Abstract
Purpose
Toll-like receptor 2 (TLR2) is a transmembrane receptor that mediates immune responses to exogenous and endogenous ligands, and interacts with heat-shock proteins, which are reportedly involved in normal tension glaucoma (NTG). We investigated whether TLR2 polymorphisms are associated with NTG.
Methods
200 Japanese patients with NTG and 128 healthy Japanese controls were recruited. We genotyped five single-nucleotide polymorphisms (SNPs) in the TLR2 gene and assessed the allele and haplotype diversities between cases and controls for all SNPs.
Results
No significant differences in the frequency of TLR2 alleles and haplotypes in the NTG cases were detected, compared with the controls.
Conclusions
Our study showed no evidence for an association between TLR2 polymorphisms and NTG. TLR2 polymorphisms may not play an important role in NTG pathogenesis in the Japanese population.
Introduction
Normal tension glaucoma (NTG) is a type of progressive optic neuropathy. This neuropathy, combined with normal intraocular pressure, open iridocorneal angles, and no other evidence of disease, makes NTG an insidious disease. People who suffer from NTG often show no symptoms until the disease has progressed. If the disease is diagnosed before its advance in development, it can be successfully treated via medication, surgery, or laser treatment. Yet, because of this difficulty in diagnosis, it continues to be the greater cause of total blindness in people [1-3].
The difficulty in diagnosis can be partially attributed to NTG patients exhibiting intraocular pressure levels that are classified as normal when compared with the rest of the population. Even though it is thought that intra-ocular pressure (IOP) is attributable to NTG, in reality, it does not have as much involvement in NTG as does high-tension glaucoma [4]. Aside from IOP, the development and progression of NTG have carried several other risk factors, which include ischemia, genetic predisposition, refraction, and systemic illness [5-8].
In addition, despite NTG showing many signs of having a heritable nature, identifying the genes that cause NTG remains difficult. For example, 20 different loci have been linked to primary open-angle glaucoma, which is the most common type of glaucoma [9]. Yet, only two of them have been identified as disease-causing until recently. Because the hereditary forms of glaucoma are genetically heterogeneous, detecting the genes that are susceptible to glaucoma could aid in early diagnosis and subsequent treatment.
Toll-like receptor (TLR) proteins are a family of phylogenetically conserved receptors that recognize both endogenous and exogenous. They help with innate and adaptive immunity. TLRs have emerged as a major component of the immune system. Recognition of pathogen-associated molecular patterns by TLRs activates signaling events that induce the expression of effector molecules, such as cytokines and chemokines, which control the adaptive immune responses [10,11].
In addition, studies have found that TLR polymorphisms are associated with a risk of bacterial infections and/or various diseases [12-17]. Among the TLR family members, TLR2 and TLR4 are the most-characterized members, and these proteins recognize heat shock protein (HSP) and lipopolysaccharide, which were previously noted as potential candidates for NTG antigens [18-21]. Yet, although TLR4 recognizes both endogenous and exogenous, TLR2 only recognizes endogenous HSPs.
Other studies show a connection between abnormal immunity and NTG, which might show that NTG is a glaucomatous condition accelerated by deviant antibodies attacking retinal tissue and causing apoptosis, or the natural death of a cell. Increased immunoactivity to bacterial hsp60, a pathogen with a similar makeup to retinal tissue, was significantly elevated in the sera of patients with NTG [19]. In addition, in groups of American patients having glaucoma, monoclonal gammopathy [20], retinal immunoglobulin deposition [21], and elevated serum antibodies titering to retinal antigens [22] (bacterial and human HSP60, HSP27, and αB-crystallin [23]) have been prevalent.
Our previous analysis indicates that TLR4 polymorphisms have a relationship with abnormal immunity, and potentially with NTG [24]. Because TLR2 recognizes the HSPs, which are suggested to be the candidate antigen of NTG, we hypothesized that TLR2 polymorphisms may be associated with the risk of NTG. To test this hypothesis, we performed a single-nucleotide polymorphism (SNP) analysis of TLR2 in patients with NTG and healthy controls.
