Abstract
Purpose
To investigate the efficacies of different concentrations of N-acetylcysteine (NAC) in preventing hyperoxia-induced lens opacification and changes to biochemical parameters in organ-cultured rabbit lenses.
Methods
Thirty-six lenses from adult rabbits were divided into the control group (group A), the hyperoxia-exposed group (group B), and the hyperoxia-exposed, NAC-treated groups: 5 mM NAC (group C), 10 mM NAC (group D), 20 mM NAC (group E), and 40 mM NAC (group F). Groups B–F were incubated with hyperoxia (pO2>80%) for 4 h per day for 7 d. Lens transparency, histology, and enzymatic activities were measured after incubation.
Results
Gross examination of these lenses revealed some severe cortical opacification in group B, and moderate cortical opacification in the lenses of groups C and D. There was minimal cortical opacification in groups A, E, and F. The activities of Na, K-ATPase, and catalase were significantly (p<0.05) lower in group B (38.2%) than in group A (39.9%). It was also lower in group E and F lenses (p<0.05), which had higher levels of NAC-protected enzymes. The glutathione and water-soluble protein content were significantly lower in group B lenses than in group A, E, or F lenses (p<0.05). However, there was no difference between group E and F lenses (p>0.05).
Conclusions
The present data suggests that NAC (20 mM–40 mM) significantly prevented experimental lenses’ hyperoxia-induced cortical opacification, indicating NAC’s potential role in protecting lenses against cataracts induced by high oxygen levels.
Introduction
Cataract is a progressive opacity of the lens of the eye that impairs vision. It is the leading cause of human blindness worldwide, accounting for more than half of all blindness. The prevalence of cataract in developing countries is much greater than in developed ones [1]. Although the causative factors of cataract are many, and its etiology is still unclear, it has been linked to oxidation [2]. It plays a major role, not only in age-related cataract, but also in cataract after vitrectomy and hyperbaric oxygen (HBO) therapy [3-5]. Changes in the lens under hyperbaric oxygen have been used as a model for cataract research [6]. We investigated the damaging effects of hyperoxia on lens transparency and biochemistry, and the possible protective effect of N-acetylcysteine (NAC) against this damage.
The cornea and retina are highly oxygenated. However, the partial pressure of O2 in the center of the lens is normally low, approximately 10 mm Hg, or 1% [7], due in part to metal-catalyzed reactions of the molecule with glutathione (GSH) [8]. Sakaue et al. [9] measured vitreous oxygen tension in the human vitreous body using a paleographic oxygen electrode. The mean oxygen tensions of the anterior peripheral vitreous body, central vitreous body, and posterior vitreous body were 16.7±3.7 mm Hg, 15.9±2.8 mm Hg, and 19.9±4.8 mm Hg, respectively, while the preretinal oxygen tension of the detached retina was 30.0±4.8 mm Hg. The mean oxygen tension around the lens was almost equal to that of the posterior vitreous body. Much of the O2 that enters the anterior side of the lens from the aqueous humor is consumed by mitochondria located in the lens’s anterior epithelium [10,11]. During oxidative phosphorylation, some electron leakage to oxygen occurs, forming superoxide. Mitochondrial production of superoxide increases with age. Superoxide may diffuse throughout the lens, that is, via the lens membranes. This process could contribute to elevated hydrogen peroxide (H2O2) in the nuclei of old lenses. Hydrogen peroxide appears to contribute to lens damage, and H2O2 alone is not sufficient. H2O2 itself is not a strong oxidant; however, the hydroxyl radicals it produces in the presence of ferrous ions, through the Fenton reaction, are highly reactive, and can damage proteins, lipids, and nucleic acids, as well as small molecules [12-15]. It also induces expression of the proto-oncogenes (c-jun, c-fos, and c-myc in rabbit-lens epithelial cells), which may regulate lens crystallin genes and other genes containing activating protein-1 (AP-1) binding sites [16,17]. Elimination of hydrogen peroxide is critical in reducing oxidative stress. Glutathione peroxidase and catalase serve this purpose.
GSH plays a key role in the protection against oxidative stress, which is quantitatively the most important endogenous rechargeable antioxidant, and which functions as an essential antioxidant for maintaining the tissue’s transparency [18]. GSH is synthesized in the cortex, and intercepts those reactive intermediates. If H2O2 is formed, GSH will be oxidized to GSSG. In the presence of protein sulfhydryl (-SH) groups, GSSG can cause protein thiolation [14]. However, glutathione reductase (GR) reduces GSSG to GSH with electrons donated from the reduced form of nicotinamide-adenine dinucleotide phosphate (NADPH), allowing GSH to protect protein thiols from oxidative damage, and preventing protein cross-link formation [19].
Catalase is a key enzyme that plays a role in intracellular lens defense mechanisms. It converts H2O2 to H2O to reduce oxidative damage [14], and the catalase (CAT) of eye tissues regulates the endogenous H2O2 in eye humors at the physiologic level, which also protects the cation pump [15,20]. Na, K-ATPase is responsible for maintaining normal physiologic concentrations of sodium and potassium in lens cells, and for maintaining the ionic balance of the cells [21,22]. Decreasing both the GSH content and the catalase and Na, K-ATPase activities may increase oxygen-associated cellular damage, as well as the rate of cataract formation. In the present study, we examined GSH content and the activities of Na, K-ATPase and catalase in lenses exposed to hyperoxia, both with and without various concentrations of NAC in the culture medium.
