Abstract
Due to the aging of the populations of developed countries and a common occurrence of risk factors, it is increasingly probable that a patient may have both cancer and cardiovascular disease. In addition, cytotoxic agents and targeted therapies used to treat cancer, including classic chemotherapeutic agents, monoclonal antibodies that target tyrosine kinase receptors, small molecule tyrosine kinase inhibitors, and even antiangiogenic drugs and chemoprevention agents such as cyclooxygenase-2 inhibitors, all affect the cardiovascular system. One of the reasons is that many agents reach targets in the microenvironment and do not affect only the tumor. Combination therapy often amplifies cardiotoxicity, and radiotherapy can also cause heart problems, particularly when combined with chemotherapy. In the past, cardiotoxic risk was less evident, but it is increasingly an issue, particularly with combination therapy and adjuvant therapy. Today's oncologists must be fully aware of cardiovascular risks to avoid or prevent adverse cardiovascular effects, and cardiologists must now be ready to assist oncologists by performing evaluations relevant to the choice of therapy. There is a need for cooperation between these two areas and for the development of a novel discipline, which could be termed cardio-oncology or onco-cardiology. Here, we summarize the potential cardiovascular toxicities for a range of cancer chemotherapeutic and chemopreventive agents and emphasize the importance of evaluating cardiovascular risk when patients enter into trials and the need to develop guidelines that include collateral effects on the cardiovascular system. We also discuss mechanistic pathways and describe several potential protective agents that could be administered to patients with occult or overt risk for cardiovascular complications.
During the first decades of the last century, because of improvements in prevention and therapy of infectious diseases, better hygiene and socioeconomic conditions, and consequently increased life span, cardiovascular and cerebrovascular diseases rose to become the leading causes of death. In addition to age, there are other common risk factors for both cardiovascular disease and cancer (1). Now that efforts to prevent cardiovascular diseases have been effective, cancer is rising as the major cause of death. A patient with a neoplasm or preneoplastic condition who undergoes cancer therapy or chemoprevention is now at a substantial risk for the deterioration of his or her cardiovascular health. In the past, this risk was less evident because the life span of a patient with metastatic disease was often too short to make the cardiovascular complications a major matter of concern. However, now that progress has been made in terms of early diagnosis, therapy, and survival, targeted drugs and combinations of two or three different agents have emerged, and intervention is more and more often used at the adjuvant stage; cardiotoxicity of cancer therapy is a pivotal issue. The medical community is becoming increasingly aware of its impact, as highlighted in several recent reviews (2–5).
Here, we summarize major aspects of cardiotoxicity of anticancer therapies and strongly recommend the creation of teams of cardiologists working with oncologists to build an interdisciplinary field that could be termed cardio-oncology (6). We also suggest that cardio-oncology teams develop guidelines to protect the oncology patient from the rising risk of cardiovascular side effects among anticancer therapies.
What Exactly Does Cardiotoxicity Mean?
The National Cancer Institute defines cardiotoxicity in very general terms as “toxicity that affects the heart” (www.cancer.gov/dictionary/). However, although it is well known that several cancer chemotherapeutics adversely affect the heart and the vascular system, and that a growing number of clinical trials (registered at www.clinicaltrials.gov) are now studying long-term side effects of anticancer therapy, including cardiovascular events, a clear understanding of what cardiotoxicity is and how anticancer therapy stresses the cardiovascular system is lacking. One of the most accurate clinical definitions of cardiotoxicity has been formulated by the cardiac review and evaluation committee supervising trastuzumab clinical trials, which defined drug-associated cardiotoxicity as one or more of the following: 1) cardiomyopathy in terms of a reduction in left ventricular ejection fraction (LVEF), either global or more severe in the septum; 2) symptoms associated with heart failure (HF); 3) signs associated with HF, such as S3 gallop, tachycardia, or both; 4) reduction in LVEF from baseline that is in the range of less than or equal to 5% to less than 55% with accompanying signs or symptoms of HF, or a reduction in LVEF in the range of equal to or greater than 10% to less than 55%, without accompanying signs or symptoms (7). This definition does not include subclinical cardiovascular damage that may occur early in response to some chemotherapeutic agents; thus, to date, an ideal definition is lacking.
The use of chemotherapeutic agents, radiation therapy, and molecular targeted therapies are all approaches that can injure the cardiovascular system, both at a central level by deteriorating the heart function and in the periphery by enhancing hemodynamic flow alterations and thrombotic events often latently present in oncology patients. Unfortunately, a comprehensive analysis of published data on cardiotoxicity is difficult to perform and would be inadequate because oncology trials measuring vascular effects vary widely in their methods and definition of cardiotoxicity. Published data regarding risk factors are contradictory, longitudinal studies on well-defined cohorts are insufficient, and knowledge of the relationship between abnormalities identified by noninvasive cardiac testing and clinical status of survival are not sufficiently clear (8). Moreover, in many cases, the primary literature is lacking in regard to the rate of the cardiovascular adverse events of antineoplastic drugs, particularly for newer targeted therapies. In addition, the reported rates of cardiotoxicity were obtained from the available published literature; thus, they apply only to the available follow-up periods for each agent (5).
Cardiotoxicity can develop in a subacute, acute, or chronic manner. Acute or subacute cardiotoxicity is characterized by either the occurrence of abnormalities in ventricular repolarization and electrocardiographic QT-interval changes, by supraventricular and ventricular arrhythmias, or by acute coronary syndromes and pericarditis and/or myocarditis-like syndromes, observed any time from the initiation of therapy up to 2 weeks after termination of treatment. Chronic cardiotoxicity may be differentiated in two subtypes based on the onset of clinical symptoms. The first subtype occurs early, within 1 year after termination of chemotherapy, and the second occurs late, more than 1 year after chemotherapy. The most typical sign of chronic cardiotoxicity is asymptomatic systolic and/or diastolic left ventricular dysfunction that leads to severe congestive cardiomyopathy and that may ultimately lead to death (8,9).
How Cardiotoxicity Manifests Itself: Effects of Anticancer Therapy on the Cardiovascular System
Several publications (2–4,7–12) have focused on the cardiotoxicity of specific classes of cancer therapeutic agents and recently, we have begun to see a global analysis of the diverse classes together (5). We have provided a summary of the cardiovascular toxic effects produced by different drugs and therapeutic agents (Table 1). Briefly, anthracyclines can generate congestive heart failure (CHF) and left ventricular dysfunction. The occurrence of CHF is dose- and schedule-dependent; left ventricular dysfunction is more frequently observed in women, in patients with a personal history of cardiac disease, and after mediastinal X-ray therapy (12). The risk of cardiotoxic adverse events increases when anthracycline chemotherapy is administered concurrently or sequentially before adjuvant therapy with trastuzumab, a monoclonal antibody specific for the HER2 protein (11). Mitoxantrone, which is a derivative of anthraquinone, induces acute myocarditis and arrhythmia during infusion. Antimetabolite agents, such as capecitabine or cytarabine, can induce ischemia, pericarditis, CHF, and cardiogenic shock. In particular, cardiotoxicity that is induced by fluoropyrimidines such as 5-fluorouracil could be manifested by myocardial ischemia, as indicated by the electrocardiographic alterations that are occasionally observed during 5-fluorouracil administration and it is rate- and dose-dependent. Antimicrotubule molecules, such as paclitaxel or vinca alkaloids, can cause sinus bradycardia, atrioventricular block, ventricular tachycardia, hypotension, CHF, and ischemia [reviewed in (12)]. HF that occurs after high-dose cyclophosphamide, ifosfamide, or mitomycin treatment is manifested by neurohumoral activation without concomitant cardiomyocyte necrosis. Mild functional mitral regurgitation may also develop in cyclophosphamide-treated patients (13).
Table 1.
Potential mechanisms of cardiovascular damage induced by anticancer treatments. A summary of probable mechanisms of cardiotoxicity induced by a range of chemotherapeutics and chemoprevention agents*
| Effects |
||||||||
| Systemic-macroenvironment |
||||||||
| Mitochondrial |
Local-microenvironment |
|||||||
| Examples of chemotherapeutics | Possible cardiovascular damage | DNA damage | ATP block | Apoptotic protein release | ROS generation | Endothelial cell damage/spasms | Cell signaling/survival block | ADCC |
| Anthracyclines and anthraquinolones | CHF, LVD, acute myocarditis, arrhythmia | + | + | + | + | − | − | − |
| Capecitabine, 5-fluorouracil, cytarabine | Ischemia, pericarditis, CHF, cardiogenic shock | + | + | + | + | + | − | − |
| Paclitaxel, vinca alkaloids | Sinus bradicardia, ventricular tachycardia, atrioventricular block, hypotension, CHF, ischemia | + | ? | ? | ? | ? | − | − |
| Cyclophosphamide | Neurohumoral activation, mitral regurgitation | + | ? | ? | ? | + | − | − |
| Imatinib | Arrythmias, CHF, angioedema, LVD | − | + | + | − | < > | < > | − |
| Sorafenib | Hypertension, arrythmias | − | − | − | − | < > | < > | − |
| Sunitinib | Hypertension, arrythmias | − | − | − | − | < > | < > | − |
| SERMs | LDL/HDL modulation, thromboembolism | − | − | − | − | – | − | − |
| Trastuzumab | Arrythmias, CHF, angioedema, LVD | − | − | − | − | < > | < > | + |
| Bevacizumab | Hypertension, thromboembolism, GI tract bleeding | − | − | − | − | < > | < > | − |
| COX-2–specific inhibitors | Thromboembolism | − | − | − | − | < > | − | − |
| Thorax irradiation | Myocardial fibrosis, valvular heart disease, LVD | + | − | < > | + | + | − | − |
+ = likely; − = unlikely; ? = unknown; < > = probable. ADCC = antibody-dependent cellular cytotoxicity; CHF = congestive heart failure; COX-2 = cyclooxygenase 2; GI = gastrointestinal tract; HDL = high-density lipoprotein; LDL = low-density lipoprotein; LVD = left ventricular dysfunction; ROS = reactive oxygen species; SERMs = selective estrogen receptor modulators. For references, see text.
