target for the Actions of Chronic Lithium and Valproate
The identification of the acute, in vitro effects of a number of antimanic agents led investigators to develop criteria for relevant targets to pursue for future therapeutic agents.[20,26] The criteria include: (i) corroboration of the target at the protein and functional level; (ii) observation of effects at the target of chemically dissimilar but clinically effective agents; (iii) occurrence of the effects at a dose/plasma concentration and time-frame consistent with clinical therapeutic effects; (iv) localization of the target to brain regions implicated in the neurobiology of the disorder under consideration; and (v) when known, relevance of the target to known pathophysiology. Application of this stringent criteria strategy has led a number of independent groups[27–31] to recognize PKC as a promising direct biochemical target for developing therapeutics for bipolar disorder.
The antimanic drugs that meet the aforementioned criteria for further testing are lithium and valproate. First, it is important to note that lithium (a monovalent cation) and valproate (an 8-carbon branched fatty acid) are structurally dissimilar but have similar therapeutic effects, both being antimanic agents. Secondly, their effect takes place in brain regions that have been implicated to be critical circuits in mood disorder pathophysiology, namely in limbic and limbic-related regions. Thirdly, the effects occur at therapeutic concentrations in vivo. Fourthly, similar to the clinical therapeutic effects, the biochemical alterations occur only after chronic (and not acute) administration in a timeframe consistent with clinically therapeutic effects. Lastly, the effects are specific for these agents. As will be discussed below, both lithium and valproate bring about strikingly similar effects on the PKC signalling cascade, actions which may be most relevant to their antimanic profile.[20]
A considerable amount of biochemical data supports the potential involvement of PKC in the pathophysiology and treatment of bipolar disorder (table I). Friedman and colleagues[35] investigated PKC activity and PKC translocation in response to serotonin in platelets obtained from patients with bipolar disorder before and during lithium treatment. They reported that the ratios of platelet membrane-bound to cytosolic PKC activities were elevated in subjects in a manic episode. In addition, serotonin-elicited platelet PKC translocation was found to be enhanced in those subjects. Wang and Friedman[44] measured PKC isozyme levels, activity and translocation in post-mortem brain tissue from patients with bipolar disorder. They reported increased PKC activity and translocation in the brains of patients with bipolar disorder compared with controls, effects that were accompanied by elevated levels of selected PKC isozymes in cortices of the patients.
Table I.
Evidence implicating protein kinase C (PKC) in the pathophysiology and treatment of bipolar disorder
| Study (y) | PKC-related evidence |
|---|---|
| Cultures, animals, humans | |
| Bitran et al.[32] (1990) | Chronic (but not acute) lithium treatment attenuates agonist- and phorbol ester- and phorbol-12- myristate-13-acetate-mediated stimulation of the Na+/H+ antiporter activity in HL-60 cells, suggesting an impairment of PKC activity |
| Giambalvo[33,34] (1992) | Increased particulate PKC activity in synaptosomes incubated with amphetamine 1–10 μmol/L |
| Friedman et al.[35] (1993) | Ratios of platelet membrane-bound to cytosolic PKC activities were elevated in manic subjects. Also, serotonin-elicited platelet PKC translocation was enhanced in subjects with mania; lithium treatment resulted in a reduction in cytosolic and membrane-associated PKC activities and in an attenuated PKC translocation in response to serotonin, which normalized with lithium treatment Increased membrane/cytosol PKC partitioning in platelets from manic subjects; normalized with lithium treatment |
| Gnegy et al.[36] (1993) | Amphetamine resulted in increased phosphorylation of the neural-specific calmodulin-binding protein GAP-43 (involved in neurotransmitter release) in purified synaptic plasma membrane of female rat striatum |
| Manji et al.[37] (1993) | Chronic lithium treatment for 5 wk resulted in a 30% reduction in [3H]PDbu-binding in the subiculum and in CA1 regions of the rat hippocampus as measured by quantitative autoradiography; immunoblot analysis of hippocampal PKC with isozyme-specific antibodies showed a 30% reduction in membrane-associated PKC α |
| Chen et al.[28] (1994) | Chronic exposure (6–7 d) of rat C6 glioma cells to ‘therapeutic’ concentrations (0.6 mmol/L) of valproate resulted in decreased PKC activity in both membrane and cytosolic fractions and increased the cytosol/membrane ratio of PKC activity; Western blot analysis revealed isozyme-selective decreases in the levels of PKC α and ε in both the membrane and cytosolic fractions after long-term valproate exposure |
| Manji et al.[38] (1996) | Chronic myoinositol administration attenuated lithium-induced decreased in PKC α, and levels of MARCKS and GAP-43 in rat hippocampus and frontal cortex |
| Cervo et al.[39] (1997) | In a balanced CPP in rats, PKC was found to be involved in the mechanism underlying consolidation of CPP |
| Birnbaum et al.[40] (2004) | High levels of PKC activity in prefrontal cortex, as seen during stress exposure, markedly impair behavioural and electrophysiological measures of working memory in rat; chronic treatment with lithium or valproate for 6 wk abolished exogenous activation of PKC signalling |
| Wang and Friedman[41] (1989) | Chronic treatment with lithium in rats resulted in a significantly decreased PKC stimulation-induced release with phorbol esters in cortex, hypothalamus and hippocampus; exposure of brain slices obtained from lithium-treated rats to depolarization and PKC stimulation resulted in marked reductions in translocation of PKC from the cytoplasma to the membrane compartment |
| Lenox et al.[42] (1992) | Immunoblot analysis revealed that chronic (but not acute) lithium treatment results in reduced in vivo levels of MARCKS in rat hippocampus – effects that were not immediately reversed following lithium discontinuation |
| Friedman et al.[35] (1993) | Ratios of platelet membrane-bound to cytosolic PKC activities were elevated in 12 medication-free manic subjects. Also, serotonin-elicited platelet PKC translocation was enhanced in subjects with mania; lithium treatment resulted in a reduction in cytosolic and membrane-associated PKC activities and in an attenuated PKC translocation in response to serotonin, which normalized with lithium treatment Increased membrane/cytosol PKC partitioning in platelets from manic subjects; normalized with lithium treatment |
| Steketee[27,43] (1993, 1994) | Intra-A10 administration of a PKC inhibitor, H7, inhibited cocaine-induced behavioural sensitization in rats |
| Wang and Friedman[44] (1996) | Brain membrane-associated PKC activity was higher in bipolar subjects vs controls; PKC isozymes in cortical homogenates showed that cytosolic α and membrane-associated γ PKC isozymes were elevated in cortices of subjects with bipolar affective disorder |
| Watson and Lenox[45] (1996) | Chronic lithium treatment produces a dose- and time-dependent downregulation of MARCKS protein in immortalized rat hippocampus cells |