Since GSH is not effectively transported into cells, NAC, a precursor of cysteine, and thus of GSH, has been used effectively to replenish intracellular GSH stores directly and conveniently [23]. It is an excellent source of sulfhydryl (-SH) groups, and is converted in the body into metabolites capable of stimulating GSH synthesis. Jain’s study showed that NAC supplementation might be helpful in slowing the oxidation of rabbit-lens proteins in vitro [24]. Shattuck et al. [25] also concluded that NAC prevents oxidant stress during hyperoxic exposure, most likely by supplying cysteine as a precursor for GSH synthesis. Our previous study found that NAC eyedrops could prevent streptozotocin-induced diabetic cataract in rats [26]. Injection of NAC into the vitreous cavity protected the lens against some biochemical changes induced by the increased oxygen after vitrectomy [27]. Rathbun et al. [28] showed that L-cysteine prodrugs were effective in preventing naphthalene-induced cataract, and in maintaining near-normal hepatic GSH levels. In addition, Li et al. [16,17] have reported that NAC could upregulate the mRNAs of both c-jun and c-fos, and enhance the transactivity of activating protein-1 (AP-1). Therefore, it is possible that NAC may have protective effects against lens damage by hyperoxia. The purpose of the present study was to investigate the efficacies of different concentrations of NAC in preventing the changes caused by hyperoxia to rabbit lenses under organ-culture conditions.
Methods
Materials
N-acetylcysteine (NAC) was purchased from Sigma Chemical Company (Beijing, China). Rabbits were provided by the Animal Laboratory of the Fourth Military Medical University (Xi’an, China). Protein and enzyme quantification kits were obtained from Jiancheng Biology Company (Nanjing, China). All other chemicals and solvents were analytic grade and obtained commercially from local companies (Xi'an, China).
Experimental groups
New Zealand rabbits (1.5–2.0 kg, 4 months old) were killed by an overdose of sodium pentobarbital. The lenses (whose weights were approximately 300 mg each) were removed from eyes that had been excised by a posterior approach. The lenses were at once immersed in Dulbecco’s Modified Eagle Medium (Sigma, St. Louis, MO), which contained calf serum (10%), streptomycin (5×104 U/l), and penicillin (5×104 U/l), to prevent microbial contamination.
All the lenses were examined after 8 h pre-experimental incubation. Only the intact clear lenses were chosen for experimentation, and those with any lens defects were rejected. In the experiments, each lens was cultured in 5 ml of medium having a liquid-gas interphase surface area of 3.6 cm2. Prior to culture, the medium was sterilized by passage through a 0.2 μm filter [6]. The selected clear lenses were divided into six groups:
A: Control group: six lenses were incubated in the medium with an atmosphere of 95% room air and 5% CO2.
B: Hyperoxia exposed group: six lenses were incubated in the medium and treated daily with oxygen (>80%) at 1.0 atm (atmospheric pressure) for 4 h.
C: 5 mM NAC-treated group: six lenses were incubated in the medium containing 5 mM NAC and treated daily with oxygen (>80%) at 1 atm for 4 h.
D: 10 mM NAC-treated group: six lenses were incubated in the medium containing 10 mM NAC and treated daily with oxygen (>80%) at 1 atm for 4 h.
E: 20 mM NAC-treated group: six lenses were incubated in the medium containing 20 mM NAC and treated daily with oxygen (>80%) at 1 atm for 4 h.
F: 40 mM NAC-treated group: six lenses were incubated in the medium containing 40 mM NAC and treated daily with oxygen (>80%) at 1 atm for 4 h.
All lenses were subjected to gross morphological examination daily. In addition, after the 7 d incubation period, quantitative analyses of enzyme activities were performed for the lenses of all groups. In a preliminary experiment, five different concentrations of NAC (5, 10, 20, 40, and 100 mM) were chosen to examine the side effects of NAC, by itself, on lenses.
Hyperoxia treatment
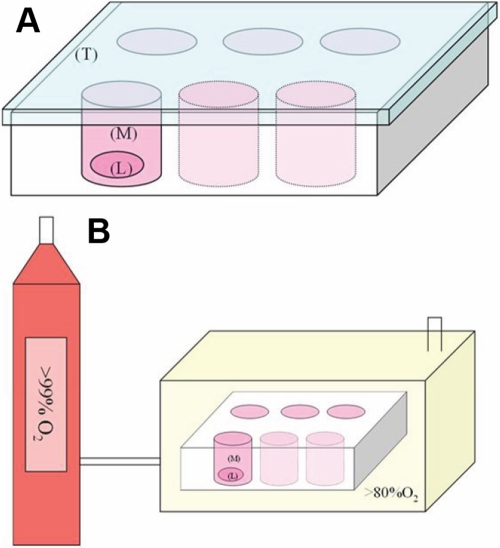
The cultured lenses were exposed to hyperoxia in a sealed chamber (Figure 1). The temperature inside the chamber was maintained at 37 °C by keeping the vessel in a temperature-controlled water bath. The concentration of oxygen was raised to 80% over a 5 min period and then maintained for 4 h daily. The pressure in the chamber was kept at 1.0 atm. The culture medium was changed daily before the hyperoxia test, and all the work was completed under a superclean bench to prevent microbial contamination. During the test, oxygen saturation inside the sealed chamber was monitored by an oxygen meter (CY-12C; Hangzhou, China) and kept constant.