Methods
Subjects
The study population was comprised of a cohort of 200 unrelated Japanese patients with NTG, which included 106 women and 94 men. Their age range was 20–60 years, with a mean age of 47.3±13.9. All study patients were part of a group of 200 patients that had been previously clinically investigated at Yokohama City University, Yamanashi University, Gifu University, Kobe University, Yamaguchi University, Kumamoto University, Hokkaido University, Tokyo University, Niigata University, Kanazawa University, Hiroshima University, and Tajimi Municipal Hospital in Japan. Selected from this group for molecular genetic analysis were 200 patients who had been followed long-term. This was to ensure diagnosis of NTG, with a maximum of certainty. The criteria applied for the diagnosis of NTG were those proposed in our previous study [24]. The mean refraction value was −3.89±3.01 diopters (D), and the mean deviation observed in the Humphrey® static visual field analyzer (HFA) C-30–2 program (Carl Zeiss Meditec, Oberkochen, Germany) was −9.82±7.96 dB.
Control DNA samples were obtained from 128 unrelated subjects of Japanese descent who did not have a family history of glaucoma. Control individuals were of Japanese ethnicity, age-matched (mean age 60.5±7.3), and were collected from the same geographic region as the probands. A diagnosis of glaucoma was ruled out based on IOP measurements and ophthalmoscopy of the optic disc. Written informed consent was obtained from all participants. This study was approved by the ethics committee of the Yokohama City University School of Medicine and complied with the guidelines of the Declaration of Helsinki.
DNA preparation and TLR2 genotype identification
Peripheral blood lymphocytes were collected, and genomic DNA was extracted from peripheral blood cells using the QIAamp DNA Blood Maxi Kit (Qiagen, Valencia, CA). TLR2 comprises two exons and has two transcript isoforms (A and B). We evaluated five SNPs: rs1898830, rs11938228, rs3804099, rs3804100, and rs7656411. These SNPs are located within TLR2, including 6 kb of the predicted 3′ UTR, with minor allele frequencies >5%, according to the National Center for Biotechnology Information dbSNP and HapMap databases (Table 1). Genotyping of all SNPs was performed by TaqMan 5′ exonuclease assay using primers supplied by ABI (Applied Biosystems, Foster City, CA). The probe fluorescence signal was detected using the TaqMan Assay for Real-Time PCR (7500 Real Time PCR System; Applied Biosystems), following the manufacturer’s instructions.
Table 1. Allele frequencies of SNPs of TLR2 among NTG patients and controls.
| dbSNP | Alleles (1/2) | Position (bp) | Gene location |
Minor allele frequency, n (%) |
p | |
|---|---|---|---|---|---|---|
| Cases (n=200) | Controls (n=128) | |||||
|
rs1898830 |
A/G |
154,827,903 |
Intron |
193 (48.3) |
122 (47.7) |
0.881 |
|
rs11938228 |
C/A |
154,841,396 |
Intron |
187 (46.8) |
119 (46.5) |
0.947 |
|
rs3804099 |
T/C |
154,844,106 |
Exon |
124 (31.0) |
71 (27.7) |
0.372 |
|
rs3804100 |
T/C, |
154,844,859 |
Exon |
115 (28.8) |
63 (24.6) |
0.245 |
| rs7656411 | G/T | 154,847,105 | 3’UTR | 180 (45.0) | 108 (42.2) | 0.479 |
In the "Alleles" column, 1 indicates the major allele and 2 indicates the minor allele. Position is distance from short arm telomere. bp, base pairs. p-values were caluculated by χ2 test 2×2 contingency table.
Statistical analysis
The Hardy-Weinberg equilibrium was tested for each SNP among controls. Differences in allele frequency between case and control were assessed by χ2 test and Fisher’s exact test. The program Haploview 3.32 was used to compute pairwise linkage disequilibrium (LD) statistics [25]. Standardized disequilibrium (D’) was plotted. LD blocks were defined according to the criteria of Gabriel et al. [26]. Haplotype frequencies were estimated using an accelerated expectation-maximization algorithm similar to the partition-ligation-expectation-maximization method [27]. All p-values were derived from a 2-sided test, and p-values < 0.05 were considered statistically significant.
Results
Five SNPs in TLR2 were genotyped. Genotype distributions of all SNPs in controls exhibited Hardy-Weinberg equilibrium (data not shown), and the minor allele frequencies of all SNPs were over 5% in controls (Table 1). We determined that the gene region could be divided into two haplotype blocks, with substantial LD among the SNPs of both blocks (block 1: D’ ≥0.96; block 2: D’ ≥0.98; Figure 1A).
Figure 1.