| Browman et al.[29] (1998) | Behavioural and tissue studies indicate that injection of a PKC inhibitor, Ro31-8220, into the nucleus acumbens in rats attenuates the acute response to amphetamine |
| Wang et al.[46] (1999) | In basal state, manic subjects had higher membrane PKC activity than depressed subjects and controls; ratio of membrane to cytosol PKC activity was significantly higher in manic subjects compared with controls, depressed or schizophrenic subjects; stimulation of platelets with serotonin in vitro resulted in greater membrane-to-cytosol ratio in manic subjects compared with other groups |
| Hahn and Friedman[47] (1999) | Long-term lithium treatment significantly reduced PKC activation in rat brains, as measured by the translocation of cytoplasmic PKC to the membrane component, or by quantitative binding of the PKC ligand, PDBu. Alterations in platelet PKC were shown in bipolar patients during the manic states of the illness. Compared with patients with major depressive disorder, schizophrenia or healthy controls, PKC activity was significantly increased in manic patients and was suppressed following mood-stabilizer treatment |
| Soares et al.[48] (2000) | Lithium-treated euthymic bipolar patients had lower levels of cytosolic PKC α compared with healthy subjects |
| Wang et al.[49] (2001) | In slices of rat brain cortex, chronic (but not acute) lithium treatment reduced phorbol-induced PKC translocation from cytosol to membrane without affecting basal membrane or cytosolic PKC activity; immunoblotting revealed that chronic lithium treatment reduced cytosolic PKC α and δ |
| Wang and Friedman[50] (2001) | Increased association of RACK1 with membrane γPKC and ζPKC was increased under basal conditions in bipolar disorder relative to control brains; stimulation with phorbol esters increased the amount of RACK1 that co-immunoprecipitated with α, β, γ, δ, ε PKC isozymes in frontal cortex of subjects with bipolar disorder |
| Einat et al.[21] (2007) | The PKC inhibitor tamoxifen significantly reduced amphetamine-induced hyperactivity in a large open field without affecting spontaneous activity, and normalized amphetamine-induced increase in visits to the centre of an open field (representing risk-taking behaviour); tamoxifen attenuated amphetamine- induced phosphorylation of GAP-43 |
| Kurita et al.[51] (2007) | Sodium valproate at therapeutic concentrations inhibited PKC in human astrocytoma cells |
| Kantor and Gnegy[30] (1998) | PKC inhibitors blocked amphetamine-mediated dopamine release in rat striatal slice |
| Pandey et al.[52] (2008) | PKC βI and PKC βII, but not PKC α or δ, were significantly decreased in both membrane as well as cytosol fractions of platelets obtained from medication-free patients with bipolar disorder compared with healthy controls; pharmacotherapy significantly increased PKC activity not PKC isozymes |
| Clinical trials | |
| Kulkarni et al.[53] (2006) | In a 28-d, three-arm, double-blind, lithium and/or valproate add-on study, the PKC inhibitor tamoxifen 40 mg/d (n = 5) was found to have antimanic effects superior to placebo (n = 4) |
| Bebchuk et al.[54] (2000) | Preliminary data from a single-blind, open-label, add-on (some patients were on no other medications) study suggest that tamoxifen may have efficacy in the treatment of acute mania |
| Zarate et al.[55] (2007) | In a 3-wk, double-blind, placebo-controlled study (n = 16), tamoxifen (up to 140 mg/d) was superior to placebo in acute mania; lorazepam up to 2 mg/d was permitted for the first 10 d of the blinded phase. Significant improvement was seen as early as d 5 in YMRS scores; no significant improvement was seen in MADRS scores |
| Yildiz et al.[56] (2008) | In a 3-wk, double-blind, placebo-controlled study (n = 66), tamoxifen (up to 80 mg/d) was superior to placebo in acute mania; lorazepam up to 5 mg/day was permitted for the entire duration of the study. Significant improvement in YMRS was reported at wk 3; significant improvement was also seen in CGI and PANSS total and positive subscale scores; no significant improvement was seen with HAMD-17 and MADRS scores; caregiver permitted as well as individualized food preferences and enriched recreational activities |
CGI = Clinical Global Impression scale; CPP = conditioned place preference; GAP-43 = growth-associated protein-43; HAMD-17 = 17-Item Hamilton Depression Rating Scale; MADRS = Montgomery-Åsberg Depression Rating Scale; MARCKS = myristoylated alanine-rich PKC substrate; PANSS = Positive and Negative Syndrome Scale; PDBu = [3H]phorbol 12,13-dibutyrate; RACK1 = receptor for activated C kinase-1; YMRS = Young Mania Rating Scale.
PKC signalling pathways are altered after treatment with lithium or valproate.[20,28,35,37,47,57] With regard to brain regions showing PKC changes, chronic treatment with lithium in rats resulted in a significantly decreased PKC stimulation-induced release with phorbol esters in the cortex, hypothalamus and hippocampus; these areas have been implicated in mood disorder pathophysiology. Regarding PKC isozymes, chronic lithium administration resulted in reduced activity of several PKC α and ε isoforms[20] in the frontal cortex and hippocampus. Similar to lithium, the other primary antimanic drug, valproate, was also found to cause an isozyme-specific decrease in PKC α and ε.[28] However, unlike lithium, the effects of valproate appear to be largely independent of myoinositol.[20] This finding that the two drugs act via different sub-pathways may explain why patients who do not respond to lithium treatment may respond to valproate treatment or have some differences in efficacy based on manic syndrome profile (e.g. manic vs mixed episode). The study of PKC substrates has also yielded information regarding PKC in bipolar disorder. Myristoylated alanine-rich protein kinase C substrate (MARCKS), a key substrate of PKC, is a protein that has been implicated in signalling and neuroplastic events associated with cytoskeletal architecture;[42,45] it is regulated by chronic lithium treatment. Valproate also has been reported to alter PKC substrates.[28,58]
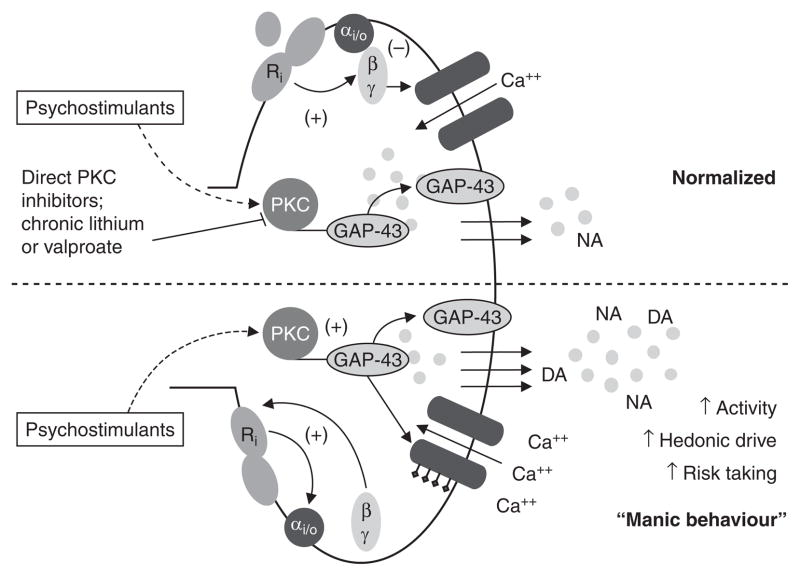
Other supportive evidence for the role of PKC in bipolar disorder is that stimulants (e.g. amphetamine), which are capable of triggering manic episodes in susceptible individuals and induce manic-like behaviours in rodents,[21] activate PKC and GAP-43 (growth-associated protein of 43 kDa) phosphorylation (implicated in neurotransmitter release)[33,36,59,60] [figure 1]. The PKC inhibitor tamoxifen significantly reduced amphetamine-induced hyperactivity in a large open field without affecting spontaneous activity, and normalized amphetamine-induced increase in visits to the centre of an open field (representing risk-taking behaviour); tamoxifen also attenuated amphetamine-induced phosphorylation of GAP-43.[21] Similarly, in addition to amphetamines, data with cocaine in animals is often used to infer the involvement of PKC in bipolar disorder. For example, increased hedonistic drive and tendency to abuse drugs are well known facets of manic behaviour. Models of such behaviours are consumption of reward and conditioned place preference (CPP).[61,62] PKC inhibition with H7 in the A10 area blocked the development of cocaine CPP[43] and intra-accumbens injections of the PKC inhibitor NPC-15437 blocked amphetamine CPP.[63]
Fig. 1.