Figure 1.
Cultured lenses exposed to hyperoxia in a sealed chamber. A: Lens culture chamber scheme: Lens (L) is totally immersed in culture medium (M). The chamber is placed vertically, with a covered top (T). B: Sealed chamber scheme: The cylinder supplied oxygen (99%) into the sealed chamber containing the culture chambers without a covered top, and the oxygen saturation inside the sealed chamber was kept above 80%.
Examination of the lenses
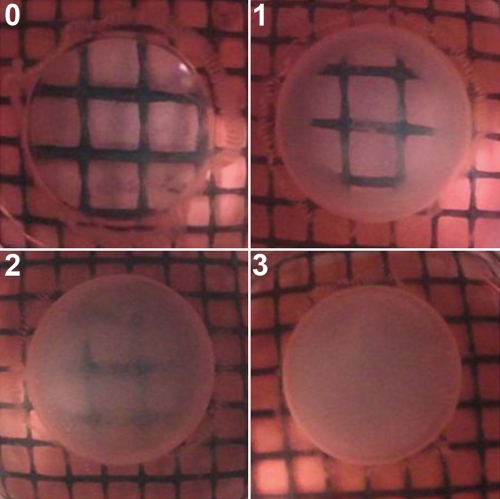
Lenses were examined by eye, as well as under the magnification of a dissecting microscope against a background of black gridlines. The degree of opacification was assessed according to Geraldine et al. [29], described as follows: grade 0, absence of opacification (gridlines clearly visible); grade 1, slight degree of opacification (minimal clouding of gridlines and gridlines still visible); grade 2, diffuse opacification involving almost the entire lens (moderate clouding of gridlines and gridlines faintly visible); grade 3, extensive thick opacification involving the entire lens (total clouding of gridlines and gridlines not seen at all; Figure 2). A double-blind method was used for classifying the degree of lens opacification.
Figure 2.
Standard photographs to illustrate the system for grading lenses. Grade 0 means the absence of opacification and the gridlines clearly visible; grade 1 means slight degree of opacification, the gridlines under the lens are mininal clouding and still visible; grade 3 means extensive thick opacification involving in the entire lens, the gridlines are total clouding and not seen at all.
Protein determination
Protein concentration was determined by the Coomassie brilliant blue method by using a protein assay kit. Each lens was ground in 0.9% physiologic saline (1 g:9 ml), homogenized using a handheld homogenizer for 15 min over ice, and then centrifuged (3,000 rpm, 10 min) in Eppendorf tubes. The clear supernatant was used for water-soluble protein determination, according to kit instructions (Jiancheng, China).
Glutathione determination
The content of GSH in each lens was determined with 5–5′ dithio-bis-(2-nitrobenzoic acid), following centrifugation (3,500 r/min) in 2 ml of 10% trichloroacetic acid for 10 min, and measured using the colorimetric method at 25 °C and at 412 nm [30].
Enzyme activities
Assay of Na, K-ATPase activity
Na, K-ATPase activity was measured by adding 0.1 ml of the clear supernatant of homogenate to 0.6 ml buffer containing 100 mM NaCl, 20 mM KCl, 5 mM MgCl2, 3 mM ATP, and 50 mM Tris (pH 7.4). After 15 min preincubation at 37 °C, ATP was used as the substrate. The liberated inorganic phosphate was estimated by spectrophotometric estimation [31]. Ouabain was used as a specific blocker of Na, K-ATPase activity. The ouabain-sensitive ATPase activity was estimated and expressed as micromoles of inorganic phosphate released per mg protein per h.
Assay of catalase activity
CAT activity was determined by the method of Beers and Sizer [32] with modification in 1952, by spectrophotometric recording of the cleavage of H2O2 at 240 nm. The reaction mixture contained 0.5 ml of 23 mM H2O2 in 1.0 ml of 0.05 M phosphate buffer (pH 7.0). The amount of H2O2 was far in excess of that of GSH. For this reason, GSH peroxide activity (for which GSH is an obligatory substrate) was assumed to be negligible, compared to catalase (whose only substrate is H2O2). The activity of catalase was expressed as units/mg protein (one unit was the amount of enzyme that used 1 mM of H2O2/min).
Assay of glutathione reductase activity
Glutathione reductase (GR) activity was measured according to the procedure of Bergmeyer in his book in 1963 [33]. The reaction was initiated by the addition of 20 μl lens homogenate. Oxidized GSH (glutathione disulfide [GSSG]), was reduced to GSH catalyzed by GR, with NADPH as the cofactor. One unit was equivalent to the oxidation of 1 mM of NADPH per min.
Statistical analysis
One-way analysis of variance (ANOVA) was used for testing statistical significances among groups. The median calculation of the lens opacity for each group was analyzed by using the Wilcoxon rank sum test. A p value of <0.05 was considered significant. All the data were analyzed using the SPSS 13.0 statistical package.