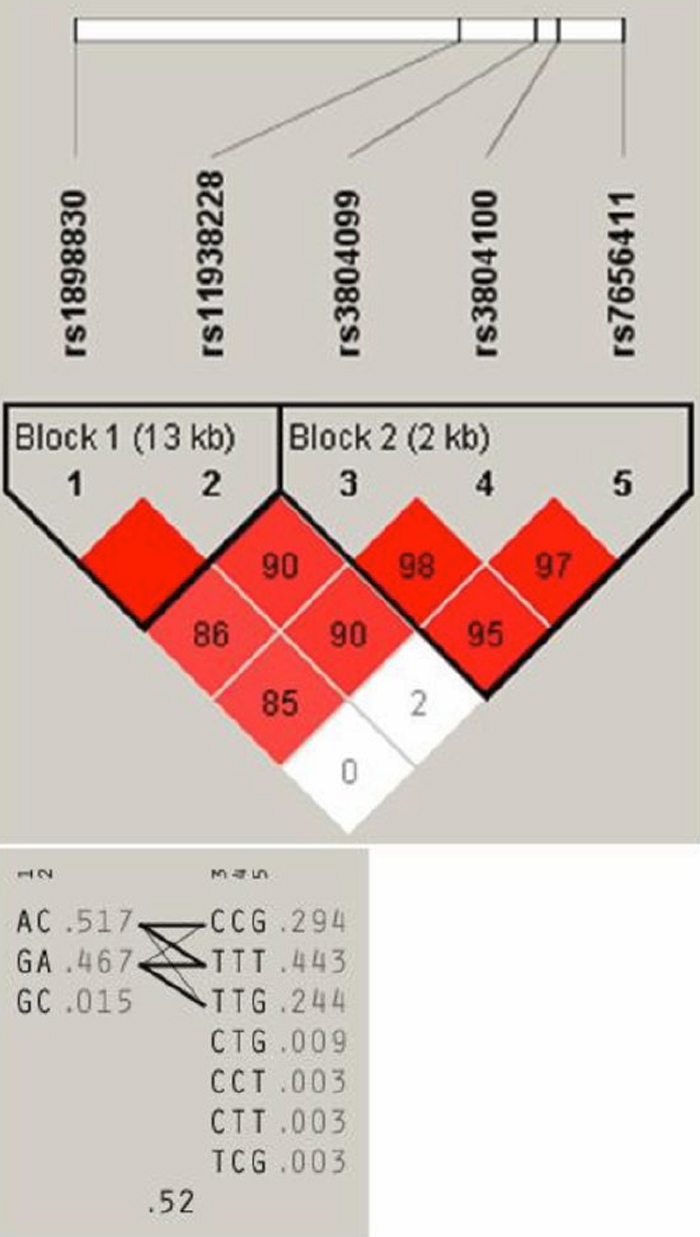
A: Linkage disequilibrium plot of five SNPs of the TLR2 gene in NTG patients and controls. A schematic of TLR2 is shown as a black line, with boxes representing its three exons. The locations of the selected SNPs are indicated by the dotted lines. The D’ value corresponding to each SNP pair is expressed as a percentage and shown within the respective square. Higher D’ values are indicated in brighter red. B: Haplotype structure and diversity of TLR2. Common haplotypes of the two blocks are listed. Haplotype frequencies observed in NTG patients and controls are given in parentheses. The line thickness reflects the frequency of adjacent block haplotype distribution (thick lines, >10%; thin lines, 1%–10%).
The allele frequencies of the five SNPs in cases and controls are listed in Table 1. No statistically significant association was observed for any of the SNPs between cases and controls (p>0.05). Haplotypes for each block and their frequencies are listed in Table 2. Two common haplotypes were observed within block 1, and three in block 2 (frequency >10%). We observed four common haplotypes of the five SNPs that extended across the two blocks (Figure 1B). As we observed, the frequencies of minor haplotypes in block 2 and two blocks, CTG and ACCTG, were decreased in cases, compared with the controls. However, this decrease did not reach statistical significance when evaluated using Bonferoni’s correction. No other significant differences in common haplotype frequencies between cases and controls were detected.