Protein kinase C (PKC) in the pathophysiology and treatment of manic behaviour. DA = dopamine; GAP-43 = growth-associated protein of 43 kDa; NA = noradrenaline (norepinephrine); ↑ indicates increased.
Although cognitive disturbance is not one of the primary components of mania, cognition certainly is disrupted in those with the disorder. Recently, Birnbaum and colleagues[23] have demonstrated that excessive activation of PKC in rodents dramatically impaired the cognitive functions of the prefrontal cortex, exposure to stress activated PKC and resulted in prefrontal dysfunction and inhibition of PKC (including indirectly with mood stabilizers) protected cognitive function.
As can be seen, pharmacological activation of PKC in animals results in many of the behavioural changes seen in mania such as hyper-activity, risk-taking behaviour and increased hedonic drive. Its inhibition attenuates these same behavioural changes in a manner similar to that of mood stabilizers in acute mania (figure 1).
Finally, the potential role of the PKC signalling pathway in the pathophysiology of bipolar disorder has been further strengthened by the exciting recent identification of a bipolar susceptibility gene, which is an upstream regulator of PKC. Two recent, independent, genome-wide association studies identified diacylglycerol kinase eta (DGKH) as a risk gene for bipolar disorder.[64,65] DGKH is a major regulator of DAG, which activates all known classical and novel isoforms of PKC.
5. PKC Inhibitors: Novel Therapeutics for Acute Mania?
As summarized in section 4, the PKC signalling pathway appears to be a relevant and important target for the antimanic actions of two structurally dissimilar antimanic agents – lithium and valproate. Do the effects of these antimanic agents on PKC signalling actually have any therapeutic significance? As discussed in section 4, the PKC signalling pathway fulfils most of the criteria for therapeutic relevance. Thus, there is an obvious need to explore the potential efficacy of a direct PKC inhibitor in the treatment of acute mania.
Up to this point, a limitation to further developing the therapeutic potential of the PKC target has been that there is only one relatively selective inhibitor of PKC available for human use that crosses the blood-brain barrier – tamoxifen. Tamoxifen, a synthetic anti-estrogen, is widely used in the treatment of breast cancer[66] and is among the least toxic of the anticancer therapeutic regimens. It has also been approved as a chemopreventive agent in women at high-risk for breast cancer. Aside from its estrogen receptor antagonism effects,[66] it has recently become apparent that tamoxifen possesses potent and selective PKC inhibitory effects at therapeutically relevant concentrations.[67,68] Furthermore, tamoxifen is brain penetrant, readily crossing the blood-brain barrier, and is fairly well tolerated even at doses 10-fold higher than routinely used in cancer (up to 200 mg/day).[69]
Based on encouraging preclinical data (section 4), we and others embarked on proof-of-concept studies with tamoxifen in acute mania.
5.1 Clinical Studies
In the first study with tamoxifen in patients with acute bipolar mania,[54] seven subjects (five males and two females) were studied. Subjects were in- or outpatients aged 18–65 years, who met a diagnosis of bipolar mood disorder – manic episode based on a structured clinical interview for DSM-IV.[70] In addition, participants were required to have a Young Mania Rating Scale (YMRS)[71] score of ≥14 at baseline and were excluded if they met diagnostic criteria for any other current psychiatric disorder and they were required to be in good physical health. Five patients received tamoxifen alone and two patients had tamoxifen added to their current treatment. In all patients, tamoxifen was started at a dosage of 10 mg twice daily and was titrated up to a maximal dosage of 80 mg/day in divided doses. Subjects received tamoxifen for a mean of 8.4 ± 4.2 days, with a mean daily dose of 57.1 mg. Assessments were done every 3–7 days by staff blind to the treatment condition. Tamoxifen resulted in a significant decrease versus baseline in manic symptoms rated by the YMRS, with a mean decrease of 10.29 points (p = 0.03). In addition, 71% met response criteria (50% decrease in the YMRS score from baseline). The Hamilton Depression Rating Scale (HAMD) scores did not show a consistent change over time. Overall, the study medication was well tolerated, one subject reported flushing.
The second study involved only women.[53] This trial consisted of a 28-day, three-arm, double-blind study involving 13 women where tamoxifen (n = 5) was compared with two other groups, medroxyprogesterone acetate (n = 4) and placebo (n = 4); all subjects were receiving concomitant treatment that consisted of either lithium (at therapeutic concentrations), valproate, or their combination. Subjects were randomized to a fixed dosage of tamoxifen 40 mg/day. Tamoxifen-treated patients showed a significantly greater improvement in symptoms of mania and positive symptoms of psychosis compared with the placebo group. Some limitations of the study include no mention on how long subjects were on lithium or valproate prior to starting the study and whether other concomitant medications were used during the study (e.g. antipsychotics or benzodiazepines). Furthermore, neither the dosage nor concentration of valproate during the study were specified. It is possible that the improvements seen could be attributed to these other factors.