Results
Grading of the lenses
In a preliminary experiment, the pH value in the 100 mM NAC medium was decreased to approximately 5 after 24 h incubation under normal oxygen conditions, and the lenses became opaque. However, lenses in the other four groups with NAC (5, 10, 20, and 40 mM) remained clear, and the pH value did not change significantly (data not shown). Therefore, we chose these four concentrations of NAC for the current study and the medium was changed daily before the hyperoxia insult.
In the present study, except for two lenses in group B, no lenses had detectable opacification under examination by dissecting microscope after 3 d. The median lens opacity is presented in Table 1. However, on the fourth day, cortical opacification of lenses was observed in group B. More than 50% of these lenses showed slight cortical opacification, whereas lenses in other groups remained clear (p<0.05; Table 1). After 7 d, all six lenses in group B exhibited total cortical opacification (Grade 3). In contrast, only a few lenses revealed lens opacification in groups C and D, with minimal opacification in groups A, E, and F (p<0.01; Figure 3). These observations indicate that NAC delayed the progression of hyperoxia-induced cortical opacification of lenses.
Table 1. Intensity of opacification in the isolated rabbit lenses (n=36, M).
| Group |
Degree of opacification (0–3) |
||||||
|---|---|---|---|---|---|---|---|
| 1 day | 2 d | 3 d | 4 d | 5 d | 6 d | 7 d | |
| A |
0 |
0 |
0 |
0 |
1 |
1 |
1 |
| B |
0 |
0 |
0 |
1* |
2* |
3** |
3** |
| C |
0 |
0 |
0 |
0 |
1 |
1 |
1 |
| D |
0 |
0 |
0 |
0 |
1 |
1 |
1 |
| E |
0 |
0 |
0 |
0# |
1# |
1## |
1## |
| F | 0 | 0 | 0 | 0# | 1# | 1## | 1## |
Values are expressed as medians, Median representation: 0, absence of opacification (gridlines clearly visible); 1, slight degree of opacification (minimal clouding of gridlines and gridlines still visible); 2, diffuse opacification involving almost the entire lens (moderate clouding of gridlines and gridlines faintly visible); 3, extensive thick opacification involving the entire lens (total clouding of gridlines and gridlines not seen at all). Statistical analyses were performed by using Wilcoxon rank sum test. The asterisk indicates a p<0.05 B versus A, the double asterisk indicates a p<0.01 B versus A, the sharp (hash mark) indicates a p<0.05 B versus E and F, and the double sharp indicates a p<0.01 B versus E and F. Group A was the control group, group B was the hyperoxia-exposed rabbit lenses group, group C-F were the hyperoxia-exposed, NAC-treated groups: 5 mM NAC (group C), 10 mM NAC (group D), 20 mM NAC (group E), and 40 mM NAC (group F).
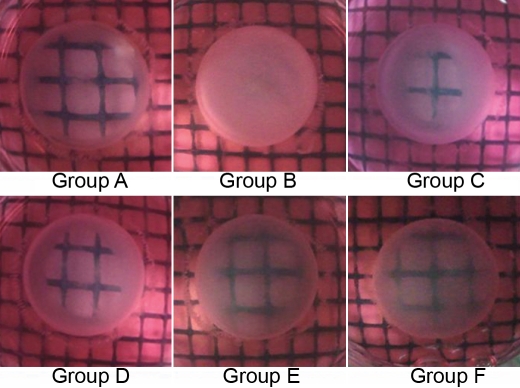
Figure 3.
Lenses’ optical clarity after 7 d incubation. Lenses in the control group (A), and those in the 5 mM and 10 mM NAC-treated groups (C and D), were in grade 1 with a slight degree of opacification (minimal clouding of gridlines and gridlines still visible). Lenses in group B were totally opaque (grade 3, gridlines not seen at all). Most lenses in 20 mM and 40 mM NAC-treated groups were kept transparent (grade 0). Group A was the control group, group B was the hyperoxia-exposed rabbit lenses group, groups C-F were the hyperoxia-exposed, NAC-treated groups: 5 mM NAC (group C), 10 mM NAC (group D), 20 mM NAC (group E), and 40 mM NAC (group F)
Protein determination
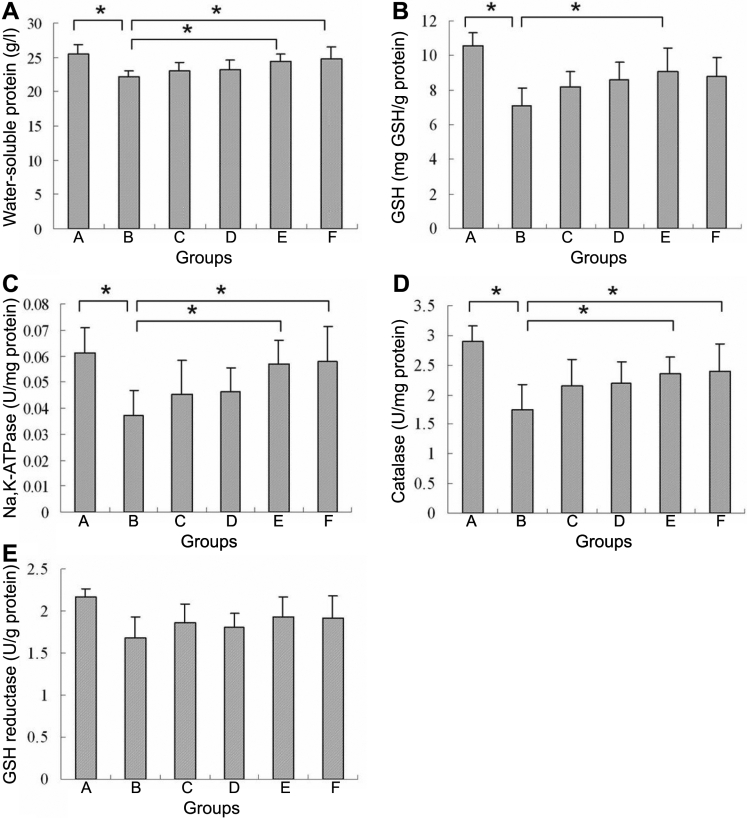
There was a significant reduction of water-soluble protein content in the lenses of group B, compared to that in group A lenses (by 13.1%; p<0.05 Figure 4A). No statistically significant difference was found between the control group and the NAC-treated groups. The four NAC-treated groups maintained their levels of water-soluble protein. Compared with the lenses in group B, the level of water-soluble protein was significantly greater in the 20 mM NAC-treated group (by 10.6%; p<0.05) and in the 40 mM NAC-treated group (by 11.9%; p<0.05).
