Abstract
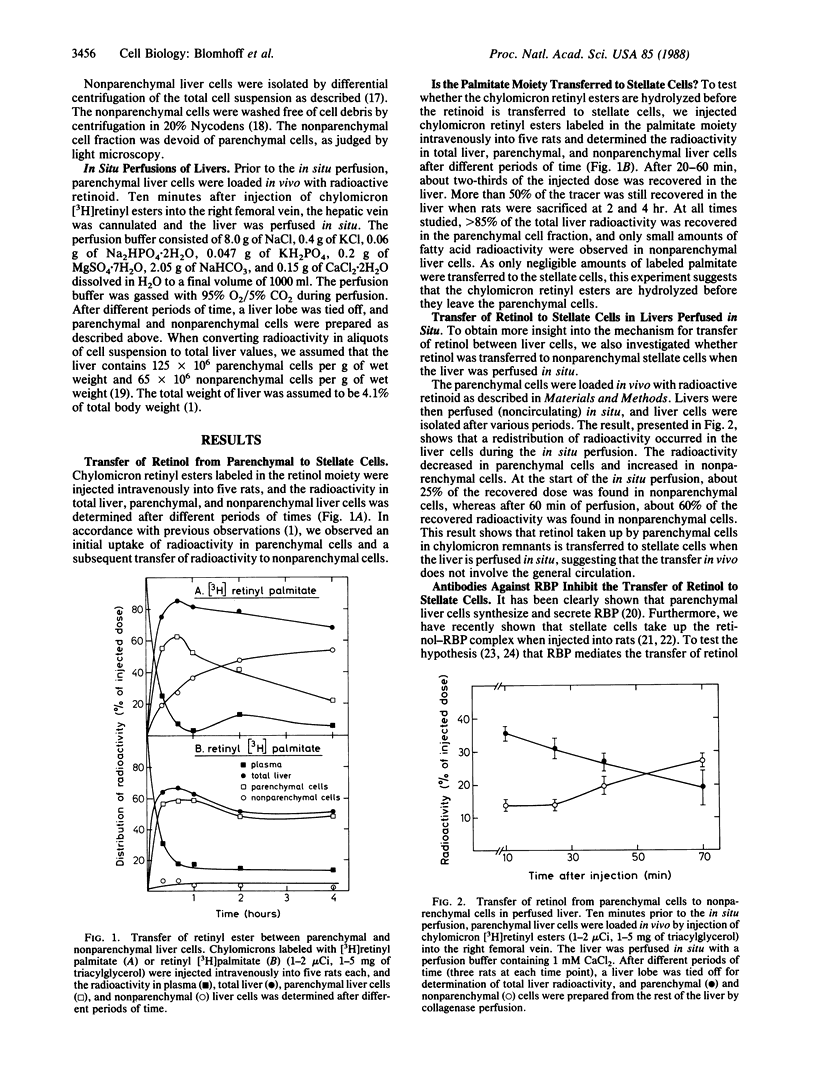
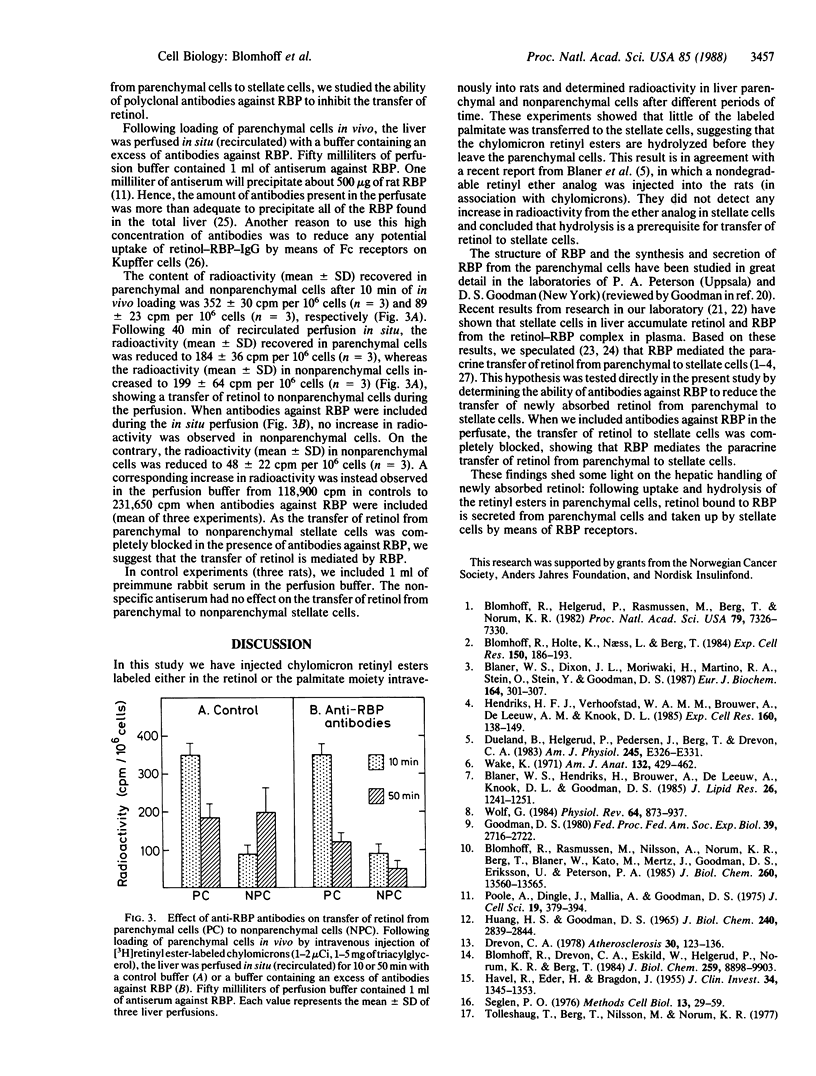
Newly absorbed chylomicron remnant retinyl ester is endocytosed by parenchymal liver cells, and retinol is subsequently transferred to perisinusoidal stellate cells in liver. In the present study we have used several approaches to elucidate the mechanism for the paracrine transfer of retinol between liver parenchymal and stellate cells. In one series of experiments, chylomicrons labeled with [3H]retinyl palmitate or with retinyl [3H]palmitate were injected intravenously into rats. It was shown that the retinol as well as the palmitate moiety were initially taken up in parenchymal liver cells. However, only the retinol moiety was detected in stellate cells, indicating that the retinyl ester is hydrolyzed before retinol is transferred to stellate cells. It is well known that parenchymal liver cells secrete retinol bound to retinol-binding protein (RBP), and we have recently found that stellate cells do have RBP receptors. Here we report that antibodies against RBP completely block the transfer of retinol from parenchymal to stellate cells. These findings indicate that following uptake of chylomicron remnant retinyl ester in parenchymal cells, the retinyl ester is hydrolyzed, and retinol secreted from parenchymal cells on RBP is taken up by stellate cells by means of RBP receptors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaner W. S., Dixon J. L., Moriwaki H., Martino R. A., Stein O., Stein Y., Goodman D. S. Studies on the in vivo transfer of retinoids from parenchymal to stellate cells in rat liver. Eur J Biochem. 1987 Apr 15;164(2):301–307. doi: 10.1111/j.1432-1033.1987.tb11058.x. [DOI] [PubMed] [Google Scholar]
- Blaner W. S., Hendriks H. F., Brouwer A., de Leeuw A. M., Knook D. L., Goodman D. S. Retinoids, retinoid-binding proteins, and retinyl palmitate hydrolase distributions in different types of rat liver cells. J Lipid Res. 1985 Oct;26(10):1241–1251. [PubMed] [Google Scholar]
- Blomhoff R., Drevon C. A., Eskild W., Helgerud P., Norum K. R., Berg T. Clearance of acetyl low density lipoprotein by rat liver endothelial cells. Implications for hepatic cholesterol metabolism. J Biol Chem. 1984 Jul 25;259(14):8898–8903. [PubMed] [Google Scholar]