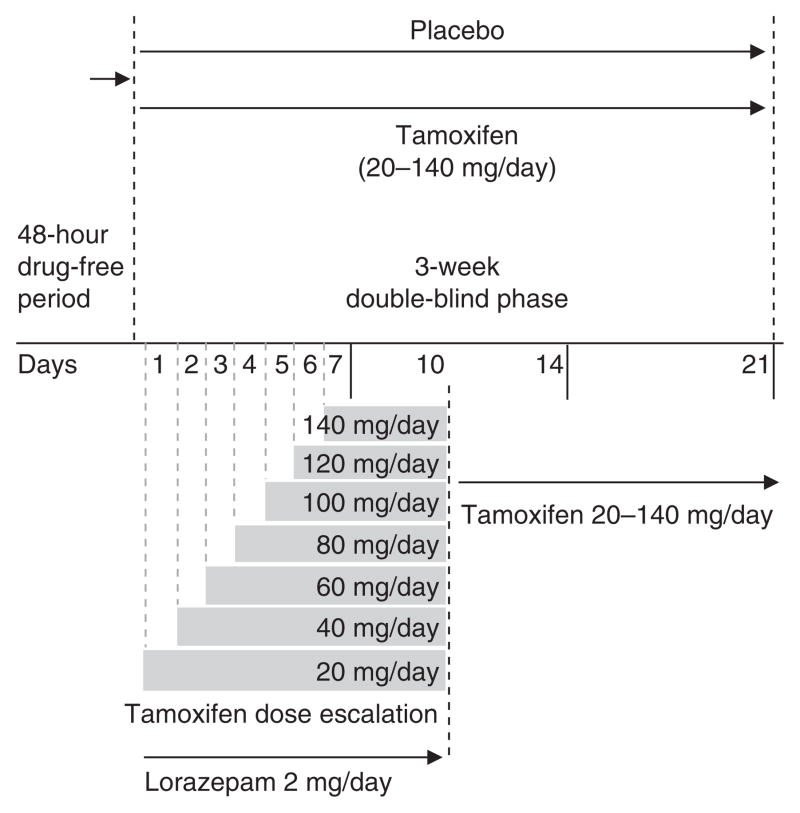
More recently, we completed a double-blind, placebo-controlled trial with tamoxifen monotherapy in patients with bipolar mania and found the drug to have significant antimanic effects as early as day 5 and throughout the 3 weeks of the trial.[55] The study characteristics were as follows: subjects were men and women in good physical health, aged 18–65 years, who were inpatients with a current diagnosis of bipolar disorder, current episode manic or mixed with or without psychotic features as diagnosed by means of the Structured Clinical Interview for Axis I DSM-IV Disorders – Patient Version (SCID-P). Subjects were required to have a score of ≥14 on the YMRS at screening and at randomization (baseline) and not to have >20% improvement in YMRS total scores between the screen and randomization visits. Subjects were required to have been previously treated with at least one trial of an antimanic agent at some point during the course of their illness (i.e. lithium, valproate, carbamazepine or an antipsychotic [typical, atypical]). All psychotropic medications, with the exception of benzodiazepines, were discontinued at least 2 days before entering the double-blind phase of the study. Subjects received flexible dosing of either tamoxifen (20–140 mg/day) or placebo up to a maximum of 140 mg/day of tamoxifen or matching placebo. Dosages as high as we studied have been reported to be well tolerated.[69] Figure 2 illustrates the study design and dose escalation used in the study. The dosage of tamoxifen was increased by 20 mg/day until one of the following endpoints was reached: (i) response criterion (defined as a 50% decrease in YMRS ratings from baseline); (ii) intolerable adverse effects; or (iii) the maximum allowable dosage of tamoxifen had been reached, 140 mg/day. Concomitant use of the benzodiazepine lorazepam (up to 2 mg/day) was allowed during the first 10 days of the double-blind phase; after that no other psychotropic medication was permitted. Subjects were rated on a daily basis for the first 7 days and then weekly thereafter (days 14 and 21) [figure 2].
Fig. 2.
Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study design and dose escalation.
Sixteen subjects were randomized. Eight subjects received tamoxifen and eight received placebo. There were 14 males and 2 females, and the mean age was 35.4 ± 7.8 years. Fifty-six percent of patients had a lifetime diagnosis of any substance abuse or dependence. Based on DSM-IV criteria using the SCID-P, 69% of patients had a manic index episode, and 50% were experiencing psychosis. The mean length of illness was approximately 16 years and the current manic episode had lasted a mean of 34 days. Most of the subjects had had significant prior treatment with antimanic agents or mood stabilizers during the course of their illness.
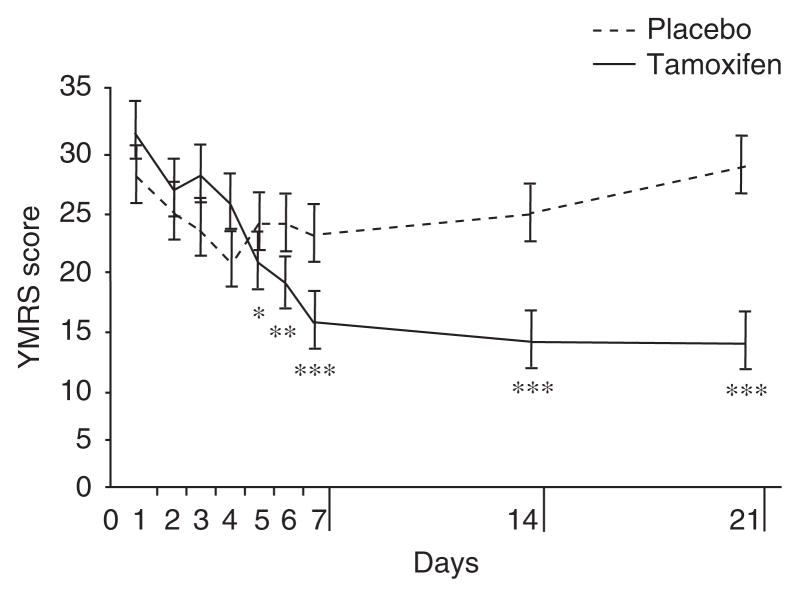
Tamoxifen-treated patients received a mean dosage of 110 mg/day. The linear mixed model for the YMRS showed a significant interaction between time and drug (p = 0.01). A significant difference between placebo and tamoxifen was found at 21 days (d = 1.08, 95% CI 0.45, 1.71). The tamoxifen group showed significant improvement from baseline as early as day 5 (figure 3). The mean change in YMRS scores on placebo at 3 weeks was 4.7 ± 4.1 and on tamoxifen was −18.3 ± 4.3. Five of eight (63%) tamoxifen-treated patients had ≥50% improvement in YMRS scores at the endpoint of the study, while one of eight (13%) had similar improvement on placebo. In the same study, remission (YMRS ≤7) rates were 25 % with tamoxifen versus 0% with placebo. Using lorazepam did not alter these results.
Fig. 3.
Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study (reproduced from Zarate et al.[55]). Tamoxifen vs placebo: * p < 0.05 after correction. Week vs baseline for tamoxifen: * p < 0.05, ** p < 0.01, *** p < 0.001 after correction. YMRS = Young Mania Rating Scale.