Hypotension or hypertension, arrhythmias, CHF, angioedema, and left ventricular dysfunction are observed in patients treated with biological agents such as monoclonal antibodies, interleukins, and interferon-α (12). Myocardial fibrosis (14), valvular heart disease, more frequently involving left-sided valves (15), and endothelial cell damage (16) can also occur following radiation therapy (Table 1). The antiangiogenic and multitarget tyrosine kinase inhibitors (TKIs) sorafenib and sunitinib are associated with hypertension and cardiotoxicity (3,10,17–19). The anti–vascular endothelial growth factor antibody bevacizumab is also associated with hypertension and instances of thromboembolism, pulmonary hemorrhage, and pulmonary edema or gastrointestinal tract bleeding (10). Thus, the antiangiogenesis class of drugs can also harbor cardiovascular toxicity, as indicated by a reduction of LVEF that over the long term may result in CHF (2–4). Another class of agents that appear to have effects on vascular system is the selective estrogen receptor modulators. It has been shown that tamoxifen induces a variation of low-density lipoprotein and high-density lipoprotein blood levels in a different manner than that induced by aromatase inhibitors (20). Tamoxifen induces a decrease in total cholesterol and low-density lipoprotein levels and an increase in triglyceride concentration, but no change has been reported for high-density lipoprotein serum concentration. Some evidence exists that tamoxifen reduces the incidence of myocardial infarction (21). However, this apparent favorable effect of tamoxifen on the lipid profile does not consistently translate into a beneficial effect on the development or progression of cardiovascular diseases. On the contrary, tamoxifen may have a detrimental effect, with a substantially increased risk of venous thromboembolism, pulmonary embolism, and stroke due to its function as a partial estrogen agonist, a characteristic that is associated per se with the increased incidence of thromboembolic events (Table 1).
Why Cardiotoxicity Happens: Mechanisms of Therapy-Induced Damage to Heart and Blood Vessels
As expected, the mechanisms of cardiotoxicity vary widely among diverse chemotherapeutics (22) (Table 1). However, when examining individual mechanisms, some common themes emerge that may provide interesting targets to block or to overcome. The cardiovascular system has numerous different targets that can be subject to damage. First, some drugs directly damage cardiomyocytes or cause inflammation of the pericardium. Second, some drugs affect the coagulation system and can promote blood clotting in the vessels that predisposes to thromboembolic events and consequent cardiovascular and cerebrovascular ischemia. The impact of antineoplastic therapies on the coagulation cascade can be basically ascribed to damage to the intima of the vessels. Damage to peripheral circulation can impair circulation and predispose to thrombosis. Antiangiogenic drugs also have an impact on the interconnections of endothelial cells and are associated with changes in vessel structure and possibly with bleeding and hemorrhage. Third, hypertension, often seen with the antiangiogenic agents, has acute and long-term effects on cardiac hypertrophy and insufficiency. Fourth, atrial fibrillation (AF), which is common in elderly individuals, can be exacerbated by anticancer treatments. Each of these four drug targets is discussed in greater detail below.
Direct Effects on the Heart.
Many drugs directly damage the cardiomyocytes or cause inflammation of the pericardium. Anthracyclines produce cardiac toxicity accompanied by an increase in myofibrillar disarray that is mediated by the signaling function of neuregulin 1β (23,24). In addition, the classic cytotoxic chemotherapeutic agents, including anthracyclines, induce mitochondrial apoptosis pathways and free radical production (25). The cardiotoxic potential of anthracycline is enhanced by the concurrent or sequential administration of antibodies that target tyrosine kinase receptors, in particular trastuzumab (26,27). Trastuzumab is cardiotoxic on its own and strikingly potentiates the cardiotoxic effects of anthracyclines. The trastuzumab target, ErbB2, is expressed on cardiomyocytes, where it exerts a protective effect on cardiac function (28). Both HER receptors and their ligands are expressed in the heart and their activation creates a hypertrophic response (29). In addition, heregulin–HER signaling promotes cell survival and growth and protects against apoptosis (29). The ability of the heart to withstand stress is due, at least in part, to a protein network leading to cell survival that is activated by HER ligands and involves the activator protein-1 and the nuclear factor κB. Activator protein-1 regulates the expression of a group of cardiac proteins that are important in the development of cardiac hypertrophy, and nuclear factor κB regulates the genes that are involved in the cellular response to stress and inflammation (29). However, not all monoclonal antibodies that target Erb proteins induce damage to the heart. In fact, lapatinib, an oral TKI that targets ErbB1 (epidermal growth factor receptor) and ErbB2 (HER2), was reported to show very little cardiotoxicity, although the different criteria used to define cardiotoxicity could have led to an underestimation of the actual incidence of cardiovascular adverse events (30). These data suggest that factors such as antibody-directed cellular cytotoxicity may contribute to adverse events when using therapeutic antibodies against cell surface receptors that are also expressed on cardiomyocytes.
A possible mechanism by which the taxane paclitaxel would cause cardiotoxicity is massive histamine release. Indeed, in animal studies, stimulation of histamine receptors in cardiac tissue has resulted in conduction disturbances and arrhythmias. Alternatively, paclitaxel-induced cardiotoxicity could be attributed to the induction of cardiac muscle damage via effects on subcellular organelles (31).
The mechanisms by which several other chemotherapy drugs produce cardiovascular toxicities have also been investigated. 5-Fluorouracil, a widely used chemotherapeutic, has direct toxic effects on vascular endothelium that involve endothelial nitric oxide (NO) synthase and lead to coronary spasms and endothelium-independent vasoconstriction via protein kinase C (32). Several new generation TKIs, for example, sorafenib and sunitinib, have been also been associated with direct cardiotoxicity (3,10). Cardiomyocytes from subjects who are being treated with imatinib appear to have endoplasmic reticula that have been activated in response to cellular stress, collapsed mitochondrial membrane potential, reduced ATP content, and to be more prone to cell death (33). However, whether the observed cardiomyocyte cell damage represents clinical cardiotoxicity is still controversial (34), as is the role of the protooncogene abl (a target of imatinib and similar compounds) in the physiology of the cardiomyocyte (33,35).
Effects on the Coagulation System.
Chemotherapy-induced effects on the coagulation system can promote blood clotting in the vessels, which is a precursor to thrombosis and thromboembolic events and sets the stage for consequent cardiovascular and/or cerebrovascular ischemia. Cancer is known to produce a prothrombotic state—Armand Trousseau diagnosed thrombosis as being related to cancer in 1865. The risk of thrombosis from cancer appears to be highest in cancer patients with metastatic disease and in those with established risk factors (5). Life-threatening hemorrhage and arterial thromboembolism have been observed in patients being treated with agents that are broad-spectrum angiogenesis inhibitors, such as thalidomide and lenalidomide (36). It is not known how angiogenesis inhibitors and vascular disrupting agents alter normal hemostasis; it is likely that they disrupt the function or the integrity of the vascular endothelium (36,37). Damage to the vessel can involve injury to the intimal layer or disruption of endothelial cell–cell communication. In either case, the loss of integrity of the vessel lining activates the coagulation cascade.
Venous thromboembolism has been associated with several categories of chemotherapeutic agents: alkylating agents, angiogenesis inhibitors, histone deacetylase inhibitors, and TKIs (38–40). In particular, the alkylating agent cisplatin can trigger platelet aggregation, enhance thromboxane formation by platelets, and activate arachidonic acid pathways in platelets (31).
The risk of hemorrhage and thromboembolisms increases with the use of drugs that modify the expression pattern of adhesion molecules, such as integrins and cadherins, on endothelial cells, producing alterations on the cell–cell and cell–matrix connections and interruption of the endothelium integrity. For example, doxycycline targets the adherens junction in vascular endothelial cells by inducing total vascular endothelial-cadherin expression while decreasing vascular endothelial-cadherin phosphorylation (41). Furthermore, it has been demonstrated that lenalidomide inhibits the vascular endothelial growth factor–induced association between cadherin 5, CD31, and β-catenin (42,43).
Hypertension.
Hypertension and cancer often coexist in the same patient, and treatment with antiangiogenic agents exacerbates hypertension, with acute and long-term effects on cardiac hypertrophy and insufficiency. In fact, high blood pressure is the most frequent comorbid condition reported in cancer registries (44,45). Hypertension is a common adverse effect in patients who are treated with bevacizumab, sorafenib, and sunitinib (5). The mechanism of antiangiogenic therapy–related hypertension is not fully understood but it is thought to be related to vascular endothelial growth factor inhibition, which, through decreased NO-synthase activity, leads to decreases in NO production in the walls of arterioles and other resistance vessels. NO is a natural vasodilator; thus, blocking its production promotes vasoconstriction and increases peripheral vascular resistance and blood pressure (46). Moreover, the decreased endothelial NO synthase activity may stimulate plasminogen activator inhibitor-1 expression, leading to an increased risk of hypertension (47).
