Abstract
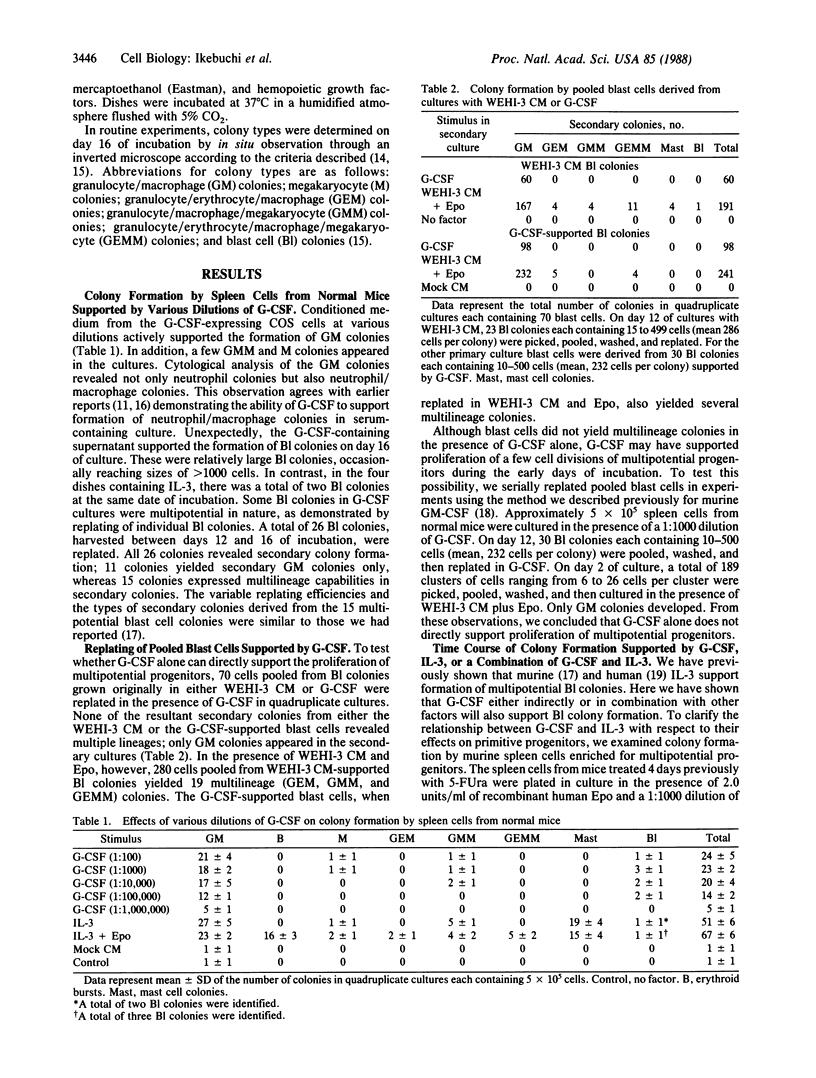
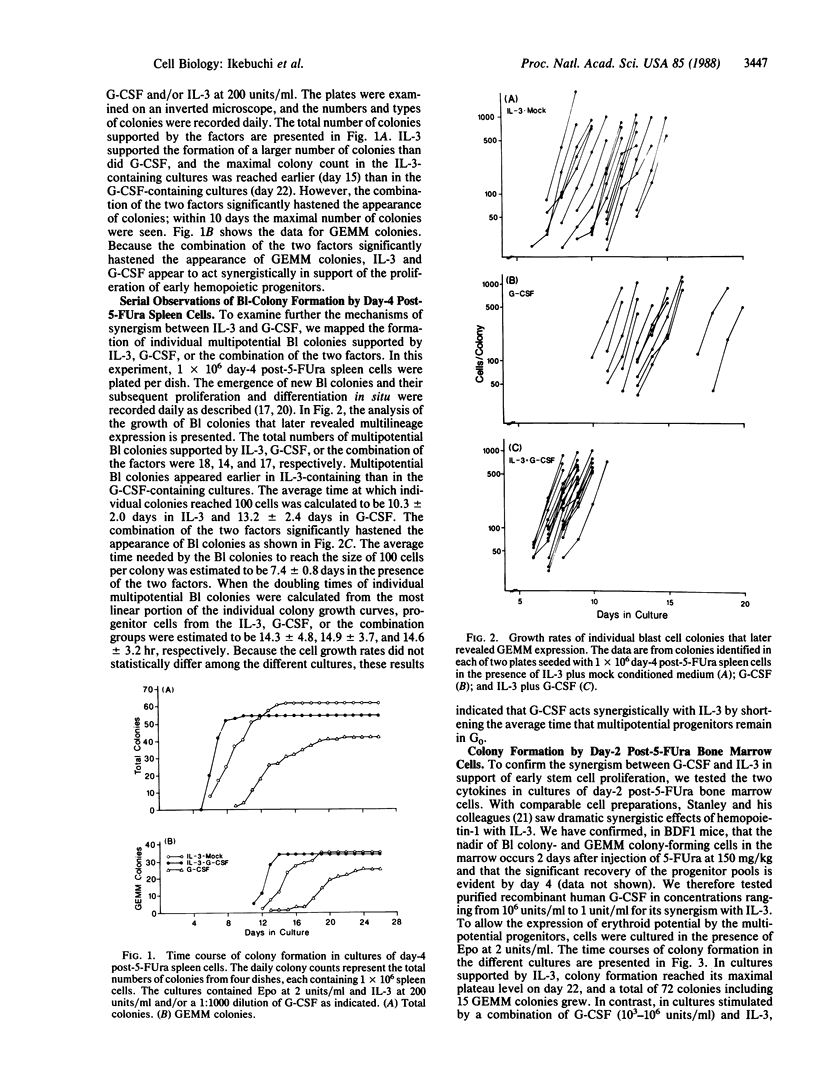
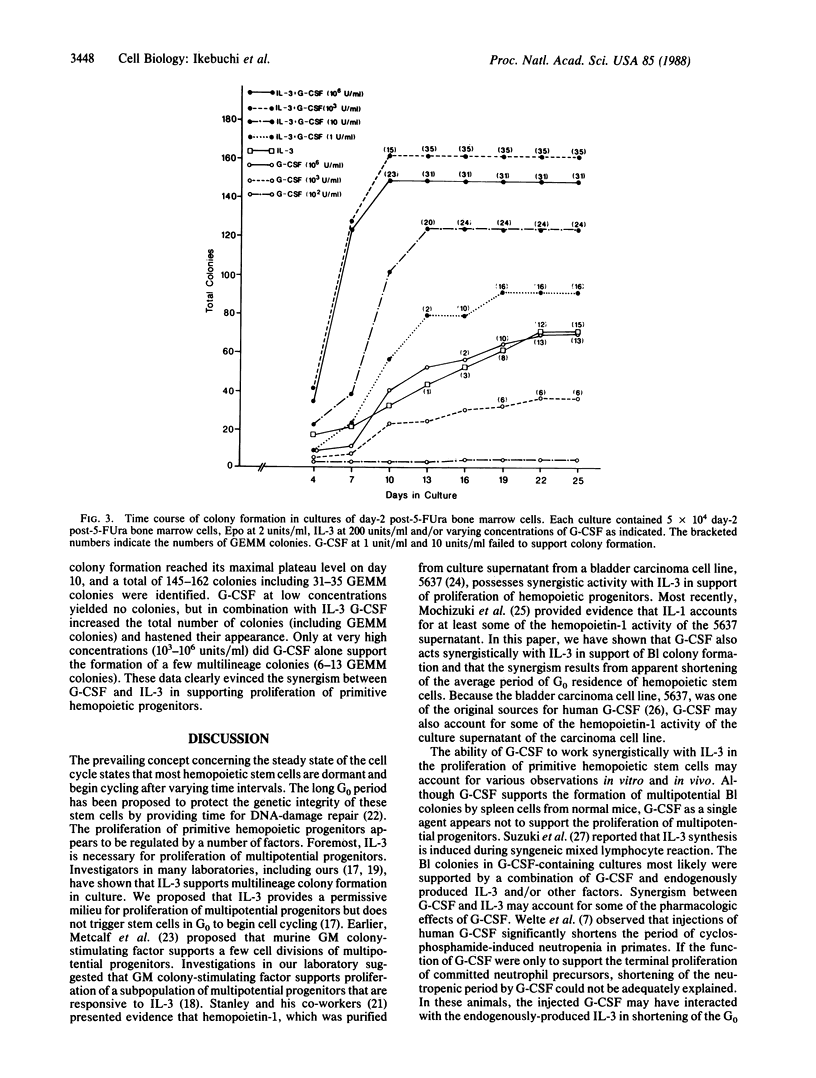
In cultures of spleen cells from normal mice, recombinant human granulocyte colony-stimulating factor (G-CSF) supported the formation of multipotential blast cell colonies. Serial replating of the blast cell colonies in the presence of G-CSF, however, failed to demonstrate any direct effect of G-CSF on murine multipotential progenitors. We therefore examined the effects of G-CSF in combination with murine interleukin 3 on proliferation of murine blast cell colony-forming cells. The time course of total colony formation and multilineage colony formation by spleen cells harvested from mice 4 days after injection of 5-fluorouracil at 150 mg/kg was significantly shortened in cultures containing both factors in contrast with cultures supported by either factor alone. Serial observations of individual multipotential blast cell colonies (mapping) revealed that blast cell colonies emerged at random time intervals in the presence of interleukin 3 or G-CSF. The appearance of blast cell colonies, however, was significantly hastened in cultures containing both factors relative to cultures grown with either factor. In cultures of day-2 post-5-fluorouracil bone marrow cells, G-CSF in concentrations as low as 1 unit/ml revealed synergism with interleukin 3 in supporting the proliferation of multipotential progenitors. This synergistic activity may explain the previous in vivo studies suggesting the effects of G-CSF on apparent multipotential stem cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Cohen A. M., Zsebo K. M., Inoue H., Hines D., Boone T. C., Chazin V. R., Tsai L., Ritch T., Souza L. M. In vivo stimulation of granulopoiesis by recombinant human granulocyte colony-stimulating factor. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2484–2488. doi: 10.1073/pnas.84.8.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliason J. F. Granulocyte-macrophage colony formation in serum-free culture: effects of purified colony-stimulating factors and modulation by hydrocortisone. J Cell Physiol. 1986 Aug;128(2):231–238. doi: 10.1002/jcp.1041280214. [DOI] [PubMed] [Google Scholar]