Tamoxifen appeared to have beneficial effects on the YMRS sub-items of elevated mood, increased motor activity or energy, decreased sleep, increased speech, increased sexual interest and appearance. No beneficial effects were noted on depressive and psychotic symptoms scores; this could be because either tamoxifen has specific antimanic effects only, or that a large sample of patients with these characteristics would be needed to discern specific effects on these other symptom domains.
Overall, tamoxifen was well tolerated during the 3-week trial. No patient discontinued treatment because of an adverse event, and the only treatment-emergent event with a statistically significantly more frequent occurrence in the tamoxifen group compared with the placebo group was loss of appetite (p = 0.03).
This double-blind, placebo-controlled, pilot trial supports our previous single-blind study[54] in demonstrating that the relatively selective PKC inhibitor tamoxifen has significant antimanic properties in individuals with bipolar disorder. Our placebo-controlled monotherapy trial with tamoxifen[55] confirmed our hypothesis, namely that directly inhibiting PKC would result in improvement in manic symptoms. The onset of the effects was fairly rapid (within 5 days), which implies that more directly targeting PKC inhibition may be associated with a short time to the onset of antimanic effects. Current data show that the effects of lithium on PKC are considerably more distal and upstream, and this may account for the prolonged lag period to the onset of effect for lithium.
A recent controlled study also found that tamoxifen was effective in acute mania and extends findings from the previous studies.[56] In this trial, 66 patients aged 18–60 years, diagnosed as having DSM-IV bipolar I disorder, currently in a manic or mixed state, with or without psychotic features, with a baseline YMRS score of >20 were randomized to treatment with tamoxifen (up to 80 mg/day) or placebo for 3 weeks. Concomitant use of oral lorazepam was permitted during the study as clinically indicated, up to a maximum of 5 mg per 24 hours. In addition, its use was avoided after the initial 12 days whenever possible and it was not given within 12 hours of scheduled ratings. A caregiver, individualized food preferences and enriched recreational activities were also permitted during the study. Intent-to-treat analysis showed greater improvements with tamoxifen than placebo. Intent-to-treat analysis of available measures on all 66 subjects indicated that tamoxifen treatment yielded mean decreases in scores on the YMRS and Clinical Global Impressions-Mania of 5.84 and 0.73 points per week, respectively, compared with mean increases of 1.50 and 0.10 points per week, respectively, with placebo. Tamoxifen was found to be superior to placebo on all individual YMRS items. Significant improvements were also seen in the Positive and Negative Syndrome Scale (PANSS) total and positive subscale scores. No significant improvements were seen in 17-item HAMD and Montgomery-Åsberg Depression Rating Scale (MADRS) scores. Rates of response were 48% (14/29) with tamoxifen versus 5% (1/21) with placebo. Remission (YMRS score ≤12) rates were 28% with tamoxifen versus 0% with placebo. No serious adverse events were reported and, overall, tamoxifen was well tolerated.
Of note, while the results from the studies of tamoxifen in mania are encouraging, they are preliminary and need to be confirmed in controlled studies involving more selective PKC inhibitors. Such future controlled studies would then provide an extra degree of confidence of the relevance of PKC in bipolar disorder.
Regarding the selectivity of the effects of tamoxifen on PKC, it is important to re-emphasise, as discussed earlier in this section, that tamoxifen is also an anti-estrogen. It is possible that some of the antimanic effects seen with tamoxifen are attributable to estrogen receptor antagonism.[53,72] Again, controlled trials with brain penetrant selective PKC inhibitors would help to clarify this issue.
Finally, the adverse effect of diminished appetite and the potential for depression merits further discussion. In our controlled study,[55] we found that a reduction in appetite was more common with tamoxifen than with placebo. The basis for this property is unknown but has been suggested to be the result of the build-up of malonyl-coenzyme A in the hypothalamus and inhibition of fatty acid synthase expression specifically in the ventromedial nucleus of the hypothalamus.[73] Depression has been reported to result from tamoxifen therapy in patients with cancer.[74,75] Whether it is a common occurrence in individuals receiving long-term tamoxifen therapy or the result of heightened distress and disrupted adjustment from cancer is the subject of ongoing study.[76] Short-term studies (≤4 weeks) in mania found no significant worsening of depression.[53–55,77] Whether this outcome occurs more commonly with chronic treatment and especially in patients with or at risk of a mood disorder is unknown.
It is important to stress that the primary aim of our studies was to determine the relevance of the PKC target for the development of drug treatments for bipolar disorder and not to develop tamoxifen per se for clinical use. The effect of tamoxifen on the course of bipolar disorder, as well as adverse effect liability when used on a long-term basis (e.g. anti-estrogen effects), would first need to be studied before the drug could be used in this indication. The evidence generated to date on both a preclinical and clinical basis supports further study with PKC inhibitors. Issues to consider in the selection of a PKC inhibitor are selectivity, brain penetrance, and short- and long-term tolerability and safety. Selective PKC inhibitors are presently in phase I–III of development to treat a variety of conditions (e.g. diabetic complications [see Gould et al.[77] for a review]) and are possible candidates to test in bipolar disorder (see table II). Much research still remains to be done to determine their tolerability, toxicity, selectivity and blood-brain barrier penetrance.
Table II.
Classification and examples of protein kinase C (PKC) inhibitors
| PKC class | Example | Isozyme affected | Route of administration | Phase of development | Comments |
|---|---|---|---|---|---|
| Indocarbazole class | Enzastaurin | PKC β | Oral | Phase I–III | |
| Midostaurin (PKC-412) | PKC α, β, γ, δ, ε, η | Oral | Phase I–II | ||
| UCN-01 | Conventional > novel PKC isoforms | Intravenous | Phase I–II | ||
| Biological agents | Bryostatin | Conventional > novel | Oral | Phase I–II | Behaves as a short-term PKC activator and long-term inhibitor |
| Curcumin | PKC isoforms | Intravenous | Phase I–III | ||
| Antisense oligonucleotides | Aprinocarsen (ISIS-3521) | Predominantly targets PKC α | Oral | Phase I–III | |
| Others | Tamoxifen | PKC α, δ, ε | Oral | Phase II, currently on the market | Positive control studies in bipolar mania; also is an anti-estrogen |
There are now compelling data to support the notion that targeting intracellular signalling cascades may have considerable utility in the treatment of bipolar disorder. The use of modulators of ubiquitous kinases in the CNS, however, still raises concerns of specificity, tolerability and safety. Is it, in fact, feasible to develop such drugs? At least 50 drugs that target kinases are in clinical development for various medical illnesses, and many more are being investigated at the preclinical level.[78] However, lithium, a mood stabilizer that targets signalling cascade molecules, has been a mainstay of treatment for bipolar disorder for almost 60 years, providing a key example of a safe and effective modulator of signalling systems in the CNS. Tamoxifen is another (nonspecific) regulator of CNS signalling molecules with an acceptable efficacy and safety profile. Although both drugs modulate signalling pathways that are involved in diverse brain functions, and possibly other CNS disorders, they demonstrate relatively circumscribed clinical effects. Developing other therapies that target the CNS is therefore entirely possible, albeit challenging. There are a number of strategies currently being explored that may be very useful in the development of CNS kinase-targeted drugs with adequate specificity and tolerability.[79,80]
6. Conclusions
The results of the clinical studies with tamoxifen, a relatively selective PKC inhibitor, clearly need to be interpreted with caution but are met with considerable enthusiasm. Our hypothesis that PKC inhibition would bring about antimanic effects in patients with bipolar disorder was confirmed in our controlled monotherapy study, as well as other studies. These findings suggest that PKC inhibition might be relevant to the antimanic effects of lithium and valproate. Future studies examining more selective PKC inhibitors would need to take advantage of existing technologies (e.g. brain imaging, genetics) in an attempt to identify surrogate measures of outcome and to increase our understanding of the pathophysiology of the disease.