Atrial Fibrillation.
AF, a common finding in the elderly, can be exacerbated by anticancer treatments and may complicate the outcome of patients with malignancies as side effects of surgical or medical therapies (48). AF may be due to patient stress but also can be induced by various cytostatic agents, such as ifosfamide, gemcitabine, melphalan, cisplatin, docetaxel, 5-fluorouracil, or etoposide, or by high doses of corticosteroids (49,50).
Inflammation plays an important role in carcinogenesis and could provide a possible explanation for a relationship between AF, inflammation, and cancer. 18.3% of patients with history of cancer had AF compared with 5.6% of patients without a history of cancer (51). A statistically significant elevation of serum levels of C-reactive protein were found in patients with AF (52) and in patients with a history of cancer (51), implying systemic inflammation. However, cancer was not found to be an independent predictor of atrial arrhythmias in multivariable analysis. Taken together, these data suggest that malignancy does not lead to AF per se, but does so through systemic inflammation.
The Sliding Doors Concept
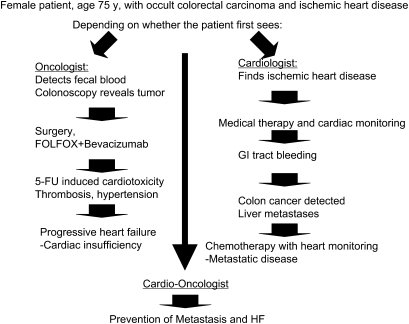
Given the variety of effects and mechanisms described above, there is an increasing awareness of the concept: “Is it time for oncologists to get to know their cardiologists?” (53). A patient who has a comorbidity of cardiovascular disease and cancer could possibly have a different outcome depending on whether he or she was first seen by an oncologist or a cardiologist. For example, if a patient with a family history of heart disease and gastrointestinal tract cancer were to have an occult cardiovascular condition and an undiagnosed colon cancer with tumor-associated thrombophilia aggravating the cardiovascular condition, he or she might encounter either of two scenarios (Figure 1). If the patient were to visit a cardiologist first, the diagnosis of cardiovascular risk would be made and appropriate medications prescribed to improve heart function and reduce thrombosis. However, the patient is at risk of being diagnosed later with tumor progression and poorer prognosis. If the patient were to go to an oncologist first, a colonoscopy examination would likely be suggested based on family history and if colon cancer were detected, the patient would undergo surgery and chemotherapy for the tumor. However, he or she might not be given a full cardiovascular workup other than a blood pressure test. The patient's cancer may respond well to the therapeutic regimen, but his or her cardiovascular system might not; cardiotoxicity from the chemotherapeutic agents used combined with inadequate control of hypertension from an antiangiogenic agent could spell incumbent disaster. This example provides the basis for the increasing necessity for communication between the cardiologist and oncologist and the creation of “cardio-oncology” interdisciplinary clinical units. Recent studies suggest that this approach can be pursued successfully (54,55).
Figure 1.
The “sliding doors” concept, an example of diverse outcomes based on first diagnosis. There is a risk for diverse outcomes depending on whether the patient with both cardiovascular disease and cancer first presents to a cardiologist or to an oncologist. The oncologist takes a tumor-centric perspective in diagnosis, and in the past might have been missing the incumbent cardiopathy of the individual. The cardiologist treats the cardiopathy correctly, but may not pick up early signs of cancer, thus the patient risks further progression and oncologic complications. Teamwork should improve patient life expectancy, treating cancer while protecting the heart, or treating the heart and providing a thorough oncologic check up. 5-FU = fluorouracil; FOLFOX = folinic acid (leucovorin), 5-FU and oxaliplatin; GI = gastrointestinal; HF = heart failure.
Cardio-Oncologist's Individual Risk Assessment
One important item needs to be addressed clinically: only a subgroup of patients who are treated with certain chemotherapy, targeted therapy, or chemoprevention regimen will develop cardiovascular complications, the risk being individual. An accurate family and anamnesis (personal history) will lead to the identification of the patients at risk. Susceptibility to the development of cardiotoxicity of any kind and severity with anticancer therapy is a multifactorial characteristic that is determined by the interaction between genetic and environmental factors. The genetic component is polygenic and different genes cooperate in an epistatic manner. The modifier effect of epigenetic factors also contributes to the genetic susceptibility to cardiotoxicity. Genetic influences have already been examined in patients taking anthracyclines (56–58). Moreover, familial risk of coronary artery disease or CHF has been associated with an additional risk of developing these conditions in the treated population (59). Age, sex, and other personal characteristics or events related to personal history, including previous or concurrent therapies, can be included.
Cardiotoxicity of cancer chemotherapeutics is a problem for patients of all ages, but it increases with age. Toxicity can also develop months after the last chemotherapy dose, and late reactions can be seen years later when they present as new-onset cardiomyopathy, often in patients who were treated for childhood neoplasms (60).
Also, men and women have differences in risk of cardiotoxicity (61). Premenopausal women are less likely than men of same age to develop atherosclerosis. However, after menopause, the levels of protective hormones drop and therefore the rate of atherosclerosis in women rapidly increases (62). Thus, the sex of the patient is important to take into account in cardio-oncology.
Early identification of patients who are at risk for cardiotoxicity should be a primary goal for oncologists in the development of personalized antineoplastic therapeutic strategies or interventions. The improvement of diagnostic tools in both cardiology and oncology has led to an increased number of patients who have been treated for cancer and diagnosed with cardiovascular disease. It may sometimes be necessary for oncology patients to undergo cardiothoracic surgery to survive heart problems, when their risk from cardiovascular disease outweighs their risk from cancer. Therefore, risk vs benefit or risk vs risk evaluations should be performed by a cardio-oncology team.
Recently published clinical cancer studies that screened participants on entry for cardiovascular conditions have revealed a high prevalence of subjects at cardiovascular risk (3,63). Schmidinger et al. (3,63) reported an observational single-center study of patients scheduled for TKI treatment with sorafenib and sunitinib who were analyzed for risk of coronary artery disease, cardiovascular history and evidence of coronary artery disease, hypertension, rhythm disturbances, or HF. The study reported a history of cardiovascular disease in 9.3% of the enrolled patients, as well as myocardial infarction (5.8%), HF (7%), rhythm disturbances (3.5%), and uncontrolled hypertension (3.5%). Many enrolled patients were at high cardiovascular risk because of hypertension (48.8%), hypercholesterolemia (26.7%), diabetes mellitus (22%), or hypertriglyceridemia (12.8%). These observations indicate that preexisting cardiac disease is often underestimated, but also that it is manageable if the patients have careful cardiovascular monitoring and cardiac treatment at the first signs of myocardial damage (3,63).
Use of on-entry screening appears to be particularly important during chemoprevention of cancer, as highlighted by studies on nonsteroidal anti-inflammatory drugs (NSAIDs) specific for cyclooxygenase 2. Although the use of aspirin is associated with a reduction in cardiovascular risk, at least in men, treatment with some COX-2 specific NSAIDs has led to increases in adverse cardiovascular effects. In the chemoprevention trial using the NSAID sulindac, 28.5% of enrolled patients were at low cardiovascular risk, 31.5% at moderate risk, and 40% at high risk (63). In this cohort, cardiovascular events were higher in the treated group (nine of 191) than in the placebo group (three of 184). However, when patients with a high baseline cardiovascular risk were excluded, the risk of cardiovascular complications became similar in the treated and placebo groups (63). This finding that patients at high cardiovascular risk were more adversely affected by these drugs is in line with a recent meta-analysis that suggests that baseline cardiovascular risk is associated with adverse cardiovascular events linked to NSAID use (64). It suggests that on-entry screening can indeed identify individuals at elevated risk of cardiotoxicity, who might then be treated either with a different, less cardiotoxic, agent or with the same agent along with cardioprotective agents.
The clinical trials that showed that celecoxib could prevent colorectal adenomas recorded many deaths from adverse cardiovascular events (hazard ratio [HR] of death = 2.3 at 200 mg of celecoxib twice daily and HR of death = 3.4 at 400 mg of celecoxib twice daily) (65–68). In these trials, patients who experienced cardiovascular problems were likely to have already had undetected high-risk conditions when they enrolled in the trial. A detailed cardiovascular examination at recruitment would have probably identified these patients and permitted the discovery of high-risk groups in which risk outweighs benefit, as well as low risk groups in which the cancer chemoprevention benefit predominates. We initially suggested that cardioprevention approaches could have been used with this class of drugs (37); here we extend the concept of assessing cardiovascular risk factor to screening in prevention trials. Another recently reported example of the importance of cardiovascular screening for cancer patients came from a trial of neoadjuvant hormonal therapy in men with prostate cancer, in which increased risk of mortality was found only in patients with multiple cardiovascular risk factors or a history of cardiovascular disease (69).