- Blomhoff R., Helgerud P., Rasmussen M., Berg T., Norum K. R. In vivo uptake of chylomicron [3H]retinyl ester by rat liver: evidence for retinol transfer from parenchymal to nonparenchymal cells. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7326–7330. doi: 10.1073/pnas.79.23.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomhoff R. Hepatic retinol metabolism: role of the various cell types. Nutr Rev. 1987 Sep;45(9):257–263. doi: 10.1111/j.1753-4887.1987.tb02696.x. [DOI] [PubMed] [Google Scholar]
- Blomhoff R., Holte K., Naess L., Berg T. Newly administered [3H]retinol is transferred from hepatocytes to stellate cells in liver for storage. Exp Cell Res. 1984 Jan;150(1):186–193. doi: 10.1016/0014-4827(84)90713-4. [DOI] [PubMed] [Google Scholar]
- Blomhoff R., Norum K. R., Berg T. Hepatic uptake of [3H]retinol bound to the serum retinol binding protein involves both parenchymal and perisinusoidal stellate cells. J Biol Chem. 1985 Nov 5;260(25):13571–13575. [PubMed] [Google Scholar]
- Blomhoff R., Rasmussen M., Nilsson A., Norum K. R., Berg T., Blaner W. S., Kato M., Mertz J. R., Goodman D. S., Eriksson U. Hepatic retinol metabolism. Distribution of retinoids, enzymes, and binding proteins in isolated rat liver cells. J Biol Chem. 1985 Nov 5;260(25):13560–13565. [PubMed] [Google Scholar]
- Blomhoff R., Smedsrød B., Eskild W., Granum P. E., Berg T. Preparation of isolated liver endothelial cells and Kupffer cells in high yield by means of an enterotoxin. Exp Cell Res. 1984 Jan;150(1):194–204. doi: 10.1016/0014-4827(84)90714-6. [DOI] [PubMed] [Google Scholar]
- Drevon C. A. Cholesteryl ester metabolism in fat- and cholesterol/fat-fed guinea pigs. Atherosclerosis. 1978 Jun;30(2):123–136. doi: 10.1016/0021-9150(78)90055-2. [DOI] [PubMed] [Google Scholar]
- Dueland S., Helgerud P., Pedersen J. I., Berg T., Drevon C. A. Plasma clearance, transfer, and distribution of vitamin D3 from intestinal lymph. Am J Physiol. 1983 Oct;245(4):E326–E331. doi: 10.1152/ajpendo.1983.245.4.E326. [DOI] [PubMed] [Google Scholar]
- Gjøen T., Bjerkelund T., Blomhoff H. K., Norum K. R., Berg T., Blomhoff R. Liver takes up retinol-binding protein from plasma. J Biol Chem. 1987 Aug 15;262(23):10926–10930. [PubMed] [Google Scholar]