Finally, regarding the future of drug trial designs in acute mania, the controlled studies[55,56] reviewed here suggest that smaller proof-of-concept studies at single sites are feasible. These studies in acute mania were conducted at academic sites with treatment-resistant patients, and similar ones should be considered in the future and are likely to be major contributors to our knowledge base in identifying novel drug targets for bipolar disorder. An editorial on clinical trials in mania and implications for study design and drug development expands on this previous point.[81]
Acknowledgments
We would like to acknowledge the support of the Intramural Research Program of the National Institute of Mental Heath, the Stanley Medical Research Institute. The authors have no conflicts of interest that are directly relevant to the content of this review. Husseini K. Manji is currently employed by Johnson & Johnson Pharmaceuticals Research and Development.
References
- 1.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64 (5):543–52. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gitlin M. Treatment-resistant bipolar disorder. Mol Psychiatry. 2006;11 (3):227–40. doi: 10.1038/sj.mp.4001793. [DOI] [PubMed] [Google Scholar]
- 3.Manji HK, Zarate CA. Molecular and cellular mechanisms underlying mood stabilization in bipolar disorder: implications for the development of improved therapeutics. Mol Psychiatry. 2002;7 (Suppl 1):S1–7. doi: 10.1038/sj.mp.4001068. [DOI] [PubMed] [Google Scholar]
- 4.Zarate CA, Jr, Singh J, Manji HK. Cellular plasticity cascades: targets for the development of novel therapeutics for bipolar disorder. Biol Psychiatry. 2006;59 (11):1006–20. doi: 10.1016/j.biopsych.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Casabona G. Intracellular signal modulation: a pivotal role for protein kinase C. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21 (3):407–25. doi: 10.1016/s0278-5846(97)00011-0. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci. 1994;17:551–67. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- 7.Serova M, Ghoul A, Benhadji KA, et al. Preclinical and clinical development of novel agents that target the protein kinase C family. Semin Oncol. 2006;33 (4):466–78. doi: 10.1053/j.seminoncol.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Toker A. Signaling through protein kinase C. Front Biosci. 1998;3:D1134–47. doi: 10.2741/a350. [DOI] [PubMed] [Google Scholar]
- 9.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332 (Pt 2):281–92. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takai Y, Kishimoto A, Inoue M, et al. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues I: purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem. 1977;252 (21):7603–9. [PubMed] [Google Scholar]
- 11.Huang KP, Nakabayashi H, Huang FL. Isozymic forms of rat brain Ca2+-activated and phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1986;83 (22):8535–9. doi: 10.1073/pnas.83.22.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackay HJ, Twelves CJ. Targeting the protein kinase C family: are we there yet? Natl Rev Cancer. 2007;7 (7):554–62. doi: 10.1038/nrc2168. [DOI] [PubMed] [Google Scholar]
- 13.Churchill E, Budas G, Vallentin A, et al. PKC Isozymes in chronic cardiac disease: possible therapeutic targets? Annu Rev Pharmacol Toxicol. 2008;48:569–99. doi: 10.1146/annurev.pharmtox.48.121806.154902. [DOI] [PubMed] [Google Scholar]
- 14.Dempsey EC, Cool CD, Littler CM. Lung disease and PKCs. Pharmacol Res. 2007;55 (6):545–59. doi: 10.1016/j.phrs.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Aksoy E, Goldman M, Willems F. Protein kinase C epsilon: a new target to control inflammation and immune-mediated disorders. Int J Biochem Cell Biol. 2004;36 (2):183–8. doi: 10.1016/s1357-2725(03)00210-3. [DOI] [PubMed] [Google Scholar]
- 16.Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007;55 (6):498–510. doi: 10.1016/j.phrs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48 (1):77–90. doi: 10.1016/j.neuron.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Craske ML, Fivaz M, Batada NN, et al. Spines and neurite branches function as geometric attractors that enhance protein kinase C action. J Cell Biol. 2005;170 (7):1147–58. doi: 10.1083/jcb.200503118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hongpaisan J, Alkon DL. A structural basis for enhancement of long-term associative memory in single dendritic spines regulated by PKC. Proc Natl Acad Sci U S A. 2007;104 (49):19571–6. doi: 10.1073/pnas.0709311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manji HK, Lenox RH. Ziskind-Somerfeld Research Award. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biol Psychiatry. 1999;46 (10):1328–51. doi: 10.1016/s0006-3223(99)00235-8. [DOI] [PubMed] [Google Scholar]
- 21.Einat H, Yuan P, Szabo ST, et al. Protein kinase C inhibition by tamoxifen antagonizes manic-like behavior in rats: implications for the development of novel therapeutics for bipolar disorder. Neuropsychobiology. 2007;55 (3–4):123–31. doi: 10.1159/000106054. [DOI] [PubMed] [Google Scholar]
- 22.Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–28. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- 23.Birnbaum SG, Yuan PX, Wang M, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–4. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- 24.Runyan JD, Moore AN, Dash PK. A role for prefrontal calcium-sensitive protein phosphatase and kinase activities in working memory. Learn Mem. 2005;12:103–10. doi: 10.1101/lm.89405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnsten AFT, Manji HK. Mania: a rational neurobiology. Future Neurol. 2008;3 (2):125–31. [Google Scholar]
- 26.Coyle JT, Duman RS. Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron. 2003;38 (2):157–60. doi: 10.1016/s0896-6273(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 27.Steketee JD. Injection of the protein kinase inhibitor H7 into the A10 dopamine region blocks the acute responses to cocaine: behavioral and in vivo microdialysis studies. Neuropharmacology. 1993;32 (12):1289–97. doi: 10.1016/0028-3908(93)90023-v. [DOI] [PubMed] [Google Scholar]