Prediagnostic Tools: Instrumental and Medicinal Chemistry
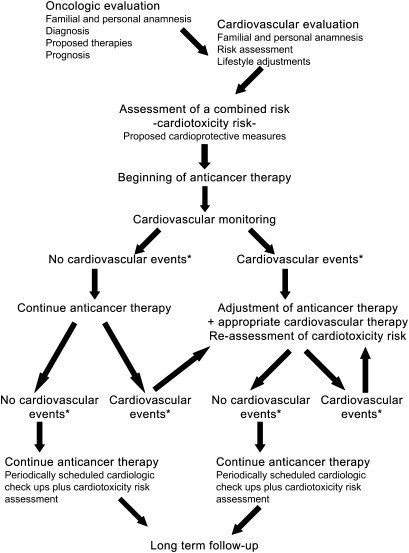
Taken together, these data suggest that a baseline cardiovascular examination along with careful cardiovascular management could not only prevent adverse events from antineoplastic drugs but could also be used to exclude their use in potentially high-risk individuals and thereby enable assessment of the effectiveness of these therapeutic or prevention agents in low- and moderate-risk individuals. In the past, oncologists were not nearly so aware of cardiovascular complications, which have become more evident with longer survival after cancer diagnosis, the use of targeted therapies, and combinations of chemotherapies and targeted therapies, as well as experience in chemoprevention trials. It is important to highlight that the choice of the therapeutic approach and the survival prognosis depend on the fine balance between cancer treatment and cardiovascular therapy. For this reason, although monitoring of cardiac functions is time-consuming and expensive, it is highly recommended before, during, and after chemotherapy to detect subclinical cardiac damage (Figure 2, Table 2). Accurate cardiovascular monitoring at regular intervals during chemotherapy is particularly important with prolonged adjuvant therapy. It would help to develop a series of diagnostic tools—instrumental, serological, or molecular—for early detection of cardiotoxicity and initiation of appropriate therapy to restore cardiac function and hemodynamics. It would also help to develop accurate guidelines for diagnosis and therapies to control cardiotoxic side effects, such as β-blockers and angiotensin-converting enzyme inhibitors (5).
Figure 2.
A possible cardio-oncology team flowchart. In a cardio-oncology team effort, the oncologist and cardiologist should work together, evaluating the patient's cardiovascular risk level as an integral part of the choice of cancer therapy. In addition, the patient is monitored throughout therapy and follow-up so that eventual cardiovascular alterations can be detected in a timely manner and treated either by intervention on the cardiovascular side or by modulation of the cancer therapy. Future trial protocols could include a series of candidate marker measurements, both instrumental and serological, in the initial evaluation stage as well as during the course of therapy to identify those most informative of risk potential. HF = heart failure. *Substantial changes in cardiovascular risk assessment; for example, a reduction in left ventricular ejection fraction (LVEF) from baseline greater than 5% to less than 55% with accompanying signs or symptoms of HF or a reduction in LVEF greater than 10% to less than 55%, without accompanying signs or symptoms.
Table 2.
Cardiovascular monitoring of cancer patients*
| Approach | Before antineoplastic therapy† | During antineoplastic therapy‡ and follow-up |
| Clinical assessment | Familial and personal anamnesis; physical examination; diagnosis; risk assessment (5); (www.acc.org/qualityandscience/clinical/statements.htm) | Physical examination; cancer therapy evaluation (ctep.info.nih.gov); risk reassessment (5); (www.acc.org/qualityandscience/clinical/statements.htm) |
| Tests | Blood pressure assessment; chest radiography; LVEF evaluation by any of these means: ECG, dynamic ECG, Eco-Doppler, MUGA scanning (5,11) | Blood pressure assessment; chest radiography; LVEF follow-up by any of these means: ECG, dynamic ECG, Eco-Doppler, MUGA scanning (5,11) |
| Serum markers | Troponin isoforms; B-type natriuretic peptide; myeloperoxidase (5,70–72) | Troponin isoforms; B-type natriuretic peptide myeloperoxidase (5,70–72) |
| Prevention-Treatment | Lifestyle adjustments; cardioprotection; ACE inhibitors; angiotensin II receptor blockers; β-blockers; prevention of thromboembolism with aspirin or anticoagulants or platelet antiaggregants (5,63); (www.acc.org/qualityandscience/clinical/statements.htm) | ACE inhibitors; angiotensin II receptor blockers; β-blockers; cardiologic therapeutic regimen titration; other appropriate therapies (ie, anticoagulant therapies); change of antineoplastic therapeutic regimen (drug, schedule, or suspension) (5,47); (www.acc.org/qualityandscience/clinical/statements.htm) |
ACE = angiotensin-converting enzyme; ECG = electrocardiogram; LVEF = left ventricular ejection fraction; MUGA = multiple gated acquisition.
In our approach, we propose to perform a preliminary evaluation 10 days before beginning of the antineoplastic therapy. Different schedules can be followed (3,73).
In the proposed protocol, we suggest a cardiovascular evaluation at 2 and 4 weeks after the beginning of the antineoplastic therapy, followed by physical and instrumental evaluation every 6 weeks throughout the course of the treatment, different schedules can be followed (3,73). In our approach, physical and instrumental evaluation could be set after 3, 6, 12, 18, and 24 months after ending antineoplastic therapy. Schedule may change depending on the clinician's judgment, different schedules can be followed (3,73).
One key and simple approach to monitor the effects of chemotherapy is arterial pressure measurement to identify hypertension. Hypertension is frequently seen in patients who are treated with several antiangiogenic agents (such as bevacizumab, sorafenib, and sunitinib) and can be severe (74). Hypertension in the cancer patient under therapy needs to be promptly and adequately treated (5,54).
The most frequently used and effective approach to monitor cardiac function and its impairment by chemotherapy is evaluation of LVEF, which is commonly measured by echocardiography, a noninvasive technique. LVEF is one of the most important predictors of prognosis; patients with substantially reduced ejection fractions typically have poorer prognosis. There is no clear international opinion on the frequency and method of LVEF assessment. Both echocardiography and Multiple Gated Acquisition scanning can be used to assess LVEF and both techniques have advantages and disadvantages to be evaluated (11). However, echocardiographic LVEF assessment and angiography with radionuclides have shown low diagnostic sensitivity and low predictive power in detecting subclinical myocardial injury. The presence of HF heralds a much more serious prognosis than a change in LVEF, and HF can occur independently of a finding of a normal LVEF (54). Other techniques, such as endomyocardial biopsy, are troublesome in clinical practice because they are invasive procedures. Thus, the quest for noninvasive and cost-effective diagnostic tools for the early identification of patients who are susceptible to developing drug-induced cardiotoxicity needs to be increasingly pursued (75).
The use of other indicators such as lipid profile and serum markers for monitoring cardiotoxicity is being investigated. Biomarkers may provide important information regarding the pathogenesis of HF or the identification of subjects at risk or may be useful in risk stratification, in the diagnosis of HF, or in monitoring therapy (76). Serum markers that detect damage of the myofibrils and of the cardiomyocytes, such as the different isoforms of troponin, are useful in detecting the acute cardiotoxicity that is mediated by therapeutic agents that induce cell death (70). However, these markers do not appear to be valuable for detecting cardiotoxicity induced by nonclassical therapeutic agents or for detecting cardiotoxicity at early stages (8). Cardiac natriuretic peptides, which are released during hemodynamic stress when the ventricles are dilated, hypertrophic, or subjected to increased wall tension, have been used as biomarkers, but definitive evidence is still lacking with regard to a diagnostic or prognostic role in predicting chemotherapy-induced cardiomyopathy (71). It is clear that the diagnostic and prognostic value of other biomarkers used to monitor cardiovascular damage, such as myeloperoxidase (72), should also be validated for clinical use in cardio-oncology. Genomics, proteomics, and/or recently identified oligoclonal B-cell repertoires (77) may provide us with genomic profiles and serological biomarkers for assessment of cardiotoxicity in the foreseeable future.
From Risk Identification to Monitoring and Rescue by the Cardio-Oncologist
As stated throughout this review, cardiologists and oncologists must work together to improve disease prognosis and patient overall survival. To date, no guidelines have been developed specifically for the definition, detection, or therapy of cardiotoxicity from antineoplastic therapy, so it is imperative that these guidelines be defined. Meanwhile, cancer patients with cardiovascular diseases should be treated based on the guidelines published by the American College of Cardiology and American Heart Association (www.acc.org/qualityandscience/clinical/statements.htm). We propose a possible flowchart of an integrated approach (Figure 2, Table 2). Prevention of cardiotoxicity begins before the initiation of cancer therapy, with the oncologist and the cardiologist working as a team, the former performing a complete history and objective evaluation of the patient regarding cancer therapy or prevention and the latter evaluating cardiovascular parameters and function. Blood hematochemical parameters will be measured together with blood pressure, electrocardiogram, dynamic electrocardiogram, Eco-Doppler to assess volume and thickness of heart chambers, LVEF, ST interval evaluation, assessment of rhythm disturbances, and presence of valvular dysfunction. Patients with LVEF less than 50%, HF, severe or instable angina, aortic-coronary bypass, previous stroke or transient ischemic attacks, thromboembolic events, hypertension poorly responding to treatment, or severe arrhythmias should receive particular attention during the course of anticancer therapy because their risk of developing adverse events due to cardiotoxicity is elevated (Figure 2, Table 2). This assessment of the cardiovascular profile by the cardiologist should then be taken into consideration by the oncologist in deciding the therapeutic approach, in terms of drug selection and schedule, for each individual patient.