Figure 4.
Effects of hyperoxia in rabbit lenses after 7 d incubation compared with controls and the NAC treatment. A: The water-soluble protein in rabbit lenses (mean±SD). Lenses exposed to hyperoxia (group B) had a significant reduction in the water-soluble protein (*p<0.05). Compared with the hyperoxia-exposed group (group B), the level of water-soluble protein was increased dramatically in the 20 mM and 40 mM NAC-treated groups (*p<0.05). No significant differences were observed between any other two groups. B: The effect of NAC on GSH content of rabbit lenses (mean±SD). Lenses exposed to hyperoxia (group B) had a significant reduction of GSH (*p<0.05). A statistically significant difference was observed between the hyperoxia group (group B) and the 20 mM NAC-treated group (group E; *p<0.05). No significant differences were observed between any other two groups. C: The effect of NAC on the activity of Na, K-ATPase in rabbit lenses (mean±SD). Lenses exposed to hyperoxia (group B) had a significant reduction of Na, K-ATPase activity (*p<0.05). Compared with the hyperoxia-exposed group (group B), the activity of Na, K-ATPase was increased dramatically in the 20 mM and 40 mM NAC-treated groups (*p<0.05), but did not fully restore it to the normal level. No significant differences were observed between any other two groups. D: The effect of NAC on the activity of CAT in rabbit lenses (mean±SD). Lenses exposed to hyperoxia (group B) had a significant reduction of CAT activity (*p<0.05); Compared with the hyperoxia-exposed group (group B), the activity of CAT was increased dramatically in the 20 mM and 40 mM NAC-treated groups (*p<0.05). No significant differences were observed between any other two groups. E: The effect of NAC on the activity of GR in rabbit lenses (mean±SD). No significant difference was noted between groups. Group A was the control group, group B was the hyperoxia-exposed rabbit lenses group, group C-F were the hyperoxia-exposed, NAC-treated groups: 5 mM NAC (group C), 10 mM NAC (group D), 20 mM NAC (group E), and 40 mM NAC (group F).
Glutathione determination
Hyperoxia lowered the level of GSH significantly (for group B, compared to the control group) by 33% (p<0.05). NAC, 20 mM NAC, and 40 mM NAC, seemed to protect GSH levels by 28.2% (p<0.05) and 23.9% respectively (Figure 4B), but no significant difference was found between the 20 mM NAC- and the 40 mM NAC-treated groups.
The activity of enzymes
The activity of Na, K-ATPase was significantly lowered (38.2%) by hyperoxia (comparing group B to group A; p<0.05). The enzymatic activity (Figure 4C) showed that hyperoxia damaged the activity of Na, K-ATPase in the lens. The reduction of Na, K-ATPase activities by hyperoxia was prevented by NAC. The Na, K-ATPase activity was protected in groups E and F (p<0.05; Figure 4C). However, there was no significant difference between groups E and F.
The results for catalase activity in the lenses after incubation are shown in Figure 4D. The activity of CAT (about 39.9% [p<0.05]) was significantly lower in hyperoxia-exposed lenses than in the control lenses. The treatment by NAC resulted in a partial protection of CAT activity, by 34.7% (p<0.05) in group E, and by 37.5% (p<0.05) in group F.
The activity of GR was decreased by 18% in the lenses of group B, and was higher in the four NAC-treated groups, compared to the untreated group, about 8.0% medially. However, there were no statistically significant differences (Figure 4E).
Discussion
The lens is avascular, and depends on diffusion for its oxygen supply. The vitreous humor impedes the flow of oxygen from the retina, and therefore normally keeps the concentration of O2 at the surface of the lens low. With age, liquefaction of the human vitreous humor may damage the oxygen tension gradient in the vitreous cavity, permitting increased migration of O2 from the retinal circulation through the vitreous space to the posterior side of the lens. In addition, loss of protein sulfhydryl groups, oxidation of methionine residues, and damage to enzymatic antioxidant defense systems in the lens possibly accelerate the formation of nuclear cataract [34]. A study by Harocopos et al. [35] showed that the correlation between the extent of nuclear opacification and the percent liquefaction was highly significant, after adjusting for age. Age was also significantly associated with nuclear opacification. Oxidative damage to lens proteins and lipids is a hallmark of age-related cataract. Therefore, vitreous degeneration in the eye may contribute to increased oxidative stress and formation of nuclear cataract.