- Garman R. D., Jacobs K. A., Clark S. C., Raulet D. H. B-cell-stimulatory factor 2 (beta 2 interferon) functions as a second signal for interleukin 2 production by mature murine T cells. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7629–7633. doi: 10.1073/pnas.84.21.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Hodgson G. S., Bradley T. R. Properties of haematopoietic stem cells surviving 5-fluorouracil treatment: evidence for a pre-CFU-S cell? Nature. 1979 Oct 4;281(5730):381–382. doi: 10.1038/281381a0. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Ikebuchi K., Wong G. G., Clark S. C., Ihle J. N., Hirai Y., Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9035–9039. doi: 10.1073/pnas.84.24.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubinsky P. T., Stanley E. R. Purification of hemopoietin 1: a multilineage hemopoietic growth factor. Proc Natl Acad Sci U S A. 1985 May;82(9):2764–2768. doi: 10.1073/pnas.82.9.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K., Ogawa M., Ihle J. N., Miyake T., Shimizu T., Miyajima A., Yokota T., Arai K. Recombinant murine granulocyte-macrophage (GM) colony-stimulating factor supports formation of GM and multipotential blast cell colonies in culture: comparison with the effects of interleukin-3. J Cell Physiol. 1987 Jun;131(3):458–464. doi: 10.1002/jcp.1041310319. [DOI] [PubMed] [Google Scholar]
- Lajtha L. G. Stem cell concepts. Differentiation. 1979;14(1-2):23–34. doi: 10.1111/j.1432-0436.1979.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Leary A. G., Yang Y. C., Clark S. C., Gasson J. C., Golde D. W., Ogawa M. Recombinant gibbon interleukin 3 supports formation of human multilineage colonies and blast cell colonies in culture: comparison with recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1987 Nov;70(5):1343–1348. [PubMed] [Google Scholar]
- Metcalf D., Johnson G. R., Burgess A. W. Direct stimulation by purified GM-CSF of the proliferation of multipotential and erythroid precursor cells. Blood. 1980 Jan;55(1):138–147. [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A. Proliferative effects of purified granulocyte colony-stimulating factor (G-CSF) on normal mouse hemopoietic cells. J Cell Physiol. 1983 Aug;116(2):198–206. doi: 10.1002/jcp.1041160211. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H., Suda T., Miura Y., Kubota K., Takaku F. Hemopoietic stem cells in nude mice transplanted with colony-stimulating-factor-producing tumors. Exp Hematol. 1982 Nov;10(10):874–880. [PubMed] [Google Scholar]