The dose of the anticancer drug administered during each session, cumulative dose, schedule of delivery, route of administration, combination of drugs given, and sequence of drug administration are important factors to be considered to avoid cardiotoxicity (12). Drug formulation is also important. For example, paclitaxel is formulated in a Cremophor EL vehicle to enhance the drug solubility, and it has been suggested that the vehicle and not the cytotoxic drug itself is responsible for cardiac rhythm disturbances, possibly via histamine release causing conduction disturbances and arrhythmias, as shown in animal models, or alternatively by affecting subcellular organelles and inducing cardiac muscle damage (31). However, cardiac rhythm disturbances are not reported with use of other drugs containing Cremophor EL, such as cyclosporine (31). Changes of drug administration and dosage have been also suggested to prevent cardiotoxicity. One approach for trastuzumab therapy is to schedule short-course treatment concurrently with nonanthracycline-based chemotherapy followed by anthracyclines in a block-sequential design (78). Others use nonanthracycline-based regimens or targeted anthracyclines [reviewed in (11)].
Because cardiovascular function changes in response to stress and other external influences on the individual patient, we feel that a cardiovascular follow-up of the treated patients should be periodically scheduled; for example, 2 weeks after the beginning of the cancer therapy, followed by another after 4 weeks, then regular examinations every 6 weeks for the duration of therapy, and later during follow-up. The follow-up ideally would include a complete reevaluation of the cardiovascular parameters as in the baseline exam and a reassessment of the anticancer regimen by the oncologist.
If a cardiovascular event occurs during therapy, drug withdrawal, with or without additional therapy directed at the HF, such as angiotensin-converting enzyme inhibitors and/or angiotensin II receptor antagonists and/or β-blockers, should be considered. This type of approach has been found effective in treating drug-induced cardiotoxicity in most patients undergoing therapy with anthracyclines, but not those in therapy with targeted agents (5,79,80). There are reports that antihypertensive, antiarrhythmic, and cardioprotective drugs defend against drug-induced cardiotoxicity with a dual mechanism, protecting cardiovascular function while inhibiting tumor angiogenesis (81–87). Careful clinical approaches will allow for eventual adjustment of the follow-up approach, in particular with regard to baseline patient risk and the specific therapeutic agent(s) and schedule used.
Long-term surveillance in cancer survivors is also indicated. The survivors of childhood cancer are more likely than survivors of adult cancer to experience cardiac damage and to require management (88). Moreover, a Swedish retrospective study on long-term risk of cardiovascular disease in Hodgkin lymphoma survivors (54) showed that Hodgkin lymphoma survivors had an increased risk for being admitted to the hospital for cardiovascular diseases occurring after more than 20 years since their diagnosis with lymphoma, as compared with the general Swedish population.
It is clear that the oncology community needs to work toward developing guidelines for early assessment of cardiac risk as well as identification and treatment of cardiotoxicity in the oncologic patient. Clinical studies are needed to define these approaches and their application.
Future Perspectives: Chemoprevention of Cardiotoxicity Induced by Cancer Treatments
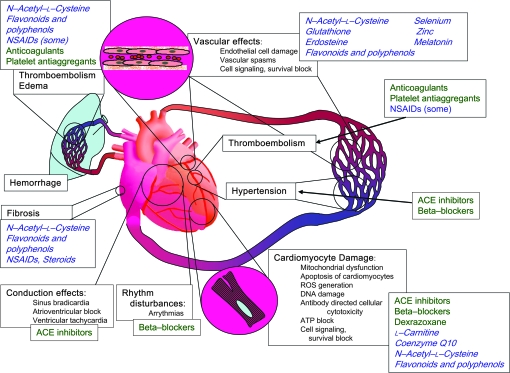
Moving toward a protective chemoprevention approach, several drugs may be useful to flank chemotherapy to reduce cardiotoxicity without losing antitumor activity, and possibly even to enhance antitumor activity (Figure 3). Most of these approaches are still experimental; however, some could easily be considered for clinical trials. Dexrazoxane, a cyclic derivative of EDTA that chelates iron and thereby prevents the generation of cardiotoxic reactive oxygen species, is a drug used to protect the myocardium from anthracycline-induced cardiotoxicity (89). At high concentrations, dexrazoxane also inhibits topoisomerase II, which may also be involved in its cardioprotective activities (89). Data from nine clinical studies that had enrolled a total of 1403 patients who received anthracycline-based therapy for advanced breast cancer showed an overall benefit from dexrazoxane in protecting cancer patients from anthracycline-induced HF (90). However, this drug may increase the risk of adverse effects, such as a slightly higher incidence of myelosuppression, infection, and fever. An early dropout rate was also higher in patients who received dexrazoxane (91).
Figure 3.
Examples of major mechanisms causing cardiotoxicity of anticancer treatments (black text), clinically used therapeutic agents (green text), and potential protective agents (blue cursive text). ROS = reactive oxygen species; ACE = angiotensin-converting enzyme, NSAIDs = nonsteroidal anti-inflammatory drugs.
Preexisting medical conditions and therapies modify the risk of cardiotoxicity in cancer patients. Lipid-lowering agents have been indicated as protective agents against anthracycline-mediated cardiotoxicity (92), and, in particular, statins seem to have a chemopreventive and direct antitumor effect (93,94). Whether statins may have protective or harmful effects on cancer risk is still a matter of debate, but the most recent reviews of the literature suggest that these drugs do not have short-term negative consequences on cancer risk (95); moreover, they can have an antithrombotic effect (96) that could lower the risk of thrombosis induced by anticancer treatment.
Antioxidants such as the glutathione precursor and analogue N-acetyl-L-cysteine (NAC) can be given to patients at relatively high levels and have an excellent toxicity profile. NAC showed protective (97) and synergistic antitumor and antimetastatic (98,99) effects in murine models, but it had no effect in reversing long-standing doxorubicin-induced cardiomyopathy in a trial that evaluated 19 sarcoma patients (100). Numerous antioxidants and free radical scavengers found in dietary components such as polyphenols and flavonoids, vitamins, micronutrients, enzymes, and hormones, such as selenium, zinc, coenzyme Q10, and melatonin, also show promise. Several plant derivatives, such as extracts from Gingko biloba (101) and grape seed (102) as well as polyphenols that were previously investigated for their antiangiogenic, anti-inflammatory, and anticancer activities (Epigallocatechin-3-gallate from green tea, resveratrol from red wine, and curcumin from curry) (103–108), have been found to have cardioprotective activity in experimental studies. Preclinical and clinical studies suggested that the anthracycline-induced cardiotoxicity could be prevented by administering coenzyme Q10 (109). L-Carnitine, a quaternary ammonium compound synthesized from methionine and lysine that is required for oxidative metabolism in mitochondria, has been proposed as a supplement to reduce the cardiotoxicity of epirubicin (110). However, in spite of theoretical expectations and encouraging results obtained in experimental models, all randomized controlled trials in which potential cardioprotective agents (coenzyme Q10, L-carnitine, carvedilol, NAC, and combinations of vitamins E and C with NAC) (90) were tested in cancer patients receiving anthracyclines had methodological limitations. Furthermore, the limited data from clinical trials on the use of vitamin E did not lead to firm conclusions (90).
Because damage to the endothelium appears to be the underlying mechanism for the cardiotoxicity of a variety of chemotherapeutic drugs, agents that prevent endothelial cell apoptosis should provide protective effects. Investigation of some antiangiogenic compounds, in particular those that target the nuclear factor κB pathway, have also been found to render endothelial cells more resistant to apoptosis induced by external stimuli. These include NAC (111,112) and deguelin (113). However, xanthohumol (114) and triterpenoids (115) did not perturb endothelial cell viability in vitro. The concept is that these agents force the endothelial cell into a quiescent state that leads to increased resistance to apoptosis (116–118).
Because the pathogenesis of both cardiovascular disease and cancer is frequently linked to inflammation, it follows that prevention of inflammation should protect from both diseases. NSAIDs such as aspirin are known to provide cardioprotective benefit particularly in men; aspirin use has also been found to reduce risk for colorectal cancer (119), breast cancer (120,121), and other cancers (122). Development of cardioprotection during and after cancer therapy is another area that needs to be focused on to translate laboratory discoveries into clinical trials and to develop consensus protocols.
Conclusions
Cardiotoxicity is becoming one of the most important complications of cancer chemotherapy and, sometimes, of cancer chemoprevention. Identification of those patients at higher risk will be one key strategy to reduce the morbidity and mortality from cardiotoxicity. However, current screening methods are cumbersome and lack sufficient predictive power. Thus, the discovery of new biomarkers to identify patients at a high risk for the development of these complications is a high priority. Guidelines for cancer treatment that take cardiologic conditions into account are currently lacking and need to be developed. Furthermore, because many major chemotherapeutic agents used in the treatment of cancer are associated with strong adverse effects on the cardiovascular system, a key goal will be identification of new compounds such as antioxidants and endothelial- or cardiomyocyte-protective agents that can prevent cardiotoxicity. Last but not least, assessment of cardiotoxicity should become part of phase I trials to develop agents with less risk.
It is important to consider that the approach of cardiologists to the definition of disease is quite different from that of the oncologists. Therefore, to perform interdisciplinary medicine, there is a risk that many concepts and observations may well be “lost in translation” (123). We feel that there is a need to train a generation of “cardio-oncologist” or “onco-cardiologist” investigators and clinicians to overcome these communication gaps. Finally, we need to take into consideration risk–benefit (or in this case, risk–risk) profiles. It is clear that for cancers with very poor prognosis, even with very aggressive therapy, cardiovascular risks fall to a lower priority. In cancers with high probability of long-term survival, such as breast and prostate cancers, it is very important to consider cardiovascular risks. For the vast majority of cancers that lie in-between, cardio-oncology needs to be brought increasingly into practice.