High oxygen tension after vitrectomy is also associated with a high risk of progressive nuclear sclerosis [3,34]. Clinical studies have reported that lens opacities progress in 41%–80% of operated eyes, after removal of idiopathic epiretinal membranes using vitrectomy [36,37]. In elderly individuals undergoing vitrectomy surgery, 60% to 98% will develop clinically significant nuclear sclerosis within two years [38]. Holekamp et al. also found that vitrectomy surgery increased intraocular oxygen tension during, and for prolonged periods after, surgery. This exposes the crystalline lens to abnormally high oxygen, and may lead to nuclear cataract formation [3]. These observations may provide an insight into the development of nuclear cataract after vitrectomy.
Additional evidence to link O2 with the formation of nuclear cataract comes from the therapeutic treatment of patients with hyperbaric oxygen (HBO). One study showed that of 15 patients who had clear lens nuclei before exposure to hyperbaric oxygen treatment, seven patients later showed development of nuclear cataract that decreased visual acuity; others showed increased nuclear light scattering [4]. Similar results regarding the detrimental effects of hyperbaric oxygen on the lens nucleus have also been reported in animal models. In Schocket’s [39] study, in which mice were treated with HBO, one half of the surviving animals had nuclear cataracts within eight months. Therefore, hyperoxia has been linked to the development of human nuclear cataract. As in the present study, these studies found no evidence of nuclear opacities after milder hyperoxia treatment. This may have been due to the short duration and normal oxygen pressure used for incubation. The present study only showed the lens changes induced by hyperoxia at an earlier stage.
The past clinical findings just described have led some laboratories to investigate the impact of antioxygen agents on the transparency or optical quality of the lenses of experimental animals [40]. In the present study, we investigated the preventive and ameliorating effects of NAC on lenses exposed to hyperoxia, as reflected by the activities of two major enzymes. One is catalase, a key enzyme that neutralizes formation of reactive oxygen species. The other is Na, K-ATPase, which is responsible for the ionic balance of the cells. In addition, the contents of GSH and the water-soluble protein were changed in this study. GSH is the most important endogenous rechargeable antioxidant. The content of water-soluble protein indicates lens damage by hyperoxia: the greater the damage to the lens, the lower the content of water-soluble protein. Based on that observation, we conclude that the observed lens opacification in the present study was the result of the high oxygen and oxidative load. The level of GSH was decreased in lenses exposed to hyperoxia, and it increased in NAC-treated groups; 20 mM NAC appeared to protect the thiols (from GSH) in four treated groups, compared to the control group. That means NAC corrected the reduction in GSH concentration.
A reduction in CAT activity in the hyperoxia-exposed group was observed. Hydrogen peroxide is one of the molecules responsible for oxidative injury to the lens. CAT is a key enzyme against relatively high concentrations of peroxide [41]. The lenses with NAC showed lower damage from catalase, which suggests that NAC results in significant protection of the activities of catalase.
ATPase activity was clearly lower in the lenses exposed to hyperoxia without NAC, when compared to the control lenses. The extracellular and intracellular cation balance is the result of permeability properties of the lens cell membranes, and of the activity of the Na, K-ATPase pump. Inhibition of Na, K-ATPase results in loss of cation balance and elevation of water content in the lens [42]. A previous study suggested that membrane permeability is increased with cataract development, and that oxidative stress can damage membrane permeability and stability [43]. Therefore, the enzyme activities decreased in the hyperoxia-exposed group. NAC has mucolytic and anticollagenolytic properties: it can keep the cell membrane and ionic pump stable [23]. The oxygen levels in this study may seem high, being four or five times the normal atmospheric level, but they are much lower than has been used in hyperbaric oxygen experiments. Furthermore, there is the usual problem: that we have to study changes within a reasonable time in the laboratory, whereas cataract in vivo takes years to form. Even after vitrectomy, it takes months.
In conclusion, oxygen plays a key role in cataract formation. It is also believed to be one of the potential causative agents for the development of nuclear cataract, following vitrectomy, and after HBO treatment [3,6,7]. The present study’s results are the first report on a possible role for NAC in the prevention of hyperoxia-induced damage to rabbit lenses. It appears that NAC attenuates oxidative lenticular damage and the protective effects of NAC against oxidative damage through its favorable effect on GSH, catalase, and Na, K-ATPase activities. The observed antioxidant protective effects of NAC could result from intra-lenticular action. Given our current state of knowledge, we are unable to specify whether this constriction has the same effects. The results of the present investigation suggest that NAC is able to significantly retard experimental hyperoxia-induced lens damage.
Acknowledgments
We are grateful to the National Natural Science Foundation of China (No. 30872837) support for Prof. H. Yan and the Faculty Fund from the Fourth Military Medical University, Xi’an, China. Many Thanks to Dr. Ying-Bo Shui of Washington University for many helpful suggestions and constructive comments about the manuscript.