- Mochizuki D. Y., Eisenman J. R., Conlon P. J., Larsen A. D., Tushinski R. J. Interleukin 1 regulates hematopoietic activity, a role previously ascribed to hemopoietin 1. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5267–5271. doi: 10.1073/pnas.84.15.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S., Tsuchiya M., Asano S., Kaziro Y., Yamazaki T., Yamamoto O., Hirata Y., Kubota N., Oheda M., Nomura H. Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. 1986 Jan 30-Feb 5Nature. 319(6052):415–418. doi: 10.1038/319415a0. [DOI] [PubMed] [Google Scholar]
- Nakahata T., Ogawa M. Clonal origin of murine hemopoietic colonies with apparent restriction to granuclocyte-macrophage-megakaryocyte (GMM) differentiation. J Cell Physiol. 1982 Jun;111(3):239–246. doi: 10.1002/jcp.1041110304. [DOI] [PubMed] [Google Scholar]
- Nakahata T., Ogawa M. Identification in culture of a class of hemopoietic colony-forming units with extensive capability to self-renew and generate multipotential hemopoietic colonies. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3843–3847. doi: 10.1073/pnas.79.12.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., Matsumoto M., Johnson G. R. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem. 1983 Jul 25;258(14):9017–9023. [PubMed] [Google Scholar]
- Shimamura M., Kobayashi Y., Yuo A., Urabe A., Okabe T., Komatsu Y., Itoh S., Takaku F. Effect of human recombinant granulocyte colony-stimulating factor on hematopoietic injury in mice induced by 5-fluorouracil. Blood. 1987 Jan;69(1):353–355. [PubMed] [Google Scholar]
- Sieff C. A. Hematopoietic growth factors. J Clin Invest. 