Funding
The AIRC (Associazione Italiana per la Ricerca sul Cancro) (to A.A. and D.M.N.), the Ministero della Salute (to A.A. and D.M.N.), the Compagnia di San Paolo (to A.A.), the Ministero dell’Università e della Ricerca (to D.M.N.), and the Università degli Studi dell’Insubria (to D.M.N.)
Footnotes
S. D. Flora and D. M. Noonan contributed equally to this work.
R. Cammarota is a PhD student at the Department of Molecular Medicine, University of Milan, Via Fratelli Cervi, Segrate.
The sponsors had no role in the preparation of the manuscript or the decision to submit the manuscript for publication.
We thank Paola Corradino and Luca Generoso for bibliographical searches. We are grateful to Ornella Gottardi and the Oncology Department of MultiMedica for helpful discussion and Alessandra Panvini Rosati (MultiMedica) for secretarial assistance.
References
- 1.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7(2):139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 2.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7(5):332–344. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 3.Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26(32):5204–5212. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 4.Sereno M, Brunello A, Chiappori A, et al. Cardiac toxicity: old and new issues in anti-cancer drugs. Clin Transl Oncol. 2008;10(1):35–46. doi: 10.1007/s12094-008-0150-8. [DOI] [PubMed] [Google Scholar]
- 5.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 6.Cardinale D. A new frontier: cardio-oncology. Cardiologia. 1996;41(9):887–891. [PubMed] [Google Scholar]
- 7.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20(5):1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 8.Dolci A, Dominici R, Cardinale D, Sandri MT, Panteghini M. Biochemical markers for prediction of chemotherapy-induced cardiotoxicity: systematic review of the literature and recommendations for use. Am J Clin Pathol. 2008;130(5):688–695. doi: 10.1309/AJCPB66LRIIVMQDR. [DOI] [PubMed] [Google Scholar]
- 9.Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22(4):263–302. doi: 10.2165/00002018-200022040-00002. [DOI] [PubMed] [Google Scholar]
- 10.Elice F, Jacoub J, Rickles FR, Falanga A, Rodeghiero F. Hemostatic complications of angiogenesis inhibitors in cancer patients. Am J Hematol. 2008;83(11):862–870. doi: 10.1002/ajh.21277. [DOI] [PubMed] [Google Scholar]
- 11.Popat S, Smith IE. Therapy insight: anthracyclines and trastuzumab—the optimal management of cardiotoxic side effects. Nat Clin Pract Oncol. 2008;5(6):324–335. doi: 10.1038/ncponc1090. [DOI] [PubMed] [Google Scholar]
- 12.Yeh ET, Tong AT, Lenihan DJ, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109(25):3122–3131. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- 13.Zver S, Zadnik V, Bunc M, Rogel P, Cernelc P, Kozelj M. Cardiac toxicity of high-dose cyclophosphamide in patients with multiple myeloma undergoing autologous hematopoietic stem cell transplantation. Int J Hematol. 2007;85(5):408–414. doi: 10.1532/IJH97.E0620. [DOI] [PubMed] [Google Scholar]
- 14.Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol. 1996;27(8):766–773. doi: 10.1016/s0046-8177(96)90447-5. [DOI] [PubMed] [Google Scholar]
- 15.Brosius FC, III, Waller BF, Roberts WC. Radiation heart disease. Analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am J Med. 1981;70(3):519–530. doi: 10.1016/0002-9343(81)90574-x. [DOI] [PubMed] [Google Scholar]
- 16.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293(5528):293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 17.Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370(9604):2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khakoo AY, Kassiotis CM, Tannir N, et al. Heart failure associated with sunitinib malate: a multitargeted receptor tyrosine kinase inhibitor. Cancer. 2008;112(11):2500–2508. doi: 10.1002/cncr.23460. [DOI] [PubMed] [Google Scholar]
- 19.Telli ML, Witteles RM, Fisher GA, Srinivas S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol. 2008;19(9):1613–1618. doi: 10.1093/annonc/mdn168. [DOI] [PubMed] [Google Scholar]
- 20.Mouridsen H, Keshaviah A, Coates AS, et al. Cardiovascular adverse events during adjuvant endocrine therapy for early breast cancer using letrozole or tamoxifen: safety analysis of BIG 1-98 trial. J Clin Oncol. 2007;25(36):5715–5722. doi: 10.1200/JCO.2007.12.1665. [DOI] [PubMed] [Google Scholar]
- 21.Filippatos TD, Liberopoulos EN, Pavlidis N, Elisaf MS, Mikhailidis DP. Effects of hormonal treatment on lipids in patients with cancer. Cancer Treat Rev. 2009;35(2):175–184. doi: 10.1016/j.ctrv.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Kang YJ. Molecular and cellular mechanisms of cardiotoxicity. Environ Health Perspect. 2001;109(suppl 1):27–34. doi: 10.1289/ehp.01109s127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Billingham ME, Mason JW, Bristow MR, Daniels JR. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat Rep. 1978;62(6):865–872. [PubMed] [Google Scholar]
- 24.Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105(13):1551–1554. doi: 10.1161/01.cir.0000013839.41224.1c. [DOI] [PubMed] [Google Scholar]
- 25.Elliott P. Pathogenesis of cardiotoxicity induced by anthracyclines. Semin Oncol. 2006;33(3)(suppl 8):S2–S7. doi: 10.1053/j.seminoncol.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, Sawyer DB. Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol. 2008;26(22):3777–3784. doi: 10.1200/JCO.2007.14.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56(2):185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 28.Crone SA, Zhao YY, Fan L, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8(5):459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 29.Feldman AM, Lorell BH, Reis SE. Trastuzumab in the treatment of metastatic breast cancer: anticancer therapy versus cardiotoxicity. Circulation. 2000;102(3):272–274. doi: 10.1161/01.cir.102.3.272. [DOI] [PubMed] [Google Scholar]
- 30.Perez EA, Koehler M, Byrne J, Preston AJ, Rappold E, Ewer MS. Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc. 2008;83(6):679–686. doi: 10.4065/83.6.679. [DOI] [PubMed] [Google Scholar]
- 31.Schimmel KJ, Richel DJ, van den Brink RB, Guchelaar HJ. Cardiotoxicity of cytotoxic drugs. Cancer Treat Rev. 2004;30(2):181–191. doi: 10.1016/j.ctrv.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Alter P, Herzum M, Soufi M, Schaefer JR, Maisch B. Cardiotoxicity of 5-fluorouracil. Cardiovasc Hematol Agents Med Chem. 2006;4(1):1–5. doi: 10.2174/187152506775268785. [DOI] [PubMed] [Google Scholar]
- 33.Kerkela R, Grazette L, Yacobi R, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12(8):908–916. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro AL, Marcolino MS, Bittencourt HN, et al. An evaluation of the cardiotoxicity of imatinib mesylate. Leuk Res. 2008;32(12):1809–1814. doi: 10.1016/j.leukres.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Gambacorti-Passerini C, Tornaghi L, Franceschino A, Piazza R, Corneo G, Pogliani E. In reply to ‘Cardiotoxicity of the cancer therapeutic agent imatinib mesylate’. Nat Med. 2007;13(1):13–14. doi: 10.1038/nm0107-13b. author reply 15–16. [DOI] [PubMed] [Google Scholar]
- 36.van Heeckeren WJ, Sanborn SL, Narayan A, et al. Complications from vascular disrupting agents and angiogenesis inhibitors: aberrant control of hemostasis and thrombosis. Curr Opin Hematol. 2007;14(5):468–480. doi: 10.1097/MOH.0b013e3282a6457f. [DOI] [PubMed] [Google Scholar]
- 37.Albini A, Noonan DM. Rescuing COX-2 inhibitors from the waste bin. J Natl Cancer Inst. 2005;97(11):859–860. doi: 10.1093/jnci/dji149. [DOI] [PubMed] [Google Scholar]
- 38.Rodeghiero F, Elice F. Thalidomide and thrombosis. Pathophysiol Haemost Thromb. 2003;33(suppl 1):15–18. doi: 10.1159/000073282. [DOI] [PubMed] [Google Scholar]
- 39.Zangari M, Elice F, Fink L, Tricot G. Thrombosis in multiple myeloma. Expert Rev Anticancer Ther. 2007;7(3):307–315. doi: 10.1586/14737140.7.3.307. [DOI] [PubMed] [Google Scholar]
- 40.Czaykowski PM, Moore MJ, Tannock IF. High risk of vascular events in patients with urothelial transitional cell carcinoma treated with cisplatin based chemotherapy. J Urol. 1998;160(6, pt 1):2021–2024. doi: 10.1097/00005392-199812010-00022. [DOI] [PubMed] [Google Scholar]
- 41.Fainaru O, Adini I, Benny O, et al. Doxycycline induces membrane expression of VE-cadherin on endothelial cells and prevents vascular hyperpermeability. FASEB J. 2008;22(10):3728–3735. doi: 10.1096/fj.08-110494. [DOI] [PubMed] [Google Scholar]
- 42.Matsumura T, Wolff K, Petzelbauer P. Endothelial cell tube formation depends on cadherin 5 and CD31 interactions with filamentous actin. J Immunol. 1997;158(7):3408–3416. [PubMed] [Google Scholar]