References
- 1.Brian G, Taylor H. Cataract blindness: challenges for the 21st century. Bull World Health Organ. 2001;79:249–56. [PMC free article] [PubMed] [Google Scholar]
- 2.Spector A, Garner WH. Hydrogen peroxide and human cataract. Exp Eye Res. 1981;33:673–81. doi: 10.1016/s0014-4835(81)80107-8. [DOI] [PubMed] [Google Scholar]
- 3.Simpanya MF, Ansari RR, Suh KI, Leverenz VR, Giblin FJ. Aggregation of lens crystallins in an in vivo hyperbaric oxygen guinea pig model of nuclear cataract: dynamic light-scattering and HPLC analysis. Invest Ophthalmol Vis Sci. 2005;46:4641–51. doi: 10.1167/iovs.05-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmquist BM, Philipson B, Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol. 1984;68:113–7. doi: 10.1136/bjo.68.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80:709–25. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Giblin FJ, Schrimscher L, Chakrapani B, Reddy VN. Exposure of rabbit lens to hyperbaric oxygen in vitro: regional effects on GSH level. Invest Ophthalmol Vis Sci. 1988;29:1312–9. [PubMed] [Google Scholar]
- 7.Barbazetto IA, Liang J, Chang S, Zheng L, Spector A, Dillon JP. Oxygen tension in the rabbit lens and vitreous before and after vitrectomy. Exp Eye Res. 2004;78:917–24. doi: 10.1016/j.exer.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Giblin FJ. Glutathione: a vital lens antioxidant. J Ocul Pharmacol Ther. 2000;16:121–35. doi: 10.1089/jop.2000.16.121. [DOI] [PubMed] [Google Scholar]
- 9.Sakaue H, Negi A, Honda Y. Comparative study of vitreous oxygen tension in human and rabbit eye. Invest Ophthalmol Vis Sci. 1989;30:1933–7. [PubMed] [Google Scholar]
- 10.McNulty R, Wang H, Mathias RT, Ortwerth BJ, Truscott RJ, Bassnett S. Regulation of tissue oxygen levels in the mammalian lens. J Physiol. 2004;559:883–98. doi: 10.1113/jphysiol.2004.068619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spector A, Ma W, Wang RR. The aqueous humor is capable of generating and degrading H2O2. Invest Ophthalmol Vis Sci. 1998;39:1188–97. [PubMed] [Google Scholar]
- 12.McGahan MC, Harned J, Grimes AM, Fleisher LN. Regulation of ferritin levels in cultured lens epithelial cells. Exp Eye Res. 1994;59:551–5. doi: 10.1006/exer.1994.1140. [DOI] [PubMed] [Google Scholar]
- 13.Moffat BA, Landman KA, Truscott RJ, Sweeney MH, Pope JM. Age-related changes in the kinetics of water transport in normal human lenses. Exp Eye Res. 1999;69:663–9. doi: 10.1006/exer.1999.0747. [DOI] [PubMed] [Google Scholar]
- 14.Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995;9:1173–82. [PubMed] [Google Scholar]
- 15.Varma SD, Kumar S, Richards RD. Light-induced damage to ocular lens cation pump: prevention by vitamin C. Proc Natl Acad Sci USA. 1979;76:3504–6. doi: 10.1073/pnas.76.7.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li WC, Wang GM, Wang RR, Spector A. The redox active components H2O2 and N-acetyl-L-cysteine regulate expression of c-jun and c-fos in lens systems. Exp Eye Res. 1994;59:179–90. doi: 10.1006/exer.1994.1096. [DOI] [PubMed] [Google Scholar]
- 17.Li DW, Spector A. Hydrogen peroxide-induced expression of the proto-oncogenes, c-jun, c-fos and c-myc in rabbit lens epithelial cells. Mol Cell Biochem. 1997;173:59–69. doi: 10.1023/a:1006828402225. [DOI] [PubMed] [Google Scholar]
- 18.Lou MF. Redox regulation in the lens. Prog Retin Eye Res. 2003;22:657–82. doi: 10.1016/s1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- 19.Ganea E, Harding JJ. Glutathione-related enzymes and the eye. Curr Eye Res. 2006;31:1–11. doi: 10.1080/02713680500477347. [DOI] [PubMed] [Google Scholar]
- 20.Bhuyan KC, Bhuyan DK. Regulation of hydrogen peroxide in eye humors. Effect of 3-amino-1H–1,2,4-triazole on catalase and glutathione peroxidase of rabbit eye. Biochim Biophys Acta. 1977;497:641–51. doi: 10.1016/0304-4165(77)90284-7. [DOI] [PubMed] [Google Scholar]
- 21.Delamere NA, Tamiya S. Expression, regulation and function of Na, K-ATPase in the lens. Prog Retin Eye Res. 2004;23:593–615. doi: 10.1016/j.preteyeres.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Paterson CA, Delamere NA. ATPases and lens ion balance. Exp Eye Res. 2004;78:699–703. doi: 10.1016/j.exer.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev. 1998;3:114–27. [PubMed] [Google Scholar]