- 28.Chen G, Manji HK, Hawver DB, et al. Chronic sodium valproate selectively decreases protein kinase C alpha and epsilon in vitro. J Neurochem. 1994;63 (6):2361–4. doi: 10.1046/j.1471-4159.1994.63062361.x. [DOI] [PubMed] [Google Scholar]
- 29.Browman KE, Kantor L, Richardson S, et al. Injection of the protein kinase C inhibitor Ro31-8220 into the nucleus accumbens attenuates the acute response to amphetamine: tissue and behavioral studies. Brain Res. 1998;814 (1–2):112–9. doi: 10.1016/s0006-8993(98)01040-3. [DOI] [PubMed] [Google Scholar]
- 30.Kantor L, Gnegy ME. Protein kinase C inhibitors block amphetamine-mediated dopamine release in rat striatal slices. J Pharmacol Exp Ther. 1998;284 (2):592–8. [PubMed] [Google Scholar]
- 31.Jope RS. Anti-bipolar therapy: mechanism of action of lithium. Mol Psychiatry. 1999;4 (2):117–28. doi: 10.1038/sj.mp.4000494. [DOI] [PubMed] [Google Scholar]
- 32.Bitran JA, Potter WZ, Manji HK, et al. Chronic Li+ attenuates agonist- and phorbol ester-mediated Na+/H+ antiporter activity in HL-60 cells. Eur J Pharmacol. 1990;188 (4–5):193–202. doi: 10.1016/0922-4106(90)90002-f. [DOI] [PubMed] [Google Scholar]
- 33.Giambalvo CT. Protein kinase C and dopamine transport: 2. Effects of amphetamine in vitro. Neuropharmacology. 1992;31 (12):1211–22. doi: 10.1016/0028-3908(92)90049-u. [DOI] [PubMed] [Google Scholar]
- 34.Giambalvo CT. Protein kinase C and dopamine transport: 1. Effects of amphetamine in vivo. Neuropharmacology. 1992;31 (12):1201–10. doi: 10.1016/0028-3908(92)90048-t. [DOI] [PubMed] [Google Scholar]
- 35.Friedman E, Hoau-Yan-Wang, Levinson D, et al. Altered platelet protein kinase C activity in bipolar affective disorder, manic episode. Biol Psychiatry. 1993;33 (7):520–5. doi: 10.1016/0006-3223(93)90006-y. [DOI] [PubMed] [Google Scholar]
- 36.Gnegy ME, Hong P, Ferrell ST. Phosphorylation of neuromodulin in rat striatum after acute and repeated, intermittent amphetamine. Brain Res Mol Brain Res. 1993;20 (4):289–98. doi: 10.1016/0169-328x(93)90055-t. [DOI] [PubMed] [Google Scholar]
- 37.Manji HK, Etcheberrigaray R, Chen G, et al. Lithium decreases membrane-associated protein kinase C in hippocampus: selectivity for the alpha isozyme. J Neurochem. 1993;61 (6):2303–10. doi: 10.1111/j.1471-4159.1993.tb07474.x. [DOI] [PubMed] [Google Scholar]
- 38.Manji HK, Bersudsky Y, Chen G, et al. Modulation of protein kinase C isozymes and substrates by lithium: the role of myoinositol. Neuropsychopharmacology. 1996;15 (4):370–81. doi: 10.1016/0893-133X(95)00243-7. [DOI] [PubMed] [Google Scholar]
- 39.Cervo L, Mukherjee S, Bertaglia A, et al. Protein kinases A and C are involved in the mechanisms underlying consolidation of cocaine place conditioning. Brain Res. 1997;775 (1–2):30–6. doi: 10.1016/s0006-8993(97)00866-4. [DOI] [PubMed] [Google Scholar]
- 40.Birnbaum SG, Yuan PX, Wang M, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306 (5697):882–4. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- 41.Wang HY, Friedman E. Lithium inhibition of protein kinase C activation-induced serotonin release. Psychopharmacology (Berl) 1989;99 (2):213–8. doi: 10.1007/BF00442810. [DOI] [PubMed] [Google Scholar]
- 42.Lenox RH, Watson DG, Patel J, et al. Chronic lithium administration alters a prominent PKC substrate in rat hippocampus. Brain Res. 1992;570 (1–2):333–40. doi: 10.1016/0006-8993(92)90598-4. [DOI] [PubMed] [Google Scholar]
- 43.Steketee JD. Intra-A10 injection of H7 blocks the development of sensitization to cocaine. Neuroreport. 1994;6 (1):69–72. doi: 10.1097/00001756-199412300-00019. [DOI] [PubMed] [Google Scholar]
- 44.Wang HY, Friedman E. Enhanced protein kinase C activity and translocation in bipolar affective disorder brains. Biol Psychiatry. 1996;40 (7):568–75. doi: 10.1016/0006-3223(95)00611-7. [DOI] [PubMed] [Google Scholar]
- 45.Watson DG, Lenox RH. Chronic lithium-induced down-regulation of MARCKS in immortalized hippocampal cells: potentiation by muscarinic receptor activation. J Neurochem. 1996;67 (2):767–77. doi: 10.1046/j.1471-4159.1996.67020767.x. [DOI] [PubMed] [Google Scholar]
- 46.Wang HY, Markowitz P, Levinson D, et al. Increased membrane-associated protein kinase C activity and translocation in blood platelets from bipolar affective disorder patients. J Psychiatr Res. 1999;33 (2):171–9. doi: 10.1016/s0022-3956(98)90057-7. [DOI] [PubMed] [Google Scholar]
- 47.Hahn CG, Friedman E. Abnormalities in protein kinase C signaling and the pathophysiology of bipolar disorder. Bipolar Disord. 1999;1 (2):81–6. doi: 10.1034/j.1399-5618.1999.010204.x. [DOI] [PubMed] [Google Scholar]
- 48.Soares JC, Chen G, Dippold CS, et al. Concurrent measures of protein kinase C and phosphoinositides in lithium-treated bipolar patients and healthy individuals: a preliminary study. Psychiatry Res. 2000;95 (2):109–18. doi: 10.1016/s0165-1781(00)00175-x. [DOI] [PubMed] [Google Scholar]
- 49.Wang HY, Johnson GP, Friedman E. Lithium treatment inhibits protein kinase C translocation in rat brain cortex. Psychopharmacology (Berl) 2001;158 (1):80–6. doi: 10.1007/s002130100834. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Friedman E. Increased association of brain protein kinase C with the receptor for activated C kinase-1 (RACK1) in bipolar affective disorder. Biol Psychiatry. 2001;50 (5):364–70. doi: 10.1016/s0006-3223(01)01147-7. [DOI] [PubMed] [Google Scholar]
- 51.Kurita M, Nishino S, Ohtomo K, et al. Sodium valproate at therapeutic concentrations changes Ca2+ response accompanied with its weak inhibition of protein kinase C in human astrocytoma cells. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31 (3):600–4. doi: 10.1016/j.pnpbp.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 52.Pandey GN, Ren X, Dwivedi Y, et al. Decreased protein kinase C (PKC) in platelets of pediatric bipolar patients: effect of treatment with mood stabilizing drugs. J Psychiatr Res. 2008;42 (2):106–16. doi: 10.1016/j.jpsychires.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kulkarni J, Garland KA, Scaffidi A, et al. A pilot study of hormone modulation as a new treatment for mania in women with bipolar affective disorder. Psychoneuroendocrinology. 2006;31 (4):543–7. doi: 10.1016/j.psyneuen.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 54.Bebchuk JM, Arfken CL, Dolan-Manji S, et al. A preliminary investigation of a protein kinase C inhibitor in the treatment of acute mania. Arch Gen Psychiatry. 2000;57 (1):95–7. doi: 10.1001/archpsyc.57.1.95. [DOI] [PubMed] [Google Scholar]