- 43.Lu L, Payvandi F, Wu L, et al. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc Res. 2009;77(2):78–86. doi: 10.1016/j.mvr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Jain M, Townsend RR. Chemotherapy agents and hypertension: a focus on angiogenesis blockade. Curr Hypertens Rep. 2007;9(4):320–328. doi: 10.1007/s11906-007-0058-7. [DOI] [PubMed] [Google Scholar]
- 45.Watson G, Kugel M, Shih H, Tak Piech C, McKenzie R. Cardiac comorbidities in women with metastatic breast cancer treated with doxorubicin-based and non-doxorubicin-based chemotherapy. J Clin Oncol. 2009;27(15S):1052. [Google Scholar]
- 46.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96(12):1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dincer M, Altundag K. Angiotensin-converting enzyme inhibitors for bevacizumab-induced hypertension. Ann Pharmacother. 2006;40(12):2278–2279. doi: 10.1345/aph.1H244. [DOI] [PubMed] [Google Scholar]
- 48.Lainscak M, Dagres N, Filippatos GS, Anker SD, Kremastinos DT. Atrial fibrillation in chronic non-cardiac disease: where do we stand? Int J Cardiol. 2008;128(3):311–315. doi: 10.1016/j.ijcard.2007.12.078. [DOI] [PubMed] [Google Scholar]
- 49.van der Hooft CS, Heeringa J, van Herpen G, Kors JA, Kingma JH, Stricker BH. Drug-induced atrial fibrillation. J Am Coll Cardiol. 2004;44(11):2117–2124. doi: 10.1016/j.jacc.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 50.van der Hooft CS, Heeringa J, Brusselle GG, et al. Corticosteroids and the risk of atrial fibrillation. Arch Intern Med. 2006;166(9):1016–1020. doi: 10.1001/archinte.166.9.1016. [DOI] [PubMed] [Google Scholar]
- 51.Guzzetti S, Costantino G, Fundaro C. Systemic inflammation, atrial fibrillation, and cancer. Circulation. 2002;106(9) doi: 10.1161/01.cir.0000028399.42411.13. e40; author reply e40. [DOI] [PubMed] [Google Scholar]
- 52.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104(24):2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 53.van Heeckeren WJ, Bhakta S, Ortiz J, et al. Promise of new vascular-disrupting agents balanced with cardiac toxicity: is it time for oncologists to get to know their cardiologists? J Clin Oncol. 2006;24(10):1485–1488. doi: 10.1200/JCO.2005.04.8801. [DOI] [PubMed] [Google Scholar]
- 54.Lenihan DJ. Tyrosine kinase inhibitors: can promising new therapy associated with cardiac toxicity strengthen the concept of teamwork? J Clin Oncol. 2008;26(32):5154–5155. doi: 10.1200/JCO.2008.18.5439. [DOI] [PubMed] [Google Scholar]
- 55.Lenihan DJ, Esteva FJ. Multidisciplinary strategy for managing cardiovascular risks when treating patients with early breast cancer. Oncologist. 2008;13(12):1224–1234. doi: 10.1634/theoncologist.2008-0112. [DOI] [PubMed] [Google Scholar]
- 56.Deng S, Wojnowski L. Genotyping the risk of anthracycline-induced cardiotoxicity. Cardiovasc Toxicol. 2007;7(2):129–134. doi: 10.1007/s12012-007-0024-2. [DOI] [PubMed] [Google Scholar]
- 57.Duan S, Bleibel WK, Huang RS, et al. Mapping genes that contribute to daunorubicin-induced cytotoxicity. Cancer Res. 2007;67(11):5425–5433. doi: 10.1158/0008-5472.CAN-06-4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang RS, Duan S, Kistner EO, et al. Genetic variants contributing to daunorubicin-induced cytotoxicity. Cancer Res. 2008;68(9):3161–3168. doi: 10.1158/0008-5472.CAN-07-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andersson A, Naslund U, Tavelin B, Enblad G, Gustavsson A, Malmer B. Long-term risk of cardiovascular disease in Hodgkin lymphoma survivors—retrospective cohort analyses and a concept for prospective intervention. Int J Cancer. 2009;124(8):1914–1917. doi: 10.1002/ijc.24147. [DOI] [PubMed] [Google Scholar]
- 60.Broder H, Gottlieb R, Lepor N. Chemotherapy and cardiotoxicity. Rev Cardiovasc Med. 2008;9(2):75–83. [PMC free article] [PubMed] [Google Scholar]
- 61.Allender S, Scarborough P, Peto V, et al. Brussels, Belgium: European Heart Network; 2008. European cardiovascular disease statistics. www.ehnheart.org. Accessed July 14, 2009. [Google Scholar]
- 62.Mendelsohn M, Karas R. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308(5728):1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 63.Zell JA, Pelot D, Chen WP, McLaren CE, Gerner EW, Meyskens FL. Risk of cardiovascular events in a randomized placebo-controlled, double-blind trial of difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas. Cancer Prev Res (Phila Pa) 2009;2(3):209–212. doi: 10.1158/1940-6207.CAPR-08-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355(9):885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 66.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 67.Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352(11):1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 68.Solomon SD, Pfeffer MA, McMurray JJ, et al. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation. 2006;114(10):1028–1035. doi: 10.1161/CIRCULATIONAHA.106.636746. [DOI] [PubMed] [Google Scholar]
- 69.Nanda A, Chen MH, Braccioforte MH, Moran BJ, D’Amico AV. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA. 2009;302(8):866–873. doi: 10.1001/jama.2009.1137. [DOI] [PubMed] [Google Scholar]
- 70.Cardinale D, Sandri M, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109(22):2749–2754. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 71.Sandri MT, Salvatici M, Cardinale D, et al. N-terminal pro-B-type natriuretic peptide after high-dose chemotherapy: a marker predictive of cardiac dysfunction? Clin Chem. 2005;51(8):1405–1410. doi: 10.1373/clinchem.2005.050153. [DOI] [PubMed] [Google Scholar]
- 72.Penn M. The role of leukocyte-generated oxidants in left ventricular remodeling. Am J Cardiol. 2008;101(10A):30D–33D. doi: 10.1016/j.amjcard.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 73.Jones A, Barlow M, Barrett-Lee P, et al. Management of cardiac health in trastuzumab-treated patients with breast cancer: updated United Kingdom National Cancer Research Institute recommendations for monitoring. Br J Cancer. 2009;100(5):684–692. doi: 10.1038/sj.bjc.6604909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Daher IN, Yeh ET. Vascular complications of selected cancer therapies. Nat Clin Pract Cardiovasc Med. 2008;5(12):797–805. doi: 10.1038/ncpcardio1375. [DOI] [PubMed] [Google Scholar]
- 75.Altena R, Perik P, van Veldhuisen D, de Vries E, Gietema J. Cardiovascular toxicity caused by cancer treatment: strategies for early detection. Lancet Oncol. 2009;10(4):391–399. doi: 10.1016/S1470-2045(09)70042-7. [DOI] [PubMed] [Google Scholar]
- 76.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358(20):2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 77.Burioni R, Canducci F, Saita D, et al. Antigen-driven evolution of B lymphocytes in coronary atherosclerotic plaques. J Immunol. 2009;183(4):2537–2544. doi: 10.4049/jimmunol.0901076. [DOI] [PubMed] [Google Scholar]
- 78.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354(8):809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 79.Ewer MS, Vooletich MT, Durand JB, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23(31):7820–7826. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 80.Telli ML, Hunt SA, Carlson RW, Guardino AE. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol. 2007;25(23):3525–3533. doi: 10.1200/JCO.2007.11.0106. [DOI] [PubMed] [Google Scholar]
- 81.Christian JB, Lapane KL, Hume AL, Eaton CB, Weinstock MA. Association of ACE inhibitors and angiotensin receptor blockers with keratinocyte cancer prevention in the randomized VATTC trial. J Natl Cancer Inst. 2008;100(17):1223–1232. doi: 10.1093/jnci/djn262. [DOI] [PubMed] [Google Scholar]
- 82.Silber JH, Cnaan A, Clark BJ, et al. Design and baseline characteristics for the ACE Inhibitor After Anthracycline (AAA) study of cardiac dysfunction in long-term pediatric cancer survivors. Am Heart J. 2001;142(4):577–585. doi: 10.1067/mhj.2001.118115. [DOI] [PubMed] [Google Scholar]
- 83.Simbre IV, Adams MJ, Deshpande SS, Duffy SA, Miller TL, Lipshultz SE. Cardiomyopathy caused by antineoplastic therapies. Curr Treat Options Cardiovasc Med. 2001;3(6):493–505. doi: 10.1007/s11936-001-0023-8. [DOI] [PubMed] [Google Scholar]
- 84.Bagshaw SM, Galbraith PD, Mitchell LB, Sauve R, Exner DV, Ghali WA. Prophylactic amiodarone for prevention of atrial fibrillation after cardiac surgery: a meta-analysis. Ann Thorac Surg. 2006;82(5):1927–1937. doi: 10.1016/j.athoracsur.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 85.Bangalore S, Messerli FH, Cohen JD, et al. Verapamil-sustained release-based treatment strategy is equivalent to atenolol-based treatment strategy at reducing cardiovascular events in patients with prior myocardial infarction: an INternational VErapamil SR-Trandolapril (INVEST) substudy. Am Heart J. 2008;156(2):241–247. doi: 10.1016/j.ahj.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 86.Carreira RS, Monteiro P, Gon Alves LM, Providencia LA. Carvedilol: just another beta-blocker or a powerful cardioprotector? Cardiovasc Hematol Disord Drug Targets. 2006;6(4):257–266. doi: 10.2174/187152906779010746. [DOI] [PubMed] [Google Scholar]