Table 2. Haplotype frequencies of tag SNPs of TLR2 among NTG patients and controls.
| Haplotype |
Frequency, % |
p | Pc | |
|---|---|---|---|---|
| Cases (n=200) | Controls (n=128) | |||
| Block 1 (tag SNPs rs1898830, rs11938228) | ||||
| AC |
51.8 |
52.3 |
0.88 |
|
| GA |
46.8 |
46.5 |
0.95 |
|
| GC |
1.5 |
1.2 |
0.72 |
|
| Block 2 (tag SNPs rs3804099, rs3804100, and rs7656411) | ||||
| TTT |
44.3 |
41.7 |
0.50 |
|
| CCG |
29.4 |
24.1 |
0.14 |
|
| TTG |
24.4 |
30.6 |
0.081 |
|
| CTG |
1.0 |
3.1 |
0.047 |
0.19 |
| Block 1+2 (tag SNPs rs1898830, rs11938228, rs3804099, rs3804100, and rs7656411) | ||||
| GATTG |
22.9 |
29.0 |
0.081 |
|
| ACCCG |
26.6 |
21.2 |
0.12 |
|
| ACTTT |
22.2 |
26.0 |
0.25 |
|
| GATTT |
22.1 |
15.7 |
0.043 |
0.34 |
| ACCTG |
1.0 |
3.1 |
0.044 |
0.35 |
| GACCG |
1.7 |
1.8 |
0.93 |
|
| ACTTG |
1.0 |
1.4 |
|
|
| GCCCG | 1.0 | 1.2 | 0.83 | |
Haplotypes with frequency less than 1% are not listed. p values were caluculated by χ2 test 2×2 contingency table. We corrected these p values (Pc) for multiple testing by Bonferoni's correction.
Discussion
The purpose of this study was to determine whether or not TLR2 polymorphisms are associated with the risk of NTG, based on recent findings of increased immunoactivity in TLR2, after learning that TLR4 has a relationship with NTG. Previously, we analyzed the relationship between TLR4 polymorphisms and the development of NTG [24]. This analysis infers that the polymorphisms of rs7037117, located in the 3′-untranslated lesion, have a strong association with the clinical characteristics of NTG. To compare these results with our previous results, we genotyped five single-nucleotide polymorphisms (SNPs) in TLR2, and assessed the allele and haplotype diversities between cases and controls for all SNPs. Here, we report a lack of association between TLR2 polymorphisms and NTG in Japanese patients, suggesting that the abnormal function found and TLR2 polymorphisms do not contribute to NTG, due to the results of the test comparing subjects and controls.
Recently, it has been suggested that the immune system and heat shock proteins (HSPs) play important roles in glaucoma [18]. HSPs are highly immunogenic molecules that are widely distributed in nature; they perform important functions relating to the folding and assembly of protein complexes. Human HSPs are expressed on cell membranes in response to stress, such as physiologic shock and microbial challenge. Wax and Tezel et al. [19] observed that NTG patients have increased serum immunoreactivity to bacterial and human HSP60. They also showed that direct application of antibodies to HSPs resulted in neuronal apoptosis, and NTG patients had higher titers of antibodies to HSPs, including HSP27 and HSP60, compared both to patients with high IOP and to healthy controls [23]. Furthermore, they observed increased expression of HSP27 and HSP60 in the glaucomatous retina and/or optic nerve head, and proposed that immune regulation of these HSPs is an important component of glaucomatous optic neuropathy [18].
In retrospect, TLR4 has the ability to distinguish between exogenous ligands and endogenous ligands. Many different exogenous and endogenous ligands bind to TLR4, thereby activating an innate immune response. For example, TLR4 can distinguish between the two different types of HSP60 (self and Chlamydia pneumoniae). Yet TLR2 cannot process the bacterial (foreign) form of HSP60. Bacterial HSP60 was shown to have sparked serum immunoreactivity in previous studies [19], and thus bears significance when introduced to TLR2 and TLR4. Yet, as was deduced from our study, there was not a significant relationship between TLR2 and NTG. Therefore, because TLR2 cannot recognize bacterial HSP60, perhaps the abnormal recognition of HSP60 by TLR4 is the beginning of normal autoimmunity in NTG. Studies to confirm this may prove essential to determining autoimmune effects in glaucoma optic neuropathy.
In NTG patients, due to the delayed ability to diagnose the progression of the disease, it was thought that identifying a susceptibility gene and elucidating pathogenic mechanisms was the best course of action to take. Through other studies, it has been concluded that polymorphisms in TLRs are associated with blunted immune responses to microbial pathogens. Our advanced analysis indicates that the common polymorphisms in TLR2 are not associated with NTG in Japanese patients. Though the possibility of TLR2 polymorphisms being primarily associated with the pathogenesis of NTG is deemed low, the TLR2 ligands, HSP, and lipopolysaccharide, may act as risk factors for NTG, and changes in cytokine secretion induced by the ligands may contribute to the induction of pathological immune reaction in NTG [28-33]. In conclusion, further studies are essential for a more detailed investigation of TLR signaling pathways related to the ligands in NTG patients.