- Goodman D. S. Vitamin A metabolism. Fed Proc. 1980 Aug;39(10):2716–2722. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG H. S., GOODMAN D. S. VITAMIN A AND CAROTENOIDS. I. INTESTINAL ABSORPTION AND METABOLISM OF 14C-LABELLED VITAMIN A ALCOHOL AND BETA-CAROTENE IN THE RAT. J Biol Chem. 1965 Jul;240:2839–2844. [PubMed] [Google Scholar]
- Hendriks H. F., Verhoofstad W. A., Brouwer A., de Leeuw A. M., Knook D. L. Perisinusoidal fat-storing cells are the main vitamin A storage sites in rat liver. Exp Cell Res. 1985 Sep;160(1):138–149. doi: 10.1016/0014-4827(85)90243-5. [DOI] [PubMed] [Google Scholar]
- Mallia A. K., Smith J. E., Goodman D. W. Metabolism of retinol-binding protein and vitamin A during hypervitaminosis A in the rat. J Lipid Res. 1975 May;16(3):180–188. [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Berg T., Seljelid R. Distribution of lysosomal enzymes in different types of rat liver cells. Exp Cell Res. 1976 Apr;99(1):146–154. doi: 10.1016/0014-4827(76)90689-3. [DOI] [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Kaplan G., Seljelid R. On the mechanism of internalization of opsonized particles by rat Kupffer cells in vitro. Exp Cell Res. 1976 Nov;103(1):201–212. doi: 10.1016/0014-4827(76)90256-1. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Dingle J. T., Mallia A. K., Goodman D. S. The localization of retinol-binding protein in rat liver by immunofluorescence microscopy. J Cell Sci. 1975 Nov;19(2):379–394. doi: 10.1242/jcs.19.2.379. [DOI] [PubMed] [Google Scholar]
- Redgrave T. G., Vakakis N. Hepatic vitamin A fat-storage cells and the metabolism of chylomicron cholesterol. Aust J Exp Biol Med Sci. 1976 Dec;54(6):519–525. doi: 10.1038/icb.1976.53. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Wake K. "Sternzellen" in the liver: perisinusoidal cells with special reference to storage of vitamin A. Am J Anat. 1971 Dec;132(4):429–462. doi: 10.1002/aja.1001320404. [DOI] [PubMed] [Google Scholar]
- Wolf G. Multiple functions of vitamin A. Physiol Rev. 1984 Jul;64(3):873–937. doi: 10.1152/physrev.1984.64.3.873. [DOI] [PubMed] [Google Scholar]