- 87.Kalay N, Basar E, Ozdogru I, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48(11):2258–2262. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 88.Bryant J, Picot J, Levitt G, Sullivan I, Baxter L, Clegg A. Cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review. Health Technol Assess. 2007;11(27) doi: 10.3310/hta11270. iii, ix–x, 1–84. [DOI] [PubMed] [Google Scholar]
- 89.Hasinoff BB, Herman EH. Dexrazoxane: how it works in cardiac and tumor cells. Is it a prodrug or is it a drug? Cardiovasc Toxicol. 2007;7(2):140–144. doi: 10.1007/s12012-007-0023-3. [DOI] [PubMed] [Google Scholar]
- 90.van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2008;(2) doi: 10.1002/14651858.CD003917.pub3. CD003917. [DOI] [PubMed] [Google Scholar]
- 91.Langer SW. Dexrazoxane for anthracycline extravasation. Expert Rev Anticancer Ther. 2007;7(8):1081–1088. doi: 10.1586/14737140.7.8.1081. [DOI] [PubMed] [Google Scholar]
- 92.Iliskovic N, Singal PK. Lipid lowering: an important factor in preventing adriamycin-induced heart failure. Am J Pathol. 1997;150(2):727–734. [PMC free article] [PubMed] [Google Scholar]
- 93.Cauley JA, McTiernan A, Rodabough RJ, et al. Statin use and breast cancer: prospective results from the Women's Health Initiative. J Natl Cancer Inst. 2006;98(10):700–707. doi: 10.1093/jnci/djj188. [DOI] [PubMed] [Google Scholar]
- 94.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5(12):930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 95.Kuoppala J, Lamminpaa A, Pukkala E. Statins and cancer: a systematic review and meta-analysis. Eur J Cancer. 2008;44(15):2122–2132. doi: 10.1016/j.ejca.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 96.Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360(18):1851–1861. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Doroshow JH, Locker GY, Ifrim I, Myers CE. Prevention of doxorubicin cardiac toxicity in the mouse by N-acetylcysteine. J Clin Invest. 1981;68(4):1053–1064. doi: 10.1172/JCI110328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.D’Agostini F, Bagnasco M, Giunciuglio D, Albini A, De Flora S. Inhibition by oral N-acetylcysteine of doxorubicin-induced clastogenicity and alopecia, and prevention of primary tumors and lung micrometastases in mice. Int J Oncol. 1998;13(2):217–224. doi: 10.3892/ijo.13.2.217. [DOI] [PubMed] [Google Scholar]
- 99.De Flora S, D’Agostini F, Masiello L, Giunciuglio D, Albini A. Synergism between N-acetylcysteine and doxorubicin in the prevention of tumorigenicity and metastasis in murine models. Int J Cancer. 1996;67(6):842–848. doi: 10.1002/(SICI)1097-0215(19960917)67:6<842::AID-IJC14>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 100.Dresdale AR, Barr LH, Bonow RO, et al. Prospective randomized study of the role of N-acetyl cysteine in reversing doxorubicin-induced cardiomyopathy. Am J Clin Oncol. 1982;5(6):657–663. doi: 10.1097/00000421-198212000-00015. [DOI] [PubMed] [Google Scholar]
- 101.Agha AM, El-Fattah AA, Al-Zuhair HH, Al-Rikabi AC. Chemopreventive effect of Ginkgo biloba extract against benzo(a)pyrene-induced forestomach carcinogenesis in mice: amelioration of doxorubicin cardiotoxicity. J Exp Clin Cancer Res. 2001;20(1):39–50. [PubMed] [Google Scholar]
- 102.Ray SD, Patel D, Wong V, Bagchi D. In vivo protection of dna damage associated apoptotic and necrotic cell deaths during acetaminophen-induced nephrotoxicity, amiodarone-induced lung toxicity and doxorubicin-induced cardiotoxicity by a novel IH636 grape seed proanthocyanidin extract. Res Commun Mol Pathol Pharmacol. 2000;107(1–2):137–166. [PubMed] [Google Scholar]
- 103.Danz ED, Skramsted J, Henry N, Bennett JA, Keller RS. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic Biol Med. 2009;46(12):1589–1597. doi: 10.1016/j.freeradbiomed.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 104.Devika PT, Stanely Mainzen Prince P. (-)Epigallocatechingallate protects the mitochondria against the deleterious effects of lipids, calcium and adenosine triphosphate in isoproterenol induced myocardial infarcted male Wistar rats. J Appl Toxicol. 2008;28(8):938–944. doi: 10.1002/jat.1357. [DOI] [PubMed] [Google Scholar]
- 105.Devika PT, Stanely Mainzen Prince P. (-)Epigallocatechin-gallate (EGCG) prevents mitochondrial damage in isoproterenol-induced cardiac toxicity in albino Wistar rats: a transmission electron microscopic and in vitro study. Pharmacol Res. 2008;57(5):351–357. doi: 10.1016/j.phrs.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 106.Hrelia S, Bordoni A, Angeloni C, Leoncini E, Biagi P. Nutritional interventions to counteract oxidative stress in cardiac cells. Ital J Biochem. 2004;53(4):157–163. [PubMed] [Google Scholar]
- 107.Tatlidede E, Sehirli O, Velioglu-Ogunc A, et al. Resveratrol treatment protects against doxorubicin-induced cardiotoxicity by alleviating oxidative damage. Free Radic Res. 2009;43(3):195–205. doi: 10.1080/10715760802673008. [DOI] [PubMed] [Google Scholar]
- 108.Zhao XY, Li GY, Liu Y, et al. Resveratrol protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. Br J Pharmacol. 2008;154(1):105–113. doi: 10.1038/bjp.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Conklin KA. Coenzyme q10 for prevention of anthracycline-induced cardiotoxicity. Integr Cancer Ther. 2005;4(2):110–130. doi: 10.1177/1534735405276191. [DOI] [PubMed] [Google Scholar]
- 110.Selcoki Y, Uz E, Bayrak R, et al. The protective effect of erdosteine against cyclosporine A-induced cardiotoxicity in rats. Toxicology. 2007;239(1–2):53–59. doi: 10.1016/j.tox.2007.06.096. [DOI] [PubMed] [Google Scholar]
- 111.Aluigi MG, De Flora S, D’Agostini F, Albini A, Fassina G. Antiapoptotic and antigenotoxic effects of N-acetylcysteine in human cells of endothelial origin. Anticancer Res. 2000;20(5A):3183–3187. [PubMed] [Google Scholar]
- 112.Cai T, Fassina G, Morini M, et al. N-acetylcysteine inhibits endothelial cell invasion and angiogenesis. Lab Invest. 1999;79(9):1151–1159. [PubMed] [Google Scholar]
- 113.Dell’Eva R, Ambrosini C, Minghelli S, Noonan DM, Albini A, Ferrari N. The Akt inhibitor deguelin, is an angiopreventive agent also acting on the NF-kappaB pathway. Carcinogenesis. 2007;28(2):404–413. doi: 10.1093/carcin/bgl162. [DOI] [PubMed] [Google Scholar]
- 114.Albini A, Dell’Eva R, Vene R, et al. Mechanisms of the antiangiogenic activity by the hop flavonoid xanthohumol: NF-kappaB and Akt as targets. FASEB J. 2006;20(3):527–529. doi: 10.1096/fj.05-5128fje. [DOI] [PubMed] [Google Scholar]
- 115.Vannini N, Lorusso G, Cammarota R, et al. The synthetic oleanane triterpenoid, CDDO-methyl ester, is a potent antiangiogenic agent. Mol Cancer Ther. 2007;6(12, pt 1):3139–3146. doi: 10.1158/1535-7163.MCT-07-0451. [DOI] [PubMed] [Google Scholar]
- 116.Sartippour MR, Heber D, Henning S, et al. cDNA microarray analysis of endothelial cells in response to green tea reveals a suppressive phenotype. Int J Oncol. 2004;25(1):193–202. [PubMed] [Google Scholar]
- 117.Pfeffer U, Ferrari N, Dell’eva R, et al. Molecular mechanisms of action of angiopreventive anti-oxidants on endothelial cells: microarray gene expression analyses. Mutat Res. 2005;591(1–2):198–211. doi: 10.1016/j.mrfmmm.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 118.Vannini N, Pfeffer U, Lorusso G, Noonan DM, Albini A. Endothelial cell aging and apoptosis in prevention and disease: E-selectin expression and modulation as a model. Curr Pharm Des. 2008;14(3):221–225. doi: 10.2174/138161208783413248. [DOI] [PubMed] [Google Scholar]
- 119.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23(12):2840–2855. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 120.Harris RE, Beebe-Donk J, Alshafie GA. Reduction in the risk of human breast cancer by selective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer. 2006;6:27. doi: 10.1186/1471-2407-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Takkouche B, Regueira-Mendez C, Etminan M. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. J Natl Cancer Inst. 2008;100(20):1439–1447. doi: 10.1093/jnci/djn324. [DOI] [PubMed] [Google Scholar]
- 122.Bosetti C, Gallus S, La Vecchia C. Aspirin and cancer risk: an updated quantitative review to 2005. Cancer Causes Control. 2006;17(7):871–888. doi: 10.1007/s10552-006-0033-7. [DOI] [PubMed] [Google Scholar]
- 123.Mankoff S, Brander C, Ferrone S, Marincola F. Lost in translation: obstacles to translational medicine. J Transl Med. 2004;2(1):14. doi: 10.1186/1479-5876-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]