- 24.Jain AK, Lim G, Langford M, Jain SK. Effect of high-glucose levels on protein oxidation in cultured lens cells, and in crystallin and albumin solution and its inhibition by vitamin B6 and N-acetylcysteine: its possible relevance to cataract.formation in diabetes. Free Radic Biol Med. 2002;33:1615–21. doi: 10.1016/s0891-5849(02)01109-7. [DOI] [PubMed] [Google Scholar]
- 25.Shattuck KE, Rassin DK, Grinnell CD. N-acetylcysteine protects from glutathione depletion in rats exposed to hyperoxia. JPEN J Parenter Enteral Nutr. 1998;22:228–33. doi: 10.1177/0148607198022004228. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Chai FY, Yan H, Guo Y, Harding JJ. Effects of N-acetylcysteine and glutathione ethylester drops on streptozotocin-induced diabetic cataract in rats. Mol Vis. 2008;14:862–70. [PMC free article] [PubMed] [Google Scholar]
- 27.Liu XC, Wang P, Yan H. A rabbit model to study biochemical damage to the lens after virectomy: effects of N-acetylcysteine. Exp Eye Res. 2009;88:1165–70. doi: 10.1016/j.exer.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Rathbun WB, Nagasawa HT, Killen CE. Prevention of naphthalene-induced cataract and hepatic glutathione loss by the L-cysteine prodrugs, MTCA and PTCA. Exp Eye Res. 1996;62:433–41. doi: 10.1006/exer.1996.0048. [DOI] [PubMed] [Google Scholar]
- 29.Geraldine P, Sneha BB, Elanchezhian R, Ramesh E, Kalavathy CM, Kaliamurthy J, Thomas PA. Prevention of selenite-induced cataractogenesis by acetyl-L-carnitine: an experimental study. Exp Eye Res. 2006;83:1340–9. doi: 10.1016/j.exer.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Reddy VN, Varma SD, Chakrapani B. Transport and metabolism of glutathione in the lens. Exp Eye Res. 1973;16:105–14. doi: 10.1016/0014-4835(73)90305-9. [DOI] [PubMed] [Google Scholar]
- 31.Secchi GC, Ambrosi L, Rezzonico A. Studies on Na, K-ATPase and the acetylcholinesterase activity in erythrocyte membranes in saturnine anemia. Med Lav. 1968;59:593–8. [PubMed] [Google Scholar]
- 32.Beers RF, Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–40. [PubMed] [Google Scholar]
- 33.Bergmeyer HU. Methods of Enzymatic Analysis. New York: Academic Press 1963; 1064. [Google Scholar]
- 34.Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139:302–10. doi: 10.1016/j.ajo.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 35.Harocopos GJ, Shui YB, McKinnon M, Holekamp NM, Gordon MO, Beebe DC. Importance of vitreous liquefaction in age-related cataract. Invest Ophthalmol Vis Sci. 2004;45:77–85. doi: 10.1167/iovs.03-0820. [DOI] [PubMed] [Google Scholar]
- 36.Cherfan GM, Michels RG, de Bustros S, Enger C, Glaser BM. Nuclear sclerotic cataract after vitrectomy for idiopathic epiretinal membranes causing macular pucker. Am J Ophthalmol. 1991;111:434–8. doi: 10.1016/s0002-9394(14)72377-3. [DOI] [PubMed] [Google Scholar]
- 37.Smiddy WE, Michels RG, Glaser BM, deBustros S. Vitrectomy for macular traction caused by incomplete vitreous separation. Arch Ophthalmol. 1988;106:624–8. doi: 10.1001/archopht.1988.01060130678025. [DOI] [PubMed] [Google Scholar]
- 38.Cheng L, Azen SP, El-Bradey MH, Scholz BM, Chaidhawangul S, Toyoguchi M, Freeman WR. Duration of vitrectomy and postoperative cataract in the vitrectomy for macular hole study. Am J Ophthalmol. 2001;132:881–7. doi: 10.1016/s0002-9394(01)01263-6. [DOI] [PubMed] [Google Scholar]
- 39.Schocket SS, Esterson J, Bradford B, Michaelis M, Richards RD. Induction of cataracts in mice by exposure to oxygen. Isr J Med Sci. 1972;8:1596–601. [PubMed] [Google Scholar]
- 40.Schaal S, Beiran I, Bormusov E, Chevion M, Dovrat A. Zinc-desferrioxamine reduces damage to lenses exposed to hyperbaric oxygen and has an ameliorative effect on catalase and Na, K-ATPase activities. Exp Eye Res. 2007;84:455–63. doi: 10.1016/j.exer.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 41.Ma W, Li D, Sun F, Kleiman NJ, Spector A. The effect of stress withdrawal on gene expression and certain biochemical and cell biological properties of peroxide-conditioned cell lines. FASEB J. 2004;18:480–8. doi: 10.1096/fj.03-0732com. [DOI] [PubMed] [Google Scholar]
- 42.Huang WH, Wang Y, Askari A. Na, K-ATPase: inactivation and degradation induced by oxygen radicals. Int J Biochem. 1992;24:621–6. doi: 10.1016/0020-711x(92)90337-z. [DOI] [PubMed] [Google Scholar]
- 43.Yan H, Harding JJ, Xing K, Lou MF. Revival of glutathione reductase in human cataractous and clear lens extracts by thioredoxin and thioredoxin ductase, in conjunction with α-crystallin or thioltransferase. Curr Eye Res. 2007;32:455–63. doi: 10.1080/02713680701257837. [DOI] [PubMed] [Google Scholar]