- 55.Zarate CA, Jr, Singh JB, Carlson PJ, et al. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9 (6):561–70. doi: 10.1111/j.1399-5618.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 56.Yildiz A, Guleryuz S, Ankerst DP, et al. Protein kinase C inhibition in the treatment of mania: a double-blind, placebo-controlled trial of tamoxifen. Arch Gen Psychiatry. 2008;65 (3):255–63. doi: 10.1001/archgenpsychiatry.2007.43. [DOI] [PubMed] [Google Scholar]
- 57.Seelan RS, Khalyfa A, Lakshmanan J, et al. Deciphering the lithium transcriptome: microarray profiling of lithium-modulated gene expression in human neuronal cells. Neuroscience. 2008;151 (4):1184–97. doi: 10.1016/j.neuroscience.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 58.Lenox RH, et al. Myristoylated alanine-rich C kinase substrate (MARCKS): a molecular target for the therapeutic action of mood stabilizers in the brain? J Clin Psychiatry. 1996;57 (Suppl 13):23–31. [PubMed] [Google Scholar]
- 59.Iwata S, Hewlett GH, Gnegy ME. Amphetamine increases the phosphorylation of neuromodulin and synapsin I in rat striatal synaptosomes. Synapse. 1997;26 (3):281–91. doi: 10.1002/(SICI)1098-2396(199707)26:3<281::AID-SYN9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 60.Iwata SI, Hewlett GH, Ferrell ST, et al. Enhanced dopamine release and phosphorylation of synapsin I and neuromodulin in striatal synaptosomes after repeated amphetamine. J Pharmacol Exp Ther. 1997;283 (3):1445–52. [PubMed] [Google Scholar]
- 61.Johnson DN. Effect of diazepam on food consumption in rats. Psychopharmacology (Berl) 1978;56 (1):111–2. doi: 10.1007/BF00571417. [DOI] [PubMed] [Google Scholar]
- 62.Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104 (2):255–9. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- 63.Aujla H, Beninger RJ. Intra-accumbens protein kinase C inhibitor NPC 15437 blocks amphetamine-produced conditioned place preference in rats. Behav Brain Res. 2003;147 (1–2):41–8. doi: 10.1016/s0166-4328(03)00136-0. [DOI] [PubMed] [Google Scholar]
- 64.Baum AE, Akula N, Cabanero M, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008;13 (2):197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447 (7145):661–78. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jordan VC. Molecular mechanisms of antiestrogen action in breast cancer. Breast Cancer Res Treat. 1994;31 (1):41–52. doi: 10.1007/BF00689675. [DOI] [PubMed] [Google Scholar]
- 67.Horgan K, Cooke E, Hallett MB, et al. Inhibition of protein kinase C mediated signal transduction by tamoxifen: importance for antitumour activity. Biochem Pharmacol. 1986;35 (24):4463–5. doi: 10.1016/0006-2952(86)90764-1. [DOI] [PubMed] [Google Scholar]
- 68.Couldwell WT, Weiss MH, DeGiorgio CM, et al. Clinical and radiographic response in a minority of patients with recurrent malignant gliomas treated with high-dose tamoxifen. Neurosurgery. 1993;32 (3):485–9. doi: 10.1227/00006123-199303000-00034. [DOI] [PubMed] [Google Scholar]
- 69.Tang P, Roldan G, Brasher PM, et al. A phase II study of carboplatin and chronic high-dose tamoxifen in patients with recurrent malignant glioma. J Neurooncol. 2006;78 (3):311–6. doi: 10.1007/s11060-005-9104-y. [DOI] [PubMed] [Google Scholar]
- 70.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 71.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 72.Goldstein JA. Danazol and the rapid-cycling patient. J Clin Psychiatry. 1986;47 (3):153–4. [PubMed] [Google Scholar]
- 73.Lopez M, Lelliott CJ, Tovar S, et al. Tamoxifen-induced anorexia is associated with fatty acid synthase inhibition in the ventromedial nucleus of the hypothalamus and accumulation of malonyl-CoA. Diabetes. 2006;55 (5):1327–36. doi: 10.2337/db05-1356. [DOI] [PubMed] [Google Scholar]
- 74.Cathcart CK, Jones SE, Pumroy CS, et al. Clinical recognition and management of depression in node negative breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 1993;27 (3):277–81. doi: 10.1007/BF00665698. [DOI] [PubMed] [Google Scholar]
- 75.Legha SS. Tamoxifen in the treatment of breast cancer. Ann Intern Med. 1988;109 (3):219–28. doi: 10.7326/0003-4819-109-3-219. [DOI] [PubMed] [Google Scholar]
- 76.Costanzo ES, Lutgendorf SK, Mattes ML, et al. Adjusting to life after treatment: distress and quality of life following treatment for breast cancer. Br J Cancer. 2007;97 (12):1625–31. doi: 10.1038/sj.bjc.6604091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gould TD, Quiroz JA, Singh J, et al. Emerging experimental therapeutics for bipolar disorder: insights from the molecular and cellular actions of current mood stabilizers. Mol Psychiatry. 2004;9 (8):734–55. doi: 10.1038/sj.mp.4001518. [DOI] [PubMed] [Google Scholar]
- 78.Catapano L, Manji H. Kinases as drug targets in the treatment of bipolar disorder. Drug Development Today. 2008;13:295–302. doi: 10.1016/j.drudis.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 79.Podar K, Raab MS, Chauhan D, et al. The therapeutic role of targeting protein kinase C in solid and hematologic malignancies. Expert Opin Investig Drugs. 2007;16 (10):1693–707. doi: 10.1517/13543784.16.10.1693. [DOI] [PubMed] [Google Scholar]
- 80.Martinez A, Castro A, Medina M. Glycogen synthase kinase 3 (GSK-3) and its inhibitors. Hoboken (NJ): John Wiley & Sons, Inc; 2006. [Google Scholar]
- 81.Tohen M. Clinical trials in bipolar mania: implications in study design and drug development. Arch Gen Psychiatry. 2008;65 (3):252–3. doi: 10.1001/archgenpsychiatry.2007.44. [DOI] [PubMed] [Google Scholar]