1987 Jun;79(6):1549–1557. doi: 10.1172/JCI112988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza L. M., Boone T. C., Gabrilove J., Lai P. H., Zsebo K. M., Murdock D. C., Chazin V. R., Bruszewski J., Lu H., Chen K. K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986 Apr 4;232(4746):61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Bartocci A., Patinkin D., Rosendaal M., Bradley T. R. Regulation of very primitive, multipotent, hemopoietic cells by hemopoietin-1. Cell. 1986 Jun 6;45(5):667–674. doi: 10.1016/0092-8674(86)90781-6. [DOI] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M., Ihle J. N. Permissive role of interleukin 3 (IL-3) in proliferation and differentiation of multipotential hemopoietic progenitors in culture. J Cell Physiol. 1985 Aug;124(2):182–190. doi: 10.1002/jcp.1041240203. [DOI] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Proliferative kinetics and differentiation of murine blast cell colonies in culture: evidence for variable G0 periods and constant doubling rates of early pluripotent hemopoietic progenitors. J Cell Physiol. 1983 Dec;117(3):308–318. doi: 10.1002/jcp.1041170305. [DOI] [PubMed] [Google Scholar]
- Suzuki R., Suzuki S., Igarashi M., Kumagai K. Induction of interleukin 3 but not interleukin 2 or interferon production in the syngeneic mixed lymphocyte reaction. J Immunol. 1986 Sep 1;137(5):1564–1572. [PubMed] [Google Scholar]
- Tsuchiya M., Asano S., Kaziro Y., Nagata S. Isolation and characterization of the cDNA for murine granulocyte colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7633–7637. doi: 10.1073/pnas.83.20.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte K., Bonilla M. A., Gillio A. P., Boone T. C., Potter G. K., Gabrilove J. L., Moore M. A., O'Reilly R. J., Souza L. M. Recombinant human granulocyte colony-stimulating factor. Effects on hematopoiesis in normal and cyclophosphamide-treated primates. J Exp Med. 1987 Apr 1;165(4):941–948. doi: 10.1084/jem.165.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte K., Platzer E., Lu L., Gabrilove J. L., Levi E., Mertelsmann R., Moore M. A. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1526–1530. doi: 10.1073/pnas.82.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein A., Ruggieri R., Korn J. H., Revel M. Structure and expression of cDNA and genes for human interferon-beta-2, a distinct species inducible by growth-stimulatory cytokines. EMBO J. 1986 Oct;5(10):2529–2537. doi: 10.1002/j.1460-2075.1986.tb04531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