Acknowledgments
This study was supported by grants-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan; a grant from the Ministry of Health, Labor, and Welfare, Japan; and a grant from the Johnson & Johnson KK Vision Care Company.
References
- 1.Hitchings RA, Anderson SA. A comparative study of visual field defects seen in patients with low-tension glaucoma and chronic simple glaucoma. Br J Ophthalmol. 1983;67:818–21. doi: 10.1136/bjo.67.12.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hitchings RA. Low-tension glaucoma – its place in modern glaucoma practice. Br J Ophthalmol. 1992;76:494–6. doi: 10.1136/bjo.76.8.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werner EB. Normal-tension glaucoma. In: Ritch R, Shields MB, Krupin T, editors. The Glaucomas. 2nd ed. St. Louis: Mosby; 1996. p. 769–97. [Google Scholar]
- 4.Quigley HA. Open-angle glaucoma. N Engl J Med. 1993;328:1097–106. doi: 10.1056/NEJM199304153281507. [DOI] [PubMed] [Google Scholar]
- 5.Broadway DC, Drance SM. Glaucoma and vasospasm. Br J Ophthalmol. 1998;82:862–70. doi: 10.1136/bjo.82.8.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontana L, Poinoosawmy D, Bunce CV, O'Brien C, Hitchings RA. Pulsatile ocular blood flow investigation in asymmetric normal tension glaucoma and normal subjects. Br J Ophthalmol. 1998;82:731–6. doi: 10.1136/bjo.82.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishida K, Yamamoto T, Kitazawa Y. Clinical factors associated with progression of normal-tension glaucoma. J Glaucoma. 1998;7:372–7. [PubMed] [Google Scholar]
- 8.Drance S, Anderson DR, Schulzer M.for theCollaborative Normal-Tension Glaucoma Study Group. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol 2001131699–708. [DOI] [PubMed] [Google Scholar]
- 9.Fan BJ, Wang DY, Lam DS, Pang CP. Gene mapping for primary open angle glaucoma. Clin Biochem. 2006;39:249–58. doi: 10.1016/j.clinbiochem.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 11.Andreakos E, Foxwell B, Feldmann M. Is targeting Toll-like receptors and their signaling pathway a useful therapeutic approach to modulating cytokine-driven inflammation? Immunol Rev. 2004;202:250–65. doi: 10.1111/j.0105-2896.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 12.Kang SSW, Kauls LS, Gaspari AA. Toll-like receptors: Applications to dermatologic disease. J Am Acad Dermatol. 2006;54:951–83. doi: 10.1016/j.jaad.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Lorenz E, Maria JP, Cornish KL, Arbour NC, Schwartz DA. A novel polymorphism in the toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect Immun. 2000;68:6398–401. doi: 10.1128/iai.68.11.6398-6401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang TJ, Chae GT. Detection of Toll-like receptor 2 (TLR2) mutations in the lepromatous leprosy patients. FEMS Immunol Med Microbiol. 2001;31:53–8. doi: 10.1111/j.1574-695X.2001.tb01586.x. [DOI] [PubMed] [Google Scholar]
- 15.Park Y, Park S, Yoo E, Kim D, Shin H. Association of the polymorphism for Toll-like receptor 2 with type 1 diabetes susceptibility. Ann N Y Acad Sci. 2004;1037:170–4. doi: 10.1196/annals.1337.028. [DOI] [PubMed] [Google Scholar]
- 16.Boraska Jelavic T, Barisic M, Drmic Hofman I, Boraska V, Vrdoljak E, Peruzovic M, Hozo I, Puljiz Z, Terzić J. Microsatellite GT polymorphism in the Toll-like receptor 2 is associated with colorectal cancer. Clin Genet. 2006;70:156–60. doi: 10.1111/j.1399-0004.2006.00651.x. [DOI] [PubMed] [Google Scholar]
- 17.Tabel Y, Berdeli A, Mir S. Association of TLR2 gene Arg753Gln polymorphism with urinary tract infection in children. Int J Immunogenet. 2007;34:399–405. doi: 10.1111/j.1744-313X.2007.00709.x. [DOI] [PubMed] [Google Scholar]
- 18.Tezel G, Hernandez R, Wax MB. Immunostaining of heat shock proteins in the retina and optic nerve head of normal and glaucomatous eyes. Arch Ophthalmol. 2000;118:511–8. doi: 10.1001/archopht.118.4.511. [DOI] [PubMed] [Google Scholar]
- 19.Wax MB, Tezel G, Saito I, Gupta RS, Harley JB, Li Z, Romano C. Anti-Ro/SS-A positivity and heat shock protein antibodies in patients with normal-pressure glaucoma. Am J Ophthalmol. 1998;125:145–57. doi: 10.1016/s0002-9394(99)80084-1. [DOI] [PubMed] [Google Scholar]
- 20.Wax MB. Barrett and A. Pestronk. Increased incidence of paraproteinemia and autoantibodies in patients with normal-pressure glaucoma. Am J Ophthalmol. 1994;117:561–8. doi: 10.1016/s0002-9394(14)70059-5. [DOI] [PubMed] [Google Scholar]
- 21.Wax MB, Tezel G, Edward PD. Clinical and histopathological findings of a patient with normal pressure glaucoma. Arch Ophthalmol. 1998;116:993–1001. doi: 10.1001/archopht.116.8.993. [DOI] [PubMed] [Google Scholar]
- 22.Romano C, Barrett DA, Li Z, Pestronk A, Wax MB. Anti-Rhodopsin antibodies in sera from patients with normal-pressure gluaucoma. Invest Ophthalmol Vis Sci. 1995;36:1968–75. [PubMed] [Google Scholar]
- 23.Tezel G, Seigel GM, Wax MB. Autoantibodies to small heat shock proteins in glaucoma. Invest Ophthalmol Vis Sci. 1998;39:2277–87. [PubMed] [Google Scholar]
- 24.Shibuya E, Meguro A, Ota M, Kashiwagi K, Mabuchi F, Iijima H, Kawase K, Yamamoto T, Nakamura M, Negi A, Sagara T, Nishida T, Inatani M, Tanihara H, Aihara M, Araie M, Fukuchi T, Abe H, Higashide T, Sugiyama K, Kanamoto T, Kiuchi Y, Iwase A, Ohno S, Inoko H, Mizuki N. Association of Toll-like receptor 4 gene polymorphisms with normal tension glaucoma. Invest Ophthalmol Vis Sci. 2008;49:4453–7. doi: 10.1167/iovs.07-1575. [DOI] [PubMed] [Google Scholar]
- 25.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 26.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 27.Qin ZS, Niu T, Liu J. Partition-ligation-expectation-maximization algorithm for haplotype inference with single-nucleotide polymorphisms. Am J Hum Genet. 2002;71:1242–7. doi: 10.1086/344207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoneda S, Tanihara H, Kido N, Honda Y, Goto W, Hara H, Miyawaki N. Interleukin-1β mediates ischemic injury in the rat retina. Exp Eye Res. 2001;73:661–7. doi: 10.1006/exer.2001.1072. [DOI] [PubMed] [Google Scholar]
- 29.Wang CY, Shen YC, Lo FY, Su CH, Lee SH, Tsai HY, Fan SS. Normal tension glaucoma is not associated with the interleukin-1α (−889) genetic polymorphism. J Glaucoma. 2007;16:230–3. doi: 10.1097/IJG.0b013e3180300818. [DOI] [PubMed] [Google Scholar]
- 30.Wang CY, Shen YC, Su CH, Lo FY, Lee SH, Tsai HY, Fan SS. Investigation of the association between interleukin-1β polymorphism and normal tension glaucoma. Mol Vis. 2007;13:719–23. [PMC free article] [PubMed] [Google Scholar]
- 31.How ACS, Aung T, Chew X, Yong VH, Lim MC, Lee KY, Toh JY, Li Y, Liu J, Vithana EN. Lack of association between interleukin-1 genes cluster polymorphisms and glaucoma in Chinese subjects. Invest Ophthalmol Vis Sci. 2007;48:2123–6. doi: 10.1167/iovs.06-1213. [DOI] [PubMed] [Google Scholar]
- 32.Wang CY, Shen YC, Lo FY, Su CH, Lee SH, Lin KH, Tsai HY, Kuo NW, Fan SS. Polymorphism in the IL-1α (−889) locus associated with elevated risk of primary open angle glaucoma. Mol Vis. 2006;12:1380–5. [PubMed] [Google Scholar]
- 33.Baudouin C, Hamard P, Liang H, Creuzot-Garcher C, Bensoussan L, Brignole F. Conjunctival epithelial cell expression of interleukins and inflammatory markers in glaucoma patients treated over the long term. Ophthalmology. 2004;111:2186–92. doi: 10.1016/j.ophtha.2004.06.023. [DOI] [PubMed] [Google Scholar]


